Abstract
Neurocysticercosis, an endemic parasitic disease in most developing countries, is caused by Taenia solium and compromises the human central nervous system. Cathepsin L-like proteases are secreted by several parasites including T. solium and constitute important antigens for immunodiagnostics. A protein fraction with cathepsin L-like activity was purified from the cysticercus fluid by size exclusion and ion exchange chromatography. Cathepsin L-like activity was measured fluorometrically by detecting the hydrolysis of the fluorogenic substrate Z-Phe-Arg-AMC. The purified protein fraction included antigens of 53 and 25 kD that were tested in a Western immunoblot and in an enzyme-linked immunosorbent assay (ELISA) for detection of human cysticercosis. The sensitivity of the Western immunoblot was 96% for patients infected with multiple cysts and 78% for patients with a single cyst. Specificity was 98%. The sensitivity of the ELISA was 98% in patients with multiple cysts and 84% in patients with a single cyst. Specificity was 92.7%.
INTRODUCTION
Taeniasis/cysticercosis caused by the cestode Taenia solium is an important public health problem in developing countries, where spread of the tapeworm persists in communities with poor sanitation.1 This condition has been recognized as an increasing cause of human disease,2 where its major complication, neurocysticercosis (NCC), is an important cause of morbidity,3 accounting for more cases of epilepsy than any other pathology in these areas.4
The standard diagnosis of NCC requires expensive imaging methods such as computed-tomography or magnetic resonance, which are difficult to access in disease-endemic areas. The highly sensitive and specific enzyme-linked immunotransfer blot (EITB) is the immunodiagnostic test of choice for confirming a clinical and radiologic presumptive diagnosis of NCC.5 The EITB is the Centers for Disease Control and Prevention (CDC) Western immunoblot for cysticercosis. This assay is based on detection of antibody to one or more of the seven lentil-lectin glycoproteins (LLGPs) of the T. solium cysticercus. This test is nearly 100% specific and is highly sensitive in cases of cysticercosis with more than one cyst.
The enzyme-linked immunosorbent assay (ELISA) format is simpler to perform in most laboratories and is less costly than the EITB. The ELISA is a quantitative test that can be used in high throughput studies and is more objective than the EITB because it does not rely on individual interpretation to determine a positive case of disease.
In recent years, several ELISAs have been evaluated for their efficacy in diagnosing cysticercosis. Over time, the antigenic proteins used in these tests have become more sophisticated; they have evolved from crude cysticercus fluid, to purified fractions, to recombinant proteins and synthetic peptides.6-15 Most parasites secrete proteases to facilitate tissue penetration and infection. Cathepsin L is such a protease that is secreted by several parasites, and is an important immunodiagnostic antigen for the trematode Fasciola hepatica.16-21 In a previous report, we demonstrated that cathepsin L is secreted by T. solium oncospheres and is a relatively abundant antigen among serine and cysteine proteases in the cyst fluid.22
In the present study, we purified a 53/25-kD protein fraction with cathepsin L-like activity from cysticercus fluid and then evaluated its utility as an immunodiagnostic antigen for human cysticercosis in Western immunoblot and ELISA.
MATERIALS AND METHODS
Cysticercus fluid
Eight pigs naturally infected with cysticercosis were selected from a disease-endemic area in the central highlands of Peru and were confirmed by tongue test.23 Selected pigs had 6–7 positive bands to the standard CDC Western immunoblot test. The carcasses were dissected and T. solium cysts were identified. Without extracting the cystic erci, the cyst fluid content was recovered by aspiration with a syringe, carefully avoiding contamination with the animal blood. Approximately 400 mL of cyst fluid was recovered from 4,000 viable cysts and stored at −70°C.
Protein purification
Ethanol precipitation and concentration
Particulate material was removed from the cyst fluid by centrifugation (10,397 × g for 30 minutes at 4°C). Cold absolute ethanol (−20°C), was added to the supernatant (30% [v/v]), and this was mixed with an inhibitor cocktail (0.01 mM trans-epoxysuccinyl-L-leucylamido-(4-guanidino)butane [E64], 0.1 mM chymostatin, 0.1 mM leupeptin, 1 μM bestatin, 0.1 mM tosylphenylalanylchloromethane, 0.1 mM antipain, 1 mM 4-[2-aminoethyl] benzenesulfonyl fluoride, 1.5 mM pepstatin, 10 mM EDTA) to prevent protein degradation. After incubation for 20 minutes at −20°C, precipitated proteins were eliminated by centrifugation (10,397 × g for 20 minutes at 4°C). The supernatant was concentrated to one-fifth of its original volume by using an Amicon system with a 10-kD (YM-10) membrane (Millipore, Billerica, MA). The final protein concentration of four different batches was 6–8 mg/mL.
Size exclusion chromatography
The concentrated batches of supernatant from the ethanol precipitation (500 μL) were passed through a column packed with G75 Sephadex (120 mL, 75 cm length), equilibrated with citrate buffer (0.1 M, pH 4, 0.1 mM HgCl2) at 4°C. Buffer at 4°C (300 mL) was passed at a flow rate of 0.2 mL/minute and fractions of 1 mL were collected. Fractions were analyzed for cathepsin L-like activity using the fluorometric assay described below. The fraction with the highest activity was selected for ion exchange chromatography.
Ion exchange chromatography
The selected fraction (1 mL) was loaded into a 15-mL column packed with SP Sephadex, equilibrated with citrate buffer (50 mM, pH 4, 0.1 mM HgCl2) at 4°C. After washing (30 mL, 50 mM citrate pH 4, 0.1 mM HgCl2), protein fractions were eluted with 20 mL of a NaCl gradient (0–2 M) in the same citrate buffer at a flow rate of 0.2 mL/minute. The fraction showing the highest cathepsin L-like activity was selected for being tested as an immunodiagnostic antigen for cysticercosis.
Fluorometric assay for cathepsin L-like activity
Cathespin L-like activity of cyst supernatant protein fractions was detected by the hydrolysis of the peptide substrate coupled to the fluorogen 7-amino-4-methylcoumarin (AMC), Z-Phe-Arg-AMC (MP Biomedicals, Aurora, OH).24 The hydrolysis reaction was performed in 100 mM citrate buffer, pH 5, with 5 mM dithiothreitol (DTT) to reduce trypsin-like interference. Urea at a final concentration of 4 M was included in the reaction buffer to reduce cathepsin B-like interference.22,25 Reaction buffer (92 mL), purified T. solium cyst protein (4 μL), and the fluorogenic substrate Z-Phe-Arg-AMC (4 μL, 5 mM), were incubated at 37°C in a 0.2-mL tube and followed for 4 hours and 24 hours.22,25 The AMC fluorogen released after the cleavage of the peptide substrate was measured by fluorescence in a TD-700 fluorometer (Turner Designs, Sunnyvale, CA) as the excess of absolute fluorescence units above the blank reaction with buffer replacing the purified protein. Excitation was performed at 360 nm and emission was detected at 460 nm using a 70-μL microcuvette. The supernatant protein fraction with the highest cathepsin L-like activity was selected for testing as an immunodiagnostic antigen for cysticercosis.
Cathepsin L-like protein identification
Protein sizing
An alternate purification was as described above but in the absence of protease inhibitors except HgCl2. Fractions with cathepsin L-like activity purified from 10 runs were pooled and concentrated to approximately 1 mg/mL, and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (12% acrylamide gel, 1.2 μg protein/5-mm well). To determine the location of the proteins on the gel, one lane was excised and stained with silver nitrate. Resolved proteins in the matrix of the unstained gel that co-migrated with the stained proteins were excised according to molecular weight, minced, and tested for cathepsin L-like activity in the fluorometric assay. The control reaction for the cathepsin L-like activity was performed in the fluorometric assay using a similar amount of minced gel corresponding to one lane free of common-stainable protein.
Gelatin zymography
To confirm the proteolytic activity and to estimate the molecular weight of the protease associated, the ion exchange chromatography cathepsin L-like purified antigen was tested in a gelatin zymography.26 The purified antigen was resolved by electrophoresis on an 11% polyacrylamide gel co-polymerized with 0.12 mg/mL of gelatin (buffer 25 mM, Tris, pH 7.2, 190 mM glycine, 0.1% SDS). After electrophoresis, the gel was washed in 2.5% Triton X for 1 hour, rinsed twice in 100 mM citrate buffer, pH 5.0, and incubated at 37°C for approximately 48 hours in 100 mM citrate buffer, pH 5, 5 mM DTT. The gelatinolytic activity was revealed as clear bands against a blue background by a single step stain-destain method using 0.02% Coomassie blue at an acetic acid: methanol: water ratio of 1:3:6.
Inhibition assays
To confirm the specific cathepsin L-like activity, inhibition with 10 μM E64 and 100 μM chymostatin was tested. Cathepsin L-like activity is inhibited by E64 but not by chymostatin.27,28
The total purified protein and the minced gels corresponding to each band revealed by SDS-PAGE were pre-incubated with 0.01 mM E-64 or 0.1 mM chymostatin for 10 minutes at room temperature. After pre-incubation with inhibitors, each sample was tested for proteolytic inhibition in the fluorometric hydrolysis reaction and in the gelatin-zymography described above. In gelatin zymography, proteolytic inhibition was detected by a lack of gelatin degradation shown by the absence of a revealed band in the gel.
Sample sera
Serum samples were selected from the repository of the Cysticercosis Working Group in Peru. All patient and healthy control sera were collected under appropriate informed consent processes including permission for future use and approved by the Institutional Review Board of the Universidad Peruana Cayetano Heredia (FWA 00000525). These included 100 samples from clinically defined NCC cases (50 from patients with a single viable cyst and 50 from patients with more than one viable cyst, range = 2–53; 20 patients had more than 10 cysts), 48 NCC-negative control sera, and 109 sera from patients with different helminth infections to determine cross-reactions.
Cases were defined by the identification of active lesions in the brain using computed tomography and/or magnetic resonance imaging. Cases could have additional calcified or inactive lesions and although they were selected on the basis of imaging characteristics alone, all had positive LLGP-EITB results. Negative control sera were collected from volunteers in Iquitos, a city in the Amazon basin in northern Peru where NCC is not endemic and other helminth infections are common. All negative control sera were tested by LLGP-EITB to confirm that they were not infected with cysticerci, but we did not rule out other helminth infections by using other serologic assays.
To determine cross-reactions, LLGP-EITB-negative sera (n = 109) from patients who were infected with a single helminth were tested. These sera included samples from patients proven by positive serologic results to have infections with Ancylostoma duodenale (n = 9), Ascaris lumbricoides (n = 3), Enterobius vermicularis (n = 14), Hymenolepis nana (n = 34), Strongyloides stercoralis (n = 13), Terranova trichiuris (n = 6), Taenia saginata (n = 10), and Echinococcus granulosus (n = 10). Additionally, sera from patients who had negative serologic results for E. granulosis , but had hydatid cysts recovered from surgery (n = 10) were also used as NCC-negative controls. Five sera positive for T. saginata were also positive in the LLGP-EITB.
Western immunoblot assay
The ion exchange chromatography–purified antigen with cathepsin L-like activity was resolved by SDS-PAGE in gradient polyacrylamide gels (5–22.5%) at a concentration of 0.024 μg/mm of gel width. The fraction was heated at 65°C for 15 minutes in sample buffer (0.1% SDS, 0.025% [w/v] bromophenol blue, 1% glycerol, 0.0025 M Tris-glycine-HCl, pH 8, and 5 mM DTT). Electrophoresis was performed at 5 mA for stacking and at 25 mA for resolving. Proteins were electrically transferred onto a nitrocellulose membrane at 2 A for one hour in a Transfer-Blot Cell (Bio-Rad, Hercules CA) in buffer (20% methanol, 0.212 M Tris glycine-HCl). Nitrocellulose membranes were cut into small strips to be tested using individual serum samples. A blocking solution of phosphate-buffered saline (PBS), pH 7.4, 0.3% Tween 20, 5% nonfat dry milk was prepared as a diluent for the serum samples. Each sera at a final dilution 1:300 was incubated with a nitrocellulose strip overnight at 4°C.5 The proteins were visualized using a peroxidase-labeled anti-human IgG, followed by the addition of 3,3′-diaminobenzadine plus hydrogen peroxide as the substrate. When a positive reaction was present, it was further classified as weak positive, positive, or strong positive on the basis of intensity of the band measured by simple observation.
ELISA
The ion exchange chromatography–purified antigen with cathepsin L-like activity was diluted in PBS (0.8 μg/mL) and was used to coat the wells (100 μL each) of Immulon 1 microtiter plates (Dynatech Laboratories, Chantilly, VA). After incubation for two hours with shaking, the 96-well plates were washed five times with PBS, 0.1% Tween using a manual washer. The wells were blocked with 100 μL of PBS, 0.1% Tween at room temperature for 30 minutes with shaking. After washing, the wells were incubated in duplicate with 25 μL of serum diluted in 75 μL of inactivated fetal bovine serum (Invitrogen, Carlsbad, CA) at room temperature for one hour with shaking. The plates were washed and antibodies were detected with anti-human IgG labeled with peroxidase (Kirkegaard and Perry, Gaithersburg, MD) diluted 1:20,000 in a 5% milk solution of PBS, 0.3% Tween and incubated at room temperature for one hour with shaking. The reaction was developed after incubation with tetramethyl benzidine SureBlue substrate (Kirkegaard and Perry) (100 μL/well) at room temperature for 30 minutes with shaking. The optical density (OD) was determined at 650 nm using a spectrophotometer (Molecular Devices, Toronto, Ontario, Canada).
To ensure ELISA validity, each plate included two strong positive control wells, two weak positive wells, and six negative control wells. The positive controls were prepared from a cysticercosis-positive serum pool diluted in a negative serum pool at 1:8 (strong positive) and 1:128 (weak positive). The six negative control wells corresponded to six individual serum samples from persons who were negative for cysticercosis. If any of the two weak positive wells did not give a positive reaction, results from the entire plate were discarded.
Every test sample was run in duplicate. An ELISA result was rejected if the difference of the ODs in the duplicate wells was greater than 15% or if the positive/negative reactivity in the duplicate wells were different, in which case the sample was tested again. For testing the ELISA, 21 additional NCC-negative control serum samples became available and were included. A total of 69 NCC-negative control serum samples were used to test the specificity.
Statistical analysis
To control for inter-plate variability of the OD absolute values, the percentage of positivity (PP), was calculated for each tested sera by dividing its mean OD by the mean OD of the strong positive controls of the corresponding plate. A simple logistic regression to model the disease condition (positive/negative) using the PP of all samples as the predictor covariate was performed. A linear score to estimate the probability of the positive-disease condition was developed based on the PP and the regression coefficient. A sample was considered positive if the probability was greater than the best cutoff (Pr-cutoff/PP). A receiver operating characteristic curve (ROC) was calculated and the Pr-cutoff/PP was estimated to maximize the Youden's J-index (sensitivity + specificity − 1).29 The same Pr-cutoff/PP was used to test every sample in all plates.
As an alternative method of calculating a positive result, a sample was considered positive if the mean OD of the tested serum sample was greater than the mean OD plus three standard deviations of the six negative controls, which defines the classification cutoff (NC-cutoff) of a plate.
The 95% confidence intervals (CIs) for estimated sensitivities and specificities were calculated for proportions following a binomial distribution. All analysis was performed using Stata 10 statistical software (Stata Corp., College Station, TX).
RESULTS
Protein purification
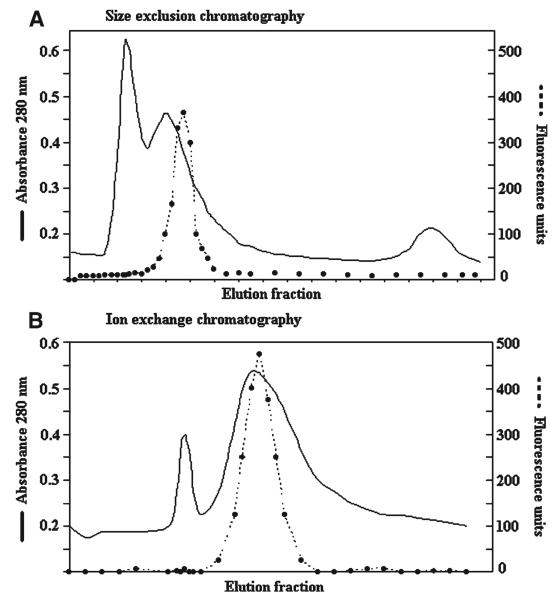
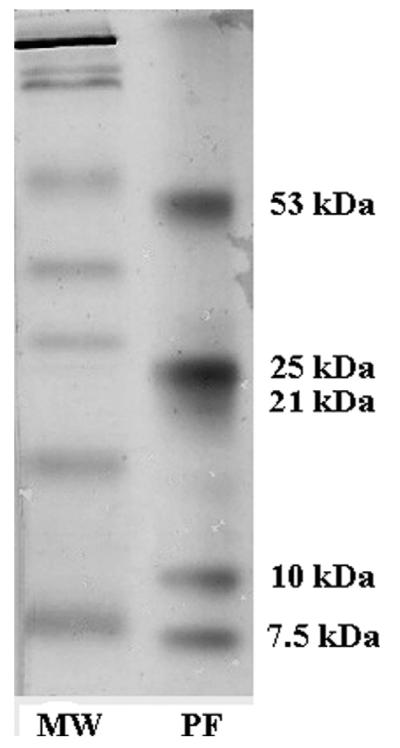
The size exclusion and the ion exchange chromatography patterns were similar and showed two major peaks of protein (Figure 1). The specific cathepsin L-like activity after each purification step is shown in Table 1. After the ion exchange chromatography, the final purification factor was 42.5. The silver-stained SDS-PAGE of the ion exchange chromatography–purified antigen processed without inhibitors showed a partially purified complex with four intense bands of 53, 25, 10, and 7.5 kD plus a weak and smeared band of 21 kD (Figure 2). In contrast, the SDS-PAGE pattern of the purified antigens processed with inhibitors showed the only three intense bands (53, 25, and 21 kD).
Figure 1.
Chromatographic and Z-Phe-Arg-AMC fluorometric patterns during A. Size exclusion chromatography, followed by B, ion exchange chromatography. The continuous line represents the total protein concentration, and the dotted line represents the cathepsin L-like activity.
Table 1.
Purification efficiency during the steps used to recover the 53/25-kD cathepsin L-like fraction from Taenia solium cyst fluid
| Purification step | Total protein (μg/mL) |
Specific activity (μmol substrate/minute/mg of protein) × 10−9 |
Purification factor |
|---|---|---|---|
| Crude cyst fluid | 4,933 | 1.32 | 1 |
| Ethanol precipitate | 3,033 | 1.32 | 1.00 |
| Size exclusion G75 | 278 | 6.44 | 4.88 |
| Ion exchange | 70 | 56.00 | 42.46 |
Figure 2.
Silver-stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis of the concentrated purified fraction (PF) with cathepsin ion exchange chromatography–purified cysticercus fraction with cathepsin L-like activity (no protease inhibitor added). MW = molecular weight marker.
Cathepsin L-like activity in purified antigens and inhibition assays
The fluorometric assay from the slices of gel showed that the protein fraction of 53 kD is associated with a cathepsin L-like activity; the 25-kD band was only associated to a marginal activity. The 21-, 10-, and 7.5-kD bands were not associated with cathepsin L-like activity. These reactions were inhibited by E64 but not by chymostatin, which confirms the cathepsin L-like nature of the proteolytic activity. We observed systematically that pre-incubation with chymostatin enhanced the cathepsin L-like activity.
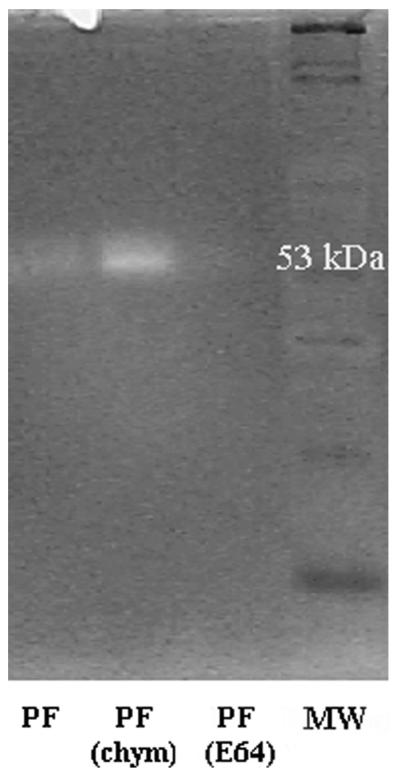
The gelatin zymography showed a strong gelatinolytic activity at 53 kD; no gelatinolytic activity was observed in the 25-kD band. The gelatinolytic activity of the 53-kD protein fraction was partially inhibited by E64, but not by chymostatin (Figure 3). Similarly, as in the fluorometric assay, pre-incubation with chymostatin enhanced the gelatinolytic activity.
Figure 3.
Gelatinolytic zymography of cathepsin L-like fraction purified without using protease inhibitors (PF). To confirm the cathepsin L-like activity, the purified fraction was incubated with E64 (PF(E64)) and chymostatin (PF(chym)) before electrophoresis. MW = molecular weight marker.
Western immunoblot assay
The ion exchange chromatography–purified antigen showed a Western immunoblot pattern similar to the silver-stained SDS-PAGE. Among the 50 positive samples with multiple viable cysts, 48 reacted positively against the 53-kD and 25-kD bands, giving a sensitivity of 96% (95% CI = 86.3–99.5%). Serum samples from patients with a single cyst showed a sensitivity of 78% (95% CI = 64.0–88.5%) against the 53-kD band and a sensitivity of 76% (95% CI = 61.8–86.9%) against the 25-kD band. Negative controls (47/48) had no detectable reactivity with the 53-kD band, giving a specificity of 97.9% (95% CI = 88.9–99.9%). Negative controls (45/48) did not show reactivity with the 25-kD band, giving a specificity of 93.8% (95% CI = 82.8–98.7%). Positive bands were classified as weak positive, positive, and strong positive depending on the visual intensity of the staining. Serum samples from patients with many cysts had visibly stronger reactions than patients with fewer numbers of cysts (Table 2). The sensitivity of the 21-, 10.5-, and 7.5-kD bands were lower than 70% with either the 53-kD or the 25-kD bands.
Table 2.
Distribution of the Taenia solium cathepsin L Western immunoblot band intensity according to sera tested
| Protein | Result | Negative controls (%) (n = 48) |
Patients with a single viable cyst (%) (n = 50) |
Patients with multiple viable cysts (%) (n = 50) |
|---|---|---|---|---|
| 25 kD | Negative | 45 (94) | 12 (24) | 2 (4) |
| Weak positive | 2 | 11 | 6 | |
| Positive | 1 | 21 | 18 | |
| Strong positive | 0 | 6 | 24 | |
| % positive | 6 | 76 | 96 | |
| 53 kD | Negative | 47 (98) | 11 (22) | 2 (4) |
| Weak positive | 0 | 13 | 6 | |
| Positive | 1 | 21 | 18 | |
| Strong positive | 0 | 5 | 24 | |
| % positive | 2 | 78 | 96 |
Cross-reactions
In the Western immunoblot, 11 of 109 serum samples from persons with other helminthic infections had a cross-reaction. However all were weak reactions and not easily detected visually: 1 of 14 serum samples with E. vermicularis cross-reacted with the 53-kD band, 1 of 13 serum samples with S. stercoralis cross-reacted with the 53-kD and 25-kD bands, 3 of 33 serum samples with H. nana cross-reacted with the 53-kD band; 1 of 10 serum samples with T. saginata cross-reacted with the 53-kD band (however, this serum sample was also LLGP-EITB positive), and 5 of 20 serum samples with E. granulosus cross-reacted with the 53-kD and 25-kD bands. None of the serum samples with A. duodenale, A. lumbricoides, or T. trichiura cross-reacted with the 53-kD or 25-kD bands.
ELISA
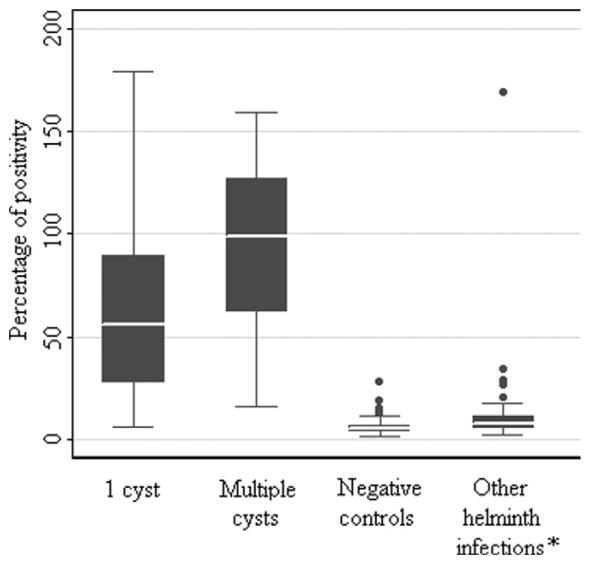
The average OD of the positive sera samples was approximately 15-fold greater than the OD of the negative controls. On the basis of the Pr-cutoff/PP (18.5%), the sensitivity of the cathepsin L ELISA was 84% (42 of 50, 95% CI = 70.9–92.8%) in patients with one cyst, and 98% (49 of 50, 95% CI = 89.4–99.9%) in patients with more than one cyst. The specificity of the cathepsin L ELISA was 92.7% (64 of 69, 95% CI = 86.3–95.7%). The assay efficacy, measured by Youden's J index, was 0.87, and the area under the ROC curve was 0.975. As shown in Figure 4, the PP values for the group of patients with multiple cysts are higher than those with a single cyst. The PP values of the cross-reactions against other helminths are slightly higher than PP values of the NCC-negative controls. When we excluded serum samples from NCC-positive patients with a single cyst and used only serum samples with high antibody responses from patients with multiple cysts, a new Pr-cutoff/PP was calculated (34.5%). The estimated sensitivity of the cathepsin L ELISA was 98% and the estimated specificity was 98.5%, with an assay efficacy of 0.965 and an area under the ROC curve of 0.9903.
Figure 4.
Box plot distribution of the percentage of positivity (PP) of the cathepsin L enzyme-linked immunosorbent assay in patients with a single cyst infection, patients with more than 1 cyst, negative controls, and patients with other helminth infections.
Classification of the serum samples with the NC-cutoff (mean = 0.16, SD = 0.05) had a sensitivity of 94% (95% CI = 87.4–97.8%) for patients with one cyst and a sensitivity of 100% (95% CI = 96.4–100%) for patients with more than one cyst, but the specificity was only 88.8% (95% CI = 83.0–93.7%).
Cross-reactions
On the basis of the PP test, 8 of 109 serum samples with other helminth infections showed a cross-reaction: 1 of 14 serum samples with E. vermicularis, 3 of 34 serum samples with H. nana, 2 of 10 serum samples with T. saginata (however, these were also LLGP-EITB positive), and 2 of 20 serum samples with E. granulosus showed cross-reactions. The PP values of the cross-reactions were lower than those of the NCC-positive samples and slightly higher than those of the NCC-negative controls (Figure 4). Serum samples that showed cross-reactions in the Western immunoblot and the ELISA were not the same, but approximately 50% of them overlapped.
DISCUSSION
This study demonstrates that a partially purified antigen from T. solium cysticercus fluid that contains two protein fractions of 53 kD and 25 kD associated with cathepsin L-like activity performed well in the immunodiagnostics of human cysticercosis in an ELISA and a Western immunoblot assay.
It has been demonstrated that the T. crassiceps metacestode secretes a number of proteolytic enzymes including metallo-aminopeptidases and cysteine proteases that degrade human IgG,30 suggesting that this parasite may be using these proteases to modulate the host immune response. Cathepsin L proteases that degrade human IgG have been isolated from T. solium cysticerci in two prior studies; a 48-kD protein thought to be involved in immune response evasion31 and a 29-kD protein that also degraded collagen and bovine serum albumin.32 The 29-kD cathepsin L protease was shown to be reactive against sera from cysticercosis patients in an immunoblot format, but neither of these proteases were fully evaluated for their immunogenicity or diagnostic potential.
In this study, the 53-kD, and to a lesser extent, the 25-kD partially purified protein fractions were able to hydrolyze the fluorogenic substrate Z-Phe-Arg-AMC. This activity was inhibited by E64 but not by chymostatin, confirming that the protein fraction includes at least one cathepsin L-like peptidase. The relatively low total purification factor of 42.4 suggests that the 53/25-kD cathepsin L-like fraction is highly abundant in the cyst fluid or that other proteins are present in this fraction.
The 53-kD and 25-kD protein fractions performed well in the Western immunoblot and in the ELISA, with a sensitivity and specificity similar to that of the LLGP-EITB. The sensitivities of the cathepsin L Western immunoblot and the cathepsin L ELISA were higher in NCC patients with multiple cysts compared with patients with a single cyst, which suggests that patients with multiple cysts have a higher antibody response than patients with a single cyst infection. Remarkably, the sensitivities of these two tests for patients with one cyst were similar to the sensitivity of the LLGP-EITB in the same group of patients.
It is possible that the immunoreactive antigen recognized in the ELISA is the cathepsin L-like peptidase. However, further studies are required to confirm this hypothesis. Further analyses are needed to identity the cathepsin L-like protease in the 53/25-kD purified fraction and its relationship with the 48-kD and 29-kD cathepsin L-like proteases previously identified.
Currently, on the basis of T. solium metacestode lentil-lectin structural glycoproteins,5 the best immunologic test to diagnose human cysticercosis is the LLGP-EITB. However, this assay is expensive, requires specialized trained personnel, and is not quantitative. An ELISA is a simpler assay and can be performed in most laboratories at a lower cost than a Western immunoblot. The ELISA is also quantitative and lends itself to high throughput studies, and its results are not subject to individual interpretation as is the case with blot analyses. We have not tested the seven LLGPs used in the CDC Western immunoblot for cathepsin L-like activity. However, neither of the amino acid sequences of the LLGP (GenBank accession nos. AAF25005.1, AAM00206.1, AAM00207.1, AAM00208.1, AAD51763.2, AAD51767.2, AAD51768.1, AAD51769.1, and AAF60975.1) showed a protease motif in a protein BLAST against the entire GenBank within an e value from 1e-42 to 0.020).33
The major limitation of our cathepsin L ELISA is the difficulty in obtaining and processing the cyst fluid. Despite its high sensitivity and specificity, our cathepsin L ELISA requires purification of antigens from cyst fluid, which is time-consuming and requires infected pigs. As disease control measures increase, the ability to obtain large amounts of antigen may become more difficult. To make this test practical, it will be necessary to produce reliable recombinant antigens.
Another limitation of the cathepsin L ELISA is its cross-reactivity with E. granulosus (5 of 20, 25%), T. saginata (1 of 10, 10%), and H. nana (3 of 33, 9%), which are common parasites in developing countries, and to a lesser extent its cross-reactivity with E. vermicularis (1 of 14, 7%). However E. granulosus and T. saginata are close related to T. solium and have always shown high cross-reactivity in immunodiagnostic tests for cysticercosis.34
The cathepsin L-based ELISA for the diagnosis of human cysticercosis may have a significant clinical impact. The high sensitivity of the assay implies that it can be used for detection of NCC in patients, even those with one cyst who make up a large proportion of the infected population. The ELISAs relatively low cost and ease of use suggests that this assay can be used as a bedside diagnostic test. The use of the antigen has the potential to be further expanded for its use in a bedside diagnostic test and as a vaccine candidate. Further studies are required to produce these antigens as recombinant proteins.
Acknowledgments
We thank Dr. Clinton White, Dr. Philip LoVerde, Dr. Manuela Verástegui, and Dr. Jeanette Velasquez for their advice and review of the manuscript. We are also grateful to Myra Flores and Patricia Arias for their hard work on sample processing.
Financial support: This study was partially supported by research grants P01 AI51976 TMRC from the National Institutes of Health; 23981 from The Bill and Melinda Gates Foundation; U01 AI35894, TW05562, K24 A10668903, R01 TW005860, and D43 TW007120 from the National Institutes of Health; 01107 from the Food and Drug Administration; and 063109 from The Wellcome Trust, United Kingdom.
Footnotes
Disclaimer: The sponsors had no role in the design or writing of this manuscript.
REFERENCES
- 1.Garcia HH, Gonzalez AE, Evans CA, Gilman RH. Taenia solium cysticercosis. Lancet. 2003;362:547–556. doi: 10.1016/S0140-6736(03)14117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schantz PM. Surveillance and control programs for cestode diseases. In: Miller MJ, Love EJ, editors. Parasitic Diseases: Treatment and Control. CRC Press, Inc.; Boca Raton, FL: 1989. pp. 275–290. [Google Scholar]
- 3.White AC, Garcia HH. Recent developments in the epidemiology, diagnosis, treatment, and prevention of neurocysticercosis. Curr Infect Dis Rep. 1999;1:434–440. doi: 10.1007/s11908-999-0055-x. [DOI] [PubMed] [Google Scholar]
- 4.Garcia HH, Gilman R, Martinez M, Tsang VC, Pilcher JB, Herrera G, Diaz F, Alvarado M, Miranda E. Cysticercosis as a major cause of epilepsy in Peru. The Cysticercosis Working Group in Peru (CWG) Lancet. 1993;341:197–200. doi: 10.1016/0140-6736(93)90064-n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium) J Infect Dis. 1989;159:50–59. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Arruda GC, da Silva AD, Quagliato EM, Maretti MA, Rossi CL. Evaluation of Taenia solium and Taenia crassiceps cysticercal antigens for the serodiagnosis of neurocysticercosis. Trop Med Int Health. 2005;10:1005–1012. doi: 10.1111/j.1365-3156.2005.01480.x. [DOI] [PubMed] [Google Scholar]
- 7.Bueno EC, Scheel CM, Vaz AJ, Machado LR, Livramento JA, Takayanagui OM, Tsang VC, Hancock K. Application of synthetic 8-kD and recombinant GP50 antigens in the diagnosis of neurocysticercosis by enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 2005;72:278–283. [PubMed] [Google Scholar]
- 8.Dorny P, Brandt J, Zoli A, Geerts S. Immunodiagnostic tools for human and porcine cysticercosis. Acta Trop. 2003;87:79–86. doi: 10.1016/s0001-706x(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 9.Espindola NM, Iha AH, Fernandes I, Takayanagui OM, Machado Ldos R, Livramento JA, Mendes Maia AA, Peralta JM, Vaz AJ. Cysticercosis immunodiagnosis using 18- and 14-kilodalton proteins from Taenia crassiceps cysticercus antigens obtained by immunoaffinity chromatography. J Clin Microbiol. 2005;43:3178–3184. doi: 10.1128/JCM.43.7.3178-3184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrer E, Gonzalez LM, Martinez-Escribano JA, Gonzalez-Barderas ME, Cortez MM, Davila I, Harrison LJ, Parkhouse RM, Garate T. Evaluation of recombinant HP6-Tsag, an 18 kDa Taenia saginata oncospheral adhesion protein, for the diagnosis of cysticercosis. Parasitol Res. 2007;101:517–525. doi: 10.1007/s00436-007-0507-x. [DOI] [PubMed] [Google Scholar]
- 11.Fleury A, Beltran C, Ferrer E, Garate T, Harrison LJ, Parkhouse RM, Garcia E, Fragoso G, Costa-Cruz J, Biondi G, Agapejev S, Sciutto E. Application of synthetic peptides to the diagnosis of neurocysticercosis. Trop Med Int Health. 2003;8:1124–1130. doi: 10.1046/j.1360-2276.2003.01132.x. [DOI] [PubMed] [Google Scholar]
- 12.Hancock K, Khan A, Williams FB, Yushak ML, Pattabhi S, Noh J, Tsang VC. Characterization of the 8-kilodalton antigens of Taenia solium metacestodes and evaluation of their use in an enzyme-linked immunosorbent assay for serodiagnosis. J Clin Microbiol. 2003;41:2577–2586. doi: 10.1128/JCM.41.6.2577-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez M, Beltran C, Garcia E, Fragoso G, Gevorkian G, Fleury A, Parkhouse M, Harrison L, Sotelo J, Sciutto E. Cysticercosis: towards the design of a diagnostic kit based on synthetic peptides. Immunol Lett. 2000;71:13–17. doi: 10.1016/s0165-2478(99)00166-2. [DOI] [PubMed] [Google Scholar]
- 14.Sako Y, Nakao M, Nakaya K, Yamasaki H, Ito A. Recombinant antigens for serodiagnosis of cysticercosis and echinococcosis. Parasitol Int. 2006;55(Suppl):S69–S73. doi: 10.1016/j.parint.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Scheel CM, Khan A, Hancock K, Garcia HH, Gonzalez AE, Gilman RH, Tsang VC. Serodiagnosis of neurocysticercosis using synthetic 8-kD proteins: comparison of assay formats. Am J Trop Med Hyg. 2005;73:771–776. [PubMed] [Google Scholar]
- 16.Harmsen MM, Cornelissen JB, Buijs HE, Boersma WJ, Jeurissen SH, van Milligen FJ. Identification of a novel Fasciola hepatica cathepsin L protease containing protective epitopes within the propeptide. Int J Parasitol. 2004;34:675–682. doi: 10.1016/j.ijpara.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Tantrawatpan C, Maleewong W, Wongkham C, Wongkham S, Intapan PM, Nakashima K. Serodiagnosis of human fascioliasis by a cystatin capture enzyme-linked immunosorbent assay with recombinant Fasciola gigantica cathepsin L antigen. Am J Trop Med Hyg. 2005;72:82–86. [PubMed] [Google Scholar]
- 18.Cornelissen JB, Gaasenbeek CP, Boersma W, Borgsteede FH, van Milligen FJ. Use of a pre-selected epitope of cathepsin-L1 in a highly specific peptide-based immunoassay for the diagnosis of Fasciola hepatica infections in cattle. Int J Parasitol. 1999;29:685–696. doi: 10.1016/s0020-7519(99)00017-x. [DOI] [PubMed] [Google Scholar]
- 19.Collins PR, Stack CM, O'Neill SM, Doyle S, Ryan T, Brennan GP, Mousley A, Stewart M, Maule AG, Dalton JP, Donnelly S. Cathepsin L1, the major protease involved in liver fluke (Fasciola hepatica) virulence: propetide cleavage sites and auto-activation of the zymogen secreted from gastrodermal cells. J Biol Chem. 2004;279:17038–17046. doi: 10.1074/jbc.M308831200. [DOI] [PubMed] [Google Scholar]
- 20.Cordova M, Herrera P, Nopo L, Bellatin J, Naquira C, Guerra H, Espinoza JR. Fasciola hepatica cysteine proteinases: immunodominant antigens in human fascioliasis. Am J Trop Med Hyg. 1997;57:660–666. doi: 10.4269/ajtmh.1997.57.660. [DOI] [PubMed] [Google Scholar]
- 21.Cordova M, Reategui L, Espinoza JR. Immunodiagnosis of human fascioliasis with Fasciola hepatica cysteine proteinases. Trans R Soc Trop Med Hyg. 1999;93:54–57. doi: 10.1016/s0035-9203(99)90178-5. [DOI] [PubMed] [Google Scholar]
- 22.Zimic MJ, Infantes J, Lopez C, Velasquez J, Farfan M, Pajuelo M, Sheen P, Verastegui M, Gonzalez A, Garcia HH, Gilman R. Comparison of the peptidase activity in the oncospheres excretory/secretory products of Taenia solium and Taenia saginata. J Parasitol. 2007;93:727–734. doi: 10.1645/GE-959R.1. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez AE, Cama V, Gilman RH, Tsang VC, Pilcher JB, Chavera A, Castro M, Montenegro T, Verastegui M, Miranda E. Prevalence and comparison of serologic assays, necropsy, and tongue examination for the diagnosis of porcine cysticercosis in Peru. Am J Trop Med Hyg. 1990;43:194–199. doi: 10.4269/ajtmh.1990.43.194. [DOI] [PubMed] [Google Scholar]
- 24.Werle B, Staib A, Julke B, Ebert W, Zladoidsky P, Sekirnik A, Kos J, Spiess E. Fluorometric microassays for the determination of cathepsin L and cathepsin S activities in tissue extracts. Biol Chem. 1999;380:1109–1116. doi: 10.1515/BC.1999.138. [DOI] [PubMed] [Google Scholar]
- 25.Sriveny D, Raina OK, Yadav SC, Chandra D, Jayraw AK, Singh M, Velusamy R, Singh BP. Cathepsin L cysteine proteinase in the diagnosis of bovine Fasciola gigantica infection. Vet Parasitol. 2006;135:25–31. doi: 10.1016/j.vetpar.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Kaewpitoon N, Laha T, Kaewkes S, Yongvanit P, Brindley PJ, Loukas A, Sripa B. Characterization of cysteine proteases from the carcinogenic liver fluke, Opisthorchis viverrini. Parasitol Res. 2008;102:757–764. doi: 10.1007/s00436-007-0831-1. [DOI] [PubMed] [Google Scholar]
- 27.Schirmeister T, Klockow A. Cysteine protease inhibitors containing small rings. Mini Rev Med Chem. 2003;3:585–596. doi: 10.2174/1389557033487935. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto K, Mizoue K, Kitamura K, Tse WC, Huber CP, Ishida T. Structural basis of inhibition of cysteine proteases by E-64 and its derivatives. Biopolymers. 1999;51:99–107. doi: 10.1002/(SICI)1097-0282(1999)51:1<99::AID-BIP11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 29.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Baig S, Damian RT, Morales-Montor J, Olecki P, Talhouk J, Hashmey R, White AC Jr. Characterization of excretory/secretory endopeptidase and metallo-aminopeptidases from Taenia crassiceps metacestodes. J Parasitol. 2005;91:983–987. doi: 10.1645/GE-200R1.1. [DOI] [PubMed] [Google Scholar]
- 31.Baig S, Damian RT, Molinari JL, Tato P, Morales-Montor J, Welch M, Talhouk J, Hashmeys R, White AC., Jr Purification and characterization of a metacestode cysteine proteinase from Taenia solium involved in the breakdown of human IgG. Parasitology. 2005;131:411–416. doi: 10.1017/s0031182005007821. [DOI] [PubMed] [Google Scholar]
- 32.Li AH, Moon SU, Park YK, Na BK, Hwang MG, Oh CM, Cho SH, Kong Y, Kim TS, Chung PR. Identification and characterization of a cathepsin L-like cysteine protease from Taenia solium metacestode. Vet Parasitol. 2006;141:251–259. doi: 10.1016/j.vetpar.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parkhouse RM, Harrison LJ. Cyst fluid and surface associated glycoprotein antigens of Taenia sp. metacestodes. Parasite Immunol. 1987;9:263–268. doi: 10.1111/j.1365-3024.1987.tb00505.x. [DOI] [PubMed] [Google Scholar]