Abstract
It has been widely reported that gonadal hormones influence the display of aggression in Syrian hamsters; conversely, much less is known about whether gonadal hormones modulate submissive/defensive behaviors in these animals. Following social defeat, male hamsters no longer display normal territorial aggression but instead display submissive/defensive behavior in the presence of a smaller opponent, a phenomenon we have termed conditioned defeat (CD). The purpose of the present study was to examine the effect of gonadal hormones on the display of CD in male hamsters. In Experiment 1, males were castrated or sham-operated. The castrated males were significantly more submissive following social defeat relative to their intact counterparts. The increased submissive behavior in the castrated males during CD testing was particularly surprising, given the fact that they were attacked significantly less during CD training. In Experiment 2a, males were castrated and given hormone replacement. Castrated males treated with testosterone or dihydrotestosterone displayed significantly less submissive behavior following social defeat than did those treated with and cholesterol or estradiol. Finally, in Experiment 2b, there was no effect of hormone replacement on aggressive behavior in non-defeated hamsters suggesting that the decrease in submissive behavior in males treated with dihydrotestosterone or testosterone is specific to being previously defeated. Taken together the data indicate that the presence of androgens reduces the display of submission in defeated male hamsters. More importantly, these findings suggest that androgens may have a protective effect against the development of depression-like or anxiety-like behaviors following exposure to an ethologically relevant stressor.
Keywords: anxiety, testosterone, social defeat, aggression, subordination, gonadectomy, estradiol, depression
Introduction
Agonistic behavior includes all behaviors that are exhibited during social conflict, including aggressive, submissive/defensive and communicative (e.g., scent marking) behaviors. In most rodent species, agonistic behavior has been studied almost exclusively in males, as females display little aggression or fighting except in defense of their offspring; (see Lonstein and Gammie, 2002). Conversely, agonistic behavior has been widely studied in both male and female Syrian hamsters, as both display high levels of aggressive behaviors in the laboratory (Grelk, Papson, Cole, and Rowe, 1974; Huhman, Solomon, Janicki, Harmon, Lin, Israel, and Jasnow, 2003; Meisel, Sterner, and Diekman, 1988; Payne, 1973; Payne, 1974; Solomon, Karom, and Huhman, 2007; Takahashi and Lisk, 1984; Whitsett, 1975).
Several factors influence the outcome of social conflict in Syrian hamsters. For example, body weight, environment and gonadal hormones are key predictors of success during agonistic interactions. In male-male interactions, heavier males dominate smaller opponents over 60% of the time (Drickamer, Vandenbergh, and Colby, 1973; Vandenbergh, 1971). The environment in which the agonistic encounters occur is also an important factor in predicting success. More specifically, Syrian hamsters are often more aggressive when defending their own home cage compared with a neutral area (Murphy, 1976; Murphy and Schneider, 1970), although it has been shown that Syrian hamsters are still aggressive when paired outside of their home cages (Payne and Swanson, 1970). In addition, gonadal hormones influence the likelihood of “winning.” For instance, intact male hamsters are more aggressive and often defeat their castrated counterparts (Payne and Swanson, 1972; Vandenbergh, 1971).
In many species, androgens facilitate aggressive behavior in males. Castration of both rats and mice reduces aggression, while androgen replacement restores aggression in both species (Albert, Walsh, Gorzalka, Siemens, and Louie, 1986; Beeman, 1947; Bevan, 1958; Christie and Barfield, 1979). Likewise, in Syrian hamsters there are reports of castration decreasing aggression and androgen replacement restoring aggression (Drickamer et al., 1973; Grelk et al., 1974; Payne, 1973; Payne and Swanson, 1971). There is also evidence demonstrating that aggression in adult Syrian hamsters is independent of gonadal hormones (Garrett and Campbell, 1980; Tiefer, 1970; Whitsett, 1975).
While much attention has been paid to the environmental and hormonal factors that may be associated with “winning,” our laboratory is interested in the behavioral and hormonal consequences of “losing.” Numerous studies suggest that the losers in social conflict situations exhibit many characteristics indicative of increased depression-like and anxiety-like behaviors including but not limited to, alterations in food intake and body weight, increased social withdrawal and/or submissiveness as well as decreased locomotor activity (for review; (Huhman, 2006). We study a phenomenon termed conditioned defeat (CD) in Syrian hamsters. During the CD acquisition period, the experimental animal is defeated by a larger aggressor in the aggressor's home cage. On the following day, the previously defeated animal displays increased submissive/defensive behavior and increased social withdrawal when paired with a smaller opponent. This increase in submissive behavior is particularly notable because the experimental animal is tested in its own home cage and is heavier than the intruder. As mentioned previously, both home cage testing and increased body weight are key predictors in the success of agonistic encounters. Following defeat, however, the influence of these factors is offset by the previous defeat experience.
We have previously demonstrated that gonadal hormones modulate the display of CD in female hamsters (Faruzzi, Solomon, Demas, and Huhman, 2005; Solomon et al., 2007), but we have not tested the hypothesis that gonadal hormones alter CD in males. The present study was designed to assess the effects of gonadal hormones on the display of CD in male hamsters. Here, we report that the presence of androgens decreases the display of submission in defeated male hamsters.
Methods
Subjects
Subjects were adult male Syrian hamsters (Mesocricetus auratus, Charles River Laboratories) that weighed approximately 120-130 g (10 weeks) upon arrival. Hamsters were individually housed in a temperature-controlled colony room on a 14:10 hr light: dark cycle with lights off at 1100 h. Additional singly-housed male hamsters weighing >180 g (>6 months old) were used as resident aggressors during defeat training. We routinely use older trained resident aggressors that are larger to ensure that the experimental animals experience defeat in a “threatening” environment. Group-housed male hamsters (five per cage) weighing 110-120 g (7 weeks old) were used as non-aggressive intruders (NAI) during conditioned defeat testing. All animals were housed in polycarbonate cages (20×40×20cm) with wire mesh tops, corn cob bedding and cotton nesting materials. Food and water were available ad libitum. All procedures and protocols were approved by the Georgia State University Institutional Care and Use Committee and are in accordance with the Public Health Service and US Department of Agriculture guidelines.
Surgery/Hormone Replacement
Experiment 1
All male hamsters were anesthetized deeply with sodium pentobarbitol (90mg/kg). In Experiment 1, hamsters were castrated or sham operated and divided into the following groups no-defeat castrated (n=8), no defeat sham-operated (n=7) and defeat castrated (n=8) and defeat sham-operated (n=8). All hamsters were individually housed for 4 weeks prior to behavioral testing. All hamsters were defeated one time for 15 minutes by a larger resident aggressor (CD training) in the aggressor's home cage. On the following day, hamsters were paired in their own home cage for five minutes with a non-aggressive intruder (CD testing).
Experiment 2a
Male hamsters were castrated and then surgically implanted (subcutaneously) with Silastic capsules containing 17-β estradiol benzoate (E2) (n=9), dihydrotestosterone (DHT) (n=9), testosterone (T) (n=14) or cholesterol (C) (n=11). Following surgery and capsule implantation, all animals were individually housed for four weeks. Hamsters were then defeated once for 15 minutes by a larger resident aggressor (CD training) in the aggressor's home cage. On the following day, these hamsters were paired in their own home cage for five minutes with a smaller non-aggressive intruder (CD testing).
Experiment 2b
A separate group of male hamsters treated with E2 (n=9) DHT (n=7), T (n=8), and C (n=6) and singly housed for four weeks served as no defeat controls who were handled and remained in their home cage until testing with a smaller non-aggressive intruder.
Hormonal Capsules
E2 capsules were made of Silastic Brand medical-grade tubing (0.078in. i.d. × 0.125 in. o.d.), 10 mm in length, sealed with Factor II 6382 RTV Silicone and Elastomer, and filled with 5 mm of 17-β-estradiol (Sigma, cat. number E1024 St. Louis, MO). T, DHT and C capsules were made of Silastic tubing (0.078 in. i.d. × 0.125 in. o.d.). T capsules were 28 mm long and filled 20 mm with testosterone propionate (Sigma, cat. number T-1500, St. Louis, MO). DHT capsules were 30 mm long and filled 20 mm with dihydrotestosterone (Sigma, cat. number A-8380, St. Louis, MO) and C capsules were 30 mm long and filled 20 mm with cholesterol (Sigma, St. Louis, MO).
Conditioned Defeat Training/Testing
All behavioral training and testing took place during the first two hours of the daily light:dark cycle. On the day of conditioned defeat training, male hamsters were transported from the colony room to the behavioral testing room. Conditioned defeat training consisted of a single resident/intruder pairing in which experimental animals were placed into the home cage of a larger resident aggressor for 15 minutes. During the 15 minute defeat session, experimental animals were routinely attacked by the resident aggressors and displayed submissive and defensive behavior towards resident aggressors. On the following day, a resident/intruder pairing was used in which the experimental animals were paired in their own home cage with a smaller non-aggressive intruder. All training and testing sessions were recorded on VHS tape, transferred to CD-ROM, and scored by a trained observer blind to the experimental condition using Noldus Observer (version 5) (Noldus Information Technology, Wageningen, Netherlands). The following classes of behaviors were scored as total duration in seconds: 1) Non-social: locomotor/exploratory, self-groom, nesting, feeding, pouching, sleeping; 2) Social: attend, approach, investigate, sniff, touching nose; 3) Submissive/defensive: upright and side defense, rearing, flee, full submissive posture; 4) Aggressive: upright and side offense, chase, attack, bite.
Hormonal Assays
Nonextracted hamster serum samples were assayed in duplicate for E2, T, and DHT using commercial radioimmunoassay kits (Active® Estradiol RIA DSL-43100, Active® Testosterone RIA DSL-4000 and Dihydrotestosterone Non Extraction RIA DSL-96100 (Diagnostic Systems Laboratories, Inc., Webster TX), respectively. All kits were validated with hamster serum. E2, T and DHT detection ranges were 0.52-1033.76 pg/ml, 0.01-21.69 ng/ml and 1.59-1250.70 pg/ml, respectively. The specificity for the E2 kit for 17-β estradiol was 100%, with a negligible cross reactivity (< 0.01%) for testosterone. The specificity for the T kit for T was 100%, with a 5.8% cross reactivity with DHT. This kit also had insignificant cross-reactivity with 17-β estradiol (< 0.01%). The specificity for the DHT kit for DHT was 100%, with a 0.6% cross-reactivity for T and a negligible cross reactivity (<0.01%) for E2. Samples from each experiment were run together in one assay, thereby eliminating inter-assay variability. Intra-assay variability for all assays was less than 10%.
Statistical Analyses
In Experiment 1 behavioral data were analyzed using a 2-way analyses of variance (ANOVA) with social experience (i.e., defeat vs. no defeat) and hormonal condition (i.e., castration vs. sham-operated) as the independent variables. Submissive/defensive, aggressive, social and nonsocial behaviors were dependent variables. In Experiment 2, behavioral data were analyzed using one-way ANOVA with social experience (i.e., defeat or no defeat) as the independent variable and submissive/defensive, aggressive, social and nonsocial behaviors as the dependent variables. Hormonal data for Experiment 1 were analyzed using an independent sample t-test comparing castrated males vs. sham-operated males and for Experiment 2, data were analyzed using an independent sample t-test comparing the respective hormone treatment group (i.e., DHT, T, E2) vs. the cholesterol treated group. All t-tests were two-tailed. When necessary, statistically significant differences for ANOVA were further analyzed using Fisher's post hoc analyses, and significance for all analyses was ascribed at p ≤ 0.05.
Exclusion of Animals
In Experiment 1, two animals from the defeat sham group were eliminated from behavioral analyses due to injury during CD training. Another animal from the defeat sham group was not included in the training analyses because of problems with the videotape but because the observers' notes on the training session indicated a normal defeat during the training session, this animal was included in the testing analyses. In Experiment 2a, 2 animals from the C group, 4 animals from the T group and 1 animal from the DHT group were eliminated from behavioral analyses due to an insufficient amount of collected serum. An additional animal from the C group was not included due to high amounts of circulating gonadal hormones, which is suggestive of incomplete gonadectomy.
Results
Behavioral Analyses
Experiment 1
There was a significant main effect of hormonal status (F(1,12)=16.95, p < 0.01) (Figure 1a) on aggressive behavior produced by resident aggressors towards experimental animals that were individually housed for four weeks prior to behavioral testing in that resident aggressors were significantly more aggressive during CD training towards sham-operated intruders than they were towards castrated intruders. There was no difference in the display of submissive/defensive, social or nonsocial behaviors displayed by resident aggressors in the presence of these two groups, (p > 0.05). Despite the fact that castrated intruders were attacked significantly less than sham-operated animals, there was no difference between these groups in the display of submissive/defensive, aggressive, social or nonsocial behaviors in response to the resident aggressor, (p > 0.05) (Figure 1b).
Figure 1.
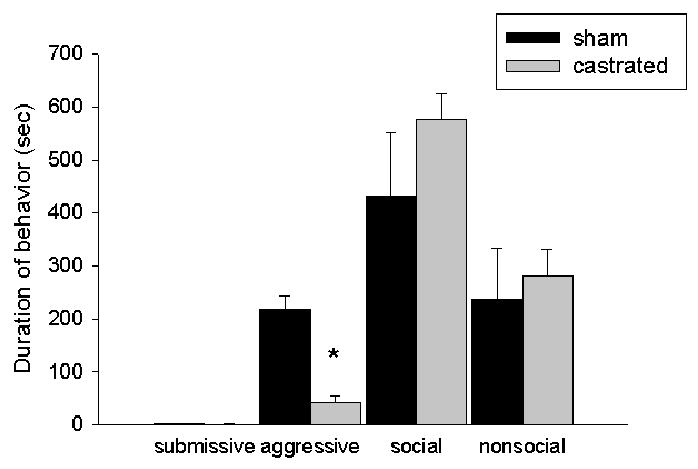
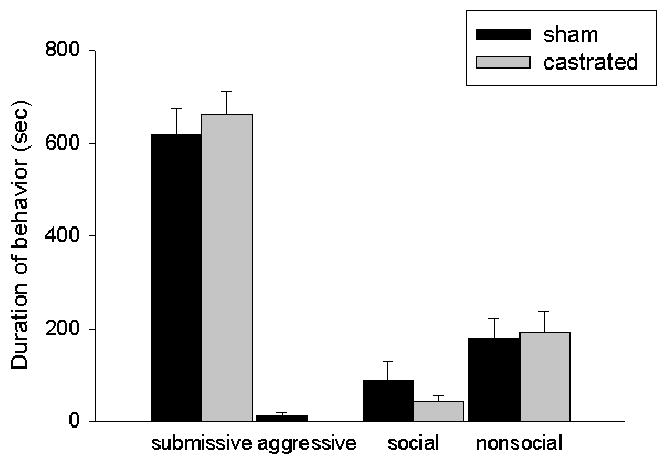
(a) Average duration (mean +/-SEM in seconds) during a 15- minute training episode of submissive/defensive, aggressive, social and nonsocial behavior of resident aggressors towards sham-operated and castrated male hamsters. MANOVA revealed that resident aggressors were significantly more aggressive towards sham-operated (n=5) males compared with castrated males (n=8) (* p < 0.01). (b) Average duration (mean +/-SEM in seconds) of submissive, aggressive, social and nonsocial behavior of experimental hamsters during a 15-minute training episode with a resident aggressor.
During CD testing (Figure 2), there was a significant main effect of social experience (i.e., defeat vs. no defeat) (F(1,28)=13.67, p < 0.01), a significant main effect of hormonal status (F(1,28)=32.57, p < 0.01) and significant social experience × hormonal status interaction (F(1,28)=5.34, p< 0.05) on the display of submissive behavior in experimental animals towards a non-aggressive intruder. Specifically, defeated castrated males displayed significantly more submissive behavior relative to all groups. Non-defeated, castrated males displayed more submissive behavior compared with the defeated sham and non-defeated sham males. There was no difference in submissive behavior between defeated sham and non-defeated male hamsters that were individually housed for four weeks prior to behavioral testing. There was a significant main effect of social experience (F(1,28)=19.80, p < 0.01) on the display of aggressive behavior. When paired with a NAI, non-defeated males were significantly more aggressive compared with previously defeated males. There was no significant main effect of hormonal status or significant social experience × hormonal status interaction on aggressive behavior (p >0.05). There was a significant main effect of hormonal status (F(1,28)=17.83, p < 0.01), no significant main effect of social experience (p >0.05) and a significant social experience × hormonal status interaction (F(1,28)=7.93, p < 0.01) on social behavior. Previously defeated, castrated males were significantly less social towards a NAI compared with all other groups. Non-defeated, castrated males were significantly less social than were defeated sham males. There was no significant difference in social behavior between non-defeated shams and non-defeated castrated males. Finally, there was no difference in nonsocial behavior among groups (p > 0.05).
Figure 2.
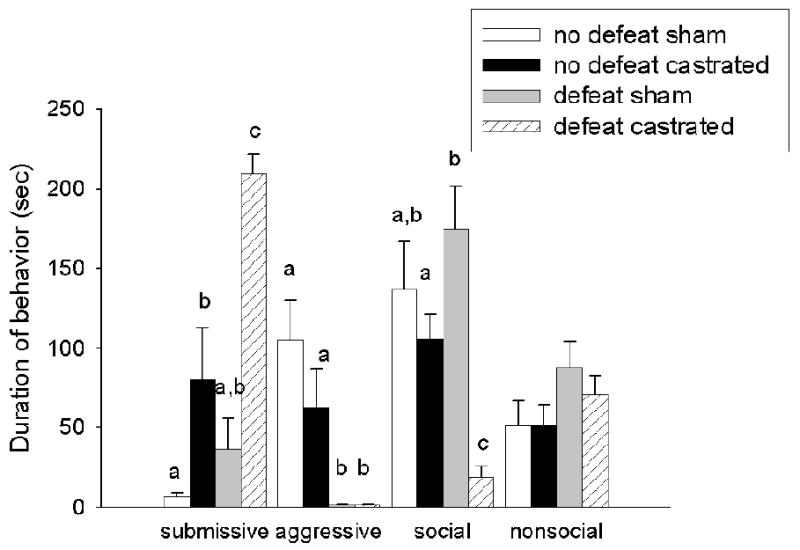
Average duration (mean +/-SEM in seconds) of submissive/defensive, aggressive, social and nonsocial behavior in defeated sham-operated (n=6), defeated castrated (n=8), non-defeated sham-operated (n=7) and non-defeated castrated (n=8) male hamsters during testing with a non-aggressive intruder. Analyses were run for each specific behavior; therefore, differences are compared only within one category of behavior and not between behavioral categories. Non-shared letters depict significant difference (p < 0.05) as determined by Fisher's post hoc analyses.
Experiments 2a and 2b
There was a significant main effect of hormonal status on aggressive (F(3,34)=3.48, p<0.01), social (F(3,34)=3.76, p<0.05) and nonsocial behaviors (F(3,34)=3.17, p <0.05) of resident aggressors in response to experimental animals during CD training (Figure 3a). Post hoc analyses revealed that resident aggressors attacked the DHT-treated group significantly more than they did groups treated with C, T or E2. Resident aggressors spent more time sniffing (scored as social behavior) experimental males treated with C and E2 compared with males treated with DHT and T. In addition, the resident aggressors were significantly less interactive (i.e., nonsocial) with the group treated with T compared with those treated with C, DHT and E2. There was no difference in submissive behavior of resident aggressors in the presence of experimental animals (p>0.05). All experimental animals behaved similarly in response to exposure to a resident aggressor during CD training (Figure 3b).
Figure 3.
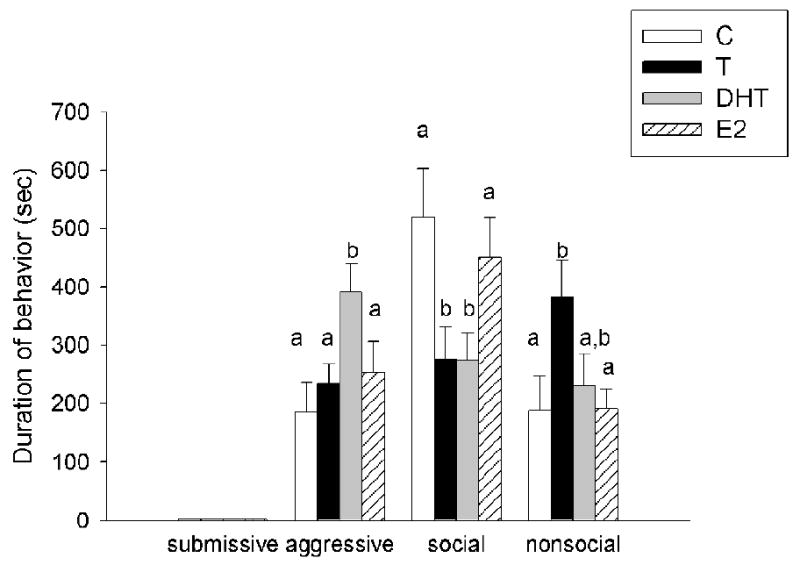
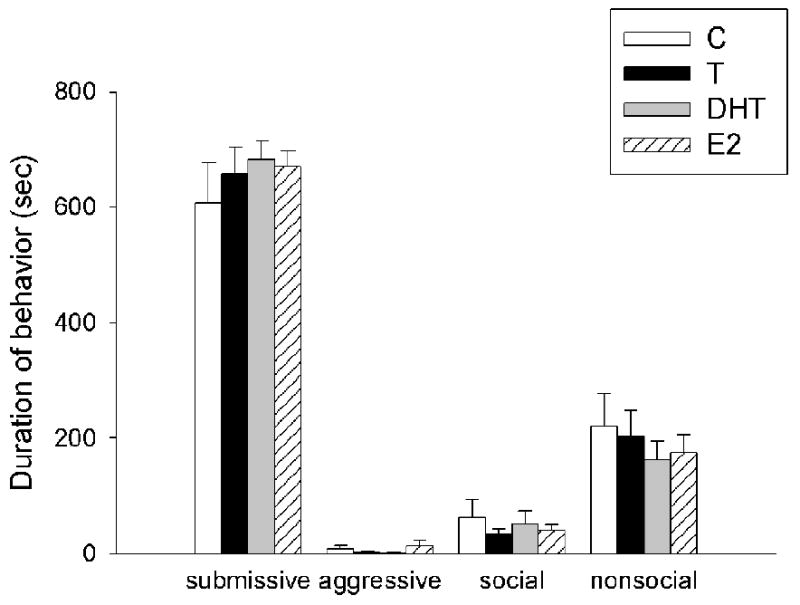
(a) Average duration (mean +/-SEM in seconds) during a 15-minute training episode of submissive/defensive, aggressive, social and nonsocial behavior of resident aggressors towards gonadectomized males treated with cholesterol (C)(n=8), testosterone (T) (n=10), dihydrotestosterone (DHT) (n=8) and estradiol (E2)(n=9). Analyses were run for each specific behavior; therefore, differences are compared only within one category of behavior and not between behavioral categories. Non-shared letters depict significant difference (p < 0.05) as determined by Fisher's post hoc analyses. (b) Average duration (mean +/-SEM in seconds) of submissive, aggressive, social and nonsocial behavior of experimental hamsters during a 15-minute training episode with a resident aggressor.
During CD testing (Figure 4a), there was a significant main effect of hormonal status on submissive (F(3,34)=5.64, p<0.01) and social behavior (F(3,34)=3.45, p<0.05) in previously defeated males towards non-aggressive intruders. Previously defeated males treated with C and E2 were significantly more submissive and significantly less social than were males treated with T and DHT. There was no difference in aggressive and nonsocial behavior among hormonal groups (p>0.05). Finally in Experiment 2b (Figure 4b), there was no significant difference among groups in submissive, aggressive, social and nonsocial behavior in non-defeated hamsters towards non-aggressive intruders (p >0.05).
Figure 4.
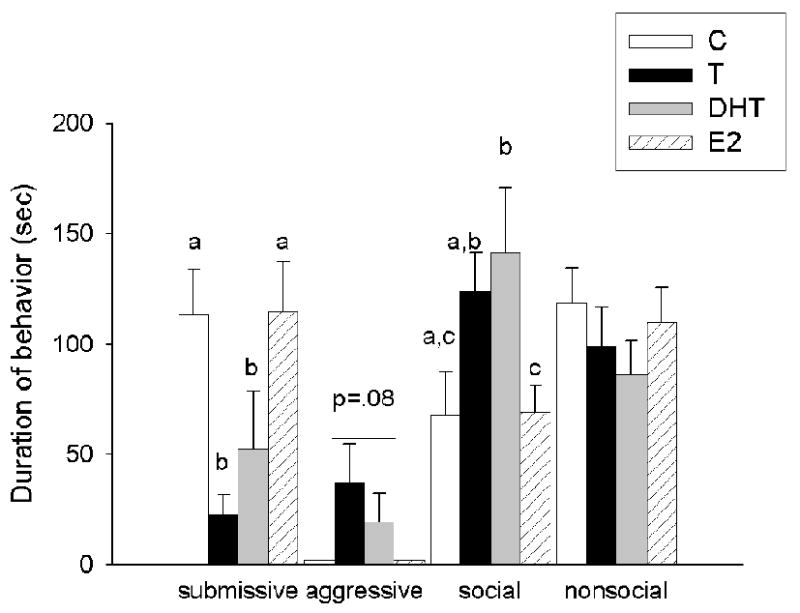
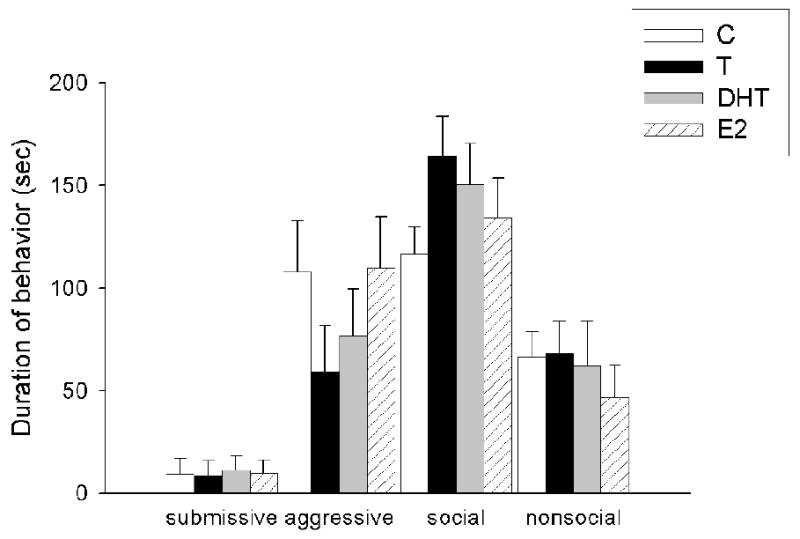
(a) Average duration (mean +/-SEM in seconds) of submissive/defensive, aggressive, social and nonsocial behavior in defeated male hamsters administered C (n=8), T (n=10), DHT (n=8) and E2 (n=9) and (b) non-defeated male hamsters administered C (n=6), T (n=8), DHT (n=7) and E2 (n=9) during a test encounter with a non-aggressive intruder. Analyses were run for each specific behavior; therefore, differences are compared only within one category of behavior and not between behavioral categories. Non-shared letters depict significant differences (p < 0.05) as determined by Fisher's post hoc analyses.
Hormonal Analyses
In Experiment 1, castration greatly reduced serum concentrations of T (t(27)= -15.29, p < 0.01), DHT (t(27)=-12.71. p < 0.01) and E2 (t(27)=-6.37, p < 0.01) in male hamsters that were hormonally manipulated and individually housed for 4 weeks prior to behavioral testing (Table 1). In Experiment 2 (Table 2), hormonal treatment influenced serum hormonal concentrations. Male hamsters treated with T had significantly higher serum T concentrations compared with those given C capsules (t(30)=4.52, p < 0.01). In addition, males treated with DHT had significantly higher serum DHT concentrations compared with those given C capsules (t(27)=5.63, p < 0.01). Likewise, males treated with E2 had significantly higher serum E2 concentrations compared with those given C capsules (t(30)=4.52, p < 0.01).
Table 1.
Serum levels of T, DHT and E2 in sham-operated and castrated male hamsters individually housed for 4 weeks prior to behavioral testing for Experiment 1. Data are presented as (mean +/-SEM).
| Experiment 1 Hormonal Group |
n | Serum Levels of Hormone |
|---|---|---|
| Castrate T (ng/ml) | 16 | 0.00+/-0.02* |
| Sham | 13 | 5.94+/-.43 |
| Castrate DHT (pg/ml) | 16 | 4.35+/-2.53* |
| Sham | 13 | 624.77+/-54.29 |
| Castrate E (pg/ml) | 16 | 96.40+/-5.98* |
| Sham | 13 | 171.79.48+/-11.19 |
indicates statistically different from sham-operated hamsters, p < 0.05.
Table 2.
Serum levels of T, DHT and E2 in hormonally treated male hamsters that were individually housed for 4 weeks prior to behavioral testing for Experiment 2. Analyses were run such that each hormone–treated group is compared against the cholesterol group only for the specific hormone of interest. The hormonal data includes both the defeated and non-defeated groups. Data are presented as (mean +/-SEM).
| Experiment 2 Hormonal Group |
n | T (ng/ml) | DHT (pg/ml) | E (pg/ml) |
|---|---|---|---|---|
| T Treated | 18 | 3.62+/-.29* | ||
| DHT Treated | 15 | 1041.54+/-129.69* | ||
| E Treated | 18 | 247.69+/-21.24* | ||
| C Treated | 14 | .19+/-.10 | 253.69+/-41.97 | 117.38+/-17.87 |
indicates statistical significance, p < 0.05.
Discussion
The purpose of the present study was to examine the impact of gonadal hormones on agonistic behavior, particularly submission, in male hamsters. The data presented here are novel in that previous reports in the literature have focused primarily on the effect of gonadal hormones on aggression and not submission. We found that sham-castrated males exhibit significantly lower levels of submission following social defeat than do castrated males. Similarly, in the hormone replacement study hamsters given T or DHT were significantly less submissive than were hamsters given C or E2.
Another interesting finding of the present study is that the intensity (duration) of the defeat experience does not necessarily predict the strength of the behavioral response of defeated animals either to the aggressor (during defeat training) or to a later non-aggressive intruder (during CD testing). In Experiment 1, castrated males were attacked significantly less by the resident aggressors than were intact males, and yet there was no difference in the duration of submission produced in response to the resident aggressors during training. Furthermore, despite the decrease in aggression received, castrated males were significantly more submissive than were intact males during subsequent CD testing. Conversely, in Experiment 2, castrated hamsters given DHT capsules were attacked significantly more than were hamsters given cholesterol, T or E2 capsules, yet the DHT-treated animals actually exhibited significantly less submission during testing. As in Experiment 1, all groups produced similar levels of submission during defeat training despite the variability in the behavior of the resident aggressors. This difference in aggression produced by resident aggressors towards intruders of differing hormonal status is not surprising, and has, in fact, been recognized for a long time (Evans and Brain, 1974; Payne, 1974). This effect is thought to be induced, at least in part, by hormone-dependent cues emitted by the experimental animals. What we would like to stress here is that the overall experience of defeat as well as characteristics of the subjects (e.g., hormonal status or past social experience) appear to more important to the behavioral outcome of defeat than is the actual intensity of the defeat experience.
In both experiments, non-defeated males, regardless of hormonal status, displayed robust territorial aggression toward intruders. This finding is consistent with earlier studies reporting that both castrated and intact male hamsters will exhibit aggression towards conspecifics (Potegal, Blau, Black, and Glusman, 1980; Whitsett, 1975). Interestingly, in Experiment 1 although non-defeated, castrated males displayed aggression towards intruders, they also displayed increased submissive behavior relative to non-defeated, intact (sham-castrated) hamsters. At first glance, these data suggest that castration might increase fear-like behaviors in male hamsters, and this speculation is consistent with previous reports of increased fear or anxiety-like behavior in castrated males relative to intact males or androgen-replaced males (Fernandez-Guasti and Martinez-Mota, 2005; Frye and Seliga, 2001; King, De Oliveira, and Patel, 2005). Enthusiasm for the possibility must be tempered, however, by the fact that we did not observe a similar increase in submissiveness in castrated, cholesterol-treated males in Experiment 2. This inconsistency indicates, at the very least, that the effect of gonadal hormones on baseline submissiveness in non-defeated male hamsters is variable.
Castrated males treated with T and DHT were significantly less submissive than were males treated with E2 or C capsules. Because both T and DHT decreased submissive behavior in castrated males, it is likely that this effect is produced by binding at androgen as opposed to estrogen receptors. In contrast, there was no significant effect of T or DHT on aggressiveness in either defeated on non-defeated hamsters. Several possibilities may account for a lack of effect of these steroids on aggression. First, it is possible that the doses were not high enough. This possibility seems more likely in the case of T as the plasma concentration of T in the hormone-replaced animals was in the mid physiological range. The plasma concentration of DHT in Experiment 2, however, was in the high physiological range, so this explanation seems less likely, at least in the case of DHT. It also does not appear that the lack of an effect of T and DHT on aggression was due to a ceiling effect on aggression. It is possible that the effects of gonadal hormones on submissiveness may actually be more robust than are the effects on aggressiveness, at least in hamsters.
The increased submissive behavior in castrated males treated with E2 is consistent with our earlier work in intact female hamsters, which illustrated that submissiveness and conditioned defeat were highest during proestrus, the phase of the cycle when endogenous levels of E2 are highest (Solomon et al., 2007). These findings are also consistent with other reports of E2- mediated increases in contextual and cued fear responses (Jasnow, Schulkin, and Pfaff, 2006). Interestingly, we have previously reported that E2 inhibits submissiveness in previously defeated ovariectomized female hamsters when the hormone treatment is limited to only 3 weeks (Faruzzi et al., 2005) suggesting that the effects of chronic E2 treatment on agonistic behavior following social defeat may be dependent upon the length of hormonal exposure or sex-dependent.
Based on the experimental data in Experiment 1, we suspect that longer duration of social isolation prior to behavioral testing may contribute to a decrease in CD in intact male hamsters. For example, sham-operated males in Experiment 1 did not display increased submissive behavior relative to our previous studies but instead displayed high levels of social or mildly aggressive behavior (i.e., following unusually close but not at the level of chasing). Typically, our intact male hamsters are individually housed approximately 10-14 days prior to behavioral testing suggesting that this increase in social isolation prior to behavioral testing may have decreased the display of submissiveness and increased the mildly aggressive behaviors in the sham-operated males. This postulate is supported by previous findings which indicate that longer periods of isolation increase aggression in Syrian hamsters (Brain, 1972; Wise, 1974). Future research should attempt to tease apart the effects of the hormonal manipulations from the effects of individual housing on the display of CD in hamsters.
Overall, the data in the present study suggest that the presence of androgens decreases CD in male hamsters. Given that androgens have been reported to decrease depression-like and anxiety-like behaviors in males with the use of standard animal models for assessing mood, (Frye, Edinger, and Sumida, 2008; Frye and Walf, 2009) these data may have important implications concerning the protective effects of androgens on anxiety-like and/or depression-like behaviors following exposure to an ethologically relevant stressor.
Acknowledgments
This research was supported by the National Institutes of Health RO1 MH62044 to K.L.H. and in part by The Center for Behavioral Neuroscience, a National Science Foundation Science and Technology Center program under agreement no. IBN-9876754.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert DJ, Walsh ML, Gorzalka BB, Siemens Y, Louie H. Testosterone removal in rats results in a decrease in social aggression and a loss of social dominance. Physiol Behav. 1986;36(3):401–7. doi: 10.1016/0031-9384(86)90305-7. [DOI] [PubMed] [Google Scholar]
- Beeman EA. The effect of male hormone on aggressive behavior in mice. Physiol Zool. 1947;20:373–405. doi: 10.1086/physzool.20.4.30151969. [DOI] [PubMed] [Google Scholar]
- Bevan JM, Bevan M, Williams BF. Spontaneous aggressiveness in young castrate C3H mice treated with three dose levels of testosterone. Physiol Zool. 1958;31:284–288. [Google Scholar]
- Brain PF. Effects of isolation on adrenocortical and gonadal function in male and female golden hamsters. J Endocrinol. 1972;53(3):62–3. [PubMed] [Google Scholar]
- Christie MH, Barfield RJ. Effects of aromatizable androgens on aggressive behaviour among rats (rattus norvegicus) J Endocrinol. 1979;83(1):17–26. doi: 10.1677/joe.0.0830017. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Vandenbergh JG, Colby DR. Predictors of dominance in the male golden hamster (Mesocricetus auratus) Anim Behav. 1973;21(3):557–63. doi: 10.1016/s0003-3472(73)80016-8. [DOI] [PubMed] [Google Scholar]
- Evans CM, Brain PF. Proceedings: Some influences of sex steroids on the aggressiveness directed towards golden hamsters (Mesocricetus auratus, Waterhouse) of both sexes by ‘trained fighter’ individuals. J Endocrinol. 1974;61(2):XLVI–XLVII. [PubMed] [Google Scholar]
- Faruzzi AN, Solomon MB, Demas GE, Huhman KL. Gonadal hormones modulate the display of submissive behavior in socially defeated female Syrian hamsters. Horm Behav. 2005;47(5):569–75. doi: 10.1016/j.yhbeh.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Martinez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30(8):762–70. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger K, Sumida K. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology. 2008;33(5):1049–61. doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci. 2001;1(4):371–81. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Depression-like behavior of aged male and female mice is ameliorated with administration of testosterone or its metabolites. Physiol Behav. 2009;97(2):266–269. doi: 10.1016/j.physbeh.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett JW, Campbell CS. Changes in social behavior of the male golden hamster accompanying photoperiodic changes in reproduction. Horm Behav. 1980;14(4):303–18. doi: 10.1016/0018-506x(80)90020-3. [DOI] [PubMed] [Google Scholar]
- Grelk DF, Papson BA, Cole JE, Rowe FA. The influence of caging conditions and hormone treatments on fighting in male and female hamsters. Horm Behav. 1974;5(4):355–66. doi: 10.1016/0018-506x(74)90021-x. [DOI] [PubMed] [Google Scholar]
- Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm Behav. 2006;50(4):640–6. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44(3):293–9. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav. 2006;49(2):197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- King JA, De Oliveira WL, Patel N. Deficits in testosterone facilitate enhanced fear response. Psychoneuroendocrinology. 2005;30(4):333–40. doi: 10.1016/j.psyneuen.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev. 2002;26(8):869–88. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Sterner MR, Diekman MA. Differential hormonal control of aggression and sexual behavior in female Syrian hamsters. Horm Behav. 1988;22(4):453–66. doi: 10.1016/0018-506x(88)90050-5. [DOI] [PubMed] [Google Scholar]
- Murphy MR. Olfactory stimulation and olfactory bulb removal: effects on territorial aggression in male Syrian golden hamsters. Brain Res. 1976;113(1):95–110. doi: 10.1016/0006-8993(76)90009-3. [DOI] [PubMed] [Google Scholar]
- Murphy MR, Schneider GE. Olfactory bulb removal eliminates mating behavior in the male golden hamster. Science. 1970;167(916):302–4. doi: 10.1126/science.167.3916.302. [DOI] [PubMed] [Google Scholar]
- Payne AP. A comparison of the aggressive behaviour of isolated intact and castrated male golden hamsters towards intruders introduced into the home cage. Physiol Behav. 1973;10(3):629–31. doi: 10.1016/0031-9384(73)90235-7. [DOI] [PubMed] [Google Scholar]
- Payne AP. The aggressive response of the male golden hamster towards males and females of differing hormonal status. Anim Behav. 1974;22(4):829–35. doi: 10.1016/0003-3472(74)90005-0. [DOI] [PubMed] [Google Scholar]
- Payne AP, Swanson HH. Agonistic behaviour between pairs of hamsters of the same and opposite sex in a neutral observation area. Behaviour. 1970;36(4):260–9. [PubMed] [Google Scholar]
- Payne AP, Swanson HH. The effect of castration and ovarian implantation on aggressive behaviour of male hamsters. J Endocrinol. 1971;51(1):217–8. doi: 10.1677/joe.0.0510217. [DOI] [PubMed] [Google Scholar]
- Payne AP, Swanson HH. The effect of sex hormones on aggression in the male golden hamster. J Endocrinol. 1972;53(3):lxi–lxii. [PubMed] [Google Scholar]
- Potegal M, Blau AD, Black M, Glusman M. Effects of castration of male golden hamsters on their aggression toward a restrained target. Behav Neural Biol. 1980;29(3):315–30. doi: 10.1016/s0163-1047(80)90202-2. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Karom MC, Huhman KL. Sex and estrous cycle differences in the display of conditioned defeat in Syrian hamsters. Horm Behav. 2007;52(2):211–9. doi: 10.1016/j.yhbeh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Lisk RD. Intrasexual interactions among female golden hamsters (Mesocricetus auratus) over the estrous cycle. J Comp Psychol. 1984;98(3):267–75. [PubMed] [Google Scholar]
- Tiefer L. Gonadal hormones and mating in the adult golden hamster. Horm Behav. 1970;1:189–202. [Google Scholar]
- Vandenbergh JG. The effects of gonadal hormones on the aggressive behaviour of adult golden hamsters (Mesocricetus auratus) Anim Behav. 1971;19(3):589–94. doi: 10.1016/s0003-3472(71)80116-1. [DOI] [PubMed] [Google Scholar]
- Whitsett JM. The development of aggressive and marking behavior in intact and castrated male hamsters. Horm Behav. 1975;6(1):47–57. doi: 10.1016/0018-506x(75)90022-7. [DOI] [PubMed] [Google Scholar]
- Wise DA. Aggression in the female golden hamster: effects of reproductive state and social isolation. Horm Behav. 1974;5(3):235–50. doi: 10.1016/0018-506x(74)90032-4. [DOI] [PubMed] [Google Scholar]


