Cervical Cancer Burden
Worldwide, there were approximately 493,243 new cases, and 273,505 deaths attributed to cervical cancer in 2002, which is about one-tenth of total female cancer deaths 1. Cervical cancer incidence 2, and mortality in the United States has declined significantly since the 1950's by more than 70% 3,4. This decline is mainly attributed to the introduction of the Papanicolaou test in the 1940's. Cervical cancer which in this country was the number one killer of women, is now ranked 12th in cancer deaths for women in the US 5,6. In the US, it is estimated that 11,150 women will be diagnosed with cervical cancer, and approximately 3,670 will die of it. However, cervical cancer is the second most frequent of all female malignancies worldwide, with 80% of the cases occurring in resource-poor countries 7. While in the United States and most developed countries, cervical cancer accounts for 7% of all female malignancies, in developing countries, it accounts for 24% of all such cancers 1. This disparity is primarily attributed to the lack of screening and treatment of pre-cancerous lesions 1.
The anatomical accessibility of the cervix to direct examination, with a long preclinical stage during which precursor lesions can be treated conservatively and successfully (∼95%) 8, make cervical neoplasia ideal for secondary prevention efforts such as screening. The Pap smear test is probably the most widely used cancer screening test, even though it has never been evaluated in a randomized controlled trial, and will not be because it is been accepted as an effective screening tool. Additionally there are numerous convincing epidemiological data which show that since the introduction of Pap in countries with well organized screening programs, and with wide population coverage, both incidence of, and mortality from cervical cancer has significantly decreased. The best data to support these observations come from Nordic countries. Incidence of cervical cancer has fallen by more than 50% in Finland, Sweden, Denmark, and Iceland, where organized cervical screening programs were established in the 1960s 9. Interestingly, despite the availability of a Pap screening program in England, cervical cancer incidence remained relatively constant, until the introduction of an organized screening program in 1988, which led to a dramatic reduction in subsequent years 9.
Since the introduction of organized cervical screening in the 1960's in the U.S., incidence and mortality from cervical cancer have declined by 75%, however, this decline is not uniform. In the 1990s U.S. women experienced at least 33% higher incidence of, and 71% higher mortality from cervical cancer in high poverty counties than in low poverty counties 10. The Healthy People 2010 objective of 90% Pap screening for women aged 18 or older has not yet been achieved by any State or territory 11. Unscreened populations of women in the U.S. historically include older women, uninsured and impoverished women, migrant and minority women, and those residing in rural areas 12. Data from the 1994 and 2003 U.S. National Health Interview Survey showed that screening trends have remained unchanged from the 1994 survey to the 2003 survey - 77% and 76% of women reported having had a Pap test in the past 3 years, respectively 13,14. Additionally, they show that age is still a risk factor for inadequate screening; in the 1994 survey screening was higher among 18-44 year old women (82%) compared to 57% among women 65 or older. This age difference may be due to a cohort effect. Pap testing was still gaining acceptance in the late 1950's and 1960's. Women who were in the reproductive age group at that time (and are now over 65 years) never became accustomed to regular cytology screening. This trend remained virtually unchanged in the 2003 survey; 81% of the 18-44 and 57% of 65 or older women had a Pap in the previous 3 years. The 2003 survey showed that while screening rates among African-Americans and Whites were similar, screening rates were much lower in other race groups (76%, 80%, and 69% for Whites, African-Americans, and other race groups, respectively).
Organizing screening programs in developing countries where the burden of cervical cancer is the greatest has remained a challenge. There are many obstacles to cervical cancer screening in resource poor countries, generally attributed to a lack of infrastructure and resources – technical, medical, and financial - and a lack of awareness and education about cervical cancer among women and health-care providers. Moreover, in Africa and South America which bear the biggest cervical cancer burden, there are competing health care needs such as HIV/AIDS, infectious diseases such as malaria, tuberculosis, and high infant and maternal mortality rates. In addition there are not many trained clinicians, and there is a lack of adequate laboratory supplies and personnel, and treatment facilities 5. We refer interested readers to an excellent review specific to this topic 15.
Furthermore, there are considerable cultural barriers to routine pelvic screening, especially in the absence of any symptoms, underscoring the profound need for an acceptable and reliable screening method that focuses on timely detection of early lesions, and treatment of the lesions so as to reduce cervical cancer burden. Although cytology has proven to be extremely effective in detection of abnormal cervical cells in developed countries, it is still under-utilized by many even in regions with successful screening programs. An important aspect of the success of cytology screening in developed countries is attributed to repeated screening of women during the long natural history of cervical cancer development. The repeated nature of the screening makes it cost-prohibitive for resource-poor countries. Moreover, the need for multiple visits – one to perform the test, a second to obtain results, and a possible third for treatment – can lead to loss of follow-up of women who may be at greatest risk of cervical cancer, further compounding a complex issue. Screening programs in resource-poor settings must consider these limitations and develop approaches sustainable and suitable for those settings such considering screening methods that target the etiologic agent HPV, appropriate age to initiate screening, and screening interval, and one designed to encompass screening and treatment at one time.
A range of abnormal cellular changes occur in the transformation zone of the cervical epithelium. There are two embryologically distinct cell types that make up the cervical epithelium. 1) The ectocervix, the part of the cervix that extends into the vagina, is made of nonkeratinized stratified squamous epithelium, similar to the lining of the vagina; 2) the endocervix, the part of the cervix that leads to the uterus, is made of mucus secreting, columnar epithelium 16. The junction of the columnar and the stratified squamous epithelial cells is called the squamocolumnar junction, which recedes towards the endocervix with age, replacing columnar cells with stratified squamous epithelium. This process is called squamous metaplasia, and leads to the formation of the ‘transformation zone’ from the original squamocolumnar junction to the current squamocolumnar junction. Due to the rapid turn over of cells in the transformation zone, it is very susceptible to carcinogens and carcinogenesis, and HPV infections. Most precancerous cervical lesions and cancers arise in the transformation zone. Approximately 75 to 80% of all cervical cancers are squamous cell carcinoma (SCC) 17. Adenocarcinomas account for the remaining 25%.
C. Screening Test Characteristics
There are important concepts of risk to consider when discussing screening and quality of screening tests and diagnostics, and in separating out people with and without the disease. The simple 2×2 screening table (Table 1) holds key concepts to understanding clinical diagnostics.
Table 1.
The 2*2 table
| Cancer Risk | No Cancer | |
|---|---|---|
| Screening test (eg. Pap)+ | A | B |
| Screening test (eg. Pap)- | C | D |
Although the validity of a screening test, measured by sensitivity and specificity are important from a public health view in clinical settings, a different set of questions is of importance to the clinician. First, if the test results are positive in a patient, what is the probability or risk of disease for that patient? This is the positive predictive value of the test. Similarly, if a patient's test results are negative, what is the probability that the patient does not have the disease? This is the negative predictive value of a test and provides reassurance against disease among test negative women. Another important concept is the disease endpoint considered. Cervical cancer prevention specialists study surrogate endpoints to evaluate risk since no one would willingly permit a woman to develop cervical cancer under observation. Histologically-confirmed CIN3 is the best surrogate for cervical cancer, however, risk of CIN2/CIN3 is also studied since that is the threshold for treatment.
Table 1 shows results of a dichotomous screening test compared to the disease state of the population screened: for example, HSIL or above (HSIL+) versus not (Pap+ versus Pap-) for CIN3 or cancer in this example. Four outcomes are possible. True-positives (positive results in individuals with CIN3/cancer); D is true-negatives (negative results in individuals without CIN3/cancer); B reflects false-positives (positive results in individuals without CIN3/cancer); and C corresponds to false-negatives (negative results in individuals with CIN3/cancer).
Sensitivity is the ability of the test to correctly identify those who have the disease [A/(A+C)],. Specificity is the ability of the test to correctly identify those who do not have the disease [D/(B + D)]. Positive predictive value [A/(A + B)] of any abnormal result is the risk of cervical cancer among women with that result. Negative predictive value [D/(C + D)] is the reassurance that a woman with a normal test result is not at risk of cancer until the next expected visit.
The sensitivity of the Papanicolaou test for high-grade lesions or cancer is estimated to be up to 70—80% with a specificity between 94-97% 18,19. Test sensitivity must be distinguished from program sensitivity. The former is a measure of the sensitivity of a single test at one point in time. The latter is the sensitivity of a series of tests at intervals determined by the screening program to detect an abnormality at any single test event. Repeat screening at regular intervals therefore compensates somewhat for the limitations of the sensitivity of the technique.
In the context of' cervical screening, two main types of errors contribute to lower sensitivity. Sampling error occurs when a cervical lesion is present but cells representative of the abnormality are not present on the glass slide specimen. Sampling error may occur if either the lesion is not sampled or if abnormal cells collected on the sampling implement are not transferred to the slide. Factors that contribute to sampling error include small size of the lesion, inaccessible location of the lesion (high in the endocervical canal, for example), or inappropriate sampling technique. Laboratory error occurs when cells indicative of an intraepithelial lesion or carcinoma are present in the specimen but are not identified as abnormal when the result is reported. Factors that may contribute to laboratory error include presence of only a few abnormal cells, small size of the abnormal cells, presence of inflammation or blood obscuring cells, or diagnostic misinterpretation of the significance of identified cell abnormalities. Even under optimal screening conditions, sampling and laboratory error cannot be entirely eliminated.
Screening based on Cytology
Conventional Papanicolaou (Pap) Test & Liquid Based Cytology
Cytological evaluation of the cervical cells was introduced by George Papanicolaou in 1940's after whom the Pap test is named. It is probably the most widely used cancer screening technique in the U.S. and in other developed countries. The test involves gently scraping cells from the surface of the cervix and microscopic examination of the fixed and stained cells for abnormal morphologic cell changes.
While the cervical/vaginal Pap smear has been tremendously successful in reducing the incidence and mortality rate from cervical cancer, it has limitations, particularly with respect to false-negative screening results. Hence, interest has focused on development of technologies to enhance the accuracy of cervical cancer screening. The liquid based cytology (LBC) collection technique is directed at improving cytology sampling and specimen quality.
Specimen collection
Cervical sample collection for cytology involves the clinician visualizing the cervix and identifying the sqaumocolumnar junction which as described earlier is the junction where the smooth squamous surface of the ectocervix changes to the columnar lining of the encdocervix leading to the uterine cavity. Sampling should be directed at this tissue, as this is the region where majority of cervical lesions occur. It has been demonstrated that using either a combination of spatula and cervical brush or broom-shaped device that samples both the ectocervix and endocervix simultaneously results in increased detection of abnormalities 20.
a. Conventional Pap
In the conventional Pap, the cellular sample collected with either a spatula or brush is quickly and evenly spread over the surface of a glass slide, so as to thin out the large clumps, while avoiding excessive manipulation that can damage the cells. Studies have shown that more than half of the material collected on the collection instruments remain on the device which is discarded and are not transferred to the glass slide and thus lost for microscopic analysis 21. In order to preserve morphologic details, after smearing the cells on the slide, specimens are fixed by immersion in alcohol or sprayed with a fixative. Air-drying of the sample may limit the interpretability of the specimens.
At the laboratory, the conventional Pap slides are stained using a polychrome process that was developed by George Papanicolaou which in optimal circumstances, results in excellent nuclear detail and cytoplasmic transparency that allows visualization through areas of overlapping cells.
Specimen adequacy depends on a number of parameters including number and types of epithelial cells present and morphologic preservation. Additionally, the smear must not be obscured by factors such as blood, neutrophils, inflammation, or air-drying, that may limit microscopic visualization of the cells 22. The adequacy of the smear depends on sampling of the transformation zone, with “adequate” specimen consisting of well-preserved, evenly distributed squamous and glandular cells. The presence of both epithelial cell types provides indirect evidence that the squamocolumnar junction has been sampled.
The process of diagnostic evaluation of a Pap test is labor-intensive and subjective. The slide may consist of over 100,000 cells of which only a small number may be abnormal. This process of microscopic screening is performed by trained cytotechnologists who must be able to detect the rare abnormal cells amongst thousands of cytologically normal cells. Any identified abnormal or questionable cytologic changes are then referred to a pathologist for diagnostic interpretation.
b. Liquid Based Cytology
As mentioned earlier, with conventional smear techniques only a fraction of the cellular material collected from the cervix is transferred to the glass slide. Instead of spreading the exfoliated cervical cells on the glass slide, with liquid based cytology (LBC), the sampling device is vigorously rinsed or stirred in a vial of preservative/fixative, producing a suspension of cells that are filtered before a slide is made. In principle there are several advantages to these modifications in sampling over conventional Pap. This ensures more of the collected cervical cells to be captured in the suspension, and transfers most of the collected cellular material into the collection media for further processing 21 23. These techniques also allow for removal of extraneous material such as blood, ensuring the slides are composed of a uniform layer. The details of the equipment and techniques for transferring cellular material to the glass slides varies from one manufacturer to another but the central principle is shared by the various manufacturers.
Below are descriptions of some of the products available commercially:
ThinPrep by Cytyc Corporation (Boxborough, MA) was approved by the U.S. FDA in May 1996. It provides either a semi-automated single-sample processing (T2000, processes samples individually) or fully automated (T3000, fully automated, can process up to 80 samples per cycle) sample preparation. Cellular materials are collected in a vial of proprietary PreservCyt solution, and further processed by the T2000 or T3000 machine.
SurePath, formerly known as CytoRich or AutoCyte, now manufactured by TriPath Imaging (Burlington NC) is also FDA approved and claims to be the only test where 100% of the collected cellular material will be used by the labs for processing and analysis. It requires that the collection device (broom-like, or endocervical brush or spatula) with defined heads, be detached from the handle, and placed into the proprietary SurePath collection vial which contains the transport solution, hence ensuring all cells collected will be sent to the laboratory for processing, where they are centrifuged, and sedimentation preformed through a density gradient and cells are then allowed to settle on a glass slide.
Although in the US and many other countries LBC has replaced conventional Pap, few randomized trials have formally compared LBC to conventional cytology. Results of a recently published randomized clinical trial conducted in Italy, showed that while LBC had comparable sensitivity compared to conventional Pap for detection of cervical intraepithelial neoplasia of grade 2 (CIN2) or worse lesions, it resulted in more positive finding thus leading to lower positive predictive value. However, they found fewer unsatisfactory smears with LBC compared with conventional Pap 24.
Another added benefit of LBC is that the residual specimens would be available for additional testing such as ‘reflex’ HPV testing in cases of low-grade or equivocal cytology results 25,26. This ability to test for HPV from the same specimen eliminates the need for an additional patient visit to collect a separate sample.
Computerized Screening Technologies
To reduce false negative results associated with diagnostic evaluation of Pap slides, i.e. the human visual system used to identify abnormal cells, a number of new approaches involving automation of slide analysis which use computer image analysis technology have been developed to assist the cytotechnologists to focus on areas of the slide deemed not normal by the program. Slides are first converted to digitized images, making them suitable for analysis by computers. The commercially available FocalPoint (formerly known as AutoPap System, TriPath Imaging, Inc., Burlington NC.). It is intended to be used on both conventionally prepared and SurePath (formerly AutoCyte) slides.
It uses a high speed video microscope, imaging interpretation software, and morphology computers to image and analyze images on the slide. The program is designed to detect morphologic changes associated with epithelial abnormalities, specimen adequacy, and benign cellular changes and infections. Slides are classified as No Further Review, Review, or QC Review 27. FocalPoint was FDA approved for initial (primary) screening of conventional Pap slides in 1998, and in 2001 it was FDA approved for initial screening of slides with the SurePath LBC. It has also received FDA approval for secondary screening of previously evaluated negative specimens by routine manual screening.
In secondary screening Focalpoint (AutoPap) identifies approximately 20% of previously diagnosed “negative” cases as most likely to contain an abnormality, which then undergo repeat manual screening by the cytotechnologists.
Using 100% rescreening as the reference standard, FocalPoint (AutoPap), set to select 20% of slides for review, identified 77% of LSIL and above 28 (7.7 times more than a random 10% review). However, this increased sensitivity is primarily for ASCUS and LSIL diagnoses and comes at significant cost. Used in a secondary screening mode, these technologies are cost-effective only if incorporated into a less frequent screening strategy 29.
Operating as a primary screener, the FocalPoint (AutoPap) computer identifies approximately 25% of cases - those with the lowest rank score - as least likely to contain an abnormality; these slides are not reviewed by a cytotechnologist. The remaining 75% of specimens undergo manual microscopic screening. In addition, of those cases reviewed as “negative” by the cytotechnologist, a subset with the highest rank score as determined by the computer are then subjected to a second round of manual screening.
Another automated imaging device is the ThinPrep Imager, which is intended to be used with the ThinPrep LBC system. It can be used on either the ThinPrep 2000 or ThinPrep 3000 processor. The imager rapidly scans every cell and cell cluster and identifies 22 areas of interest or field of view (FOV) for every slide. The cytotechnologist can focus on and review the 22 FOV of the slides that the imager selected as likely lesions, hence reducing the amount of time needed to screen each slide. A recent report from Australia compared the ThinPrep imager with manually read conventional cytology and showed that the Imager detected 1.29 more cases of histologic high-grade squamous disease per 1000 women screened compared to manually read slides, and more of the imager read slides were satisfactory for examination and contained more low-grade cytological abnormalities 30. Similarly, results of a recent randomized trial in Finland compared the Papnet imager (which is no longer commercially available) to manually read conventional method, and showed that cytologic class III grade (which includes LSIL, ASC-H and some HSIL) and histologically confirmed CIN1 or worse lesions were more common with Papnet imaging compared to conventional method performed well. Results were similar when compared HSIL and cancer cases detected. They also showed that it may take several years for a new technique to achieve quality performance and can initially increase costs without improving efficacy 31.
A recent systematic review by the UK Health Technology Assessment Programme concluded that presently there is insufficient evidence on performance, impact on process and cost-effectiveness to recommend use of automated image analysis systems.
Evaluation of Cytology Results
Pap test results may be reported using a variety of terminology systems. A translation table (Table 2) is helpful to convert from one nomenclature to another. At the time of the emergence of cytology as a diagnostic discipline in the 1940-1950s, Dr. Papanicolaou devised a numeric classification (I-V) to communicate the degree of confidence that cancer cells were present in a specimen. As used initially by Papanicolaou, the numeric designations represented the following: Class I – benign; Class II – minor cellular abnormalities considered benign; Class III – cells suspicious for but not diagnostic of cancer; Class IV – cells fairly conclusive for malignancy; and Class V – cells diagnostic of cancer.
Table 2.
Cervical Diagnostic Terminology
| Dysplasia | Atypia | HPV | Mild Dysplasia | Moderate Dysplasia | Severe Dysplasia | CIS |
| CIN | Atypia | HPV | CIN1 | CIN2 | CIN3 | |
| Bethesda | ASCUS | LSIL | HSIL |
As the field of cytology expanded, this numeric diagnostics gave way to terminology systems that included a designation of the degree of abnormality identified, for example four grades of dysplasia (mild, moderate, severe, and carcinoma-in situ (CIS)). Richart introduced the term cervical intraepithelial neoplasia (CIN), grades 1, 2, and 3, to promote the concept of a disease continuum of precursors to invasive cancer 32. The CIN system is based on morphologic criteria and tissue architecture: the proportional thickness of the epithelium involved by disorderly growth and cytologic atypia. Mild and moderate dysplasia roughly correspond to CIN 1 and CIN 2, respectively. However, CIN 3 encompasses severe dysplasia and CIS, thus eliminating a difficult and sometimes arbitrary diagnostic distinction between almost vs. complete full-thickness abnormality.
Koilocytosis, a diagnostic term indicating cellular changes of perinuclear cytoplasmic cavitation, was recognized by Meisels to be a manifestation of genital human papillmoavirus (HPV) infection 33. Initially, HPV cellular changes were considered distinct from ‘true’ dysplasia or CIN and not part of the precursor pathway to cervical cancer. However, as techniques for identifying HPV became more sensitive, HPV DNA was found in the greater than 99% of cervical neoplasia studied 34,35. The pathogenesis of cervical neoplasia and cervical cancer is now known to be due to HPV, based on epidemiologic, virologic, and experimental evidence. Therefore, isolation of ‘koilocytotic atypia’ or ‘HPV effect’ as a distinct entity from dysplasia/CIN is no longer biologically valid.
The Bethesda System (TBS), developed at a National Cancer Institute workshop in 1988 36 and refined in 1991 37, collapses the cytologic diagnostic subcategories of intraepithelial lesions into low- and high-grade squamous intraepirhelial lesions, abbreviated as LSIL and HSIL, respectively. This division is based on the concept of HPV-induced cellular changes as discrete processes of (1) LSIL as acute infection with any HPV type resulting in mild, usually transient cytologic effects, and (2) HSIL as the result of persistent infection with predominantly oncogenic HPV types and the interplay of a variety of factors, including host immune response, that poses a substantial risk of invasion 38. While the CIN classification remains widely used in cervical histopathology, TBS is more commonly used to report Pap test results.
The Bethesda System also introduced the term atypical squamous cells of undetermined significance” (ASCUS) to reflect equivocal, abnormal changes that are quantitatively or qualitatively insufficient to establish a definitive diagnosis of SIL. ASCUS is not a single diagnostic entity and is therefore associated with highly variable clinical outcomes. It does represent an improvement, however, over older classifications that used “atypia” to encompass reactive changes and HPV associated cell changes in addition to equivocal findings. In the Bethesda System, reactive changes are categorized as “benign” and HPV changes are subsumed under SIL.
Abnormal Pap test results are not evenly distributed among the diagnostic categories described above. In a well-screened population such as the U.S., there are millions of low-grade and equivocal lesions diagnosed with relatively few cancers at the top. In the U.S., cancers represent less that one-tenth of 1% of diagnoses and high-grade lesions constitute approximately six-tenths of 1% [Solomon Chapter 20,21]. By contrast, LSIL and ASCUS account for an estimated 6% of all Pap test results, which translates to 3 million women in the U.S. annually.
Follow-up and management of Abnormalities
Screening without treatment is unethical, and it cannot be effective without follow-up of abnormal results and treatment of lesions as appropriate. Loss to follow-up is a significant problem. In two studies 39,40 13 and 15% of cervical cancers that occurred in women who had ever had a Pap test were attributed to lack of either patient notification or patient compliance with recommended treatment. ‘One-stop’ screening, diagnosis, and treatment clinics have been established in a few high-risk areas to address this problem 41,42. However, this is a labor-intensive approach to screening that cannot feasibly be applied as yet on a large scale.
The Atypical Squamous Cells of Undetermined Significance – Low-Grade Squamous Intraepithelial Lesion Triage Study (ALTS) was a randomized clinical trial launched by the U.S. National Cancer Institute, to provide empirical evidence for the best clinical management of women with minimally abnormal cervical cytology (ASCUS and LSIL). It showed that among women with ASCUS, HPV testing for carcinogenic HPV (HPV triage) was at least as sensitive for identifying women with CIN3 or worse, and referred half as many women to colposcopy, compared with universal immediate colposcopy. Among women with LSIL, HPV DNA triage was not as useful because most LSIL lesions are HPV-positive 43. It further showed that the 2-year risk of CIN2 or worse for HPV-positive ASCUS and LSIL were virtually identical, hence for the first time indicating that HPV-positive ASCUS is biologically comparable to LSIL. Further analysis of ALTS data has shown that women with HPV-negative ASCUS have a very low risk of subsequently detected CIN3 or worse lesions in the subsequent 2 years (1.4%), similar to women with negative cytology in the absence of HPV testing, suggesting they return to routine screening intervals which may be longer than 1 year depending on the patient's age and past screening history 44.
Based on the ALTS data, women with HPV-positive ASCUS and those with LSIL lesions or worse are managed by referral to colposcopy with directed biopsy. Women with histologically confirmed CIN2 or worse lesions are managed by destruction or removal of the lesion and the transformation zone of the cervix.
New Cervical Cancer Screening Techniques
Despite the success of cytology programs in developed countries to reduce the burden of cervical cancer, there is interest on development of technologies to enhance the accuracy of cervical cancer screening and thereby make it more cost-effective. As described above, some of these efforts have been directed at improving the quality of cytology, e.g., LBC methods, while others have focused on improving the laboratory microscopic screening process e.g., computerized imaging. Additionally, molecular assays, based on detecting HPV, the etiologic agent for cervical cancer are also being considered.
HPV Testing
In the 1990's data from multiple, international epidemiologic studies established that infection with a group of ∼15 ‘carcinogenic’ HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68) is a necessary cause of cervical cancer 34,45 and its immediate precursor, cervical intraepithelial neoplasia grade 3 (CIN3) (also know as carcinoma in situ or precancer). Other types are classified as ‘low-risk’ since they are not associated with cervical cancer although HPV6 and HPV11 cause 90% of genital warts.
HPV is the most common acute viral sexually transmitted infection in the United States 46 and internationally (REF), although there can be significant regional variation due to different culture, social, and sexual norms. Most infections, even by carcinogenic HPV types, are benign and clear within 1-2 years. Women who do not clear their carcinogenic HPV infection, i.e., HPV persistence, are at elevated risk of developing precancer and, if not detected and treated, then cancer. 47.
Testing based on detection of HPV DNA in cervical specimens has been introduced in the U.S. and in some European countries to improve the efficiency, and maximize the sensitivity of cervical cancer screening. As mentioned earlier, results of the ALTS trial and other studies showed that testing for carcinogenic HPV is cost-effective and more sensitive than conventional cytology for detection of pre-cancer in women with ASCUS 48-53. Carcinogenic HPV testing is approved as an adjunctive test with cytology for primary cervical cancer screening for women 30 and older and is more sensitive, with very high negative predictive value (hence providing reassurance that test negatives are at low risk for developing cervical precancer and cancer) but less specific than cytology 48,54 because HPV infections are very common.
HPV testing is now also added to follow-up of women post-colposcopy when cancer is not detected 55 and used as a follow-up strategy among women who have undergone treatment of a lesion 56-58.
At present there are no gold-standard tests for HPV detection, however, there is one FDA approved commercially available DNA based molecular assay, the Hybrid Capture 2 assay (HC2; Digene Corporation, Gaithersburg, MD), which collectively targets detection of 13 carcinogenic HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). HPV DNA testing can be performed directly from residual liquid-based cytology specimens, or a separately collected sample. HC2 is a signal amplification assay that uses a technique which combines antibody capture of HPV DNA and RNA probe hybrids and chemiluminescent signal for detection.
Because HPV incidence and prevalence is associated with age, age plays a crucial role in determining target population for HPV DNA screening. The high prevalence of HPV infection among young sexually active women, often is not associated with significant cervical disease, since at that age HPV infection and HPV associated mild lesions always almost clear spontaneously, hence precluding use of HPV testing as a primary screening strategy among young women close to onset of sexual activity. However, as HPV DNA prevalence declines sharply with age, viral persistence increases, while the sensitivity of HPV DNA for cervical neoplasia remains high. Furthermore, the incidence of cervical lesions starts to increase in the late twenties to early thirties or 10-15 years since onset of sexual activity, and cervical cancer incidence increases starting in the late thirties, hence the positive predictive value of a HPV DNA test for cervical precancer and cancer rises with age. Moreover, the accuracy of Pap smear cytology declines with age due to poor sampling of the squamocolumnar junction which migrates into the endocervical canal with age and becomes more difficult to sample, coupled with the increased detection of morphologic look-alikes that are unrelated to cervical carcinogenesis. Taken together, these suggest that HPV testing at younger ages is inefficient; however, it is a cost-effective, primary screening strategy in older women provided that the screening interval is lengthened among HPV-negative women.
Several companies are currently conducting clinical trials of new HPV diagnostic tests, results of which should be available shortly.
New Biomarkers
There is a demand for newer screening tests that may be more specific and with better predictive values for cervical cancer, to compensate for the high false-negative rates associated with Pap, and the high false-positive rate associated with the HPV DNA tests. Some of the new tests are modifications of the existing technologies (not a new biomarker) and some are based on new biomarkers of disease (HPV and host interaction). Although many assays are continually being developed, only completely standardized assays should be used in clinical practice, as lack of rigorous research and standardization can influence analytic performance of the tests 59.
Type-Specific HPV testing
An important goal of new applications of HPV testing is to improve specificity while maintaining clinical sensitivity. It is now known that HPV 16 is the most important HPV type worldwide. It is present in about 50% of cervical cancers and is the most prevalent HPV type present in invasive cervical cancers, followed by type 18 and together they are represented in approximately two-thirds of cervical cancers 60,61. It is also the most common genotype in the general population accounting for approximately 20% of the infections among cytologically normal women, 20% among women with equivocal lesions, and approximately a quarter of women with mild abnormalities 62. Hence if HPV 16 could reliably be identified, and its cytopathologic manifestations treated, then theoretically 50% of cervical cancers could be prevented. Longitudinal studies have shown that type-specific detection of HPV 16 and 18 identifies women at the greatest risk of cervical pre-cancer and cancer 63-65. These type-specific HPV detection assays are based on detection of the viral DNA from the tissue or exfoliated cells collected from the site of infection by polymerase chain reaction (PCR) amplification and detection of HPV types in the amplified products. There are several primer sets designed to allow amplification of many HPV types, however, the detection part of the assays are able to identify between 27-41 different types. At least four (MY09/MY11; PGMY09/PGMY11; GP5+/GP5+; and SPF10) primers give roughly similar results. Although currently there are no commercially available HPV genotyping assays, several companies are evaluating HPV genotype specific assays in clinical trials.
Cellular Markers
The new generation of screening tests are aimed at identifying cellular markers which can help discriminate between the rare infection that have the potential for progression to pre-cancer and cancer, and the majority of the infections that will regress. Two novel biomarkers – mRNA expression of E6/E7 transcripts and p16INK4a (referred to as p16 henceforth) – are markers of disease progression and show promising results in initial studies, however, large scale evaluations are lacking comparing them to other markers such as HPV typing 66. Both E6/E7 mRNA expression and p16 rely on understanding the basic molecular events involved in cervical carcinogenesis. E6 and E7 are two main HPV oncoproteins and their persistent expression is a necessary step for in HPV-induced carcinogenesis 66,67. They are expressed early on in the viral lifecycle are important in inducing cellular transformation and target many cellular functions most importantly degradation of human tumor suppressor genes p53 by E6 and inactivation of retinoblastoma (pRB) by E7. Therefore detection of E6/E7 mRNA of high-risk HPV types may indicate a further step on progression to cancer 66 in addition to HPV infection.
The biomarker p16, a cyclin dependent kinase inhibitor is expressed at very low levels in normal cells, while it is over-expressed in pre-cancer and cancer, indicating progressive steps from a productive HPV infection towards a transforming infection. Its over-expression can be detected by immunoassays designed to detect the protein on cytology slides 68 or in cellular lysates using an ELISA format 68.
While the above two biomarkers have been evaluated by different groups, none have been evaluated in large, formal epidemiologic studies, so their utility as primary screening methods needs further studies. There are other ‘candidate’ biomarkers being developed as screening tools, but so far data on those are based on few pilot projects. We refer the interested readers to two excellent, comprehensive recent review articles by Cuzick 66 and Wentzensen 68.
Cervical cancer screening in resource poor settings
As mentioned earlier of the 500,000 new cases of cervical cancer diagnosed worldwide, more than 80% occur in developing countries. Additionally, despite the advances in cervical cancer screening and treatment in the U.S., there are still disparities in cervical cancer rates among minority populations. The age-adjusted incidence rates between 200-2004 were 8.5/100,000 women among white women, compared to 11.4/100,000 among African-American women, and 13.8/100,000 Hispanic women 6. Other minority ethnic groups in the US also experience higher cervical cancer incidence and mortality than the average population including some Asian populations 69. Mortality from cervical cancer among women in developing countries and minority populations in the U.S. follows the same trend as incidence. These disparities in incidence and mortality are mainly attributed to poverty, lack of services and resources, cultural barriers for seeking healthcare, and disenfranchisement of women. As a result of lack of infrastructure and expense, cervical screening programs are unavailable in most developing settings and countries, and hence, declines in cervical cancer have not been observed in resource-poor countries and settings. Additionally, a constraint to screening in many developing countries is the cultural reticence to seek routine pelvic examination; even in some countries with established national screening protocols, women do not seek pelvic examinations and therefore often present with advanced disease 70-72. To increase screening coverage and overcome possible reticence among women reluctant to undergo pelvic examination, the use of alternative screening approaches to cytology have been widely investigated. Some are discussed below.
Self-collected samples for HPV detection
Self-sampling of the cervico-vaginal canal has been widely evaluated in the literature. It allows the use of molecular testing of the self-collected samples for presence of HPV DNA. With this approach, women are able to self-collect samples by inserting a Dacron swab in the vagina up to the vault and rotating it in the vaginal vault. The swab is then place in transport media and collected for further testing for HPV DNA. Several studies have evaluated the diagnostic accuracy of self-collection using swabs, tampons, or brushes. A meta-analysis of studies comparing utility of self-collected samples to clinician-collected samples (mostly performed in less-developed countries) showed that self-collection had an overall sensitivity of 74% and specificity of 84% compared to clinician-samples 66,73. Although not as good as clinician-collected samples, this sensitivity is comparable to cytology for CIN2 or worse lesions where cytology sensitivity is less than 70% 66. This lower sensitivity of self-sampling may be sufficient if women who otherwise would not get screened are encouraged to seek screening 74.
Visual Techniques
Visual inspection with acetic acid (VIA) is also known as direct visual inspection, or acetic acid test, is a very low-cost approach to screening that may be an option for areas that do not have access to comprehensive cervical cytological screening 75. VIA, at its most basic, consists of unmagnified evaluation of the uterine after the application of 3-5% dilute acetic acid for visual signs of a high grade lesion or cancer. The test is considered positive if clear and well-defined acetowhite areas are detected near the squamocolumnar junction (transformation zone). A similar technique, visual inspection with magnification (VIAM), employs low power magnification device (e.g, Aviscope) when inspecting the cervix after treating it with acetic acid. An advantage of VIA is that it gives immediate results, making it possible to treat abnormal lesions at the same visit (see below). Several cross-sectional studies, mostly performed in less-developed countries have evaluated VIA with mixed results. Sensitivity of VIA to detect high-grade lesions and cervical cancer have ranged from 49% to 96% and specificity from 49% to 98% 15,76. However, there were limitations to the studies. Most suffered from verification bias, first because the true disease status for a large majority of the individuals in the studies were not known; and second, most used colposcopy as the ‘gold-standard’ for disease verification, which is also been shown to have low sensitivity 77, and because VIA and colposcopy are based on the same visual technique, pre-cancerous lesions missed by VIA are likely to be missed by colposcopy. However, VIA may prove to be a cost-effective approach to decrease cervical cancer incidence and mortality in countries with lack of resources that cannot afford a comprehensive cytological screening program 78.
See and treat options
The current cervical cancer screening programs practiced in high-resource settings include at least a three visit intervention: screening; triage of equivocal results; colposcopy directed biopsy for diagnostic purposes; treatment; and post-treatment follow-up. To overcome the obstacles with establishing the infrastructure for cytology based screenings in resource-poor setting, many studies have investigated screening and treatment in a single visit or delayed to a short time after screening. A randomized trial was performed in South Africa of 6555 nonpregnant women between 35-65 years to determine the safety and efficacy of 2 screen-and-treat options. All women were screened with both HPV and VIA and subsequently randomized to cryotherapy if she was HPV-positive, cryotherapy if she had a positive VIA test, or delayed treatment. They showed that the prevalence of high-grade CIN defined histologically was significantly lower in both of the screen-and-treat arms at 6 and 12 months post-randomization compared to the delayed evaluation group 79.
Summary
It is important to realize that no screening test is 100% effective in detecting all cervical cancer cases. Secondary prevention of cervical cancer, as is practiced in high-resource regions includes screening, triage of equivocal lesions, colposcopically-guided biopsy of abnormal results, treatment, and follow-up post-treatment, and return to routine screening. The U.S. screening program costs 6 billion dollars annually, necessitating the need for tests with better characteristics, less visits per screening cycle, and fewer screening cycles per lifetime. New technologies are in development and if used wisely can improve the efficiency of the cervical cancer prevention program and reduce the over-treatment.
Whichever validated screening method is chosen, the key to success of cervical cancer screening (i.e. reducing cervical cancer incidence) programs is to ensure broad coverage of the services, and follow-up of the abnormalities. Different countries and settings may adopt the screening program details such as what age to initiate screening, the screening interval, and when to stop screening suitable for them based on cost-effectiveness, and societal priorities for cancer prevention.
Many of these new technologies are well beyond the financial capabilities of developing countries that are seeking to establish or improve existing screening programs. However, cost-effectiveness analyses will assist in developing a more rationally based screening program that may improve sensitivity at no/little extra cost. Using a new technology or a combination of technologies will increase the cost of a screening event; however, the gain in sensitivity may allow less frequent screening that theoretically could result in cost-neutral implementation.
A cost-effectiveness analysis of the three FDA-approved technologies to improve the accuracy of cytology screening pointed out that as technologies evolve and/or less frequent screening strategies are considered, the cost-effectiveness ratios may shift in favor of new approaches to screening 29.
Equally important is considering screening practices given the development of effective prophylactic vaccines against HPV types 16 and 18, and the two low risk types 6 and 11 (discussed in more detail in the following chapter). Preventing 16 and 18 infections can theoretically cut cervical cancer rates by 70% worldwide. However, at present, these vaccines are expensive and require three vaccination schedules, which may make it very difficult to implement in resource poor settings. In developed countries the impact of vaccination may be to reduce the number of screen detected abnormalities. Because HPV 16 and 18 by far cause the most obvious severe cytologic abnormalities, and given than vaccination would decrease the small portion of abnormal tests that are HSIL or cancer, the positive predictive value of an abnormal cytology for predicting CIN3 and cancer would decrease in an era where there is large vaccine coverage of the population and vaccine effectiveness lasts for a long period. Hence screening algorithms will then need to be re-evaluated in vaccinated populations.
Figure 1.
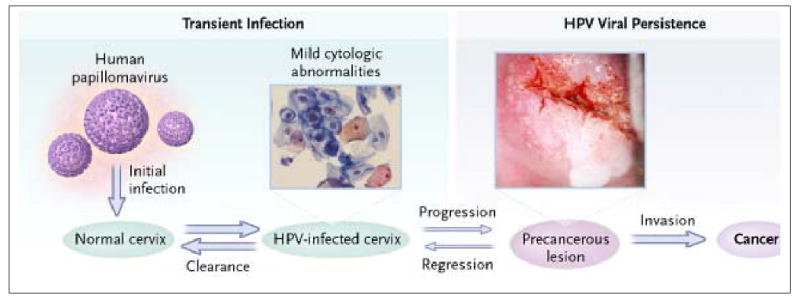
80: Steps in cervical carcinogenesis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Saraiya M, Ahmed F, Krishnan S, Richards TB, Unger ER, Lawson HW. Cervical cancer incidence in a prevaccine era in the United States, 1998-2002. Obstet Gynecol. 2007;109:360–370. doi: 10.1097/01.AOG.0000254165.92653.e8. [DOI] [PubMed] [Google Scholar]
- 3.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. 1. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930-1998. Cancer. 2003;97:3133–3275. doi: 10.1002/cncr.11380. [DOI] [PubMed] [Google Scholar]
- 5.Solomon D, Schiffman MH. Cervical Cancer Screening. In: Goldman MB, Hatch MC, editors. Women and Health. Academic Press; 2000. pp. 942–8. [Google Scholar]
- 6.Surveillance, Epidemiology, and End Results (SEER) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2006. Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Public-Use With State, Total U.S. (1969-2003) released April 2006. Underlying mortality data provided by NCHS ( www.cdc.gov/nchs). www.seer.cancer.gov. [Google Scholar]
- 7.Atlanta: American Cancer Society. American Cancer Society; 2007. Cancer Facts and Figures 2007. [Google Scholar]
- 8.Dobbs SP, Asmussen T, Nunns D, Hollingworth J, Brown LJ, Ireland D. Does histological incomplete excision of cervical intraepithelial neoplasia following large loop excision of transformation zone increase recurrence rates? A six year cytological follow up. BJOG. 2000;107:1298–1301. doi: 10.1111/j.1471-0528.2000.tb11623.x. [DOI] [PubMed] [Google Scholar]
- 9.Kitchener HC, Castle PE, Cox JT. Chapter 7: Achievements and limitations of cervical cytology screening. Vaccine. 2006;24 3:S63–S70. doi: 10.1016/j.vaccine.2006.05.113. [DOI] [PubMed] [Google Scholar]
- 10.Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975-2000. Cancer. 2004;101:1051–1057. doi: 10.1002/cncr.20467. [DOI] [PubMed] [Google Scholar]
- 11.Hughes E, McCracken M, Roberts H, et al. Surveillance for certain health behaviors among states and selected local areas--behavioral risk factor surveillance system, United States, 2004. MMWR Surveill Summ. 2006;55:1–124. [PubMed] [Google Scholar]
- 12.Brown CL. Screening patterns for cervical cancer: how best to reach the unscreened population. J Natl Cancer Inst Monogr. 1996:7–11. [PubMed] [Google Scholar]
- 13.Cucinelli J. 1994 National Health Interview Survey public use data-file. Morbidity and Mortality Weekly Report 1996 Programmer. [Google Scholar]
- 14.National Center for Health Statistics. 2003 NATIONAL HEALTH INTERVIEW SURVEY (NHIS) 2004. [Google Scholar]
- 15.Denny L, Quinn M, Sankaranarayanan R. Chapter 8: Screening for cervical cancer in developing countries. Vaccine. 2006;24 3:S71–S77. doi: 10.1016/j.vaccine.2006.05.121. [DOI] [PubMed] [Google Scholar]
- 16.Schiffman MH, Brinton LA, Devesa SS, Fraumeni JF. Cervical Cancer. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. Second. Oxford University Press; 1996. pp. 1090–116. [Google Scholar]
- 17.Sheets E, Goodman H. The Cervix. In: Berkowitz R, Barbieri R, editors. Kistner's Gynecology Principles and Practice. 6th. St. Louis: Mosby; 1995. pp. 103–36. [Google Scholar]
- 18.Sherman ME, Schiffman M, Herrero R, et al. Performance of a semiautomated Papanicolaou smear screening system: results of a population-based study conducted in Guanacaste, Costa Rica. Cancer. 1998;84:273–280. [PubMed] [Google Scholar]
- 19.Myers ER, et al. Technical Assess. No. 5, 99-E010. Agency for Health Care Policy and Research (AHCPR); 2007. Evaluation of Cervical Cytolgoy. [Google Scholar]
- 20.Boon ME, de Graaff Guilloud JC, Rietveld WJ. Analysis of five sampling methods for the preparation of cervical smears. Acta Cytol. 1989;33:843–848. [PubMed] [Google Scholar]
- 21.Hutchinson ML, Isenstein LM, Goodman A, et al. Homogeneous sampling accounts for the increased diagnostic accuracy using the ThinPrep Processor. Am J Clin Pathol. 1994;101:215–219. doi: 10.1093/ajcp/101.2.215. [DOI] [PubMed] [Google Scholar]
- 22.Solomon D, Henry M. Compendium on Quality Assurance, Proficiency Testing and Workload Limitations in Clinical Cytology. Chicago: Tutorials of Cytology; 1995. Specimen adequacy; pp. 90–4. [Google Scholar]
- 23.Watts G. Commentary: Liquid automation refreshes Dr Papanicolaou. BMJ. 2007;335:35–36. doi: 10.1136/bmj.39260.482616.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ronco G, Cuzick J, Pierotti P, et al. Accuracy of liquid based versus conventional cytology: overall results of new technologies for cervical cancer screening: randomised controlled trial. BMJ. 2007;335:28. doi: 10.1136/bmj.39196.740995.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferenczy A, Franco E, Arseneau J, Wright TC, Richart RM. Diagnostic performance of Hybrid Capture human papillomavirus deoxyribonucleic acid assay combined with liquid-based cytologic study. Am J Obstet Gynecol. 1996;175:651–656. doi: 10.1053/ob.1996.v175.a73868. [DOI] [PubMed] [Google Scholar]
- 26.Sherman ME, Schiffman MH, Lorincz AT, et al. Cervical specimens collected in liquid buffer are suitable for both cytologic screening and ancillary human papillomavirus testing. Cancer. 1997;81:89–97. [PubMed] [Google Scholar]
- 27.Tripath Inc. FocalPoint (TM) slide profiler - System Product Insert. 2007 [Google Scholar]
- 28.Colgan TJ, Patten SF, Jr, Lee JS. A clinical trial of the AutoPap 300 QC system for quality control of cervicovaginal cytology in the clinical laboratory. Acta Cytol. 1995;39:1191–1198. [PubMed] [Google Scholar]
- 29.Brown AD, Garber AM. Cost-effectiveness of 3 methods to enhance the sensitivity of Papanicolaou testing. JAMA. 1999;281:347–353. doi: 10.1001/jama.281.4.347. [DOI] [PubMed] [Google Scholar]
- 30.Davey E, d'Assuncao J, Irwig L, et al. Accuracy of reading liquid based cytology slides using the ThinPrep Imager compared with conventional cytology: prospective study. BMJ. 2007;335:31. doi: 10.1136/bmj.39219.645475.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nieminen P, Kotaniemi-Talonen L, Hakama M, et al. Randomized evaluation trial on automation-assisted screening for cervical cancer: results after 777,000 invitations. J Med Screen. 2007;14:23–28. doi: 10.1258/096914107780154468. [DOI] [PubMed] [Google Scholar]
- 32.Richart RM. Natural History of cervical intraepithelial neoplasia. Clinical Obstetrics and Gynecology. 1968;5:748. [Google Scholar]
- 33.Meisels A, Fortin A. Condylomatous lesions of the cervix and vagina. I. Cytologic patterns. Acta Cytol. 1976;20:505–509. [PubMed] [Google Scholar]
- 34.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 35.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 36.Solomon D. The 1988 Bethesda system for reporting cervical/vaginal cytologic diagnoses. JAMA. 1989;262:931–934. [PubMed] [Google Scholar]
- 37.Luff RD. The Bethesda System for reporting cervical/vaginal cytologic diagnoses. Report of the 1991 Bethesda workshop. Am J Clin Pathol. 1992;98:152–154. doi: 10.1093/ajcp/98.2.152. [DOI] [PubMed] [Google Scholar]
- 38.Schiffman MH, Liaw KL, Herrero R, Sherman M, Hildesheim A, Solomon D. Epidemiologic support for a simplified view of cervical carcinogenesis. Eur Bull. 1998;1:2–6. [Google Scholar]
- 39.Janerich DT, Hadjimichael O, Schwartz PE, et al. The screening histories of women with invasive cervical cancer, Connecticut. Am J Public Health. 1995;85:791–794. doi: 10.2105/ajph.85.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carmichael JA, Jeffrey JF, Steele HD, Ohlke ID. The cytologic history of 245 patients developing invasive cervical carcinoma. Am J Obstet Gynecol. 1984;148:685–690. doi: 10.1016/0002-9378(84)90774-9. [DOI] [PubMed] [Google Scholar]
- 41.Burger RA, Monk BJ, Van Nostrand KM, Greep N, nton-Culver H, Manetta A. Single-visit program for cervical cancer prevention in a high-risk population. Obstet Gynecol. 1995;86:491–498. doi: 10.1016/0029-7844(95)00204-5. [DOI] [PubMed] [Google Scholar]
- 42.Megevand E, Van WW, Knight B, Bloch B. Can cervical cancer be prevented by a see, screen, and treat program? A pilot study. Am J Obstet Gynecol. 1996;174:923–928. doi: 10.1016/s0002-9378(96)70327-7. [DOI] [PubMed] [Google Scholar]
- 43.Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS) Arch Pathol Lab Med. 2003;127:946–949. doi: 10.5858/2003-127-946-FTDFTA. [DOI] [PubMed] [Google Scholar]
- 44.Safaeian M, Solomon D, Wacholder S, Schiffman M, Castle P. Risk of precancer and follow-up management strategies for women with human papillomavirus-negative atypical squamous cells of undetermined significance. Obstet Gynecol. 2007;109:1325–1331. doi: 10.1097/01.AOG.0000263461.71732.40. [DOI] [PubMed] [Google Scholar]
- 45.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 46.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 47.Schiffman MH, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. The natural history and prevention of HPV infection and cervical cancer. Lancet (Seminar) In press. [Google Scholar]
- 48.Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: Clinical applications of HPV testing: A summary of meta-analyses. Vaccine. 2006;24 3:S78–S89. doi: 10.1016/j.vaccine.2006.05.117. [DOI] [PubMed] [Google Scholar]
- 49.Solomon D, Schiffman M, Tarone R. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001;93:293–299. doi: 10.1093/jnci/93.4.293. [DOI] [PubMed] [Google Scholar]
- 50.Arbyn M, Buntinx F, Van RM, Paraskevaidis E, Martin-Hirsch P, Dillner J. Virologic versus cytologic triage of women with equivocal Pap smears: a meta-analysis of the accuracy to detect high-grade intraepithelial neoplasia. J Natl Cancer Inst. 2004;96:280–293. doi: 10.1093/jnci/djh037. [DOI] [PubMed] [Google Scholar]
- 51.Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281:1605–1610. doi: 10.1001/jama.281.17.1605. [DOI] [PubMed] [Google Scholar]
- 52.Kulasingam SL, Kim JJ, Lawrence WF, et al. Cost-effectiveness analysis based on the atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesion Triage Study (ALTS) J Natl Cancer Inst. 2006;98:92–100. doi: 10.1093/jnci/djj009. [DOI] [PubMed] [Google Scholar]
- 53.Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of human papillomavirus DNA testing in the United Kingdom, The Netherlands, France, and Italy. J Natl Cancer Inst. 2005;97:888–895. doi: 10.1093/jnci/dji162. [DOI] [PubMed] [Google Scholar]
- 54.Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 55.Walker JL, Wang SS, Schiffman M, Solomon D. Predicting absolute risk of CIN3 during post-colposcopic follow-up: results from the ASCUS-LSIL Triage Study (ALTS) Am J Obstet Gynecol. 2006;195:341–348. doi: 10.1016/j.ajog.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 56.Kreimer AR, Guido RS, Solomon D, et al. Human papillomavirus testing following loop electrosurgical excision procedure identifies women at risk for posttreatment cervical intraepithelial neoplasia grade 2 or 3 disease. Cancer Epidemiol Biomarkers Prev. 2006;15:908–914. doi: 10.1158/1055-9965.EPI-05-0845. [DOI] [PubMed] [Google Scholar]
- 57.Arbyn M, Paraskevaidis E, Martin-Hirsch P, Prendiville W, Dillner J. Clinical utility of HPV-DNA detection: triage of minor cervical lesions, follow-up of women treated for high-grade CIN: an update of pooled evidence. Gynecol Oncol. 2005;99:S7–11. doi: 10.1016/j.ygyno.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 58.Gok M, Coupe VM, Berkhof J, et al. HPV16 and increased risk of recurrence after treatment for CIN. Gynecol Oncol. 2007;104:273–275. doi: 10.1016/j.ygyno.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 59.Dunn ST, Allen RA, Wang S, Walker J, Schiffman M. DNA extraction: an understudied and important aspect of HPV genotyping using PCR-based methods. J Virol Methods. 2007;143:45–54. doi: 10.1016/j.jviromet.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24 3:S11–S25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 61.Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 62.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 63.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 64.Castle PE, Solomon D, Schiffman M, Wheeler CM. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst. 2005;97:1066–1071. doi: 10.1093/jnci/dji186. [DOI] [PubMed] [Google Scholar]
- 65.Bulk S, Berkhof J, Bulkmans NW, et al. Preferential risk of HPV16 for squamous cell carcinoma and of HPV18 for adenocarcinoma of the cervix compared to women with normal cytology in The Netherlands. Br J Cancer. 2006;94:171–175. doi: 10.1038/sj.bjc.6602915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cuzick J, Mayrand MH, Ronco G, Snijders P, Wardle J. Chapter 10: New dimensions in cervical cancer screening. Vaccine. 2006;24 3:S90–S97. doi: 10.1016/j.vaccine.2006.05.122. [DOI] [PubMed] [Google Scholar]
- 67.zur HH, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]
- 68.Wentzensen N, von Knebel DM. Biomarkers in cervical cancer screening. Dis Markers. 2007;23:315–330. doi: 10.1155/2007/678793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saslow D, Castle PE, Cox JT, et al. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57:7–28. doi: 10.3322/canjclin.57.1.7. [DOI] [PubMed] [Google Scholar]
- 70.Sankaranarayanan R, Black RJ, Swaminathan R, Parkin DM. An overview of cancer survival in developing countries. IARC Sci Publ; 1998. pp. 135–173. [PubMed] [Google Scholar]
- 71.Hernandez-Avila M, Lazcano-Ponce EC, de Ruiz PA, Romieu I. Evaluation of the cervical cancer screening programme in Mexico: a population-based case-control study. Int J Epidemiol. 1998;27:370–376. doi: 10.1093/ije/27.3.370. [DOI] [PubMed] [Google Scholar]
- 72.Safaeian M, Kiddugavu M, Gravitt PE, et al. Comparability of self-collected vaginal swabs and physician-collected cervical swabs for detection of human papillomavirus infections in Rakai, Uganda. Sex Transm Dis. 2007;34:429–436. doi: 10.1097/01.olq.0000243623.67673.22. [DOI] [PubMed] [Google Scholar]
- 73.Ogilvie GS, Patrick DM, Schulzer M, et al. Diagnostic accuracy of self collected vaginal specimens for human papillomavirus compared to clinician collected human papillomavirus specimens: a meta-analysis. Sex Transm Infect. 2005;81:207–212. doi: 10.1136/sti.2004.011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bais AG, van Kemenade FJ, Berkhof J, et al. Human papillomavirus testing on self-sampled cervicovaginal brushes: an effective alternative to protect nonresponders in cervical screening programs. Int J Cancer. 2007;120:1505–1510. doi: 10.1002/ijc.22484. [DOI] [PubMed] [Google Scholar]
- 75.Singh V, Sehgal A, Luthra UK. Screening for cervical cancer by direct inspection. BMJ. 1992;304:534–535. doi: 10.1136/bmj.304.6826.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sankaranarayanan R, Gaffikin L, Jacob M, Sellors J, Robles S. A critical assessment of screening methods for cervical neoplasia. Int J Gynaecol Obstet. 2005;89 2:S4–S12. doi: 10.1016/j.ijgo.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 77.Jeronimo J, Schiffman M. Colposcopy at a crossroads. Am J Obstet Gynecol. 2006;195:349–353. doi: 10.1016/j.ajog.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 78.Sankaranarayanan R, Wesley R, Somanathan T, et al. Visual inspection of the uterine cervix after the application of acetic acid in the detection of cervical carcinoma and its precursors. Cancer. 1998;83:2150–2156. [PubMed] [Google Scholar]
- 79.Denny L, Kuhn L, De SM, Pollack AE, Dupree W, Wright TC., Jr Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. JAMA. 2005;294:2173–2181. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- 80.Wright TC, Jr, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med. 2003;348:489–490. doi: 10.1056/NEJMp020178. [DOI] [PubMed] [Google Scholar]


