Abstract
PURPOSE
Cystinuria, an inherited defect of dibasic amino acid transport, causes accumulation of urinary cystine and cystine urolithiasis. In adults, penicillamine reduces stone formation but has a high incidence of dose-limiting toxicity. We sought to evaluate the effects of penicillamine in preventing stone formation and on toxicity in our cystinuria cohort at a Pediatric Biochemical Genetics clinic.
MATERIALS AND METHODS
We reviewed charts from all 11 cystinuric children treated in our clinic using a gradual dose escalation of penicillamine (mean age at diagnosis ± SD: 5.8 ± 4.3 years; range: 1.2 - 12 years). We tracked urinary cystine concentration prior to and after initiation of treatment, penicillamine side effects, and incidence of new stones while on maintenance therapy.
RESULTS
During the gradual escalation of penicillamine to the target dose, none of the 11 patients experienced toxicity, and all had improved urinary cystine concentration (mean % reduction ± SD: 54 ± 25%; range: 5 - 81%). We followed the patients for a total of 1203 months (mean duration ± SD: 109 ± 73 months; range 41-221 months), periodically assessing urinary cystine concentration, urine protein content, complete blood count, blood urea nitrogen, creatinine and liver function. During this time, only two patients experienced significant side effects and no patients developed stones or stone crises while compliant with treatment.
CONCLUSIONS
In our cohort, penicillamine was well-tolerated after gradual initiation and it reduced urinary cystine concentration. Long-term compliance with the medication appeared to protect patients from acute stone crises.
Keywords: amino acid transport disorders, inborn, urolithiasis, drug toxicity
INTRODUCTION
Cystinuria is an autosomal recessive defect in transport of the amino acids cystine, ornithine, arginine and lysine in the intestinal epithelium and proximal renal tubule. Its major morbidity, urolithiasis, results from profoundly decreased cystine resorption in the proximal renal tubule, causing the urinary cystine concentration to exceed its solubility threshold of 1200 μM1. In addition to episodic renal colic, patients are at long-term risk for loss of renal function. Cystinuria has a worldwide incidence of approximately 1:7000, and is a major metabolic cause of pediatric urolithiasis, causing 8% of all pediatric stones2, 3.
At the molecular level, cystinuria results from dysfunction of the b0,+ heteromeric amino acid transporter, which is composed of heavy and light subunits. Type I (fully recessive) cystinuria is caused by mutations in SLC3A1, the gene encoding rBAT, a heavy subunit, while non-Type I (incompletely recessive) cystinuria is usually caused by mutations in SLC7A9, encoding a light subunit3. Both types cause urolithiasis but differ in heterozygote cystine excretion (elevated in non-Type I; normal in Type I).
A variety of treatment options have been proposed to treat cystinuria. Increasing fluid intake increases urinary volume and reduces cystine crystallization somewhat4. However, the urinary volume needed to reduce cystine concentration below the solubility threshold is high, and consequently fluid therapy alone is not feasible for very young patients. A second option is to alkalinize the urine with oral agents (e.g. potassium citrate), because cystine solubility increases when urine pH exceeds 7.5. However, alkalinization therapy, even in conjunction with increased fluid intake, is insufficient to eliminate formation of new stones in most patients5, 6.
The most effective therapy for cystinuria is oral administration of thiol-containing compounds like penicillamine (β,β-dimethylcystine), which form mixed-disulfides with urinary cystine, reducing crystallization. Penicillamine's effectiveness in reducing stone formation and dissolving pre-existing stones in cystinuria has been well-documented5-8. Compliance with a medical regimen that includes penicillamine or other thiol-containing compounds has been found to protect against the formation of new stones and the need for urological interventions to remove stones9, 10. However, penicillamine's toxicities, including marrow suppression, proteinuria, arthralgias, febrile reactions, loss of taste, liver dysfunction and skin eruptions require interruption or termination of treatment in many patients. In a series of 39 adult patients, 50% of penicillamine-treated patients had rash or proteinuria8, while in another series, a variety of toxicities (especially febrile reactions, skin eruptions and proteinuria) forced discontinuation in 40% of patients11.
Although penicillamine has been used in pediatric patients for some forty years, little information exists about either its safety or efficacy in this age group. In a series of fifteen Japanese children, 85% had a side effect to the medication12. We are not aware of a similar study in an American population, and our experience has been that most children and adolescents tolerate the medication if monitored closely. We present our eighteen years’ experience in treating pediatric cystinuria with penicillamine. We employed a treatment plan featuring gradual introduction of the drug and close monitoring of side effects. In these children, penicillamine was well-tolerated and decreased urinary cystine concentration, and patients who adhered to the dosing schedule did not have acute stone crises.
MATERIALS AND METHODS
All patients consented to participate in this chart review via a consent form approved by the Institutional Review Board at The Children's Hospital of Philadelphia (CHOP). We performed a comprehensive, retrospective chart review on all children treated for cystinuria in the CHOP Section of Biochemical Genetics. Nine of the 11 patients were initially referred from urology or nephrology clinics after diagnosis of urolithiasis and high urinary cystine. Their symptoms at presentation are listed in Table 1. Three children were between 13 and 45 months old at presentation; six were between 6 and 11 years old. One child (#4) had surgery for suspected appendicitis, and several had been treated for presumptive urinary tract infections. Two others (#2 and #11) were diagnosed pre-symptomatically by screening family members of a patient, and were stone free at the time of penicillamine initiation. Patient #2 is the sibling of patient #1 and patient #11 is the sibling of patient #10. An 8 year old girl (#7) with a staghorn calculus was diagnosed serendipitously when acting as a control subject for an abdominal ultrasound study. In addition to the patients reported here, three other children were diagnosed with cystinuria in our clinic but declined treatment.
Table 1.
Baseline data for 11 children with cystinuria
| Patient | Sex | Age (y) | Symptoms | Calculi at presentation |
|---|---|---|---|---|
| 1 | F | 6 | RC, F, H | Staghorn |
| 2 | M | 1.5 | None | None |
| 3 | M | 1.2 | I, RC, PS | Four stones in right kidney, bladder and urethra. Largest 0.9 × 0.4cm |
| 4 | F | 11.8 | RC, V | Two in right ureter, size unknown |
| 5 | M | 1.2 | MH | One stone (2 cm) in right lower pole |
| 6 | F | 2 | RC, H | Unknown |
| 7 | F | 8 | None | Left staghorn calculus; also multiple foci in both kidneys. |
| 8 | M | 11 | RC | Left: three stones, including 0.9 × 0.5 cm stone in proximal ureter. |
| 9 | M | 3.9 | H, PS | Right: three stones in pelvis, 0.7-1.1cm; three in distal ureter (0.4-0.6 cm). |
| 10 | M | 7 | RC, H | Left: “Large” stone in pelvis; three “small” stones in ureter. |
| 11 | M | 12 | None | None |
Abbreviations: RC, renal colic; F, fever; H, hematuria; I, irritability; PS, passed stone; V, vomiting; MH, microscopic hematuria.
Urinary cystine concentrations were determined in the CHOP Metabolic Diagnostic Laboratory. Patients collected 24-hour urine samples and froze them until analysis. Samples were acidified with 12 N HCl until pH was 1-2. An aliquot of 500 mL was deproteinized with 15% sulfosalicylic acid and analyzed by high performance liquid chromatography on a cation-exchange column using a post-column ninhydrin detection system. Using this method, the normal range of urinary cystine concentration in our laboratory is 29-308 μM. Other evaluations, including blood tests (complete blood counts, BUN, creatinine), urine protein content and periodic renal ultrasounds were performed using standard techniques at CHOP or other facilities. In a few patients, abdominal CT scans were also performed to improve visualization of calculi and/or anatomy.
RESULTS
Penicillamine initiation
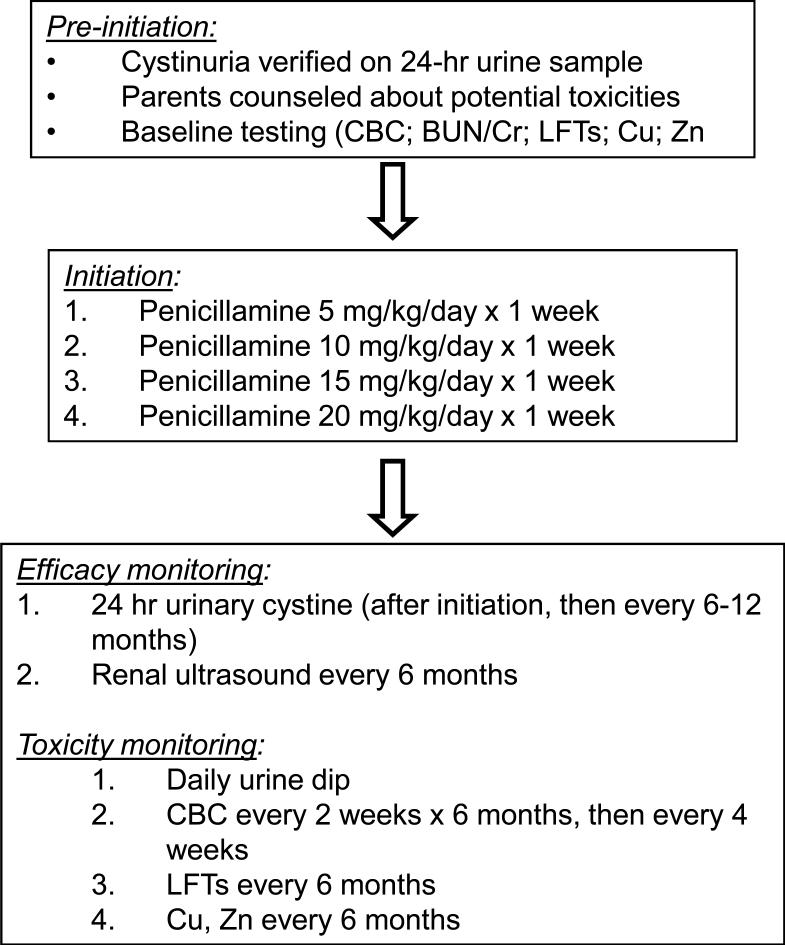
We attempted to prevent penicillamine side effects by gradually increasing the dose, a strategy used effectively in adults7. After confirming the diagnosis of cystinuria with a urine amino acid quantitation in our laboratory, we counseled parents about the utility of penicillamine and offered initiation of the medicine. For patients who agreed to start therapy, we used the protocol in Figure 1, in which the goal penicillamine dose (20 mg/kg/day) was achieved after 4-6 weeks. Prior to starting therapy, we discussed potential penicillamine toxicities and provided parents with a list of potential symptoms. We also counseled patients to increase fluid intake, including waking up overnight to drink water, and decrease dietary sodium intake.
Penicillamine treatment plan. Copper (Cu) and zinc (Zn) measurements were obtained because of penicillamine's effect on increasing losses of these metals through the urine. Abbreviations: CBC, complete blood count; BUN, blood urea nitrogen; Cr, creatinine; LFT, liver function tests.
Using this protocol, we initiated penicillamine in eleven patients (mean age at initiation ± SD: 7.4 ± 5.3 years; range 1.2 to 12.5 years), as shown in Table 2. Nine of these children (all except #2 and #11) had urolithiasis at the time of diagnosis. No patient experienced dose-limiting penicillamine-associated toxicity during the initiation protocol. We determined urine cystine concentration on a follow-up sample when the goal dose (20 mg/kg/day) was reached. All patients had decreased urinary cystine concentration on sampling after the end of the initiation period (Table 2). In two patients, the first follow-up urine cystine concentration was below 1200 μM, the theoretical solubility limit of cystine; three others had urine cystine below 1500 μM.
Table 2.
Penicillamine initiation in 11 children with cystinuria
| Patient | Sex | Age at initiation (y) | Toxicity | % decrease, [cystine]ur* |
|---|---|---|---|---|
| 1 | F | 6.9 | None | 79% |
| 2 | M | 2.8 | None | 81% |
| 3 | M | 1.2 | None | 54% |
| 4 | F | 11.8 | None | 58% |
| 5 | M | 1.5 | None | 58% |
| 6 | F | 2 | None | N/A1 |
| 7 | F | 17 | None | 38% |
| 8 | M | 12.5 | None | 36% |
| 9 | M | 4.3 | None | 51% |
| 10 | M | 7.3 | None | 5% |
| 11 | M | 12 | None | 34% |
% decrease, [cystine]ur = decrease in urinary cystine concentration between pretreatment specimen and first specimen on maintenance dose
No pre-treatment specimen available in our records.
Penicillamine maintenance
Data from long-term penicillamine maintenance are presented in Table 3. Six patients were maintained on 20 mg/kg/day, while increased doses (maximum 40 mg/kg/day) were used in five others to reduce urinary cystine concentration further. Over a total of 1203 patient-months, only two significant toxicities occurred (Table 3): generalized aminoaciduria in a 7-year-old boy after three years of therapy; and a 3-cm elastosis perforans serpiginosa (EPS) rash on the neck of a 22 year old woman after fifteen years. The boy's family opted to discontinue treatment and the amino aciduria improved, but it recurred while he was still off the medication. Penicillamine was then re-started and the child had a transient episode of aminoaciduria that resolved without discontinuation of the medicine. The woman with the EPS rash discontinued penicillamine for twelve months, then developed new stones requiring surgical removal; these were her first stones since age 6. An echocardiogram and MR angiogram showed no evidence of connective tissue abnormalities involving the great vessels, and serum copper levels were normal, so she restarted penicillamine and has since been asymptomatic and without stone crises. Several other patients complained of minor, transient problems (e.g. arthralgia) that did not require discontinuing the medication.
Table 3.
Penicillamine maintenance therapy in 11 children with cystinuria
| Pt | Sex | Duration of treatment (months) | Max dose (mg/kg/day) | Toxicity |
|---|---|---|---|---|
| 1 | F | 221 | 40 | Yes1 |
| 2 | M | 217 | 38 | No |
| 3 | M | 45 | 20 | No |
| 4 | F | 114 | 20 | No |
| 5 | M | 49 | 20 | No |
| 6 | F | 209 | 40 | No |
| 7 | F | 58 | 20 | No |
| 8 | M | 67 | 20 | No |
| 9 | M | 41 | 25 | Yes2 |
| 10 | M | 118 | 30 | No |
| 11 | M | 64 | 20 | No |
elastosis perforans serpiginosa; developed after 15 years of treatment
generalized amino aciduria; developed after 3 years treatment and recurred while off therapy.
During penicillamine treatment, six of the eleven patients had urine cystine concentrations below 1200 μM on 20-40 mg/kg/day. The other five always had urine cystine levels in the theoretical stone-forming range. In compliant patients, the penicillamine-cysteine disulfide levels were approximately equal to those of cysteine. Our follow-up regimen included renal ultrasounds every 6 months to evaluate for the appearance of new stones. In general, we observed gradual resolution of small, non-obstructing stones that were present when the patients began taking penicillamine. Several patients (#6, 7, 8, 9, 10 and 11) had ultrasound evidence of new stones and/or suffered acute stone crises at some point during the period evaluated. However, in every case these events coincided with periods of poor compliance, evidenced by history and/or lack of penicillamine disulfide in the urine. Among these events, surgery was required to remove a staghorn calculus and an obstructing 6 mm right ureteral stone in patient #6, and to place stents for bilateral stone recurrence in patient #10. Small, non-obstructing stones occurred in other patients and ultimately resolved when penicillamine was restarted. None of the patients who complied with the treatment regimen had acute stone crises or required surgery to remove stones. Renal function, monitored by measurement of BUN and creatinine, remained normal in all patients.
DISCUSSION
Cystinuria is an important cause of pediatric urolithiasis. Long-term control of cystine crystallization is necessary to reduce frequency of stone crises and the risk of renal insufficiency. We reviewed eighteen years’ experience treating pediatric cystinuria with penicillamine using a gradual introduction of the medicine and a consistent surveillance program to monitor for drug toxicities. Using these methods, our patients had just two significant side effects over 1203 patient-months of therapy: an EPS rash in a 22-year old woman and generalized amino aciduria in a 7-year old boy. EPS, an acquired abnormality of elastin, is a well-known complication of penicillamine therapy and may cause pruritis and scarring13. It manifests as a wavy, linear, arched or circular rash, measuring several centimeters in diameter. The rash is initially composed of papules, a few to several millimeters in diameter, that become dimpled and increase in number. The skin behind the papules is hypopigmented and atrophic. The most common sites are the neck (70%), upper extremities (20%), face (11%), lower extremities (6%), and trunk (3%). The pathophysiology of EPS seems to involve chelation of copper and subsequent impairment of the copper-dependent enzyme lysyl oxidase, whose function is required for proper elastin cross linking. Risk of EPS is increased by inherited connective tissue abnormalities such as Ehlers-Danlos syndrome and Marfan syndrome13; our patient lacked signs of these diseases on physical examination and echocardiography. EPS is difficult to treat medically. In the boy with generalized amino aciduria, it should be noted that this problem might have been unrelated to the medication, as it both recurred when he was off penicillamine, and resolved while on penicillamine. Over the time period of this study, none of our patients had an acute stone crisis or passed a stone while compliant with treatment.
The incidence of penicillamine toxicity in our study is much lower than that reported in other series of cystinuria patients8, 11, 14, 15, including a study of Japanese children12. The reasons for the difference may be multi-factorial, perhaps involving genetic variation among the populations studied. However, our opinion is that gradually increasing the penicillamine dose may also be an important factor. While we did not have a control group in whom penicillamine was initiated at the target dose, it is relevant that other groups have found a significant risk of toxicity, as high as 25% in one study8, within the first two weeks of therapy. In contrast, Crawhall gradually increased penicillamine dose in fifteen cystinuric adults to define a minimum effective dose for each7. Only two of his patients had serious side effects requiring permanent cessation of the medicine; several others had transient phenomena including loss of taste and rash. The need for successful initiation of thiol-containing compounds in cystinuria is underscored by the demonstration that compliance to a medical regimen containing these drugs reduces new stone formation and the need for urological intervention9, 10.
It is gratifying that no compliant patients had stone events, but also surprising, considering that five never achieved a urine cystine concentration below the supposed solubility limit. This observation suggests that urine cystine concentration alone is an imperfect predictor of crystallization risk. Other authors have come to a similar conclusion, in part because cystine solubility, a more relevant factor than concentration per se, varies widely among urine samples and is not adequately predicted by pH nomograms16, 17. Hence, periodic radiological assessments to monitor growth of preexisting stones and appearance of new ones is an important adjunct to urine amino acid analysis in monitoring treatment. A clinical assay to measure cystine supersaturation in the presence of thiol-containing medications has been developed, and may improve prediction of crystallization risk18.
CONCLUSIONS
We found penicillamine to be useful in reducing urinary cystine concentrations in our cohort of children and adolescents with cystinuria, if there was gradual initiation and careful monitoring of the medication. Our data argue that penicillamine can be considered a first-line therapy in pediatric and adolescent cystinuria, as opposed to an adjunct therapy after failure of more conservative but less effective treatments (alkalinization, fluid therapy). In addition, the low incidence of side effects suggests that gradual initiation may be useful in treating other chronic diseases with penicillamine, including Wilson disease and refractory rheumatoid arthritis.
REFERENCES
- 1.Palacin M, Goodyer P, Nunes V, Gasparini P. Cystinuria. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metaboic and Molecular Bases of Inherited Disease. 8th ed. III. McGraw-Hill; New York: pp. 4909–4932, 2001. [Google Scholar]
- 2.Levy HL. Genetics Screening. In: Harris H, Hirschhorn K, editors. Advances in Human Genetics. Vol. 4. Plenum; New York: 1973. p. 1. [Google Scholar]
- 3.Milliner DS. Cystinuria. Endocrinol Metab Clin North Am. 1990;19:889. [PubMed] [Google Scholar]
- 4.Dent CE, Friedman M, Green H, Watson LC. Treatment of Cystinuria. Br Med J. 1965;1:403. [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlberg PJ, van den Berg CJ, Kurtz SB, Wilson DM, Smith LH. Clinical features and management of cystinuria. Mayo Clin Proc. 1977;52:533. [PubMed] [Google Scholar]
- 6.Chow GK, Streem SB. Medical treatment of cystinuria: results of contemporary clinical practice. J Urol. 1996;156:1576. doi: 10.1016/s0022-5347(01)65451-x. [DOI] [PubMed] [Google Scholar]
- 7.Crawhall JC. Cystinuria--an experience in management over 18 years. Miner Electrolyte Metab. 1987;13:286. [PubMed] [Google Scholar]
- 8.Stephens AD. Cystinuria and its treatment: 25 years experience at St. Bartholomew's Hospital. J Inherit Metab Dis. 1989;12:197. doi: 10.1007/BF01800726. [DOI] [PubMed] [Google Scholar]
- 9.Dello Strologo L, Laurenzi C, Legato A, Pastore A. Cystinuria in children and young adults: success of monitoring free-cystine urine levels. Pediatr Nephrol. 2007;22:1869. doi: 10.1007/s00467-007-0575-2. [DOI] [PubMed] [Google Scholar]
- 10.Pareek G, Steele TH, Nakada SY. Urological intervention in patients with cystinuria is decreased with medical compliance. J Urol. 2005;174:2250. doi: 10.1097/01.ju.0000181817.89703.66. [DOI] [PubMed] [Google Scholar]
- 11.Lindell A, Denneberg T, Granerus G. Studies on renal function in patients with cystinuria. Nephron. 1997;77:76. doi: 10.1159/000190250. [DOI] [PubMed] [Google Scholar]
- 12.Asanuma H, Nakai H, Takeda M, Shishido S, Tajima E. Kawamura, et al., editors. [Clinical study on cystinuria in children--the stone management and the prevention of calculi recurrence]. Nippon Hinyokika Gakkai Zasshi. 1998;89:758. doi: 10.5980/jpnjurol1989.89.758. [DOI] [PubMed] [Google Scholar]
- 13.Lewis KG, Bercovitch L, Dill SW, Robinson-Bostom L. Acquired disorders of elastic tissue: Part I: Increased elastic tissue and solar elastotic syndromes. J Am Acad Dermatol. 2004;51:1. doi: 10.1016/j.jaad.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Barbey F, Joly D, Rieu P, Mejean A, Daudon M, Jungers P. Medical treatment of cystinuria: critical reappraisal of long-term results. J Urol. 2000;163:1419. doi: 10.1016/s0022-5347(05)67633-1. [DOI] [PubMed] [Google Scholar]
- 15.Akakura K, Egoshi K, Ueda T, Nozumi K, Kotake T, Masai M, et al. The long-term outcome of cystinuria in Japan. Urol Int. 1998;61:86. doi: 10.1159/000030294. [DOI] [PubMed] [Google Scholar]
- 16.Pak CY, Fuller CJ. Assessment of cystine solubility in urine and of heterogeneous nucleation. J Urol. 1983;129:1066. doi: 10.1016/s0022-5347(17)52543-4. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa Y, Asplin JR, Goldfarb DS, Parks JH, Coe FL. Clinical use of cystine supersaturation measurements. J Urol. 2000;164:1481. [PubMed] [Google Scholar]
- 18.Coe FL, Clark C, Parks JH, Asplin JR. Solid phase assay of urine cystine supersaturation in the presence of cystine binding drugs. J Urol. 2001;166:688. [PubMed] [Google Scholar]