Abstract
Glycolipid reactive CD1d restricted natural killer T (NKT) cells represent a distinct population of T cells implicated in the regulation of immune responses in a broad range of diseases including cancer. Several studies have demonstrated the capacity of NKT cells bearing an invariant T cell receptor (iNKT cells) to recruit both innate and adaptive anti-tumor immunity and mediate tumor rejection in mice. Early phase clinical studies in humans have demonstrated the capacity of dendritic cells (DCs) to mediate expansion of NKT cells in vivo. However several challenges need to be overcome in order to effectively harness the properties of these cells in the clinic.
Keywords: Innate Immunity, Natural Killer T cells, Glycolipid, Dendritic Cells, Review
2. INTRODUCTION
The immune system has several cellular components that can theoretically be recruited for protection from tumors.(1) CD4+ and CD8+ T cells recognize peptide ligands in the context of major histocompatibility complex (MHC) I and II products and are the critical adaptive components of cell-mediated immunity. In contrast, a distinct subset of T cells termed natural killer T (NKT) cells does not respond to peptide antigens but to glycolipid ligands in the context of CD1d molecules on antigen presenting cells.(2) The role of CD4 and CD8+ T cells in anti-tumor immunity is extensively studied.(1) Over the past several years, there have been considerable advances in understanding the biology of antigen presentation of glycolipids and the possible role of NKT cells in anti-tumor immunity, particularly in mice.(3) This review focuses on the potential capacity of these glycolipid reactive T cells to modulate tumor immunity. I will particularly emphasize the data from studies with human cells and studies harnessing this aspect of the immune system in the clinic.
3. DIVERSITY OF CD1D RESTRICTED NATURAL KILLER T CELLS
NKT cells were first characterized in mice as cells that express both T cell receptor (TCR) and NK1.1, a C-lectin type NK receptor.(4) This definition however does not satisfactorily delineate the spectrum of glycolipid reactive lymphocytes.(2, 5) This has led to a revised nomenclature that emphasizes the CD1d restricted nature of these cells.(2, 6–8) At least 3 distinct subsets of NKT cells exist, and these distinctions may have major implications for role of NKT cells in regulating tumor immunity.(9) Much of the current data is restricted to T cells staining with CD1d tetramers loaded with a synthetic ligand, α-galactosyl ceramide (α-GalCer). These cells express an invariant T cell receptor (Vα14 in mice and Vα24 in humans), and are termed as type I NKT cells or invariant NKT (iNKT) cells (10). These cells are also the subject of much of the discussion below, due to their anti-tumor properties (Figure 1). However, it has also become clear that several of the glycolipid reactive CD1d restricted T cells, particularly in humans, lack invariant TCR (termed type II NKT cells).(11–15) Finally, there is a heterogeneous subset of T cells with diverse TCRs that express NK markers, but are not CD1d restricted or glycolipid reactive, and are termed type III NKT cells.(16) These latter cells are diverse in their phenotypic and functional properties and not discussed here.
Figure 1.
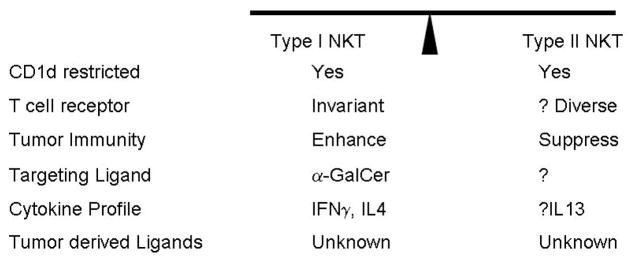
Balance of immune-regulatory NKT subpopulations. A growing body of data suggests that the balance of two distinct types of CD1d restricted NKT cells may impact the balance of tumor immunity.
4. TISSUE DISTRIBUTION AND SUBSETS OF CD1D RESTRICTED T CELLS IN HUMANS AND MICE
In mice, the primary site for iNKT cells is the liver, where they account for 20–40% of intrahepatic lymphocytes. In humans, the frequency of iNKT cells is much lower than in mice (including in the human liver), although there is considerable interindividual heterogeneity.(2, 8) In contrast, a sizeable population of CD1d restricted T cells in the human bone marrow and liver may consist of type II NKT cells.(12, 13) Human iNKT cells include at least 2 major subsets, CD4+ and double negative (CD4-CD8-), although CD8+ NKT cells have also been described.(17, 18) Functionally, the different subsets of human iNKT cells differ in terms of their ability to produce cytokines in vitro and expression of chemokine receptors. For example, IL-4 production appears to be largely restricted to the CD4+ subset, while the double negative subset can produce interferon-γ. In mice, such differences in cytokine producing capacities of different cytokine subsets are less clear. Differences in tissue distribution or cytokine production of different NKT populations may however have a major impact on the anti-tumor properties of these cells. For example, recent studies in two different mouse models suggest that the capacity of iNKT cells to stimulate anti-tumor immunity after adoptive transfer may depend on their organ of origin.(19) In contrast to liver derived NKT cells, those derived from spleen or thymus were less capable of tumor rejection.(19) Even within liver iNKT cells, CD4- iNKT cells were more potent tumor suppressors than their CD4+ counterparts. These issues may potentially be important for optimal enhancement of tumor immunity in vivo, as current approaches rely largely on systemic activation of NKT compartments concurrently in several tissues, which may be counterproductive. Whether the differences in frequency, tissue distribution, subtypes and subsets of CD1d restricted T cells in mice versus humans will have an impact on our ability to translate findings from mouse to human, remains to be determined. However they do emphasize the need to directly study humans to probe the biology of these cells, and urge caution regarding “translating” results between species.
5. ANTI-TUMOR PROPERTIES OF NKT CELLS IN MICE
Several studies have demonstrated the potential importance of NKT cells in tumor rejection in mice.(3, 8, 20, 21) Upon challenge with chemical carcinogen methylchloranthrene (MCA), mice deficient in CD1d or iNKT cells are more susceptible to tumor growth, with both an earlier onset of tumor development, as well as higher incidence of tumors.(22) These tumors exhibit an unedited phenotype, and are rejected when transplanted into wild type mice.(23) Sarcoma cell lines also grew preferentially in NKT deficient mice and could be treated by adoptive transfer of NKT cells from wild type donors (23). In these experiments, protection extends to tumors such as sarcomas that lack CD1d, indicating that direct recognition of tumors by NKT cells is not required, but instead, the mechanism depends on downstream activation of other effectors, including NK cells. NKT cells were also shown to mediate tumor rejection in a lung metastasis model of sarcoma (24), wherein they were suppressed by regulatory T cells. A fundamental question remains as to the nature of glycolipid associated antigens recognized by iNKT cells in these models.
Evidence for the anti-tumor effects of iNKT cells has also come from the experimental approaches to treat cancer. iNKT cells were found to be necessary for IL-12 mediated anti-tumor immunity in some models (25). The discovery of α-galactosylceramide (α-GalCer) as an iNKT ligand was driven largely based on its anti-tumor properties and the ability to promote NKT and CD1d dependent rejection of a broad range of tumors including melanoma, thymoma, carcinoma, and sarcoma.(26) α-GalCer can also mediate protection against chemical or oncogene dependent tumors in mice (27). The anti-tumor effects of α-GalCer involves a complex interplay of several effectors and molecules and seems to depend on its ability to induce strong production of interferon-γ in vivo.(28, 29) In addition to cytokine production and direct cytolytic function, iNKT activation can also lead to activation of other “downstream” effectors (30–33). For example, iNKT cells can cause rapid activation of NK cells in vivo in mice.(30, 31) CD1 restricted T cells, including iNKT cells can also promote DC maturation (34), and DC-NKT cross talk may allow NKT activation during microbial infections in the absence of direct recognition of microbial antigens.(35) α-GalCer mediated activation of iNKT cells may also enhance anti-tumor immunity in vivo in mice by promoting the activation of antigen presenting dendritic cells (DCs) and IL-12 production via CD40 ligand (CD40L) mediated interactions.(36–38) These adjuvant properties of iNKT cells in mice have been reviewed recently (39), and are a particularly exciting area of investigation as they suggest the possibility of harnessing the DC-NKT axis to recruit both innate and adaptive immunity. In addition to the effects on tumor and immune cells, interferon-γ mediated effects of NKT cells likely also involve inhibition of angiogenesis.(40) Consistent with the prime role for interferon-γ production by NKT cells, a C-glycoside analogue of α-GalCer that was more effective at inducing interferon production was also more effective at mediating tumor rejection as well.(41, 42) In addition to T cells, iNKT cells can also activate B cells to increase Ig secretion and therefore provide help for the generation of antibody responses.(43)
6. ENHANCEMENT OF TUMOR GROWTH BY TYPE II NKT CELLS IN MICE
Although the data discussed above support a suppressive effect of NKT cells on tumor growth, NKT cells were also shown to paradoxically enhance tumor growth in some models (44, 45). For example, experimental 15-12RM fibrosarcoma and 4T1 mammary tumors were rejected in CD1d −/− mice, but grew progressively in wild type mice. Tumor enhancement in the 15-12RM model was IL-13 dependent/IL-4 independent, and appeared to involve TGF-β production by a Gr-1+ myeloid cells, that was associated with impaired CTL responses.(46) In contrast, the effects in 4T1 model were IL-13 independent, suggesting that other mechanisms of tumor enhancement exist. CD1d dependent T cells have also been implicated in UV induced inhibition of skin carcinogenesis.(47) More recent studies have shown that the subset of NKT cells (type II) that mediates suppression of tumor immunity in at least some of these studies is different from type I or iNKT cells that suppress tumor growth. Overall, these studies suggest a cross regulation between type I and II NKT cells in regulating tumor immunity.(9, 48) As discussed earlier, the proportion of type II NKT cells may be higher in humans than in mice, although these cells have not yet been studied in the context of human tumors. It is likely that improved understanding of the cross talk between these cells may be needed to effectively translate findings made in mice to humans. Nonetheless, these studies suggest a new immune regulatory axis, between type I and II NKT cells, to regulate tumor immunity. A goal of NKT directed cancer therapies perhaps should be to boost the function of type I NKT cells (such as with α-GalCer or related ligands) relative to type II NKT cells.
7. STUDIES ON NKT CELLS IN HUMAN CANCER
The recognition that iNKT cells can mediate tumor rejection and immune surveillance in vivo and modulate the function of other downstream immune cells has led several investigators to characterize the nature of NKT cells in cancer patients.(49) Most of the effort to date has focused on iNKT cells, due to the lack of tools to monitor reliably monitor human type II NKT cells. Several studies have now reported a deficiency in iNKT numbers and/or function in the blood of patients with advanced cancers.(50, 51) In some settings such as patients with myelodysplastic syndromes, the deficiency is particularly severe, and may relate to developmental defects in the malignant clone.(52, 53) One notable exception are patients with glioma (a tumor lacking systemic metastases) who have normal numbers of circulating iNKT cells.(54) Most of the functional data suggests a deficiency of interferon-γ producing function of these cells.(50, 51) In many instances such as in multiple myeloma and prostate cancer, this appears to correlate with the clinical stage of tumors, in that the loss of NKT function is observed in patients with more progressive myeloma or hormone refractory advanced prostate cancer.(50, 51) Low levels of circulating iNKT cells were found to predict outcome in patients with head and neck cancer.(55) Nonetheless, these defects can be restored ex vivo after stimulation with α-GalCer bearing APCs suggesting that the observed defects are at least in part, reversible. Expanded NKT cells can then secrete interferon-γ, and even recognize and kill autologous tumors, if appropriately expanded in vitro using α-GalCer loaded DCs. Tumor cells in several hematologic malignancies including leukemia, lymphoma and myeloma have been shown to express CD1d, are NKT sensitive, and therefore particularly interesting targets of iNKT directed therapies.(51, 56–58)
One major caveat of most of the existing data on human NKT cells in cancer is that nearly all the data is based on NKT cells in the blood, and very few studies have analyzed iNKT cells in the tumor tissue itself.(51, 59) This is a critical issue because several aspects of NKT biology such as survival, homing and function may be altered in the tumor bed. The presence of iNKT cells infiltrating primary neuroblastoma tumors was associated with a 2 fold increase in progression free survival.(59) Another study demonstrated that NKT infiltration in primary colorectal tumors was an independent prognostic factor for survival.(60) Even less is known of the mechanisms that regulate function and survival of tumor infiltrating NKT cells. Human iNKT cells were shown to preferentially infiltrate neuroblastomas expressing the chemokine CCL2, suggesting that chemokines/other molecules secreted by tumors may recruit or modify the presence and function of iNKT cells in the tumor bed (59). Overexpression of NMYC by these tumors may repress CCL2 and provide a tumor escape mechanism by preventing NKT localization or survival.(61) Therefore activation of specific oncogenic pathways may directly help tumors escape NKT mediated surveillance. In the case of myeloma, the loss of iNKT cell function in the tumor bed correlates with the presence of clinically progressive disease.(51) The mechanism behind the observed loss of NKT effector function in cancer patients is not known. One possibility is that suppressive factors in the tumor bed or ligands expressed by tumor cells may contribute to NKT dysfunction in cancer.(62) Improved understanding of the regulation of NKT function in the tumor bed is needed to better exploit iNKT cells for therapy of cancer.
8. MANIPULATING HUMAN NKT CELLS IN VIVO
NKT cells have been implicated in regulation of a dazzling array of immune responses ranging from immunity to tumors and pathogens to autoimmunity, allergy and transplantation.(2) Therefore the ability to reliably manipulate iNKT cells in vivo in humans has broad therapeutic potential across several medical challenges, and is being actively pursued by several groups (Table 1). α-galactosylceramide (α-GalCer) is a synthetic glycolipid isolated during a screen for anti-cancer agents derived from the marine sponge Agelas mauritianus.(63–65) The discovery of α-GalCer as a potent iNKT ligand and its availability as a clinical grade reagent has permitted clinical studies to use this ligand to manipulate NKT cells in humans.(26, 66, 67) To date, all of the attempts to manipulate NKT cells in vivo in humans are based on this single ligand. However it is likely that alternate ligands will also be in the clinic in the near future.
Table 1.
Some approaches to targeting NKT cells in vivo in humans
In Clinical Trials
|
Under Development
|
8.1. Soluble alpha-GalCer therapy
As discussed above, injection of α-GalCer in vivo in mice leads to rapid iNKT activation and a “cytokine storm”, which can lead to tumor rejection in some models. In preclinical models, activation of hepatic iNKT cells was thought to carry a potential of hepatotoxicity.(68) Giaconne et al carried out a phase I trial of α-GalCer in patients with advanced cancer.(69) Overall, the infusions were very well tolerated and there was no dose limiting toxicity, including no evidence for hepatotoxicity. However the biologic effects of the infusions were also sobering and associated with only modest and transient changes in some serum cytokines, and transient NKT activation.(69) The individual responses were quite variable and seemed to depend on the baseline frequency of iNKT cells. Tolerability of this therapy has also been tested in a cohort of healthy donors. Based on the data presented at the 3rd NKT/CD1 workshop (but not yet published in a peer reviewed publication), the infusions were again well tolerated, but with only modest effects in terms of NKT activation. A major limitation of soluble α-GalCer therapy may be the induction of long term anergy by this ligand in mice.(52, 70, 71) This has not yet been convincingly documented in the context of human clinical trials of soluble α-GalCer therapy. Nonetheless, these considerations have led to alternate approaches including targeting antigen presenting cells to enhance the activation of NKT cells.
8.2. Alpha-GalCer loaded dendritic cells (DCs)
DCs are professional antigen presenting cells specialized to initiate and regulate immunity.(72, 73) The capacity to generate clinical grade DCs from precursors ex vivo has allowed clinical studies of adoptive transfer of ex vivo generated DCs.(73) Prior studies have shown that human and murine DCs are efficient APCs for the stimulation of human iNKT cells in culture and in vivo in mice (74–76). An important difference with reference to targeting antigen to DCs may relate to the induction of anergy.(70, 77) After injection of soluble glycolipid in mice, NKT cells could not be restimulated for at least a month. In contrast, injection of glycolipid loaded DCs led to prolonged NKT activation, without such induction of anergy.(76) Therefore the biologic effect of systemic injection of glycolipid is quite different from the injection of DCs loaded with the same ligand. These data suggest that the nature of antigen presenting cell (APC) presenting the glycolipid to iNKT cells has a major impact on the nature of NKT cell response in vivo. An important insight in the preclinical studies was the importance of route of administration on the effects of antigen loaded DCs. DC mediated NKT activation was observed only with intravenous, but not subcutaneous administration, which emphasizes the need to target DCs to spleen/liver/marrow (where iNKT cells are prevalent in mice), as opposed to lymph nodes. This is notably in contrast to the studies with DCs loaded with peptide antigens, wherein the subcutaneous/intradermal route has been preferred.(78)
Injection of α-GalCer loaded monocyte-derived immature DCs has been tested in two clinical trials.(79, 80) In both studies, DC injections were well tolerated and this regimen appeared to be more successful than injection of soluble α-GalCer. However, the treatment led to only modest and transient NKT activation in vivo, which was documented in only some of the patients.
Preclinical studies suggested that mature DCs were more potent than immature DCs for the activation of human iNKT cells in culture (74). Based on these data, we carried out a phase I trial to test the safety and tolerability of α-GalCer loaded mature DCs in patients with advanced cancer.(81) DC injections were well tolerated in all patients and led to > 100 fold expansion of several subsets of NKT cells in vivo. This was even true for patients who had severe iNKT deficiencies at baseline with nearly undetectable iNKT cells. DC mediated iNKT activation was sustained, lasting several months. This was a bit surprising, suggesting that the effects of iNKT cells may not be restricted to short term “innate” effects, but may be more long lasting. iNKT activation was associated with an increase in serum levels of IL-12p40 and interferon-γ inducible protein 10 (IP-10). Interestingly, DC vaccination was associated with an increase in cytomegalovirus (CMV) specific T cells, which is consistent with an adjuvant effect of iNKT activation on enhancing adaptive immunity as observed in mice.(39, 82, 83) Interestingly, despite good expansion of iNKT cells in vivo, these cells demonstrated a diminished capacity to secrete interferon-γ, when compared to NKT cells from healthy donors, consistent with prior data about defects in NKT function in cancer.(51) It is notable that the secretion of IFNγ by iNKT cells has been critically linked to their anti-tumor effects in preclinical studies.(20) Therefore an important challenge for iNKT activation in cancer relates to not only expansion of these cells, but also maintaining or improving their functional status in vivo.
9. IMPROVING NKT TARGETING IN THE CLINIC
The studies discussed above illustrate the feasibility and apparent tolerability of approaches to enhance NKT cells in the setting of human cancer. However the next major challenge is to translate these data into clinically meaningful applications. Below, we discuss some of the approaches being considered.
9.1. Patient selection
All of the initial studies discussed above were designed to evaluate safety and feasibility of targeting NKT cells in humans and involved patients with highly advanced cancer, including those with prior extensive chemotherapy. These patients by definition had multiple cancer and chemotherapy associated defects in immune system. In my view, it is critical that the next “efficacy studies” be performed in well defined patient populations, preferably with limited prior chemotherapy, in order to systematically ascertain the anti-tumor effects of enhancement of iNKT cells in humans. Although not a prerequisite, it may also be desirable to focus on CD1d expressing tumors (84), such as those with hematologic malignancies. The degree of iNKT dysfunction or deficiency at baseline, as well as the nature of tumor derived CD1d ligands may also be important considerations. For example the cross talk between type I and II NKT cells may determine the outcome of targeting type I NKT cells via α-GalCer or similar ligands.(9)
9.2. Alternate ligands
Recent structural insights into CD1d molecules, their lipid binding pockets, as well as into both canonical and non-canonical T cell receptors (TCR) have provided the framework into rational design of alternate ligands to modify the structure of α-GalCer to increase iNKT activation.(85) Recent studies have also begun to identify the biochemical properties of ligands recognized by both type I (and in the case of sulfatide, type II) NKT cells (86–89). In order to modify the functional properties of α-GalCer, all three components of the molecule (sugar residue, acyl and sphingosine chain) have been targeted.(90) Modifications in the length of the acyl or sphingosine chain can lead to altered stability of the CD1d-lipid complex and altered avidity of binding to TCR.(91) Recent data suggest that analogues with truncation of the sphingosine or the acyl chains can lead to skewing of NKT response towards a Th2 phenotype in mice.(91–93) Interestingly, some of these analogues are still able to lead to activation of dendritic cells.(94) However it is possible that the nature of DC activation and the subsequent effects on adaptive immunity may differ between these analogues and these issues need to be tested in the context of clinical studies. The analogues that induce Th2 responses may be of particular interest in the context of autoimmunity (such as type I diabetes), and may drive the induction of tolerogenic dendritic cells.(95, 96) In contrast, analogues of α-GalCer that seem to lead to greater anti-tumor effects are also being identified.(42, 97)
9.3. Combination therapies
As discussed earlier, it is possible that simply activation of iNKT cells in vivo, even with DCs, may not be sufficient to mediate clinically meaningful anti-tumor effects. This has led to possible combination approaches to enhance the anti-tumor effects of iNKT activation. For example, in the studies by Chang et al, iNKT cells expanded by DCs in vivo still had reduced capacity for secretion of interferon-γ.(81) In subsequent studies, we have observed that lenalidomide, a thalidomide analogue, can enhance costimulation of human iNKT cells, and leads to enhanced secretion of IFNγ in culture.(98) An advantage of lenalidomide is that it is orally bioavailable and well tolerated as a single agent and therefore can easily be combined with NKT targeted approaches. We hope to test this combination in the clinic in the near future. Another possible approach is to combine NKT targeted therapy with cytokines such as IL21 and IL15.(99, 100) NKT based approaches combined with anti-DR5 and anti-4-1BB mAb were also effective in eradicating murine tumors, even with previously established tumor models.(101) However the safety of these cytokines or mAbs as single agents first needs to be tested in the clinic. Cytokine dependent recruitment of NKT cells may also enhance graft versus leukemia effects and suppress graft versus host disease in the setting of allogeneic stem cell transplantation (102). These findings are consistent with the report that transplant patients with lower numbers of circulating iNKT cells have more severe GVHD.(103, 104) Another promising approach that is beginning to be tested in the clinic is adoptive transfer of iNKT cells.(105) The feasibility of such approaches has now been demonstrated and it is likely that this will also be combined with in vivo activation of transferred NKT cells.
9.4 Harnessing adjuvant properties of iNKT cells
Although much of the early work focused on the innate functions of NKT cells, more recent studies have emphasized the impact of iNKT cells on the generation of antigen specific T cell responses. The adjuvant properties of glycolipid antigens were first demonstrated in the context of enhanced immunity to a mouse malaria sporozoite vaccine.(33) α-GalCer mediated activation of iNKT cells leads to maturation of dendritic cells (DCs) in vivo, which is associated with enhanced immunostimulatory function.(82, 83) In preclinical studies, tumor immunity induced by the administration of α-GalCer with irradiated tumor cells was superior to other adjuvants such as anti-CD40 antibody or Toll receptor ligands (poly IC or LPS) (106). NKT associated adjuvant effects have also been demonstrated in the context of prime-boost vaccinations and a clinically relevant tumor antigen, NY-ESO1.(94) iNKT mediated DC activation may be synergistic with other adjuvants such as Toll receptor ligands.(94, 107, 108) The adjuvant effects of glycolipids can also be observed in the context of oral or intranasal administration, which are attractive from the perspective of clinical translation.(109, 110) In one study, murine DCs loaded concurrently with peptide and α-GalCer led to greater expansion of antigen specific T cells than DCs loaded with peptide alone. (111) Another interesting approach is loading tumor cells with α-GalCer.(112, 113) Injection of mice with α-GalCer loaded tumors was shown to mediate tumor immunity in an iNKT dependent fashion. Rejection of tumor cells by innate effectors can also lead to cross presentation of dying cells and the generation of long lived tumor immunity.(114) Together, these studies have set the stage for co-administration of glycolipid antigens with T cell based vaccines in order to enhance the induction of tumor specific adaptive immunity.
10. CHALLENGES FOR TARGETING NKT CELLS IN THE CLINIC
Although the pre-clinical and early clinical studies provide rationale and hope for targeting iNKT cells in humans, there remain several challenges before these cells can be safely and effectively targeted in the clinic. Below, I will briefly discuss some of these challenges.
10.1. Humans versus mice
Much of the impressive and elegant work on anti-tumor properties of iNKT cells comes from studies in mice. However the frequencies of iNKT cells in humans are much lower than in mice.(2) As discussed above, there may also be other differences in subsets and functional properties of these cells between species.(115) Both number and function of iNKT cells in cancer patients is further reduced.(51) Therefore the biologic effects of glycolipid ligands may be quite different, at least quantitatively between mice and humans. Although it is possible to reliably increase the number of iNKT cells in humans in vivo (e.g. by DC vaccination or adoptive transfer), there is a paucity of clinical studies specifically targeting human iNKT cells in vivo.
10.2. Type I versus II NKT cells
Recent studies have highlighted the functional diversity of CD1d restricted T cell repertoire.(6) One of the central challenges is the need to better understand the rules by which CD1d restricted T cells can boost or suppress immunity in vivo.(7) This Janus like property of NKT cells, if properly harnessed, could be very useful for a broad range of human diseases, from autoimmunity to tumors, but needs to be better understood. In contrast to mice, a significant proportion of CD1d restricted T cells in humans lack invariant T cell receptor.(2) Recent studies have shown that cross talk with these type II NKT cells can impair tumor protection mediated by iNKT cells.(48, 116) At present, very little is known about the properties of type II NKT cells in human cancer. Here again, there is a need for studies with humans to characterize the nature of ligands recognized by both type I and II NKT cells in vivo in the context of human cancer and their functional properties. Harnessing NKT cells in human cancer may require both enhancing type I and suppressing type II NKT cells.
10.3. Role of tissue and tumor microenvironments
In vivo biology of different NKT subsets and the instructive role of different tissue microenvironments on iNKT function is also poorly understood. In the setting of the tumor bed, cell derived glycolipid ligands (62, 117, 118) may in addition, have a modulating effect on the function of iNKT cells. Functional properties of NKT cells in different tissues may differ. Therefore in contrast to current approaches that lead to systemic NKT activation in multiple tissues, strategies that lead to selective NKT activation in defined tissues may be desirable. Tumor microenvironment may be less permissive to iNKT cells, and strategies to enhance homing and survival of activated iNKT cells to tumor tissues may be needed.
11. SUMMARY
With advances in the biology of antigen presentation of lipids, it is now becoming feasible to learn to harness glycolipid reactive CD1d restricted T cells. A subset of these cells, termed iNKT cells carry considerable potential for their anti-tumor properties. These cells may also provide critical link between innate and adaptive immunity via activation of DCs. However there is a need for studies in humans to both better understand the biology of these cells and to evaluate the clinical and biologic effects of manipulation of these responses.
Acknowledgments
MVD is supported in part by funds from the National Institutes of Health (NIH; CA106802, CA109465), Damon Runyon Cancer Research Fund, Dana Foundation, and Irma T Hirschl Foundation. This manuscript is dedicated to all of our patients and volunteers who have participated in our studies and clinical trials. The author thanks past and current members of Dhodapkar lab for their contributions.
Abbreviations
- α-GalCer
α-galactosylceramide
- DC
dendritic cells
- NKT
natural killer T cells
- iNKT
invariant NKT cells
- TCR
T cell receptor
- IFNγ
interferon-gamma
- IL
Interleukin
- MCA
Methylchloranthrene
References
- 1.Blattman JN, Greenberg PD. Cancer Immunotherapy: A treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 3.Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI. NKT cells - conductors of tumor immunity? Curr Opin Immunol. 2002;14:165–171. doi: 10.1016/s0952-7915(02)00316-3. [DOI] [PubMed] [Google Scholar]
- 4.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 5.Pellicci DG, Hammond KJ, Coquet J, Kyparissoudis K, Brooks AG, Kedzierska K, Keating R, Turner S, Berzins S, Smyth MJ, Godfrey DI. DX5/CD49b-positive T cells are not synonymous with CD1d-dependent NKT cells. J Immunol. 2005;175:4416–4425. doi: 10.4049/jimmunol.175.7.4416. [DOI] [PubMed] [Google Scholar]
- 6.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 7.Godfrey DI, Kronenberg M. Going both ways: Immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 9.Terabe M, Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 2007;28:491–496. doi: 10.1016/j.it.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4–8- T cells. J Exp Med. 1994;180:1171–1176. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gadola SD, Dulphy N, Salio M, Cerundolo V. Valpha24-JalphaQ-independent, CD1d-restricted recognition of alpha-galactosylceramide by human CD4 (+) and CD8alphabeta (+) T lymphocytes. J Immunol. 2002;168:5514–5520. doi: 10.4049/jimmunol.168.11.5514. [DOI] [PubMed] [Google Scholar]
- 12.Exley MA, He Q, Cheng O, Wang RJ, Cheney CP, Balk SP, Koziel MJ. Cutting edge: Compartmentalization of Th1-like noninvariant CD1d-reactive T cells in hepatitis C virus-infected liver. J Immunol. 2002;168:1519–1523. doi: 10.4049/jimmunol.168.4.1519. [DOI] [PubMed] [Google Scholar]
- 13.Exley MA, Tahir SM, Cheng O, Shaulov A, Joyce R, Avigan D, Sackstein R, Balk SP. A major fraction of human bone marrow lymphocytes are Th2-like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J Immunol. 2001;167:5531–5534. doi: 10.4049/jimmunol.167.10.5531. [DOI] [PubMed] [Google Scholar]
- 14.Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS, Mannon P, Strober W. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002;16:583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 16.Slifka MK, Pagarigan RR, Whitton JL. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells (published erratum appears in J Immunol 2000 Mar 15;164 (6):following 3444) J Immunol. 2000;164:2009–2015. doi: 10.4049/jimmunol.164.4.2009. [DOI] [PubMed] [Google Scholar]
- 17.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V (alpha)24 natural killer T cells. J Exp Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, Hayakawa Y, Godfrey DI, Smyth MJ. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smyth MJ, Godfrey DI. NKT cells and tumor immunity--a double-edged sword. Nat Immunol. 2000;1:459–460. doi: 10.1038/82698. [DOI] [PubMed] [Google Scholar]
- 21.Wilson SB, Delovitch TL. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol. 2003;3:211–222. doi: 10.1038/nri1028. [DOI] [PubMed] [Google Scholar]
- 22.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishikawa H, Kato T, Tanida K, Hiasa A, Tawara I, Ikeda H, Ikarashi Y, Wakasugi H, Kronenberg M, Nakayama T, Taniguchi M, Kuribayashi K, Old LJ, Shiku H. CD4+ CD25+ T cells responding to serologically defined autoantigens suppress antitumor immune responses. Proc Natl Acad Sci U S A. 2003;100:10902–10906. doi: 10.1073/pnas.1834479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Va 14 NKT cells by glycosylceramides. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 26.Kawano T, Nakayama T, Kamada N, Kaneko Y, Harada M, Ogura N, Akutsu Y, Motohashi S, Iizasa T, Endo H, Fujisawa T, Shinkai H, Taniguchi M. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NKT cells. Cancer Res. 1999;59:5102–5105. [PubMed] [Google Scholar]
- 27.Hayakawa Y, Rovero S, Forni G, Smyth MJ. Alpha-galactosylceramide (KRN7000) suppression of chemical- and oncogene-dependent carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:9464–9469. doi: 10.1073/pnas.1630663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayakawa Y, Takeda K, Yagita H, Kakuta S, Iwakura Y, Van Kaer L, Saiki I, Okumura K. Critical contribution of IFN-gamma and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of alpha-galactosylceramide. Eur J Immunol. 2001;31:1720–1727. [PubMed] [Google Scholar]
- 29.Sidobre S, Naidenko OV, Sim BC, Gascoigne NR, Garcia KC, Kronenberg M. The V alpha 14 NKT cell TCR exhibits high-affinity binding to a glycolipid/CD1d complex. J Immunol. 2002;169:1340–1348. doi: 10.4049/jimmunol.169.3.1340. [DOI] [PubMed] [Google Scholar]
- 30.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 31.Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000;30:985–992. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura T, Kitamura H, Iwakabe K, Yahata T, Ohta A, Sato M, Takeda K, Okumura K, Van Kaer L, Kawano T, Taniguchi M, Nakui M, Sekimoto M, Koda T. The interface between innate and acquired immunity: glycolipid antigen presentation by CD1d-expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int Immunol. 2000;12:987–994. doi: 10.1093/intimm/12.7.987. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, Nakayama T, Taniguchi M, Koezuka Y, Tsuji M. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincent MS, Leslie DS, Gumperz JE, Xiong X, Grant EP, Brenner MB. CD1-dependent dendritic cell instruction. Nat Immunol. 2002;3:1163–1168. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]
- 35.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van Kaer L, Kawano T, Taniguchi M, Nishimura T. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang YF, Tomura M, Ono S, Hamaoka T, Fujiwara H. Requirement for IFN-gamma in IL-12 production induced by collaboration between v (alpha)14 (+) NKT cells and antigen-presenting cells. Int Immunol. 2000;12:1669–1675. doi: 10.1093/intimm/12.12.1669. [DOI] [PubMed] [Google Scholar]
- 39.Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Valpha14 (+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007;220:183–198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 40.Hayakawa Y, Takeda K, Yagita H, Smyth MJ, Van Kaer L, Okumura K, Saiki I. IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood. 2002;100:1728–1733. [PubMed] [Google Scholar]
- 41.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med. 2003;198:1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujii S, Shimizu K, Hemmi H, Fukui M, Bonito AJ, Chen G, Franck RW, Tsuji M, Steinman RM. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci U S A. 2006;103:11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galli G, Nuti S, Tavarini S, Galli-Stampino L, De Lalla C, Casorati G, Dellabona P, Abrignani S. CD1d-restricted Help To B Cells By Human Invariant Natural Killer T Lymphocytes. J Exp Med. 2003;197:1051–1057. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 45.Ostrand-Rosenberg S, V, Clements K, Terabe M, Park JM, Berzofsky JA, Dissanayake SK. Resistance to metastatic disease in STAT6-deficient mice requires hemopoietic and nonhemopoietic cells and is IFN-gamma dependent. J Immunol. 2002;169:5796–5804. doi: 10.4049/jimmunol.169.10.5796. [DOI] [PubMed] [Google Scholar]
- 46.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moodycliffe AM, Nghiem D, Clydesdale G, Ullrich SE. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol. 2000;1:521–525. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- 48.Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S, Miyake S, Yamamura T, Kumar V, Berzofsky JA. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179:5126–5136. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- 49.Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI. NKT cells - conductors of tumor immunity? Curr Opin Immunol. 2002;14:165–171. doi: 10.1016/s0952-7915(02)00316-3. [DOI] [PubMed] [Google Scholar]
- 50.Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, Balk SP, Exley MA. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 51.Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S, Dhodapkar KM, Krasovsky J. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197:1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujii S, Shimizu K, Klimek V, Geller MD, Nimer SD, Dhodapkar MV. Severe and selective deficiency of interferon-gamma-producing invariant natural killer T cells in patients with myelodysplastic syndromes. Br J Haematol. 2003;122:617–622. doi: 10.1046/j.1365-2141.2003.04465.x. [DOI] [PubMed] [Google Scholar]
- 53.Zeng W, Maciejewski JP, Chen G, Risitano AM, Kirby M, Kajigaya S, Young NS. Selective reduction of natural killer T cells in the bone marrow of aplastic anaemia. Br J Haematol. 2002;119:803–809. doi: 10.1046/j.1365-2141.2002.03875.x. [DOI] [PubMed] [Google Scholar]
- 54.Dhodapkar KM, Cirignano B, Chamian F, Zagzag D, Miller DC, Finlay JL, Steinman RM. Invariant natural killer T cells are preserved in patients with glioma and exhibit antitumor lytic activity following dendritic cell-mediated expansion. Int J Cancer. 2004;109:893–899. doi: 10.1002/ijc.20050. [DOI] [PubMed] [Google Scholar]
- 55.Molling JW, Langius JA, Langendijk JA, Leemans CR, Bontkes HJ, van der Vliet HJ, von Blomberg BM, Scheper RJ, van den Eertwegh AJ. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25:862–868. doi: 10.1200/JCO.2006.08.5787. [DOI] [PubMed] [Google Scholar]
- 56.Metelitsa LS, Weinberg KI, Emanuel PD, Seeger RC. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003;17:1068–1077. doi: 10.1038/sj.leu.2402943. [DOI] [PubMed] [Google Scholar]
- 57.Fais F, Morabito F, Stelitano C, Callea V, Zanardi S, Scudeletti M, Varese P, Ciccone E, Grossi CE. CD1d is expressed on B-chronic lymphocytic leukemia cells and mediates alpha-galactosylceramide presentation to natural killer T lymphocytes. Int J Cancer. 2004;109:402–411. doi: 10.1002/ijc.11723. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi T, Haraguchi K, Chiba S, Yasukawa M, Shibata Y, Hirai H. Valpha24+ natural killer T-cell responses against T-acute lymphoblastic leukaemia cells: implications for immunotherapy. Br J Haematol. 2003;122:231–239. doi: 10.1046/j.1365-2141.2003.04429.x. [DOI] [PubMed] [Google Scholar]
- 59.Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B, Kronenberg M, Seeger RC. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167:3114–3122. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- 60.Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, Imamura M. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11:7322–7327. doi: 10.1158/1078-0432.CCR-05-0877. [DOI] [PubMed] [Google Scholar]
- 61.Song L, Ara T, Wu HW, Woo CW, Reynolds CP, Seeger RC, DeClerck YA, Thiele CJ, Sposto R, Metelitsa LS. Oncogene MYCN regulates localization of NKT cells to the site of disease in neuroblastoma. J Clin Invest. 2007;117:2702–2712. doi: 10.1172/JCI30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sriram V, Cho S, Li P, O’Donnell PW, Dunn C, Hayakawa K, Blum JS, Brutkiewicz RR. Inhibition of glycolipid shedding rescues recognition of a CD1+ T cell lymphoma by natural killer T (NKT) cells. Proc Natl Acad Sci U S A. 2002;99:8197–8202. doi: 10.1073/pnas.122636199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J Med Chem. 1995;38:2176–2187. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7:529–534. [PubMed] [Google Scholar]
- 65.Yamaguchi Y, Motoki K, Ueno H, Maeda K, Kobayashi E, Inoue H, Fukushima H, Koezuka Y. Enhancing effects of (2S,3S,4R)-1-O- (alpha-D-galactopyranosyl)-2- (N-hexacosanoylamino) -1,3,4-octadecanetriol (KRN7000) on antigen-presenting function of antigen-presenting cells and antimetastatic activity of KRN7000-pretreated antigen-presenting cells. Oncol Res. 1996;8:399–407. [PubMed] [Google Scholar]
- 66.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 67.Hayakawa Y, Godfrey DI, Smyth MJ. Alpha-galactosylceramide: potential immunomodulatory activity and future application. Curr Med Chem. 2004;11:241–252. doi: 10.2174/0929867043456115. [DOI] [PubMed] [Google Scholar]
- 68.Osman Y, Kawamura T, Naito T, Takeda K, Van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of alpha-galactosylceramide. Eur J Immunol. 2000;30:1919–1928. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 69.Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, von Blomberg BM, Scheper RJ, van der Vliet HJ, van den Eertwegh AJ, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 70.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, Bouillet P, Strasser A, Smyth MJ, Godfrey DI. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001;94:459–473. doi: 10.1002/ijc.1503. [DOI] [PubMed] [Google Scholar]
- 73.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 74.Fujii S, Shimizu K, Steinman RM, Dhodapkar MV. Detection and activation of human Valpha24+ natural killer T cells using alpha-galactosyl ceramide-pulsed dendritic cells. J Immunol Methods. 2003;272:147–159. doi: 10.1016/s0022-1759(02)00497-0. [DOI] [PubMed] [Google Scholar]
- 75.van der Vliet HJ, Molling JW, Nishi N, Masterson AJ, Kolgen W, Porcelli SA, van den Eertwegh AJ, von Blomberg BM, Pinedo HM, Giaccone G, Scheper RJ. Polarization of Valpha24+ Vbeta11+ natural killer T cells of healthy volunteers and cancer patients using alpha-galactosylceramide-loaded and environmentally instructed dendritic cells. Cancer Res. 2003;63:4101–4106. [PubMed] [Google Scholar]
- 76.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 77.Kojo S, Seino K, Harada M, Watarai H, Wakao H, Uchida T, Nakayama T, Taniguchi M. Induction of regulatory properties in dendritic cells by Valpha14 NKT cells. J Immunol. 2005;175:3648–3655. doi: 10.4049/jimmunol.175.6.3648. [DOI] [PubMed] [Google Scholar]
- 78.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 79.Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, Abraham R, Juji T, Macfarlane DJ, Nicol AJ. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 80.Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, Iizasa T, Nakayama T, Taniguchi M, Fujisawa T. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 81.Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R, Shay J, Kirchhoff K, Nishi N, Ando Y, Hayashi K, Hassoun H, Steinman RM, Dhodapkar MV. Sustained expansion of NKT cells and antigen-specific T cells after injection of {alpha}-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 84.Haraguchi K, Takahashi T, Nakahara F, Matsumoto A, Kurokawa M, Ogawa S, Oda H, Hirai H, Chiba S. CD1d expression level in tumor cells is an important determinant for anti-tumor immunity by natural killer T cells. Leuk Lymphoma. 2006;47:2218–2223. doi: 10.1080/10428190600682688. [DOI] [PubMed] [Google Scholar]
- 85.Stronge VS, Salio M, Jones EY, Cerundolo V. A closer look at CD1d molecules: new horizons in studying NKT cells. Trends Immunol. 2007;28:455–462. doi: 10.1016/j.it.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 87.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 88.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 89.Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, Sullivan B, Bodmer-Narkevitch V, Plettenburg O, Kronenberg M, Tsuji M, Ho DD, Wong CH. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc Natl Acad Sci U S A. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fujio M, Wu D, Garcia-Navarro R, Ho DD, Tsuji M, Wong CH. Structure-based discovery of glycolipids for CD1d-mediated NKT cell activation: tuning the adjuvant versus immunosuppression activity. J Am Chem Soc. 2006;128:9022–9023. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 91.McCarthy C, Shepherd D, Fleire S, Stronge VS, Koch M, Illarionov PA, Bossi G, Salio M, Denkberg G, Reddington F, Tarlton A, Reddy BG, Schmidt RR, Reiter Y, Griffiths GM, van der Merwe PA, Besra GS, Jones EY, Batista FD, Cerundolo V. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2006;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 93.Oki S, Chiba A, Yamamura T, Miyake S. The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells. J Clin Invest. 2004;113:1631–1640. doi: 10.1172/JCI20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Silk JD, I, Hermans F, Gileadi U, Chong TW, Shepherd D, Salio M, Mathew B, Schmidt RR, Lunt SJ, Williams KJ, Stratford IJ, Harris AL, Cerundolo V. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest. 2004;114:1800–1811. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen YG, Choisy-Rossi CM, Holl TM, Chapman HD, Besra GS, Porcelli SA, Shaffer DJ, Roopenian D, Wilson SB, Serreze DV. Activated NKT cells inhibit autoimmune diabetes through tolerogenic recruitment of dendritic cells to pancreatic lymph nodes. J Immunol. 2005;174:1196–1204. doi: 10.4049/jimmunol.174.3.1196. [DOI] [PubMed] [Google Scholar]
- 96.Forestier C, Takaki T, Molano A, Im JS, Baine I, Jerud ES, Illarionov P, Ndonye R, Howell AR, Santamaria P, Besra GS, Dilorenzo TP, Porcelli SA. Improved outcomes in NOD mice treated with a novel Th2 cytokine-biasing NKT cell activator. J Immunol. 2007;178:1415–1425. doi: 10.4049/jimmunol.178.3.1415. [DOI] [PubMed] [Google Scholar]
- 97.Chang YJ, Huang JR, Tsai YC, Hung JT, Wu D, Fujio M, Wong CH, Yu AL. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc Natl Acad Sci U S A. 2007;104:10299–10304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang DH, Liu N, Klimek V, Hassoun H, Mazumder A, Nimer SD, Jagannath S, Dhodapkar MV. Enhancement of ligand dependent activation of human Natural Killer T cells by Lenalidomide: Therapeutic Implications. Blood. 2006;108:618–621. doi: 10.1182/blood-2005-10-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J Exp Med. 2005;201:1973–1985. doi: 10.1084/jem.20042280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van der Vliet HJ, Nishi N, Koezuka Y, von Blomberg BM, van den Eertwegh AJ, Porcelli SA, Pinedo HM, Scheper RJ, Giaccone G. Potent expansion of human natural killer T cells using alpha-galactosylceramide (KRN7000)-loaded monocyte-derived dendritic cells, cultured in the presence of IL-7 and IL-15. J Immunol Methods. 2001;247:61–72. doi: 10.1016/s0022-1759(00)00272-6. [DOI] [PubMed] [Google Scholar]
- 101.Teng MW, Westwood JA, Darcy PK, Sharkey J, Tsuji M, Franck RW, Porcelli SA, Besra GS, Takeda K, Yagita H, Kershaw MH, Smyth MJ. Combined natural killer T-cell based immunotherapy eradicates established tumors in mice. Cancer Res. 2007;67:7495–7504. doi: 10.1158/0008-5472.CAN-07-0941. [DOI] [PubMed] [Google Scholar]
- 102.Morris ES, MacDonald KP, Rowe V, Banovic T, Kuns RD, Don AL, Bofinger HM, Burman AC, Olver SD, Kienzle N, Porcelli SA, Pellicci DG, Godfrey DI, Smyth MJ, Hill GR. NKT cell-dependent leukemia eradication following stem cell mobilization with potent G-CSF analogs. J Clin Invest. 2005;115:3093–3103. doi: 10.1172/JCI25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haraguchi K, Takahashi T, Matsumoto A, Asai T, Kanda Y, Kurokawa M, Ogawa S, Oda H, Taniguchi M, Hirai H, Chiba S. Host-residual invariant NK T cells attenuate graft-versus-host immunity. J Immunol. 2005;175:1320–1328. doi: 10.4049/jimmunol.175.2.1320. [DOI] [PubMed] [Google Scholar]
- 104.Haraguchi K, Takahashi T, Hiruma K, Kanda Y, Tanaka Y, Ogawa S, Chiba S, Miura O, Sakamaki H, Hirai H. Recovery of Valpha24+ NKT cells after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;34:595–602. doi: 10.1038/sj.bmt.1704582. [DOI] [PubMed] [Google Scholar]
- 105.Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Iizasa T, Hanaoka H, Shimizu N, Horiguchi S, Okamoto Y, Fujii S, Taniguchi M, Fujisawa T, Nakayama T. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12:6079–6086. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- 106.Liu K, Idoyaga J, Charalambous A, Fujii S, Bonito A, Mordoh J, Wainstok R, Bai XF, Liu Y, Steinman RM. Innate NKT lymphocytes confer superior adaptive immunity via tumor-capturing dendritic cells. J Exp Med. 2005;202:1507–1516. doi: 10.1084/jem.20050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hermans IF, Silk JD, Gileadi U, Masri SH, Shepherd D, Farrand KJ, Salio M, Cerundolo V. Dendritic cell function can be modulated through cooperative actions of TLR ligands and invariant NKT cells. J Immunol. 2007;178:2721–2729. doi: 10.4049/jimmunol.178.5.2721. [DOI] [PubMed] [Google Scholar]
- 108.Marschner A, Rothenfusser S, Hornung V, Prell D, Krug A, Kerkmann M, Wellisch D, Poeck H, Greinacher A, Giese T, Endres S, Hartmann G. CpG ODN enhance antigen-specific NKT cell activation via plasmacytoid dendritic cells. Eur J Immunol. 2005;35:2347–2357. doi: 10.1002/eji.200425721. [DOI] [PubMed] [Google Scholar]
- 109.Chung Y, Chang WS, Kim S, Kang CY. NKT cell ligand alpha-galactosylceramide blocks the induction of oral tolerance by triggering dendritic cell maturation. Eur J Immunol. 2004;34:2471–2479. doi: 10.1002/eji.200425027. [DOI] [PubMed] [Google Scholar]
- 110.Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005;175:3309–3317. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
- 111.Stober D, Jomantaite I, Schirmbeck R, Reimann J. NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo. J Immunol. 2003;170:2540–2548. doi: 10.4049/jimmunol.170.5.2540. [DOI] [PubMed] [Google Scholar]
- 112.Chung Y, Qin H, Kang CY, Kim S, Kwak LW, Dong C. An NKT-mediated autologous vaccine generates CD4 T-cell dependent potent antilymphoma immunity. Blood. 2007;110:2013–2019. doi: 10.1182/blood-2006-12-061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shimizu K, Goto A, Fukui M, Taniguchi M, Fujii S. Tumor cells loaded with alpha-galactosylceramide induce innate NKT and NK cell-dependent resistance to tumor implantation in mice. J Immunol. 2007;178:2853–2861. doi: 10.4049/jimmunol.178.5.2853. [DOI] [PubMed] [Google Scholar]
- 114.Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S. Cross-presentation of glycolipid from tumor cells loaded with alpha-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells. J Exp Med. 2007;204:2641–2653. doi: 10.1084/jem.20070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kenna T, Golden-Mason L, Porcelli SA, Koezuka Y, Hegarty JE, O’Farrelly C, Doherty DG. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J Immunol. 2003;171:1775–1779. doi: 10.4049/jimmunol.171.4.1775. [DOI] [PubMed] [Google Scholar]
- 116.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198:173–181. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli SA, Cardell S, Brenner MB, Behar SM. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]


