Abstract
Background
Postoperative pain was an independent predictor of postoperative delirium. Whether postoperative delirium limits patient controlled analgesia (PCA) use has not been determined.
Methods
We conducted a nested cohort study in older patients undergoing noncardiac surgery and used PCA for postoperative analgesia. Delirium was measured using the Confusion Assessment Method. We computed a structural equation model to determine the effects of pain and opioid consumption on delirium status and the effect of delirium on opioid use.
Results
Of 335 patients, 108 (32.2%) developed delirium on postoperative day (POD) 1, and 120 (35.8%) on POD 2. Postoperative delirium did not limit the use of PCA. Patients with postoperative delirium used more PCA in a 24-hour period (POD 2) compared to those and without delirium (mean dose of hydromorphone ± SE adjusted for co-variates was 2.24 ± 0.71 mg vs. 1.25 ± 0.67 mg, P = 0.02). Despite more opioid use, patients with delirium reported higher VAS scores than those without delirium (POD 1: mean Visual Analog Scale ± SE at rest 4.2 ± 0.23 vs. 3.3 ± 0.22, P = 0.0051; POD 2: 3.3 ± 0.23 vs. 2.5 ± 0.19, P = 0.004). Path coefficients from structural equation model revealed that pain and opioid use affect delirium status, but delirium does not affect subsequent opioid dose.
Conclusions
Postoperative delirium did not limit PCA use. Despite more opioids use, Visual Analog Scale scores were higher in patients with delirium. Future studies on delirium should consider the role of pain and pain management as potential etiologic factors.
Introduction
Delirium is an acute confusional state with alterations in attention and consciousness. 1 Delirium occurs in 14% to 50% of hospitalized medical patients, and its associated mortality rate is 10% to 65%. 2,3 After major noncardiac surgery, 10% to 60% of patients have delirium. 4 Delirium is a serious problem for hospitalized geriatric patients. It may be caused by an underlying medical illness, but often, the exact etiology is not identifiable. 5 In the perioperative period, the precipitating risk factors for the development of postoperative delirium in older patients include an unfamiliar environment, the stress of surgery, and exposure to medications that have the potential for profound effects on the central nervous system (CNS).
Previous studies have analyzed how patient-related factors and surgical factors contribute to postoperative delirium. 6–9 We recently demonstrate that the methods of pain relief and the severity of postoperative pain both independently increase the occurrence of postoperative delirium after controlling for factors such as age and educational level in a cohort study of patients ≥ 65 years of age undergoing elective noncardiac surgery. 10 Additionally, there was an ordered association between levels of pain and the development of postoperative delirium. This result raises an important question, that is, does postoperative delirium limit the patient’s use of on-demand patient controlled analgesia (PCA), resulting in more reportable pain? If this was true, then an alternative to PCA is indicated to provide postoperative analgesia to patients with postoperative delirium.
Accordingly, the present study aimed to determine whether patients with and without delirium differed in the amount of postoperative opioid used, adjusting for known co-variates for delirium. We hypothesized that postoperative delirium did not limit the patient’s use of on-demand PCA.
Materials and Methods
Patient Recruitment
The study was approved by the University of California, San Francisco Committee on Human Research, and informed consent was obtained preoperatively from each study patient. The study took place at the University of California, San Francisco Medical Center, from 2001–2006. Patients were selected from an ongoing larger study investigating the pathophysiology of postoperative delirium in older surgical patients. The study inclusion criteria included English-speaking patients ≥ 65 years of age undergoing noncardiac surgery requiring anesthesia and who were anticipated to stay in the hospital for longer than 48 hours. Only patients who received PCA using intravenous opioid analgesics in the postoperative period were included in this study. Excluded were those who could not provide informed consent. A subset of 215 patients in this study was included in a previous manuscript evaluating the predictors of postoperative delirium that included the measurements of pain, postoperative medications, and other co-variates. 10
Patient Assessment
The same trained research assistant conducted pre- and post-operative patient interviews in person. The preoperative interview occurred <48 hours before surgery in the preoperative clinic. During this preoperative interview, the patients were evaluated for depressive symptoms, pain, and functional status. In addition, a detailed examination was conducted to evaluate the cognitive status. The Telephone Interview for Cognitive Status, 11 modified from the Mini Mental Status Examination, which could be administered in person or over the phone, was used to measure baseline cognitive status.
Postoperative Pain Measurement and Management
The study design did not control the postoperative pain management strategy, which was determined by the attending physicians. All patients in this report used PCA as the method of postoperative pain relief, and hydromorphone was typically the opioid used. Trained research assistants measured patient pain levels during structured interviews using a verbal version of the Visual Analog Scale (VAS), in which a rating of zero corresponds to no pain, and a rating of ten corresponds to maximum pain. Patients were asked to rate their pain at rest preoperatively and on postoperative days one and two. The measurements of pain status were performed at the same time that patients were evaluated for delirium.
The daily doses of the PCA opioid analgesic administered postoperatively (hydromorphone) were recorded for the first three postoperative days.
Delirium Assessment
A trained research assistant conducted structured interviews preoperatively and on the first two postoperative days between the hours of 9 a.m. to 12 pm. to determine the presence of delirium, defined using the Confusion Assessment Method (CAM). 12 This method was developed as a screening instrument based on operationalization of the Diagnostic and Statistical Manual of Mental Disorders-III-R criteria for use by nonpsychiatric clinicians in high-risk settings. Based on a structured interview, the CAM algorithm consists of four clinical criteria: acute onset and fluctuating course, inattention, and disorganized thinking, and altered level of consciousness. In order for delirium to be defined, both the first and second criteria have to be present, plus either criterion three or four. CAM has a sensitivity of 94–100% and a specificity of 90–95% and has a high interobserver reliability, 12 and has convergent agreement with four other mental status test.
Postoperative delirium assessments were validated by a second investigator with advanced training in psychology (Dr. Sands). To ensure consistency in the evaluation, each patient was evaluated by the same research assistant for all three interviews. The research assistant was trained in the use of the CAM based on a detailed manual developed by Inouye et al. for administration of the CAM. 12 The occurrence of delirium was defined as the patient meeting CAM criteria for delirium on any of the postoperative day assessments.
Statistical Methods
Patients with delirium on either day one or day two after surgery were compared with patients who did not experience delirium on either day after surgery using t-tests for continuous valued variables, chi-square tests for categorical variables, and Fisher’s exact test when variable categories included fewer than five patients.
We sought to determine whether subjects with delirium were less consistent in their reports of pain compared to subjects without delirium. To assess this, we computed intraclass correlation coefficients based on a two-way random effects analysis of variance model. We computed two intraclass correlations, one for subjects who were and one for subjects who were not delirious on day one to assess consistency in their reports of pain at rest on postoperative day one and day two.
To investigate the temporal relationship between drug dosages given in the postoperative period and the occurrence of postoperative delirium, patients were stratified by the presence or absence of delirium on postoperative days one or two respectively. Using this method, we computed several analyses of covariance to assess whether the mean doses of hydromorphone used on each of the postoperative day differed between patients with and without delirium, after adjusting for co-variates that have potential effects on postoperative delirium using analysis of co-variance. 10 The covariates included current pain at rest, preoperative narcotic use, surgical risk, 13 and use of other medications with CNS effects. Additional analyses were performed to determine whether delirium was associated with the dose of PCA delivered opioids in the next 24 hours for those with and without delirium after adjustment for co-variates.
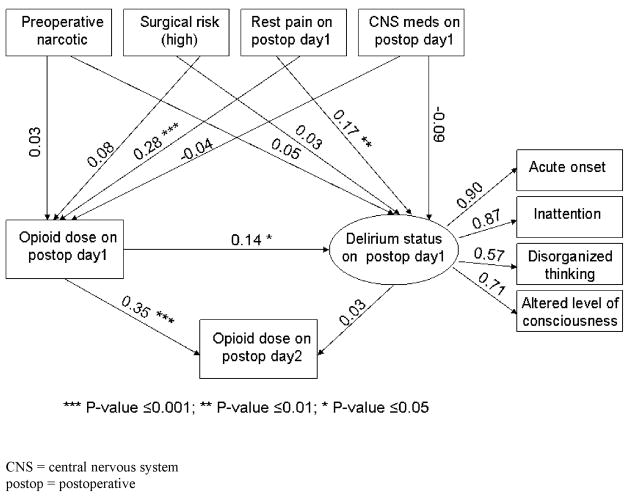
Structural equation modeling was performed to model how pain and initial postoperative opioid use affect delirium status, and how delirium status affects subsequent opioid use. Structural equation modeling is an extension of the general linear model (that includes regression) which allows estimation of latent variables and modeling associations between observed and latent variables. Latent variables are unobserved traits that can be measured with observed variables. For this study, delirium status is considered a latent variable that can be represented by four indicators that in combination are used to diagnose delirium (acute onset and fluctuating course, inattention, disorganized thinking, and altered level of consciousness). Figure 1 shows that these four variables are used to measure delirium status as illustrated by the four arrows that extend from delirium status to each of these four observed variables. We report path coefficients (factor loadings) and loadings above 0.70 are considered to be strong indicators of the latent trait. Shown in the same figure is our hypothesis that preoperative narcotic use, surgical risk, pain, use of CNS medications, and opioid use affect delirium status. Arrows that extend from each of these observed variables to delirium status show the direction of the hypothesized association. We report the standardized regression weights (path coefficients) next to each arrow. The significance of path coefficients is tested using t-tests that assess whether the coefficient is significantly different than zero. A value of zero for a coefficient would indicate no association.
Figure 1. Illustrative model of relationships between postoperative opioid use and delirium status.
This figure depicts our heuristic model for the association between postoperative delirium and opioid use. In this model, we described that delirium status was represented by the four criteria used to measure the presence of delirium. Our hypotheses were 1) rest pain on postoperative day one affected delirium status on day one, 2) opioid dose on day one affected delirium status on day one, and 3) delirium status on day one did not affect opioid dose on day two.
Shown also in figure 1 is our second hypothesis that preoperative narcotic, surgical risk resting pain, and CNS drug use, affect opioid use. Finally, figure 1 also shows the hypothesized associations between the current opioid use and delirium status on subsequent opioid use. To evaluate whether the hypothesized model shown in Figure 1 was a good fit, we computed unadjusted and adjusted goodness-of-fit index which should be above 0.90, a chi-square test, which should not be significant because it would suggest the data do not fit the model, and the root mean square residual which should be close to zero, and is typically near 0.10 for a good fitting model. All structural equation modeling computations were performed using Proc CALIS in SAS.
All statistical analysis was performed with SAS, version 9.1 (Cary, NC), except for the intraclass correlation coefficients which were computed using SPSS version 16.0 (Chicago, IL). In all tests, a P values < 0.05 (two-tailed) was considered statistically significant. All data were presented as mean ± SEM unless stated otherwise.
Results
Three hundred and thirty five patients were included in the study. The mean age (± SD) of patients was 73.5 ± 6.2 years (range, 65–96 years). Preoperative patient characteristics, surgical and medical data are shown in Table 1. The majority of patients underwent orthopaedic surgery. Preoperatively, 36% of patients received an opioid analgesic for pain. The majority of patients were intermediate surgical risk candidates, with two or more medical comorbidities.
Table 1.
Demographics and clinical characteristics (n=335)
| Demographic and clinical data | Delirious (N=185) | Non-Delirious (N=150) | P-value |
|---|---|---|---|
| Age (mean ± SD) | 74.13± 6.36 | 72.82± 5.87 | 0.053 |
| Gender | 0.0046 | ||
| Female | 115 | 70 | |
| Male | 70 | 80 | |
| Race | 0.38 | ||
| White | 158 | 133 | |
| Non-White | 27 | 17 | |
| Education | 0.24 | ||
| High school graduate or incomplete | 56 | 37 | |
| Incomplete college or above | 121 | 107 | |
| History of central nervous system disorders | 0.77 | ||
| None | 94 | 78 | |
| Yes | 90 | 70 | |
| Daily Alcohol Intake | 0.29 | ||
| None | 89 | 62 | |
| Yes | 93 | 82 | |
| Independent in performing 5 ADLs | 0.12 | ||
| No | 45 | 26 | |
| Yes | 140 | 124 | |
| Independent in performing 7 IADLs | 0.15 | ||
| No | 91 | 62 | |
| Yes | 94 | 88 | |
| Geriatric depression score (mean ± SD) | 3.33±2.97 | 2.62±2.53 | 0.023 |
| TICS SCORES (mean ± SD) | 31.47±4.33 | 32.63±3.77 | 0.016 |
| Types of Surgery | 0.90 | ||
| Spine/Knee/Hip | 128 | 107 | |
| Abdominal/Thoracic | 34 | 25 | |
| Peripheral/others | 23 | 18 | |
| Surgical Risk | 0.030 | ||
| Low | 3 | 3 | |
| Intermediate | 139 | 129 | |
| High | 43 | 18 | |
| Number of co-existent medical conditions | 0.15 | ||
| 0 | 32 | 33 | |
| 1 | 63 | 37 | |
| ≥2 | 90 | 80 | |
| ASA Classification | 0.44 | ||
| 1&2 | 92 | 81 | |
| ≥3 | 93 | 69 | |
| Preoperative opioids | 0.12 | ||
| No | 114 | 104 | |
| Yes | 71 | 45 | |
| Anesthesia type | |||
| General | 165 | 130 | 0.58 |
| Regional | 20 | 19 | |
Delirious refers to patients with delirium on either postoperative day 1 or 2
Surgical risk was estimated using the guidelines from the American College of Cardiology and American Heart Association update for the perioperative cardiovascular evaluation for noncardiac surgery, which takes into consideration the type and duration of surgery, and intraoperative blood loss, see text for citation
ADL = activities of daily living
ASA = American Society of Anesthesiologists
IADL = independent activities of daily living
TICS = telephone interview of cognitive status, a cognitive assessment tool modified from the Mini Mental Status Examination, which can be used in person or over the telephone
Of the 335 patients studied, 108 patients (32.2%) developed delirium on postoperative day one, 120 (35.8%) on postoperative day two, and 185 patients (55%) developed delirium on either day one or day two after surgery. Patients who developed postoperative delirium on either of the postoperative days tended to be older, were more likely to be female, and had more self-reported symptoms of depression as indicated on the geriatric depression score (table 1). One patient had missing data for delirium on postoperative day one due to mechanical ventilation and 13 patients had missing data for delirium on postoperative day two. The reasons for missing data for postoperative day two included early discharge or unavailable during interviews (n=6), refusal to answer questions (n=3), and too sleepy to respond (n=4). More importantly, the characteristics (demographic and surgical data) between patients with missing data on delirium were not different from those with delirium data. Similarly, the amount of missing data for VAS in our study was low (< 3% overall for both postoperative days), and the differences in missing data between those with and without delirium were not significantly different for the different postoperative days.
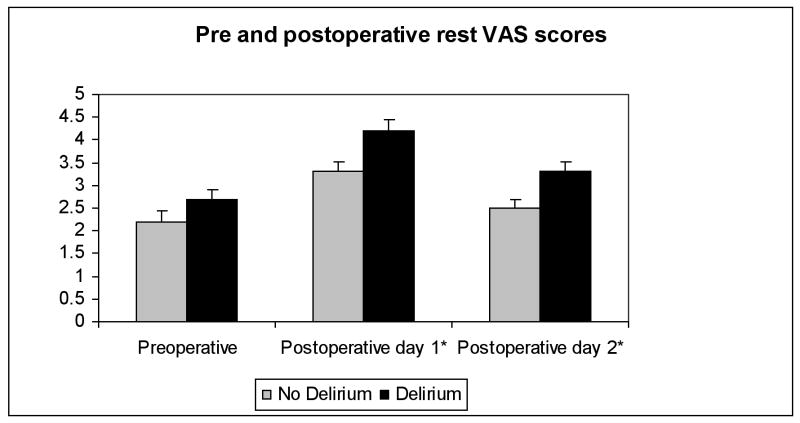
The preoperative VAS scores were not significantly different between patients who subsequently developed postoperative delirium versus those without delirium (figure 2). In contrast, patients who were delirious postoperatively experienced significantly higher VAS scores than non-delirious patients (for postoperative day one: mean postoperative VAS at rest 4.2 ± 0.23 vs. 3.3 ± 0.22, P = 0.0051; and for postoperative day two: mean postoperative VAS at rest 3.3 ± 0.23 vs. 2.5 ± 0.19, P = 0.004).
Figure 2.
Shown are the VAS pain scores plotted as mean ± SEM for patients with and without postoperative delirium in the pre- and post-operative periods
VAS – visual analog scale
* indicates P<0.05
The intraclass correlations for postoperative resting pain scores on day one and day two were significantly different than zero in patients with and without postoperative delirium (table 2). The 95% confidence intervals for the intraclass correlation coefficients suggest that patients who were delirious were equally consistent in their reports of resting pain evaluation than those who were not delirious. The difference in the magnitude of intraclass correlations could not be explained by differences in the ranges and variances of scores. On postoperative day one, 18.6% of patients with delirium and 15.7% of patients without delirium reported resting VAS pain scores of zero (P = 0.52) and on postoperative day two, 26.5% of patients with delirium and 23.1% of patients without delirium reported resting VAS pain scores of zero (P = 0.51). The variances in resting pain scores on day one and day two for patients who were and were not delirious on day one also did not differ significantly (P = 0.82 and 0.99 respectively).
Table 2.
Consistency in Reporting Pain between Postoperative Day one and Day two
| Not delirious on day 1 n=217 | Delirious on Day 1 n=102 | |
|---|---|---|
| Intraclass correlation between day one and day two resting pain scores (95% confidence interval) | 0.52 (0.37,0.63) | 0.80 (0.71,0.87) |
| P-value | <0.0001 | <0.0001 |
The intraclass correlations between postoperative day one and day two resting pain scores were significantly different than zero in patients with and without postoperative delirium. The 95% confidence intervals suggest that subjects with delirium were no less consistent than subjects without delirium in reporting pain.
The presence of postoperative delirium did not limit the use of PCA opioids as shown in tables 3 and 4. After adjusting for the VAS scores, preoperative narcotic use, surgical risk, and use of medications with CNS effects on the day of the opioid dose measurement, patients with and without delirium on postoperative day one used similar amount of hydromorphone on postoperative day one and the subsequent day (table 3). For patients with delirium on postoperative day two, they used substantially more PCA hydromorphone than those who were non-delirious on day two (adjusted mean dose 2.24 mg ± 0.71 vs. 1.25 mg ± 0.67, P = 0.02) (table 4).
Table 3.
Hydromorphone Dose by Delirium Status on POD 1 Adjusted for Current Pain at Rest, Preoperative Narcotic Use, Surgical Risk, and Use of Other Medications with CNS Effects
| Adjusted Means | |||
|---|---|---|---|
| No Delirium on POD 1 (n = 226) | Delirium on POD 1 (n = 108) | P-value | |
| Hydromorphone dose on POD 1 (mg) | 4.28±0.92 | 4.34±0.98 | 0.93 |
| Hydromorphone dose on POD 2 (mg) | 1.38±0.68 | 1.72±0.68 | 0.42 |
The results are shown in dose of hydromorphone (mg) ± SEM
POD = postoperative
Current pain refers to resting pain measured on the same day of the opioid dose, e.g. resting pain on day 1 for hydromorphone dose on day 1
The strengths of the relationship between the co-variates and the dependent variable (hydromorphone doses) are shown in figure 1
Table 4.
Hydromorphone Dose by Delirium on POD 2 Adjusted for Current Pain at Rest, Preoperative Narcotic Use, Surgery Risk, and Use of Other Medications with CNS Effects
| Adjusted Means | |||
|---|---|---|---|
| No Delirium on POD 2 (n = 202) | Delirium on POD 2 (n = 120) | P-value | |
| Hydromorphone dose on POD 2 (mg) | 1.25±0.67 | 2.24±0.71 | 0.02 |
| Hydromorphone dose on POD 3(mg) | 0.75±0.70 | 1.62±0.74 | 0.06 |
Figure 1 shows that our choice of indicator variables for measuring the latent variable, delirium status, was reasonable. Three indicator variables (acute onset, inattention, and altered level of conscious) loaded highly on the latent variable that we called delirium status. The overall model fit was assessed using statistics such as the adjusted goodness-of-fit index (0.98), chi-square (X2 =23.41; dF=25; P = 0.55), root mean square residual (0.14), root mean square error of approximation (P < 0.0001)), all of which indicated an adequate fit to the proposed model. The standardized path coefficients are displayed above their respective arrows in Figure 1. Rest pain on postoperative day one significantly affects both postoperative opioid use (P ≤ 0.001) and delirium status (P ≤ 0.01) on day one. Postoperative opioid use on day one significantly affects delirium on day one (P ≤ 0.05) and subsequent opioid use on day two (P ≤ 0.001). However, delirium status on day one does not affect opioid use on day two.
Discussion
We evaluated the association between delirium, pain and pain management to provide insight into whether treatment for postoperative pain should include the patient’s delirium status. There are several novel findings from the current study: first, we showed that postoperative delirium did not limit patient’s use of on-demand PCA; second, despite the use of PCA opioids, patients with postoperative delirium still experienced higher VAS scores than those who were not delirious.
An important question is whether patients with delirium can actually communicate their pain experience, and if so, whether the VAS scores in these patients are reproducible. Although our test-retest reliability assessment suggested that patients with delirium were consistent in reporting their pain experience, we do not have an independent method to verify the validity of VAS scores. Specifically, we cannot determine whether their self reported higher pain state actually reflects a ceiling effect of the PCA not completely mitigating their acute postoperative pain, since there is no other existing standard to determine that the VAS is actually valid in patients with delirium. As a result, the quantitative validity of the VAS in delirious patients is uncertain and needs further investigation.
Comparison with previous studies
In healthy volunteers without preexisting cognitive impairment, substantial cognitive impairment was associated with parenterally administered opioids and the impairment was dose related. 14–17 The association between delirium and opioid use has also been studied in patients with cancer and adults with acute pain. Although an association is shown between opioids and delirium in these two groups of patients, whether the relationship is causal cannot be demonstrated since these patients typically have other comorbid conditions such as organ failure, dementia or hypoxia. 18 In contrast to the several prospective studies of hospitalized patients which showed that opioid use was associated with delirium, 19–22 our study was the first to use consecutive daily assessments of resting pain, delirium, and opioid dose to show that patients who were delirious used either the same amount or more opioids even after adjusting for VAS scores, and other co-variates associated with postoperative delirium.
Which condition precipitates delirium – is it under treated pain or opioids? Several studies in both medical and surgical patients have investigated the importance of pain management on delirium. In a prospective observational study by Lynch et al, 23 higher pain scores at rest were associated with an increased risk of delirium over the first three postoperative days in patients undergoing non-cardiac surgery. In a study of patients who suffered hip fracture, Morrison et al. showed that avoiding opioids or using very low doses of opioids increased the risk of in-hospital delirium 24 In addition to our previous work 10,25 which show that both pain and opioids are contributing factors to the occurrence of postoperative delirium, the current findings show that pain is associated with postoperative delirium not because of patients’ inability to use the on-demand PCA device, rather, these patients were using the device just as frequently as their non-delirious counterparts as reflected by the amount of hydromorphone used.
Our results suggest that there is room for improvement of pain management in older patients who are at risk of developing postoperative delirium, given the higher reported VAS scores in delirious patients despite similar or even higher amount of opioids used in the postoperative period. Our present results, however, are unable to determine the “chicken or the egg” causality dilemma with respect to the relationship between pain and postoperative delirium. One way to determine if pain and/or opioids are causative factors for postoperative delirium is through an interventional trial to reduce acute postoperative pain by either non-narcotic adjuvant or by regional analgesia.
Prevention and treatment of geriatric syndromes such as postoperative delirium should focus on intervening on common sets of high impact risk factors, such as the management of pain. The illustrative model depicted in figure 1 shows the hypothesized association between postoperative opioid use, pain, and delirium. Prior studies suggest that pain relief may be associated with an improvement in cognitive performance, as shown in patients with neuropathic pain. 26 Whether this result can be extended to older patients with acute postoperative pain needs to be determined by future studies since the pathophysiology of chronic pain is different from acute pain. Although our present study did not specifically address the other factors previously shown to be associated with postoperative delirium, we need to be mindful that in addition to pain and opioids, the occurrence of postoperative delirium is likely a multifactorial phenomenon and it is not clear if the effects of various precipitating factors are additive or multiplicative.
Clinical implications
In current clinical practice, opioids are considered the “gold standard” for the treatment of acute postoperative pain despite the important side effects known to occur with opioids such as respiratory depression, mental status changes including delirium, and constipation, etc. Complete avoidance of opioids in the postoperative period is generally not feasible as nonopioid analgesics are typically not potent enough to alleviate acute surgical pain. Therefore, one possible clinical implication is that adjuvant techniques that are opioid-sparing may be good candidates to be used in patients at risk of postoperative delirium. These techniques may include adjuvant nonopioid analgesics 27 or regional techniques such as peripheral nerve blocks or epidural analgesia. Future studies investigating the role of these opioid-sparing techniques to decrease postoperative delirium are indicated. In fact, a proactive evidence-based comprehensive geriatric assessment program recognizes that postoperative pain control is an important area to target for intervention for outcomes improvement. 28
Potential limitations
We focused on measuring delirium in the early postoperative period, as subjects in this investigation were included in a larger studying examining perioperative management and delirium. As a result, incidents of later onset delirium may have been missed. Although we have described an association between postoperative opioids use, VAS scores and postoperative delirium, we cannot determine the mechanism as to how these factors interact to precipitate postoperative delirium. Future investigations are needed to determine the precise relationship.
We used pain measurement at the same time of delirium assessment to represent the pain status for each specific postoperative day. Since the measurement of pain at one point in time may not accurately represent the dynamic nature of pain over a 24 h period, our methodology of adjusting opioid usage by VAS scores may be an oversimplification of this complex relationship between opioid usage, delirium, and pain.
In addition, the use of a subjective instrument in measuring pain such as the VAS may pose substantial limitation in subjects with perceptual disturbance such as postoperative delirium, as the validity of the assessment is uncertain. Future investigations to evaluate the validity of VAS in patients with postoperative delirium are clearly indicated.
Summary
Postoperative delirium did not appear to limit the patient’s use of on-demand PCA. Despite the use of more intravenous opioids, postoperative VAS scores were significantly higher in patients with postoperative delirium compared to those who were non-delirious. These results suggest that additional studies are needed to determine the optimal way to measure and manage pain in patients with postoperative delirium.
Acknowledgments
This project was supported in part by the National Institute of Aging, National Institutes of Health, Bethesda, MD, Grant #1K24 AG00948-05 (JML) and the Anesthesia Patient Safety Foundation (Indianapolis, IN)/Anesthesia Healthcare Partners Research Award (JML). A portion of this work was presented at the 2005 Annual Meeting of the American Society of Anesthesiologists in Las Vegas, NV, on October 26, 2005
References
- 1.Lipowski Z. Delirium (acute confusional states) JAMA. 1987;258:1789–92. [PubMed] [Google Scholar]
- 2.Lipowski Z. Delirium in the elderly patient. New Engl J Med. 1989;320:578–82. doi: 10.1056/NEJM198903023200907. [DOI] [PubMed] [Google Scholar]
- 3.Inouye S. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. 1994;97:278–88. doi: 10.1016/0002-9343(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 4.Parikh S, Chung C. Postoperative delirium in the elderly. Anesth Analg. 1995;80:1223–32. doi: 10.1097/00000539-199506000-00027. [DOI] [PubMed] [Google Scholar]
- 5.Brauer C, Morrison RS, Silberzweig SB, Siu AL. The cause of delirium in patients with hip fracture. Arch Intern Med. 2000;160:1856–60. doi: 10.1001/archinte.160.12.1856. [DOI] [PubMed] [Google Scholar]
- 6.Inouye S, Charpentier P. Precipitating factors for delirium in hospitalized elderly persons: predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852–7. [PubMed] [Google Scholar]
- 7.O’Hara D, Duff A, Berlin J, Poses R, Lawrence V, Huber E, Noveck H, Strom B, Carson J. The effect of anesthetic technique on postoperative outcomes in hip fracture repair. Anesthesiology. 2000;92:947–57. doi: 10.1097/00000542-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Williams-Russo P, Sharrock N, Mattis S, Liguori G, Mancuso C, Peterson M, Hollenberg J, Ranawat C, Salvati E, Sculco T. Randomized trial of hypotensive epidural anesthesia in older adults. Anesthesiology. 1999;91:926–35. doi: 10.1097/00000542-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Williams-Russo P, Sharrock N, Mattis S, Szatrowski T, Charlson M. Cognitive effects after epidural vs general anesthesia in older adults. JAMA. 1995;274:44–50. [PubMed] [Google Scholar]
- 10.Vaurio L, Sands L, Wang Y, Mullen E, Leung J. The role of pain and medications on postoperative delirium. Anesth Analg. 2006;102:267–73. doi: 10.1213/01.ane.0000199156.59226.af. [DOI] [PubMed] [Google Scholar]
- 11.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–7. [Google Scholar]
- 12.Inouye S, van Dyke C, Alessi C, Balkin S, Siegal A, Horwitz R. Clarifying confusion: the confusion assessment method. Ann Intern Med. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 13.ACC/AHA guideline update for the perioperative cardiovascular evaluation for noncardiac surgery - executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Anesth Analg. 2002;94:1052–64. doi: 10.1097/00000539-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Hill JL, Zacny JP. Comparing the subjective, psychomotor, and physiological effects of intravenous hydromorphone and morphine in healthy volunteers. Psychopharmacology (Berl) 2000;152:31–9. doi: 10.1007/s002130000500. [DOI] [PubMed] [Google Scholar]
- 15.Kerr B, Hill H, Coda B, Calogero M, Chapman CR, Hunt E, Buffington V, Mackie A. Concentration-related effects of morphine on cognition and motor control in human subjects. Neuropsychopharmacology. 1991;5:157–66. [PubMed] [Google Scholar]
- 16.Walker DJ, Zacny JP. Subjective, psychomotor, and physiological effects of cumulative doses of opioid mu agonists in healthy volunteers. J Pharmacol Exp Ther. 1999;289:1454–64. [PubMed] [Google Scholar]
- 17.Zacny JP, Lichtor JL, Thapar P, Coalson DW, Flemming D, Thompson WK. Comparing the subjective, psychomotor and physiological effects of intravenous butorphanol and morphine in healthy volunteers. J Pharmacol Exp Ther. 1994;270:579–88. [PubMed] [Google Scholar]
- 18.Ersek M, Cherrier MM, Overman SS, Irving GA. The cognitive effects of opioids. Pain Manag Nurs. 2004;5:75–93. doi: 10.1016/j.pmn.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. Jama. 1990;263:1097–101. [PubMed] [Google Scholar]
- 20.Marcantonio ER, Juarez G, Goldman L, Mangione CM, Ludwig LE, Lind L, Katz N, Cook EF, Orav EJ, Lee TH. The relationship of postoperative delirium with psychoactive medications. Jama. 1994;272:1518–22. [PubMed] [Google Scholar]
- 21.Schor JD, Levkoff SE, Lipsitz LA, Reilly CH, Cleary PD, Rowe JW, Evans DA. Risk factors for delirium in hospitalized elderly. Jama. 1992;267:827–31. [PubMed] [Google Scholar]
- 22.Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109:2365–73. doi: 10.1002/cncr.22665. [DOI] [PubMed] [Google Scholar]
- 23.Lynch E, Lazor M, Gellis J, Orav J, Goldman L, Marcantonio E. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998;86:781–5. doi: 10.1097/00000539-199804000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Morrison RS, Magaziner J, Gilbert M, Koval KJ, McLaughlin MA, Orosz G, Strauss E, Siu AL. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76–81. doi: 10.1093/gerona/58.1.m76. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Sands LP, Vaurio L, Mullen EA, Leung JM. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15:50–9. doi: 10.1097/01.JGP.0000229792.31009.da. [DOI] [PubMed] [Google Scholar]
- 26.Rowbotham MC, Twilling L, Davies PS, Reisner L, Taylor K, Mohr D. Oral opioid therapy for chronic peripheral and central neuropathic pain. N Engl J Med. 2003;348:1223–32. doi: 10.1056/NEJMoa021420. [DOI] [PubMed] [Google Scholar]
- 27.Leung J, Sands L, Rico M, Petersen K, Rowbotham M, Dahl J, Ames C, Chou D, Weinstein P. Pilot clinical trial of gabapentin to decrease postoperative delirium in older surgical patients. Neurology. 2006;67:1–3. doi: 10.1212/01.wnl.0000233831.87781.a9. [DOI] [PubMed] [Google Scholar]
- 28.Harari D, Hopper A, Dhesi J, Babic-Illman G, Lockwood L, Martin F. Proactive care of older people undergoing surgery (‘POPS’): designing, embedding, evaluating and funding a comprehensive geriatric assessment service for older elective surgical patients. Age Ageing. 2007;36:190–6. doi: 10.1093/ageing/afl163. [DOI] [PubMed] [Google Scholar]