Summary
Multiple Myeloma (MM) remains incurable despite novel therapies, suggesting the need for further identification of factors mediating tumorigenesis and drug resistance. Using both in vitro and in vivo MM xenograft models, we show that plasmacytoid dendritic cells (pDCs) in the bone marrow (BM) microenvironment both mediate immune deficiency characteristic of MM and promote MM cell growth, survival, and drug resistance. Microarray, cell signaling, cytokine profile and immunohistochemical analysis delineate the mechanisms mediating these sequelae. Although pDCs are resistant to novel therapies, targeting Toll-like Receptors with CpG ODNs both restores pDC immune function and abrogates pDC-induced MM cell growth. Our study therefore validates targeting pDC-MM interactions as a therapeutic strategy to overcome drug resistance in MM.
Introduction
Multiple Myeloma (MM) is diagnosed in 15,000 new individuals annually. The median survival has been prolonged from 3–4 to 7 years, especially in patients less than age 50 (Brenner et al., 2008; Kumar et al., 2008). However, despite important advances including biological agents bortezomib, thalidomide, and lenalidomide, MM remains incurable due to the development of drug resistance, which manifests as relapsed/refractory disease (Anderson, 2007). The molecular mechanisms whereby MM cells evade drug-induced cytotoxicity and acquire drug-resistant phenotypes include interaction of MM cells with their bone marrow (BM) microenvironment (Dalton and Anderson, 2006; Hideshima et al., 2007). The BM milieu contains stromal cells (BMSCs), osteoclasts (Roodman, 2008), myeloid cells, and immune effector cells. BMSCs promote growth and drug resistance in MM cells; however, the functional significance of other BM cellular components is unclear.
Macrophages and dendritic cells (DCs) regulate tumor cell growth (Banchereau and Steinman, 1998; Kukreja et al., 2006; McKenna et al., 2005; Ribatti et al., 2006). DCs are BM-derived MNCs that also play an essential role in immune responses (Steinman and Cohn, 1973). In humans, two major DC subsets have been identified based on their origin, phenotype, and function (Colonna et al., 2004; O’Doherty et al., 1994): Myeloid DCs (mDCs) (CD11c+, CD123−) that include langherhans cells and interstitial DCs; and plasmacytoid DCs (pDCs) (Grouard et al., 1997). pDCs lack lineage cell markers for T, B, NK cells and monocytes; and express CD123, HLA-DR and BDCA-2 (Colonna et al., 2004; Dzionek et al., 2002; Grouard et al., 1997; O’Doherty et al., 1994). Stimulated pDCs have strong Ag-presenting potential, and regulate anti-viral innate immunity (Gilliet et al., 2008; Krieg, 2007; Siegal et al., 1999). Prior studies showed that pDCs from MM patients are defective in their antigen presenting function (Brimnes et al., 2006; Ratta et al., 2002). Indeed, loss of immune function of tumor-infiltrating DCs has been linked to the suppressive effects of the tumor microenvironment mediated via vascular endothelial growth factor (VEGF), interleukin-6 (IL-6) or macrophage stimulating factor (MCSF) in cancers (Zou, 2005) including MM (Hayashi et al., 2003). Importantly, pDCs play a role in normal B cell development into plasmablasts, their differentiation into antibody-secreting plasma cells, and their survival (Garcia De Vinuesa et al., 1999; Jego et al., 2003; Poeck et al., 2004; Tabera et al., 2008). However, the role of pDCs in regulating growth and survival of malignant plasma cells is unclear. In the present study, we have characterized the distribution/frequency and function of pDCs in MM.
Results and Discussion
Distribution and frequency of pDCs in MM
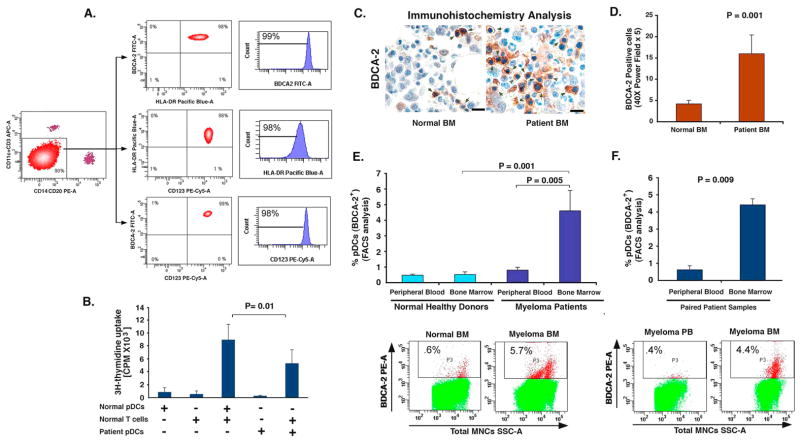
MM is characterized by immune dysfunction (Brown et al., 2001; Ratta et al., 2002). We first examined the immune function of pDCs derived from normal donors vs. MM patients (defined in Fig 1A as HLA-DR+ CD123+ BDCA-2+ cells) by their ability to stimulate allogeneic T cell response. Even though resting pDCs are known poor antigen presenting cells (Liu, 2005), a comparative analysis of MM BM pDCs versus normal pDCs shows markedly decreased ability of MM BM pDCs to trigger T cell proliferation versus normal pDCs (P = 0.01).
Figure 1. Distribution and frequency of pDCs.
(A) Total MNCs were subjected to BDCA-4 positive selection using two step purification, and then labeled with CD123 PE-Cy5, HLA-DR Pacific Blue, BDCA-2 FITC, CD14-PE, CD20 PE, CD11c and CD3 APC antibodies. Lin-, and CD11c- (90%) cells were gated and FACS sorted. Analysis of this cell population showed 99% BDCA-2+, 98% HLA-DR+, and 98% CD123+ cells. (B) DNA synthesis in allogeneic T cells stimulated by pDCs from healthy donors and MM patients (mean ± SD; n=4). (C) IHC analysis on normal donor BM and MM patient BM biopsies was performed using BDCA-2 Ab. Arrows indicate brown BDCA-2-positive pDCs. Micrographs are representative from 10 MM patients and 5 normal donors. Scale bars, 10 μM. (D) Quantification of BDCA-2-positive pDCs in MM BM vs. normal BM from C. The pDC frequency was quantified by selecting 5 random independent high power (X40) microscopic fields for each tissue sample. (E) Quantification of pDCs from normal donors PB and BM vs. MM patient PB and BM by FACS using BDCA-2-PE conjugated Ab. Upper panel: Data shown is derived from analysis of 32 MM patients and 8 normal donors. Lower panel: A representative FACS analysis showing higher percentage of BDCA-2-positive pDCs in MM BM vs. normal BM. (F) Upper panel: Quantification of pDCs from matched paired samples of 8 MM patient PB and BM by FACS using BDCA-2 Ab. Lower panel: A representative FACS analysis from 8 patients analyzed is shown. Error bars indicate standard deviation (SD).
Recent reports linked increased infiltration of pDCs in human tumors (Liu, 2005), but with unclear significance. Studies related to the distribution of pDCs in MM are limited and unclear. For example, one study observed a decreased number of pDCs in peripheral blood (PB) from MM patients compared to PB from normal donors (Brimnes et al., 2006), whereas another study showed that PB-derived pDCs from MM patients are numerically within the normal range (Brown et al., 2001). We therefore next examined the distribution and frequency of pDCs in MM patients vs. normal donors using immunohistochemical analysis of biopsy samples and FACS analysis of freshly obtained samples. For these studies, we utilized antibody against pDC specific marker BDCA-2 (Dzionek et al., 2001). We examined 10 MM patient and 5 normal donor BM biopsies for the expression of BDCA-2 by IHC analysis. Fig 1C is a representative photograph showing pDCs as BDCA-2-postively stained cells (brown cells). These cells were then quantified in all MM patient and normal samples (Fig 1D); pDCs were increased in MM BM vs. normal BM. We then utilized FACS analysis to determine the frequency of pDCs in freshly obtained samples using BDCA-2 Ab. BM samples from 32 MM patients with newly diagnosed and relapsed/refractory disease, as well as from 8 normal donors were examined. Comparative analysis shows 4.7% ± 0.8% pDCs in MM BM vs. 0.4% ± 0.2% in normal donor BM (Fig 1E). In contrast, no significant difference in pDC number was observed between normal BM vs. PB (Fig 1E, 8 normal donors). Furthermore, increased pDCs were noted in MM BM vs. MM PB. Analysis of pDCs in matched BM and PB samples from 8 patients confirmed increased numbers of pDCs in BM than in PB (4.4 ± 0.3% in BM vs. 0.62 ± 0.24% in PB, Fig 1F). These findings suggest that 1) increased numbers of pDCs are present in MM patient BM vs. normal BM; and 2) pDCs are more frequently localized in MM BM than MM PB.
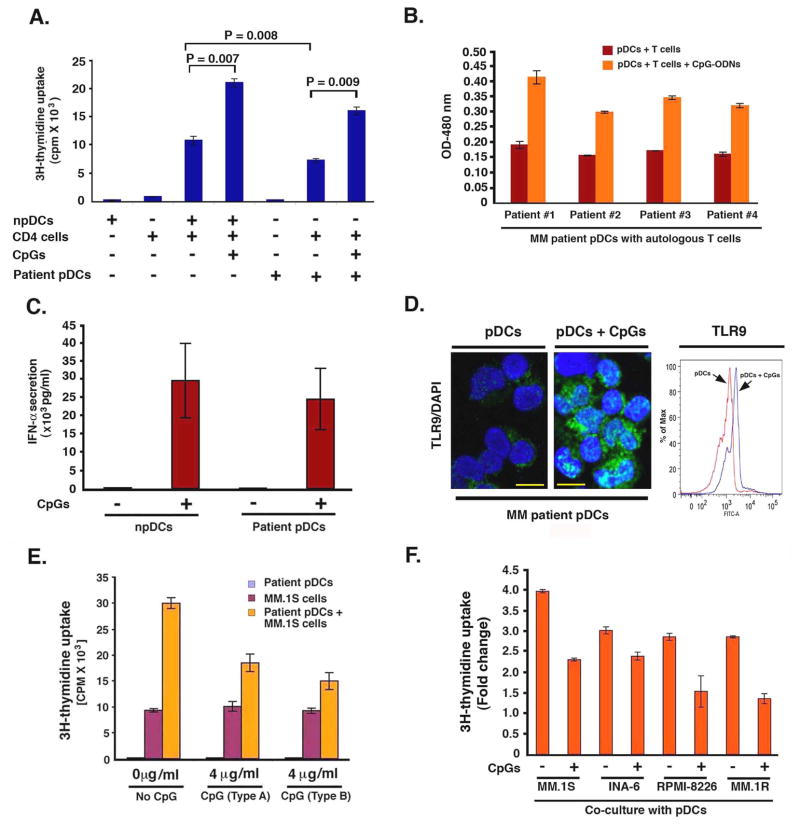
Figure 8. Therapeutic implications of pDC-MM cell interactions.
(A) Normal CD4 T cells (1 × 104) were cultured with normal pDCs or MM patient BM pDCs (1 × 105) (1:10 pDCs/T cells) in the presence or absence of CpGs for 5 days, and then analyzed for DNA synthesis (mean ± SD; n=4). (B) MM BM pDCs (1 × 104) were cultured with autologous T cells (1 × 105)(1:10 pDCs/T cells) in the presence or absence of CpGs (Type A, 4 μg/ml) for 5 days, and then analyzed for T cell proliferation using WST proliferation assay (mean ± SD; P < 0.005 for all patients). (C) CpG-ODNs (Type A, 4 μg/ml) restore IFN-α production from MM patient BM pDCs: Both normal PB pDCs and MM patient BM pDCs (105 cells) were cultured in the presence or absence of CpG-ODNs for 24h, and IFN-α levels were measured by ELISA (mean ± S.D; P = 0.01, 6 MM patient BM pDCs and 4 normal pDCs were evaluated). (D) pDCs were cultured in the presence or absence of CpG-ODNs for 12h; cytospins were prepared and subjected to immunostaining with anti-TLR9 Ab (green) and DAPI (blue). Representative micrographs from 3 experiments. Scale bars, 10 μM. Intracellular staining with TLR9 Ab followed by FACS analysis shows upregulation of TLR9 expression in CpG-treated MM pDCs. (E) CpGs block MM BM pDC-induced MM.1S cell growth: pDCs, MM.1S cells or pDCs + MM.1S cells (1:5 pDC/MM ratio) were cultured in the presence or absence of CpG-ODNs for 3 days, and DNA synthesis was measured using 3H-TdR uptake (mean ± SD; P < 0.004, n=4). (F) MM cell lines were cultured with normal PB pDCs at 1:5 pDC/MM ratio in the presence or absence of CpGs for 3 days, and then analyzed for growth by WST proliferation assay (mean ± SD; P < 0.005, n=4). Errors bars indicate standard deviation (SD).
pDCs trigger growth and prolong survival of MM cells
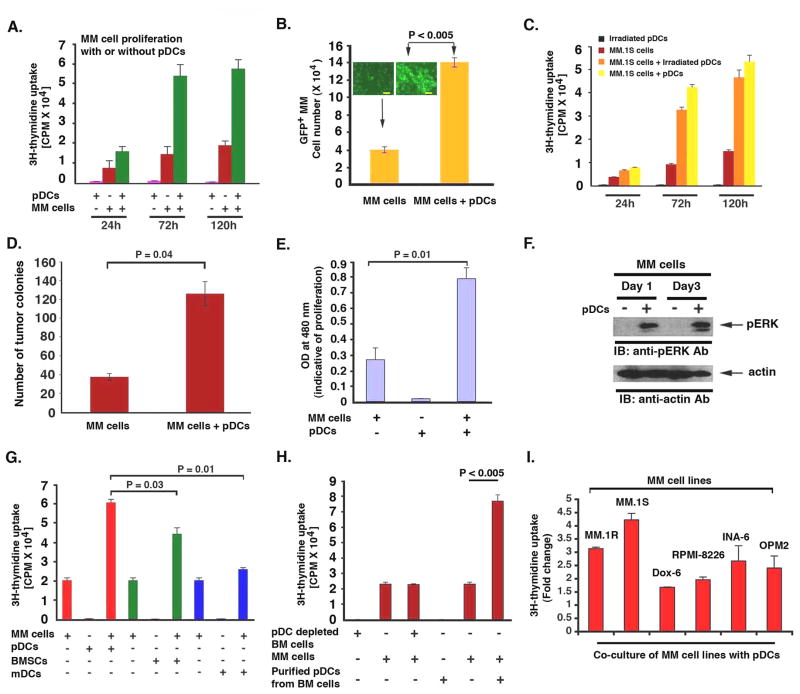
We next examined the functional significance of increased pDC numbers in MM BM. Our prior studies showed that BMSCs trigger MM cell growth; therefore, we examined whether pDCs similarly affect MM cells. MM.1S cells and normal PB pDCs were cultured either alone or together at 1:3, 1:5 or 1:7 (pDC:MM) ratio for 24h, 72h and 120h, and DNA synthesis was measured by 3H-thymidine (3H-Tdr) uptake. A significant increase in DNA synthesis was noted when MM cells were cultured with pDCs at 1:5 (pDC/MM) ratio: for example, 3–4 fold increased 3H-Tdr uptake in MM.1S cells cultured with pDCs for 72h vs. control MM cells alone, P < 0.005) (Fig 2A). No increase in DNA synthesis was observed in pDCs cultured alone (Fig 2A). Co-culture of pDCs with MM.1S cells at 1:3 or 1:7 (pDC/MM) ratio also induced MM.1S cell growth (data not shown); however, maximal growth was noted at 1:5 pDC/MM ratios. This 1:5 pDC/MM ratio is physiologically relevant, since pDC frequency in MM BM is 4.7 ± 0.8% (Fig 1E) corresponding to 23.5% ± 3% MM cells in BM.Co-culture of GFP-expressing MM.1S cells with pDCs increased number of GFP+ cells, suggesting that pDCs induce MM.1S cell growth (Fig 2B). Irradiated pDCs retain their ability to trigger MM cell proliferation (Fig 2C). To determine whether pDCs alter the clonogenic growth of MM cells, we plated MM.1S cells with pDCs in methycellulose cultures. pDCs markedly increase the number of tumor colonies vs. tumor cells alone (Fig 2D). This MM cell growth promoting activity of pDCs was further confirmed by additional assays. For example, WST proliferation assay also showed that pDCs trigger proliferation of MM cells (Fig 2E). Finally, pDC-induced MM cell growth was associated with activation of growth signaling kinase ERK in MM.1S cells (Fig 2F). Together, these findings demonstrate that pDCs can promote MM cell growth.
Figure 2. pDCs induce MM cell growth.
(A) MM.1S cells (5 × 104 cells/200 μL) and pDCs (1 × 104 cells/200 μL) were cultured either alone or together for indicated intervals; and then analyzed for DNA synthesis using 3H-TdR uptake (mean ± SD; n=3). (B) GFP+ MM.1S cells were cultured either alone or with pDCs for 3 days; GFP+ MM.1S cells were counted under fluorescent microscope (mean ± SD; n=2). (Inset: micrograph from the experiment; Scale bars, 40 μM). (C) MM.1S cells were cultured alone or together with either irradiated or non-irradiated pDCs for indicated intervals, and analyzed for growth (mean ± SD; n=2). (D) MM.1S cells were plated with or without pDCs. The numbers of tumor colonies were enumerated using microscopy after incubation for 10–14 days (mean ± SD; n=2). (E) MM.1S cells and pDCs were cultured as in “A” for 72h and analyzed for growth by WST assays (mean ± SD; n=3). (F) MM.1S cells (2.5 × 106) and pDCs (0.5 × 106) were cultured as in “A”; MM.1S cells were separated from pDC co-cultures using CD138 microbeads and analyzed for ERK activation by immunoblotting. (G) MM.1S cells, pDCs, mDCs or MM patient-BMSCs were cultured either alone or together at 1:5 (pDC/MM, mDC/MM or BMSC/MM) ratio for 3 days, and DNA synthesis was measured using 3H-TdR uptake (mean ± SD; n=3). (H) pDC-depleted MM BM cells vs. purified MM BM pDCs were examined for their stimulatory effect on MM.1S cell growth (mean ± SD; n=2). (I) MM cell lines were cultured either alone or together with pDCs for 3 days, and analyzed for DNA synthesis by 3H-TdR uptake assay. Data is presented as fold change in DNA synthesis in the presence vs. absence of pDCs (mean ± SD; P < 0.05; n=3). Normal PB pDCs were utilized in experiments shown in A-I. Co-cultures of pDCs and MM cells were performed using 1:5 (pDC/MM) ratio. Growth assays were performed using 1 × 104 pDCs and 5 × 104 MM cells in 200 microliters of media in 96 well plates. Error bars indicate standard deviation (SD).
Our prior studies showed that BMSCs trigger MM cell growth (Hideshima et al., 2007). A recent study showed that in-vitro generated mDCs enhance clonogenecity of MM cells (Kukreja et al., 2006). We therefore examined the stimulatory effect of normal PB mDCs vs. pDCs vs. MM patient BMSCs on MM cell growth. Culture of MM.1S cells with BMSCs triggered a 2.2 ± 0.7 fold increase in DNA synthesis in MM.1S cells. Importantly, pDCs triggered 3.1 ± 0.3 fold increased DNA synthesis, whereas mDCs induced only 15–20% increased DNA synthesis (Fig 2G). These data suggest that BMSCs and mDCs also stimulate DNA synthesis, albeit to a lesser extent than pDCs. The differences in the potency of pDC vs. mDCs in our study vs. a previous report (Kukreja et al., 2007) may be due to the use of different MM cell line, sources of pDCs, and/or growth assays. Nonetheless, both studies suggest a role of DCs in modulating MM cell growth. Additionally, pDCs are more robust growth stimulators of MM cells than monocytes under similar experimental conditions (Supplementary Fig 1). Importantly, the pDC-depleted BM cells did not trigger significant DNA synthesis in MM cells (Fig 2H). Similarly, co-culture of pDC-depleted BM cells with GFP-MM.1S cell showed no increase in GFP+ cell numbers (data not shown), confirming the specific MM cell growth-promoting activity of pDCs.
Genetic heterogeneity (Bergsagel and Kuehl, 2005) and drug resistance in MM impact the interaction of tumor cells with BMSCs and its functional sequelae (Hideshima et al., 2007). We examined the growth promoting activity of pDCs in a broader panel of MM cell lines, including those that are drug resistant and cytogenetically distinct. These included Dex-resistant MM.1R cell line with t14;16 translocation; parental RPMI-8266 cell line and its Doxorubicin-resistant derivative (8226/Dox-6); an IL-6-dependent cell line INA-6; and OPM-2 (t4:14) MM cell line. pDCs stimulated DNA synthesis in all MM cell lines, albeit to a differential extent (Fig 2I). These findings suggest that genetic heterogeneity in MM may be associated with differential tumor growth responses to pDCs.
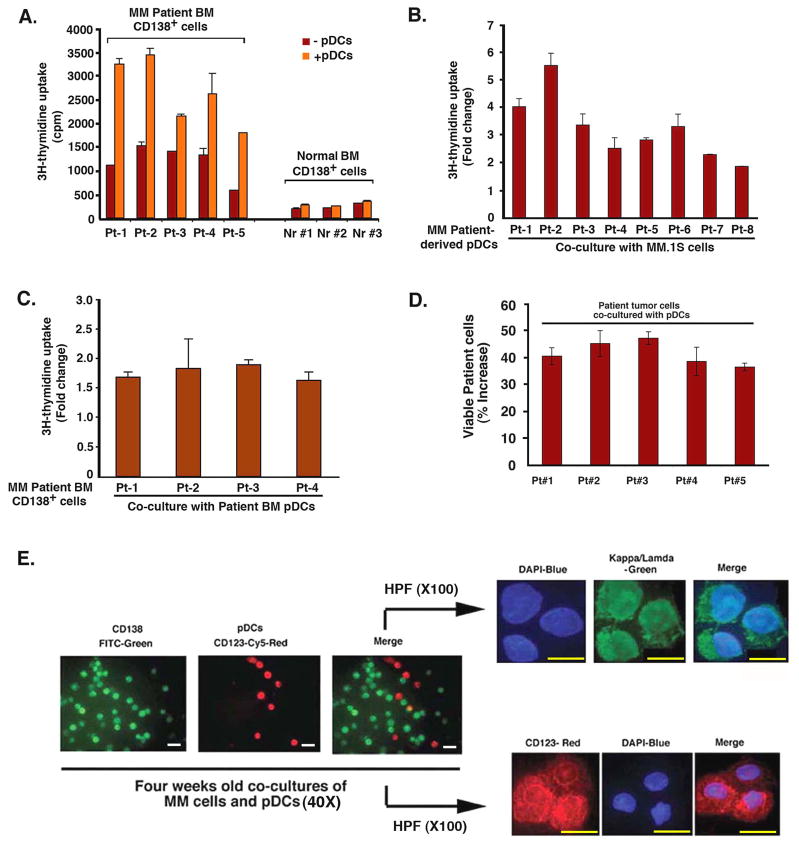
Patient MM cells have a low proliferative capacity in vitro, and we similarly examined the effects of pDCs on primary tumor cells. Our results show that pDCs trigger proliferation of patient MM cells (Fig 3A), evidenced by increase in DNA synthesis. In contrast, pDCs had little, if any, effect on DNA synthesis in normal plasma cells (Fig 3A). Similar results were obtained using viability assays (data not shown).
Figure 3. Effect of pDCs on patient MM cells.
(A) Purified patient MM cells and normal BM plasma cells from healthy donors were cultured with or without pDCs for 3 days, and DNA synthesis was measured by 3H-TdR uptake (mean ± SD of triplicate cultures; P < 0.05 for all samples; pDC/MM ratios: Pt#1–3 at 1:5; pt#4 at 1:2 and pt#5 at 1:3). (B) MM.1S cells were cultured as in Fig 2A with or without patient BM pDCs for 3 days, and analyzed for growth by 3H-TdR uptake (mean ± SD of triplicate cultures; P < 0.005 for all samples). (C) Patient MM cells were cultured with or without autologous BM pDCs for 3 days, and DNA synthesis was measured by 3H-TdR uptake (mean ± SD of triplicate cultures; P < 0.004 for all samples; pDC/MM ratio: Pt#1–4 at 1:5). Data (B and C) is presented as fold change in DNA synthesis in the presence vs. absence of patient BM pDCs. (D) Normal PB pDCs and patient MM cells were co-cultured in DCP-MM medium at 1:5 (pDC/MM) ratio for 3 weeks, and viable cells were quantified by trypan blue exclusion assay. Data is presented as percent increase in survival of tumor cells in the presence vs. absence of pDCs (mean ± SD of duplicate cultures; P < 0.05). (E) Normal PB pDCs and patient MM cells were co-cultured as in D for 4 weeks and then stained with CD138-FITC Ab, CD123-PE Ab, kappa/lamda Ab and DAPI. Micrograph shown is representative of 5 experiments with similar results. Scale bars, 20 M (left three panels) and 10 M (right, upper and lower panels). Error bars indicate standard deviation (SD).
We next examined whether MM patient pDCs, like normal donor pDCs, trigger proliferation of MM cell line and patient MM cells. MM patient-derived BM pDCs induced growth of both allogeneic MM.1S cell line (Fig 3B), and autologous tumor cells (Fig 3C). Importantly, we found a significant increase in the survival of patient MM cells when co-cultured with pDCs for 3–4 weeks (Fig 3D; 35–50% increase in 5 of 5 patients cells, P < 0.05). Immunostaining with kappa and lamda IgG Abs confirmed the clonality of MM cells in prolonged cultures with pDCs (Fig 3E).
pDCs confer drug-resistance
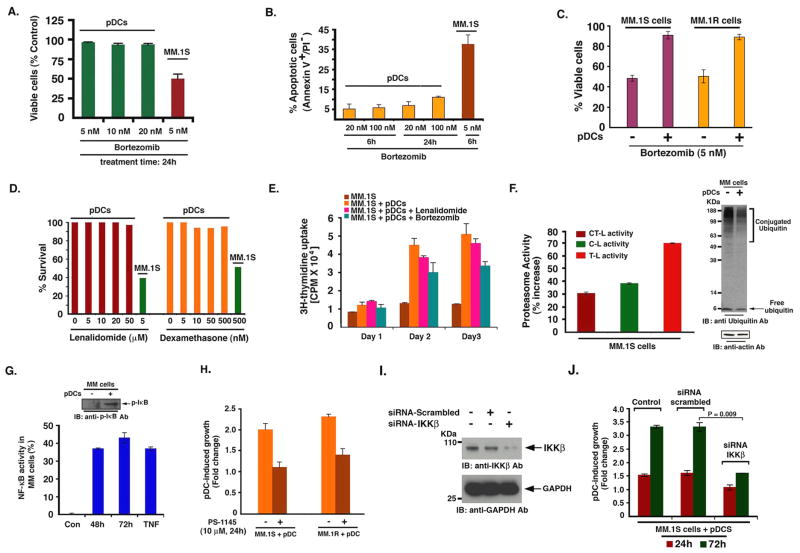
Binding of MM cells to BMSCs mediates resistance to conventional (Dexamethasone) therapies; and conversely, novel therapies (bortezomib, lenalidomide) can overcome this growth advantage conferred by BMSCs (Hideshima et al., 2007). We therefore next determined whether various anti-MM agents overcome pDC-mediated biologic sequelae in MM cells. We asked 1) whether anti-MM agent bortezomib directly affects the viability of pDCs; and 2) whether pDC-triggered growth of MM cells is affected by bortezomib. Treatment of normal PB pDCs for 24h with bortezomib (20 nM) did not significantly decrease their viability (Fig 4A). Similarly, treatment of pDCs with even higher doses of bortezomib (20–100 nM) did not trigger significant apoptosis (Fig 4B). Longer periods (48h) and higher dose of bortezomib triggered cell death of pDCs (data not shown), as also reported in a prior study (Kukreja et al., 2007). However, it is noteworthy that MM cells require many fold lesser concentrations of bortezomib and shorter periods of exposure to undergo for MM.1S cells is 5 nM at 24 h, as in Fig 4A apoptosis (e.g. IC50 and 4B), suggesting that pDCs are relatively resistant to bortezomib compared to tumor cells. Importantly, and in agreement with a previous report (Kukreja et al., 2007), we found that pDCs protect MM cells against bortezomib-induced cytotoxicity (Fig 4C). Furthermore, pDCs triggered MM cell proliferation even in the presence of bortezomib, albeit to a lesser extent than without drug (Fig 4E). As with bortezomib, treatment of pDCs with immunomodulatory drug lenalidomide does not significantly decrease their viability (Fig 4D). Moreover, pDCs triggered increased DNA synthesis in MM cells even in the presence of lenalidomide (Fig 4E). Conventional agent Dex (0.5 μM) triggered only 8–10% decreased viability of pDCs (Fig 4D), with 12–15% apoptosis. Importantly, similar concentrations of Dex triggered 70 ± 3.6% apoptosis in MM cells (data not shown). We therefore conclude that in comparison to tumor cells, pDCs are relatively resistant to botezomib, lenalidomide, and Dex therapies.
Figure 4. pDCs confer drug resistance in MM cells.
(A and B) pDCs (1 × 106 cells) were treated for the indicated time and concentrations of bortezomib and then analyzed for viability and apoptosis by MTT and Annexin V/PI staining assays (mean ± SD; P < 0.005; n=4). (C) MM.1S and MM.1R cells were treated with bortezomib in the presence or absence of pDCs for 24h, and analyzed for viability (mean ± SD; P < 0.05; n=4). (D) pDCs were treated with indicated agents for 24h, and then analyzed for viability (mean ± SD; P < 0.05; n=3). (E) MM.1S cells or MM.1S plus pDCs were cultured in the presence or absence of bortezomib (5 nM) or lenalidomide (5 μM), and analyzed for DNA synthesis by 3H-TdR uptake assay (mean ± SD; P < 0.05; n=3). (F) MM.1S cells were cultured with pDCs for 72h; separated using CD138 microbeads, and harvested; protein extracts were analyzed for proteasome activity. Data is presented as percent increase in proteasome activities in the presence vs. absence of pDCs (mean ± SD; P < 0.005; n=3). Similarly, pDC-induced alteration in protein ubiquitination in MM.1S cells was assessed by immunobloting. (G) MM.1S cells were cultured as in F and analyzed for NF- κB activity using p65 ELISA assay (mean ± SD; P < 0.05 n=3). Inset: MM.1S cells cultured with or without pDCs were analyzed for p-IκB levels by immunoblotting. (H) MM.1S or MM.1R cells were cultured with pDCs in the presence or absence of PS-1145, and then analyzed for growth (mean ± SD; P=0.01 for MM.1S and P=0.02 for MM.1R cells; n=2). (I) MM.1S cells were transfected with siRNA IKKβ (0.8 μM) or scrambled siRNA for 48h and harvested; protein extracts were subjected to immunoblot analysis with anti-IKKβ or anti-GAPDH Abs. (J) MM.1S cells were transfected with siRNA IKKβ or scrambled siRNA, followed by co-culture with pDCs for indicated times; and then analyzed for growth using WST proliferation assay (mean ± SD; n=2). Normal PB pDCs were utilized in experiments shown in A–J at 1:5 pDC/MM ratio. Culture medium is described in methods. Error bars indicate standard deviation.
We next performed microarray analysis to identify the molecular mechanisms whereby pDCs confer growth and drug resistance in MM cells. For these studies, we examined pDC-induced gene changes in MM cells. In particular, we found that pDCs triggered upregulation of transcripts mediating proteasome function in MM.1S cells, including proteasome 26S subunit and PA200 (ATPase complex) (Supplementary Fig 2). Furthermore, a marked increase in chymotrypsin-like (CT-L), caspase-like (C-L), and trypsin-like (T-L) proteasome activity, was noted in MM.1S cells cultured with pDCs vs. MM.1S cells alone (Fig 4F). In concert with these findings, pDCs triggered a decrease in protein ubiquitination in MM.1S cells (Fig 4F). Bortezomib primarily blocks CT-L and C-L, but not T-L activity, and pDCs significantly increase T-L activity in MM cells. Importantly, bortezomib-induced cytotoxicity is linked to inhibition of proteasome activity (Chauhan et al., 2005), and our data suggest that pDCs upregulate proteasome activity. It is therefore likely that pDCs, of by enhancing proteasome activity in MM cells, raise the IC50 bortezomib for MM cells. A recent study also showed that overexpression of proteasome subunit confers resistance to bortezomib (Oerlemans et al., 2008). These data suggest a likely mechanism whereby pDCs confer bortezomib resistance in MM cells.
Besides the proteasome components, pDCs also increased transcripts linked to NF-κB signaling pathway in MM cells including, NF-κB activating protein and genes containing NF-κB consensus binding sites in their promoter regions (e.g. IL-6, VEGF, IL-10). NF-κB mediates growth, survival and drug resistance in MM cells (Chauhan et al., 1996). DNA binding ELISA assays and immunoblotting confirmed pDC-induced NF-κB activation in MM.1S cells (Fig 4G and inset). Importantly, blockade of NF-κB using IKK inhibitor PS-1145 decreases pDC-triggered MM.1S and MM.1R cell growth (Fig 4H). To further confirm the role of NF-κB in mediating pDC-induced MM cell growth, we knocked down IKKβ expression in MM cells using siRNA. The functional specificity of IKKβ siRNA was evident by a marked decrease in protein levels of IKKβ (Fig 4I). Transfection of IKKβ siRNA, but not negative-control (scrambled) siRNA, significantly inhibited pDC-induced growth in MM.1S cells (Fig 4J). Together, these findings suggest an obligatory role of NF-κB during pDC-triggered MM cell growth.
Chemotaxis/migration during pDC-MM interaction
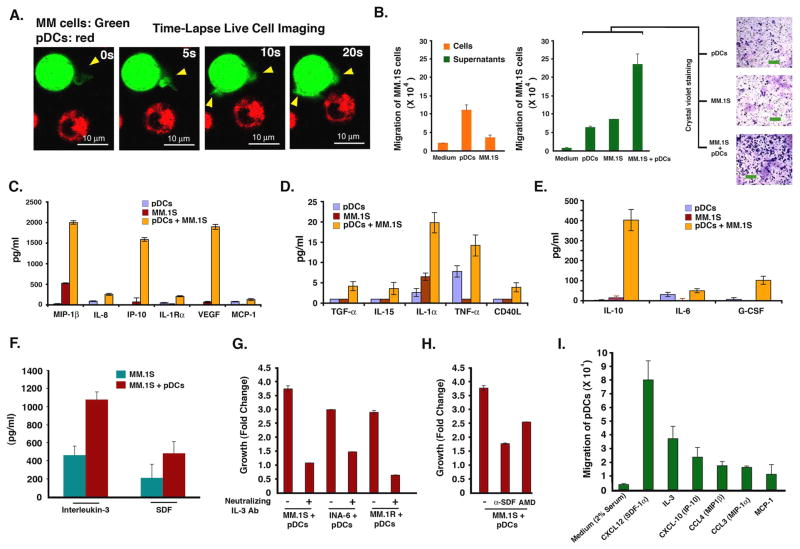
The frequent localization of pDCs in MM BM and their ability to trigger MM cell growth suggest involvement of chemotaxis between pDCs and MM cells. Time-lapse live cell imaging demonstrates increased motility of pDCs and MM cells towards each other, evidenced by formation of filopodia (Figure 5A, and movie S1). In agreement with these data, Transwell insert assays demonstrate markedly increased migration of MM.1S cells towards pDCs (Fig 5B, left panel). Importantly, 2–3 fold greater numbers of MM.1S cells migrated towards supernatants from pDC-MM cultures compared to those from cultures of pDCs or MM.1S cells alone (Fig 5B, right panel). Crystal violet staining confirms increased numbers of MM.1S cells migrating towards supernatants from pDC-MM cultures (Fig 5B, micrographs). Similarly, pDCs showed increased migration towards supernatants of pDC-MM co-cultures (data not shown). These findings demonstrate chemotaxis between pDCs and MM cells, and importantly, show that pDC-MM interaction further enhances secretion of chemotactic factors.
Figure 5. Chemotaxis/migration of MM cells and role of soluble factors in pDC-MM interactions.
(A) Representative examples of frames from time-lapse imaging of pDC-MM co-culture are shown to demonstrate chemotactic activity between pDCs and MM cells, as evidenced by formation of filopodia (yellow arrowheads). Images were taken for 30 ms interval for 5–15 mins. Video is available in supplementary file as movie S1. (B) Migration assays: MM.1S cells were seeded in the upper compartment; the lower chamber contained medium alone, pDCs, MM.1S cells, supernatants from pDCs, supernatants from MM.1S cells or supernatants from 3-day co-cultures of pDCs and MM.1S cells. The migrated cells were quantified and presented as bar graph (mean ± SD; P < 0.005; n=2). Micrographs shows crystal violet staining of migrated cells. Scale bars, 40 μM. (C-E) MM.1S cells were cultured with pDCs for 72h as described in methods; supernatants were collected and subjected to cytokine bead arrays (mean ± SD; n=4). (F) MM.1S cells were cultured with or without pDCs for 72h; supernatants were analyzed for IL-3 and SDF-1α levels using ELISA (mean ± SD; P = 0.05 for both IL-3 and SDF- 1α; n=5). (G) MM.1S, INA-6 (with rhIL6 2.5 ng/ml), and MM.1R cell lines were cultured with pDCs in the presence or absence of anti-IL-3 Ab (10 ng/ml) for 72h, and analyzed for growth by WST proliferation assays (mean ± SD; P < 0.005; n=3). (H) MM.1S cells were cultured with pDCs in the presence or absence of anti-SDF- 1α (100 ng/ml) or AMD3100 (10 μM), and analyzed for growth by WST proliferation assay (mean ± SD; P < 0.005; n=3). (I) Transwell insert assays showing migration of pDCs towards chemokines (described in methods). The indicated chemokines were added to the lower chamber of transwells, and pDcs were allowed to migrate through 5 μM pore-size filters for 2h. Migrated cells were quantified in the fluid phase of the lower chamber (mean ± S.D; n=3). Normal PB pDCs were utilized in experiments shown in A–I and cultures were at 1:5 (pDC/MM) ratio. Error bars indicate standard deviation (SD).
Role of soluble factors
We next directly analyzed secretion of cytokines and chemokines in supernatants from pDCs, MM.1S cells, and pDC-MM.1S cell co-cultures using cytokine bead arrays. pDC-MM interaction triggers secretion of many known MM cell growth and chemotactic factors (Hideshima et al., 2007) including IL-10, VEGF, CD40L, IL-8, IL-15, IL-6, and MCP-1 or IP10 (Figure 5C–5E). We also found that pDC-MM interaction increased IL-3 and SDF-1α (CXCL12) levels (Fig 5F). Both IL-3 and SDF-1α mediate autocrine growth and chemotaxis in MM cells (Hideshima et al., 2007; Lee et al., 2004). SDF-1α is involved in in vivo migration of pDCs into tumors via its ligand CXCR4 (Zou et al., 2001). Importantly, blockade of IL-3 or SDF-1α with neutralizing Abs or AMD3100 (drug targeting CXCR4-CXCL12) markedly abrogates pDC-induced MM cell growth (Fig 5G). IL-3 is a survival factor for pDCs (Grouard et al., 1997). Our finding that MM cells secrete low levels of IL-3 constitutively, which is markedly increased upon interaction with pDCs explains, at least in part, prolonged survival of pDCs when cultured with MM cells in vitro (Figure 3E) and in vivo (Fig 7B and 7C).
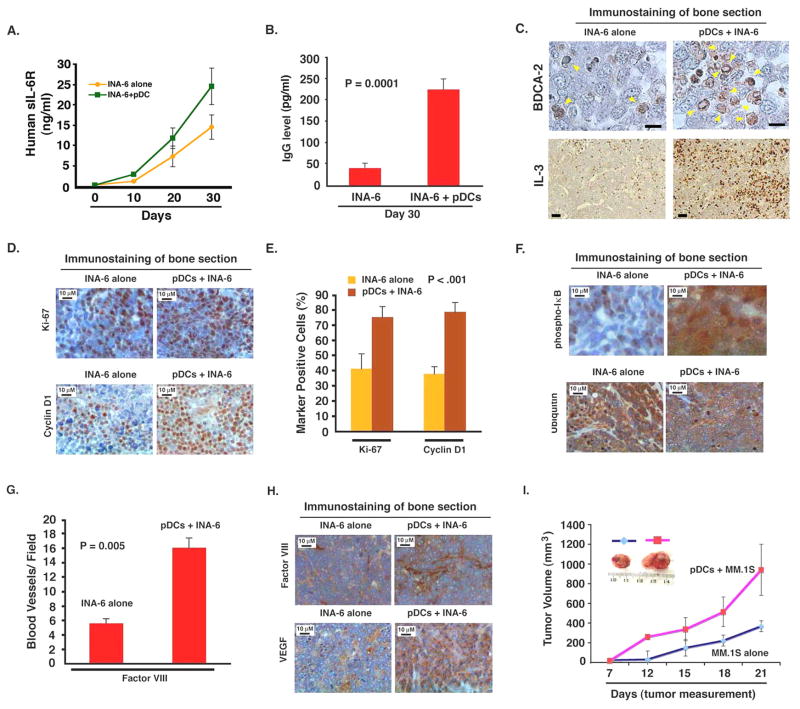
Figure 7. pDCs enhance growth of xenografted human MM cells in mice.
(A and B) INA-6 MM cells alone (2 × 106 cells without IL-6), or together with pDCs (0.4 × 106 cells) at 1:5 (pDC/MM) ratio were injected directly into the human fetal bone implant in SCID-hu mice, and mouse sera samples were analyzed for human sIL-6R and Ig by ELISA (mean ± SD; P < 0.05; n=2). C. Human bone chips were removed from mice 30 days after injection with pDCs and INA-6 cells and immunostained with anti-BDCA-2 and anti-IL-3 Abs. Scale bar, 5 μM. (D–H) Bone sections were immunostained with Abs against Ki-67, Cyclin D1, phospho-IκB, ubiquitin, and Factor VIII, and VEGFR1. Dark brown: marker-positive cells in all cases. Micrographs are representative of bone sections from two different mice in each group. (E and G) Quantification of Ki-67, Cyclin-D1 and Factor VIII-positive cells from experiments in D, and H (upper panel). (I) MM.1S cells alone (2.5 × 106) or together with pDCs (0.5 × 106) (1:5 (pDC/MM) ratio) were implanted subcutaneously in mice; tumor volume was monitored every 3rd day (P < 0.05). Inset: Tumors from mice with either MM.1S or MM.1S cells + pDC. Error bars indicate standard deviation (SD).
We next assessed whether pDCs migrate in response to chemokines identified in cytokine bead arrays. Transwell insert assays show significant chemotaxis of pDCs towards CXCL12 (SDF-1α), IL-3, CXCL10 (IP-10), CCL4 (MIP1α), CCL3 (MIP1β) and MCP-1 (Fig 5-I). These data suggest that pDC-MM interaction upregulate many chemokines that may allow for pDCs migration and homing of pDCs to MM BM.
Requirement of pDC-MM cell contact
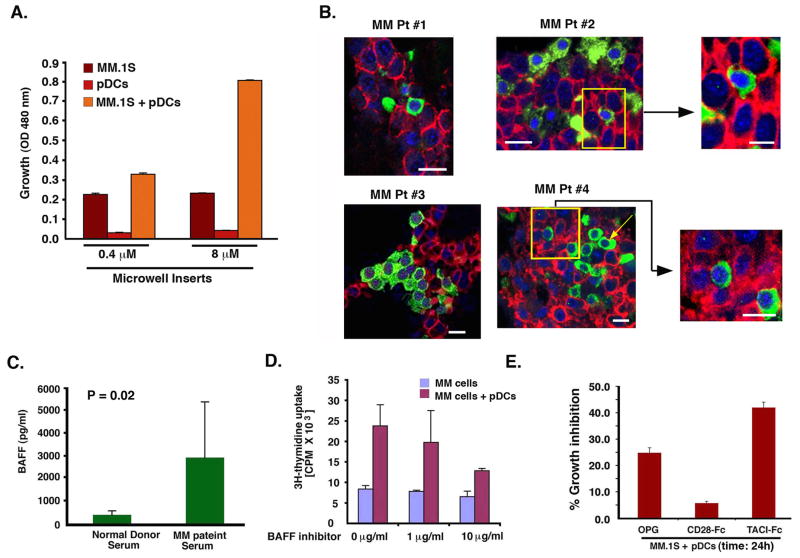
To determine whether cell-cell contact between pDCs and MM cells is required, we utilized Transwell Inserts assays, immunofluorescence (IFC) with confocal microscopy, and IHC analysis. Culture of pDCs and MM cells in the transwell system, which prevents physical interaction of pDCs with MM cells while allowing for proximity and stimulation by soluble factors, significantly attenuated the ability of pDCs to trigger MM cell proliferation (Fig 6A). These findings suggest that contact-dependent secretion of soluble factors accounts for the majority of pDC-induced MM cell growth.
Figure 6. Requirement of pDC contact with MM cells.
(A) Transwell insert assay: Two different pore size membranes were utilized to separate cells: 0.4 μM (does not allow free passage of cells) and 8 μM (allows cell passage). Normal PB pDC (2×104) and MM.1S cells (1 × 105) were added into the upper and lower chamber, respectively; and incubated for 72h at 37°C; cells from the lower chamber were then harvested and analyzed for growth by WST assays (mean ± SD; P=0.001; n=3). (B) Analysis of MM patient BM biopsies (MM #1-MM #4) to show in vivo cell-cell contact between MM BM pDCs with tumor cells. MM patients BM biopsies were subjected to IFC staining for MM cells (CD138 Ab, red) and pDCs (BDCA-2 Ab, green). Nuclear staining was performed with DAPI (blue). Slides were mounted using Vectashield with DAPI. Data shown is representative of 10 MM BM samples analyzed with similar results. Scale bars, 10 μM (MM Pt#1–4). Yellow square represents the 3X zoom areas (Scale bars, 5 μM in upper panel and 10 μM in lower panel). (C) BAFF levels were analyzed in serum obtained from 5 normal and 5 MM patients using ELISA. (D) MM.1S cells were cultured either alone or together with normal PB pDCs at 1:5 (pDC/MM) ratio in the presence or absence of BAFF inhibitor at the indicated concentrations for 72h, and then analyzed for growth (mean ± SD; P < 0.005; n=3). (E) MM.1S cells were cultured with normal PB pDCs at 1:5 pDC/MM ratio in the presence or absence of rhOPG (0.5 μg/ml), TACI-Fc (1 μg/ml) or CD28-Fc (1 μg/ml) for 24h, and then analyzed for proliferation (mean ± SD; P < 0.005; n=2). Data is presented as percent growth inhibition in pDC-induced MM.1S cell growth in the presence of agents. Error bars indicate standard deviation (SD).
To determine whether pDCs are indeed in close proximity to MM cells in vivo, we examined BM biopsy samples from MM patients. Patient BM biopsies were subjected to dual IFC staining with BDCA-2 (pDCs) and CD138 (MM cells) and DAPI. Results demonstrates direct cell-cell contact between pDCs and MM cells as well as clusters of pDCs with MM cells (Fig 6B). IHC analysis with another pDC marker CD123 confirmed these data (Supplementary Fig 3).
Adhesion of MM cells to BMSCs triggers transcription and secretion of soluble factors that mediate MM cell growth in a paracrine manner (Anderson, 2007). Similarly, we here found that pDC-MM interaction upregulates many MM cell growth, survival and chemotactic factors. Importantly, although, MM cells constitutively secrete factors, the direct cell-cell interaction between pDCs and MM cells further augments the production of cytokines and chemokines which not only confer growth, survival and drug resistance in MM cells, but also prolong survival of pDCs. Together these studies suggest a role of both soluble factors and direct pDC-MM cell-cell contact in mediating MM cell growth and pDC survival.
Role of pDC-MM cell surface receptor-ligand interactions
The above findings implicate cell surface molecules in mediating interactions that trigger MM cell growth/survival. Both pDCs and MM cells express molecules which play a role in B cell differentiation and serve as co-stimulatory molecules; e.g, BAFF/APRIL and RANK-RANKL (MacLennan and Vinuesa, 2002; Roodman and Dougall, 2008). Indeed, we found significantly increased BAFF levels in MM patient vs. normal donor serum (Fig 6C), as in our previous report (Neri et al., 2007). Importantly, blockade of BAFF with either a small molecule inhibitor or TACI-Fc significantly abrogated pDC-induced MM cell growth (Fig 6D and 6E, respectively). TACI-Fc alone did not affect the viability of MM.1S cells alone (data not shown). On the other hand, inhibition of RANK-RANKL using osteoprotegrin (OPG) also decreased pDC-induced MM cell growth, albeit to a lesser extent than inhibiting BAFF (Fig 6E). Our finding that NF-κB mediates pDC-MM functional sequelae, together with the known role of both BAFF and RANKL in activating NF-κB, suggests the therapeutic potential of targeting BAFF/RANKL in MM. However, it is very likely that other cell surface receptor ligand interactions are also involved in mediating pDC-MM interactions.
In vivo validation of pDC-induced growth of MM cells using MM xenograft models
Having defined the functional role of pDCs in regulation of MM biology in vitro, we next examined whether pDCs similarly affect MM cell growth in vivo using murine xenograft models of human MM. In order to evaluate the growth-promoting function of human pDCs within the human BM milieu, we utilized the SCID-hu model (Tassone et al., 2005), which recapitulates the human BM milieu in vivo. In this model, MM cells are injected directly into human bone chips implanted subcutaneously in SCID mice, and MM cell growth is assessed by serial measurements of circulating levels of soluble human IL-6R and Ig in mouse serum. A more robust growth of tumor occurred in mice receiving human pDCs and INA-6 MM cells than in mice injected with INA-6 cells alone (Fig 7A and 7B). pDCs alone did not induce tumors in mice (data not shown). Immunostaining of implanted human bone with BDCA-2 showed presence of viable pDCs 30 days after the initial injections (Fig 7C). As in our in vitro results, IL-3 was highly expressed in mice injected with pDCs plus INA-6 cells (Fig 7C). The observation that IL-3 is a survival factor for pDCs, coupled with our in vitro and in vivo results, suggests that IL-3, at least in part, facilitates prolonged survival of pDCs in vivo in the human MM BM milieu.
We also observed an increase in Ki-67 and Cyclin D1-positive cells in human bone sections from mice engrafted with pDCs and INA-6 MM cells compared to mice receiving INA-6 cells alone (Fig 7D, 7E). Counterstaining of bone sections with CD138 confirmed that Ki-67 and Cyclin-D1 positive cells were MM cells (data not shown). These findings are consistent with increased proliferation of MM cells in vivo triggered by pDCs. Similarly, we noted a marked increase in p-IκB, a decrease in ubiquitination and enhanced Factor VIII and VEGFR1 expression in bone sections from mice injected with pDCs plus INA-6 cells vs. INA-6 MM cells alone (Fig 7F–7H). These in vivo data confirms our in vitro data, and suggest that pDCs trigger activation of growth, survival, and angiogenic signaling pathways in MM cells.
We next utilized a subcutaneous model of human MM in SCID mice to further confirm pDC-induced MM cell growth. An early and rapid growth of tumor occurred within 12 days in mice receiving both pDCs and MM cells, whereas similar tumor growth was noted only at day 21 in mice injected with MM cells alone (Fig 7-I). Immunostaining showed increased IL-3 and presence of pDCs (BDCA-2+ cells) in tumor sections from mice receiving pDCs and MM cells vs. MM cells alone (data not shown). Together, our findings from two distinct human MM xenograft models suggest that pDCs enhance MM cell growth in vivo.
CpG-ODNs restore MM patient pDCs T cell response and block MM cell growth
MM is characterized by immune dysfunction, and pDCs may contribute, at least in part, since MM patient BM-pDCs exhibit reduced ability to induce T cell proliferation (Fig 1B). The loss of immune functioning of tumor-infiltrating DCs has been linked to the immunosuppressive effects of the tumor-host microenvironment mediated via vascular endothelial growth factor (VEGF), interleukin-6 (IL-6) or macrophage stimulating factor (MCSF) in cancers (Zou, 2005) including MM (Hayashi et al., 2003). Prior studies showed that immune function of pDCs is mediated by production of type I IFNs (Siegal et al., 1999), which in turn, is linked to expression of toll-like receptor 7 (TLR7) and TLR9 (Gilliet et al., 2008; Krieg, 2007; Moseman et al., 2004; Vollmer, 2005). Other studies have targeted TLR-9 using immunostimulatory activities of CpG-containing oligodeoxynucleotides (CpG ODNs) to activate pDCs. Indeed systemic lupus erythematous (SLE) is linked to TLR-9-activated pDCs and IFN-α release (Barrat et al., 2005; Krieg, 2007; Liu et al., 2008; Vollmer, 2005). In light of these studies, we next examined whether treatment of MM patient BM-pDCs with CpG-ODNs alters their T cell stimulatory activity. Importantly, CpG-ODNs restore the ability of MM patient BM-pDCs to trigger both allogeneic and autologus T cell proliferation (Fig 8A and 8B). Furthermore, CpGs also enhanced IFN-α secretion from MM patient BM-pDCs (Fig 8C) and upregulated TLR9 expression in MM BM pDCs (Fig 8D).
Prior studies showed that pDCs activated via TLR ligation, CD40-L engagement, or HIV can modulate regulatory T cells (Tregs) (Gilliet and Liu, 2002; Manches et al., 2008; Moseman et al., 2004). Tregs are dysfunctional and decreased in numbers in MM (Prabhalarao et al., 2006). CD4+CD25+FOXP3-high Tregs can be expanded by mDCs in vitro and after injection of cytokine-matured DCs in MM patients (Banerjee et al., 2006). It is possible that activated pDCs may restore Tregs homeostasis in MM; however, the functional significance of pDCs effect on Tregs within the human MM BM microenviroment, particularly the biologic sequelae in MM cells, remains to be examined.
We next asked whether CpG-ODNs affects pDC-induced MM cell growth. As shown in Fig 8E, CpGs inhibit patient-BM pDC-triggered DNA synthesis in MM.1S cells. CpGs also blocked pDC-induced growth of MM cells lines, as assessed by WST proliferation assays (Fig 8F). These data demonstrate that 1) activation of pDCs with CpGs both restore their ability to trigger T cell proliferation and reduces their MM cell growth promoting activity; and 2) MM cell interaction with pDCs does not require pDC activation. A recent study in the B16 mouse melanoma tumor model also showed that CpG-activated pDCs are capable of inducing a systemic anti-tumor immunity via activation of NK cells and T cells (Liu et al., 2008). It remains to be determined whether CpG-activated MM pDCs generate clinically significant effective anti-tumor immunity in MM.
In summary, we show the pathophysiologic role of pDCs in MM. Our findings demonstrate increased numbers and more frequent localization of pDCs in MM patient BM than normal BM. Both in vitro and in vivo models of human MM show that pDCs confer growth, survival, chemotaxis, and drug resistance in MM cells. Targeting Toll-like receptors with CpG ODNs both improves immune function of pDCs and abrogates pDC-induced MM cell growth. Overall, our findings identify an integral role of pDCs in MM pathogenesis and provide the basis for targeting pDC-MM interactions with CpG-ODNs as a therapeutic strategy to improve patient outcome in MM.
Experimental Procedures
Isolation, phenotypic analysis and quantification of pDCs
All studies involving human samples were performed under IRB-approved protocols at Dana-Farber Cancer Institute (Boston), Brigham and Womens Hospital (Boston), and Mayo Clinic (Rochester) through which informed consent was obtained and de-identified samples were utilized. pDCs were isolated by magnetically activated cell sorting using CD304 (BDCA-4/Neuropilin-1) microbeads kit (Miltenyi Biotec, Auburn, CA). MNCs from normal healthy donors and MM patients were isolated by Ficoll Hypaque density gradient centrifugation. Cells were then magnetically labeled with anti-BDCA-4 antibody coupled to colloidal paramagnetic microbeads and passed through a magnetic separation column twice (Mitenyi Biotec). The cells staining negative for lineage markers and CD11c were FACS sorted (Fig 1A). Polychromatic staining of cells with CD123 PE-Cy5, HLA-DR Pacific Blue, and BDCA-2 FITC confirmed the purity of pDCs (≥99%) (Dzionek et al., 2001): BDCA-4-positive pDCs derived in this way are lineage (CD3, CD14, CD20, CD11c−) negative, MHC II-positive, as well as CD123 and BDCA-2-positive. pDCs were quantified in total MNCs (2 × 106 cells) from PB or BM samples using FACS analysis (100,000 minimum events were gated) with BDCA-2 (CD303)-PE conjugated Ab (Mitenyi Biotec). For some experiments, pDCs were purified using negative depletion (miltenyi Biotec)(99% BDCA2+ CD123+ cells by FACS). To obtain pDC-depleted fraction of MM BM cells, we utilized LD columns in combination with BDCA-4 Microbeads and MACS separator (Miltenyi Biotec). FACS analysis of pDC-depleted BM cell fraction showed low to undetectable BDCA-2-positive cells (3.8% pDCs before and <0.02% pDCs after depletion). Antibody details are in the supplementary file. Flow cytometric analysis was performed on a Beckman Coulter FC 500, and the data was analyzed using CXP software (BD Biosciences, San Jose, CA).
Cell growth, viability, survival, and apoptosis assays
Cells were cultured in complete medium (RPMI-1640 media supplemented with 10% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine). DNA synthesis was measured by (3H)thymidine uptake (3H-Tdr)(PerkinElmer, Boston, MA), as previously described (Chauhan et al., 2005). Fold change in DNA synthesis was calculated using following formula: 3H-TdR uptake of cells in pDC-MM cultures minus 3H-TdR uptake of pDCs alone divided by 3H-TdR uptake of MM cells alone. Growth was also assessed by WST assay (BioVision, Mountain View, CA). Cell viability was assessed by 3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Chemicon International Inc., Temecula, CA) assay (Chauhan et al., 2005). For cell survival assays; pDCs and patient MM cells were cultured at 1:5 pDC/MM ratio in 6 well plates in DCP-MM culture medium (Mattek Corp. Ashland, MA). After each week of co-culture, tumor cells were separated with CD138 microbeads, and cultured with fresh pDCs. After four weeks of co-culture, cells were triple stained with CD138-FITC conjugated Ab, CD123-PE conjugated Ab, and DAPI, and images were taken by Zeiss LSM confocal microscope (Thornwood, NY). Staining with kappa and lamda Abs and DAPI confirmed clonality of MM cells. Apoptosis was measured by Annexin V/PI staining (Chauhan et al., 2005). T cell proliferation assays were performed as previously described (Hayashi et al., 2003). Briefly, pDCs were cultured with or without T cells, in the presence or absence of CpG-ODNS (0.4 and 4.0 μg/ml) in complete medium; and DNA synthesis was analyzed by both 3H-TdR uptake and WST assays.
Live cell imaging
pDCs were labeled with MitoFluor Red 594 (1:1000; Invitrogen, Carlsbad, CA); GFP-positive MM.1S were added to pDCs in a 24 well glass-bottom culture dish (MatTek Inc, Ashland, MA), and live cell images were taken using an Apochromat 40×/1.2W lens on a LSM-510 confocal system. Images were taken for 30ms interval for 5–15 mins, and processed using Volocity software (Improvision Inc, Waltham, MA).
Immunofluorescence and confocal microscopy
BM biopsies from MM patients and normal donors were subjected to double IFC staining with BDCA-2 and CD138 Abs. Antigen retrieval was performed by boiling the slides in DIVA Decloaker (Biocare, Concord CA) for 10 minutes in a pressure cooker. Slides were incubated with 5% normal donkey serum, followed by staining with mouse monoclonal anti-human CD138 Ab (1:100) and rabbit polyclonal BDCA-2 Ab (1:10). After TBS wash, slides were incubated with Alexa-488 conjugated Donkey anti-rabbit secondary Ab (1:200) and CY5 Donkey anti-mouse secondary Ab (1:200) for 1h at RT. Slides were mounted with Vectashield containing DAPI (Vector lab, Burlingame CA). Confocal images were taken using the Zeiss LSM510 confocal system (63×/1.4 apochromat objective) (Thornwood, NY). IHC with primary antibody specific for pDCs (BDCA-2 at 1:400 dilution) and MM cells (CD138 at 1:500 dilution) was conducted using previously described methods (Jaye et al., 2006).
Transwell migration assays
In vitro migration assays were performed using Transwell Boyden chambers (Chemicon, Billerica, MA). For pDC chemotaxis studies, we utilized 5 μM pore size membranes which allow for the free passage of pDCs. 5 × 105 pDCs were suspended in serum-free culture medium (RPMI-1640 media supplemented with antibiotics) and plated in the upper chamber; lower chamber was coated with various chemokines. After 2h incubation at 37°C, pDCs that migrated to the lower chamber were counted. pDCs and MM cell migration: Cell migration was assayed using a 24-well plate with 8-μm pore size inserts. MM cells (2 × 106 cells/ml) were plated in the upper and pDCs in the lower chamber or vice versa. Serum free culture medium (as above) was used. The plates were then incubated at 37°C for 4 h, and the non-migrating cells in the upper chamber removed from the Transwell membrane by washing with PBS. Cells migrating to the bottom face of the membrane were stained with Crystal violet. 3 randomly selected fields were examined to quantify number of cells migrating from upper to lower chambers. For cell-cell contact-dependent MM cell growth, we utilized two different pore size membranes to separate cells: 0.4 μM (that does not allow free passage of cells) and 8 μM (that allows cell passage). pDCs (2 × 104) and MM.1S cells (1 × 105) were added into the upper and lower chamber, respectively; and incubated for 72h at 37°C; cells from the lower chamber were then harvested and analyzed for growth by WST assays.
Cytokine bead arrays and ELISA
MM.1S cells (5 × 104 cells/200 μL) and pDCs (1 × 104 cells/200 μL) were cultured either alone or together at 1:5 (pDC/MM) ratio in 96 well plates for indicated intervals in triplicate; supernatants were collected (a total of 600 μL for each condition) and analyzed using Human Cytokine/Chemokine panel-29 Plex (LINCO Res, MO). Cells were cultured in complete medium. BAFF, SDF1-α and IL-3 levels were measured by Quantikine BAFF ELISA kit (R&D, MN). IFN-α levels were assessed by ELISA (PBL, NJ).
Proteasome activity assays
MM.1S cells (2.5 × 106) and pDCs (0.5 × 106) were cultured in complete medium; MM.1S cells were separated from pDC co-cultures by CD138 microbeads (purity >99% CD138+ cells), and proteasome activity assays were performed using fluorogenic peptide substrates, as previously described (Chauhan et al., 2005).
Human plasmacytoma xenograft and SCID-hu model
All animal experiments were approved by and conform to the relevant regulatory standards of the Institutional Animal Care and Use Committee at the Dana-Farber Cancer Institute. SCID-hu model has been described previously (Tassone et al., 2005). For SCID-hu model studies, we utilized an IL-6-dependent INA-6 MM cell line. Cells were propagated in vitro in complete medium, and rhIL-6 (2.5 ng/ml). INA-6 cells (2 × 106) without IL-6, pDCs alone (0.4 × 106), or INA-6 cells together with pDCs were injected directly into human bone chips implanted subcutaneously in SCID mice. Tumor growth was assessed every tenth day by measuring circulating levels of shIL-6R and IgG levels in mouse blood using ELISA (R & D Systems, MN, USA). Human plasmacytoma xenograft model: CB-17 SCID-mice were subcutaneously injected with pDCs alone, MM.1S cells alone or pDCs and MM.1S cells (5 mice/group) in plain RPMI-1640 medium, and tumor growth was measured as previously described (Chauhan et al., 2005).
Immunohistochemistry
Implanted human bone chips were excised from mice and subjected to IHC analysis using Abs against Ki-67, Cyclin-D1, p-IkB, Factor VIII, VEGFR1 and Ubiquitin (Abcam, Cambridge, MA), as previously described (Singh et al., 2006).
Statistical Analysis
Non-parametric tests and mixed models were used to analyze the data, including Wilcoxon’s signed rank and/or students t test to assess proliferation. Significance of differences observed in xenograft studies was assessed using Student t test. The minimal level of significance was P < 0.05.
Microarray data
The raw data for expression profiling and the CEL files are provided at the website Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/). Accession Number “GSE17407”.
Supplementary Material
Acknowledgments
Grant support: This investigation was supported by NIH grants SPORE-P50100707, PO1-CA078378, and RO1CA050947; and MRF funds.
Footnotes
Significance
Recent reports demonstrate infiltration of dendritic cells (DCs) at tumor sites, but with unclear significance. Here we show the pathophysiologic role of plasmacytoid DCs (pDCs) in multiple myeloma (MM). Our study shows increased numbers and more frequent localization of pDCs in MM patient BM than normal BM. Functional analysis using in vitro and in vivo models of human MM in the BM milieu shows that pDCs confer growth, survival, chemotaxis, and drug resistance. Importantly, targeting Toll-like receptors with CpG ODNs improves immune function of pDCs and abrogates pDC-induced MM cell growth. These findings identify an integral role of pDCs in MM pathogenesis and provide the basis for targeting pDC-MM interactions as therapeutic strategy to improve patient outcome.
Authors contributions: DC designed research, analyzed data and wrote the manuscript; AS designed, interpreted, and performed most experiments; MB performed growth/survival assays; GB isolated normal plasma cells; RC and DJ helped in IHC; and TH, SH, PR, YT, NR, CM, and NM provided clinical samples; and KA analyzed data and wrote the manuscript. We also thank Robert Schlossman, Bryan Ciccarelli, and Sagar Lonial for providing blood samples. We are thankful to Lay-Hong Ang for confocal microscopy; John F. Daley for FACS; Sangeetha Battar, Gaurav Chetri, and David Vasir for technical help and insightful discussions.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson KC. Targeted therapy of multiple myeloma based upon tumor-microenvironmental interactions. Exp Hematol. 2007;35:155–162. doi: 10.1016/j.exphem.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, Duramad O, Coffman RL. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsagel PL, Kuehl WM. Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol. 2005;23:6333–6338. doi: 10.1200/JCO.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111:2521–2526. doi: 10.1182/blood-2007-08-104984. [DOI] [PubMed] [Google Scholar]
- Brimnes MK, Svane IM, Johnsen HE. Impaired functionality and phenotypic profile of dendritic cells from patients with multiple myeloma. Clin Exp Immunol. 2006;144:76–84. doi: 10.1111/j.1365-2249.2006.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RD, Pope B, Murray A, Esdale W, Sze DM, Gibson J, Ho PJ, Hart D, Joshua D. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7–1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood. 2001;98:2992–2998. doi: 10.1182/blood.v98.10.2992. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Catley L, Li G, Podar K, Hideshima T, Velankar M, Mitsiades C, Mitsiades N, Yasui H, Letai A, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8:407–419. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, Anderson KC. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- Dalton W, Anderson KC. Synopsis of a roundtable on validating novel therapeutics for multiple myeloma. Clin Cancer Res. 2006;12:6603–6610. doi: 10.1158/1078-0432.CCR-06-1489. [DOI] [PubMed] [Google Scholar]
- Dzionek A, Inagaki Y, Okawa K, Nagafune J, Rock J, Sohma Y, Winkels G, Zysk M, Yamaguchi Y, Schmitz J. Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions. Hum Immunol. 2002;63:1133–1148. doi: 10.1016/s0198-8859(02)00752-8. [DOI] [PubMed] [Google Scholar]
- Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia De Vinuesa C, Gulbranson-Judge A, Khan M, O’Leary P, Cascalho M, Wabl M, Klaus GG, Owen MJ, MacLennan IC. Dendritic cells associated with plasmablast survival. Eur J Immunol. 1999;29:3712–3721. doi: 10.1002/(SICI)1521-4141(199911)29:11<3712::AID-IMMU3712>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Hideshima T, Akiyama M, Raje N, Richardson P, Chauhan D, Anderson KC. Ex vivo induction of multiple myeloma-specific cytotoxic T lymphocytes. Blood. 2003;102:1435–1442. doi: 10.1182/blood-2002-09-2828. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- Jaye DL, Geigerman CM, Herling M, Eastburn K, Waller EK, Jones D. Expression of the plasmacytoid dendritic cell marker BDCA-2 supports a spectrum of maturation among CD4+ CD56+ hematodermic neoplasms. Mod Pathol. 2006;19:1555–1562. doi: 10.1038/modpathol.3800679. [DOI] [PubMed] [Google Scholar]
- Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- Krieg AM. TLR9 and DNA ‘feel’ RAGE. Nat Immunol. 2007;8:475–477. doi: 10.1038/ni0507-475. [DOI] [PubMed] [Google Scholar]
- Kukreja A, Hutchinson A, Dhodapkar K, Mazumder A, Vesole D, Angitapalli R, Jagannath S, Dhodapkar MV. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med. 2006;203:1859–1865. doi: 10.1084/jem.20052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukreja A, Hutchinson A, Mazumder A, Vesole D, Angitapalli R, Jagannath S, O’Connor O A, Dhodapkar MV. Bortezomib disrupts tumour-dendritic cell interactions in myeloma and lymphoma: therapeutic implications. Br J Haematol. 2007;136:106–110. doi: 10.1111/j.1365-2141.2006.06369.x. [DOI] [PubMed] [Google Scholar]
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Chung HY, Ehrlich LA, Jelinek DF, Callander NS, Roodman GD, Choi SJ. IL-3 expression by myeloma cells increases both osteoclast formation and growth of myeloma cells. Blood. 2004;103:2308–2315. doi: 10.1182/blood-2003-06-1992. [DOI] [PubMed] [Google Scholar]
- Liu C, Lou Y, Lizee G, Qin H, Liu S, Rabinovich B, Kim GJ, Wang YH, Ye Y, Sikora AG, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118:1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- MacLennan I, Vinuesa C. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity. 2002;17:235–238. doi: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- Manches O, Munn D, Fallahi A, Lifson J, Chaperot L, Plumas J, Bhardwaj N. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118:3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol. 2005;79:17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- Neri P, Kumar S, Fulciniti MT, Vallet S, Chhetri S, Mukherjee S, Tai Y, Chauhan D, Tassone P, Venuta S, et al. Neutralizing B-cell activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clin Cancer Res. 2007;13:5903–5909. doi: 10.1158/1078-0432.CCR-07-0753. [DOI] [PubMed] [Google Scholar]
- O’Doherty U, Peng M, Gezelter S, Swiggard WJ, Betjes M, Bhardwaj N, Steinman RM. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- Oerlemans R, Franke NE, Assaraf YG, Cloos J, van Zantwijk I, Berkers CR, Scheffer GL, Debipersad K, Vojtekova K, Lemos C, et al. Molecular basis of bortezomib resistance: proteasome subunit beta5 (PSMB5) gene mutation and overexpression of PSMB5 protein. Blood. 2008;112:2489–2499. doi: 10.1182/blood-2007-08-104950. [DOI] [PubMed] [Google Scholar]
- Poeck H, Wagner M, Battiany J, Rothenfusser S, Wellisch D, Hornung V, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–3064. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- Prabhala RH, Neri P, Bae JE, Tassone P, Shammas MA, Allam CK, Daley JF, Chauhan D, Blanchard E, Thatte HS, et al. Dysfunctional T regulatory cells in multiple myeloma. Blood. 2006;107:301–304. doi: 10.1182/blood-2005-08-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogli M, Ferri E, Della Cuna GR, Tura S, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230–237. doi: 10.1182/blood.v100.1.230. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Vacca A. Importance of the bone marrow microenvironment in inducing the angiogenic response in multiple myeloma. Oncogene. 2006;25:4257–4266. doi: 10.1038/sj.onc.1209456. [DOI] [PubMed] [Google Scholar]
- Roodman GD. Novel targets for myeloma bone disease. Expert Opin Ther Targets. 2008;12:1377–1387. doi: 10.1517/14728222.12.11.1377. [DOI] [PubMed] [Google Scholar]
- Roodman GD, Dougall WC. RANK ligand as a therapeutic target for bone metastases and multiple myeloma. Cancer Treat Rev. 2008;34:92–101. doi: 10.1016/j.ctrv.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Singh AV, Franke AA, Blackburn GL, Zhou JR. Soy phytochemicals prevent orthotopic growth and metastasis of bladder cancer in mice by alterations of cancer cell proliferation and apoptosis and tumor angiogenesis. Cancer Res. 2006;66:1851–1858. doi: 10.1158/0008-5472.CAN-05-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabera S, Perez-Simon JA, Diez-Campelo M, Sanchez-Abarca LI, Blanco B, Lopez A, Benito A, Ocio E, Sanchez-Guijo FM, Canizo C, San Miguel JF. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica. 2008;93:1301–1309. doi: 10.3324/haematol.12857. [DOI] [PubMed] [Google Scholar]
- Tassone P, Neri P, Carrasco DR, Burger R, Goldmacher VS, Fram R, Munshi V, Shammas MA, Catley L, Jacob GS, et al. A clinically relevant SCID-hu in vivo model of human multiple myeloma. Blood. 2005;106:713–716. doi: 10.1182/blood-2005-01-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer J. Progress in drug development of immunostimulatory CpG oligodeoxynucleotide ligands for TLR9. Expert Opin Biol Ther. 2005;5:673–682. doi: 10.1517/14712598.5.5.673. [DOI] [PubMed] [Google Scholar]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, Wei S, Krzysiek R, Durand-Gasselin I, Gordon A, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.