Abstract
Stem cell specific proteins and regulatory pathways that determine self-renewal and differentiation have become of fundamental importance in understanding regenerative and reparative processes in the myocardium. One such regulatory protein named nucleostemin has been studied in the context of stem cells and several cancer cell lines, where expression is associated with proliferation and maintenance of a primitive cellular phenotype. We find nucleostemin is present in young myocardium and is also induced following cardiomyopathic injury. Nucleostemin expression in cardiomyocytes is induced by fibroblast growth factor −2 (FGF-2) and accumulates in response to Pim-1 kinase activity. Cardiac stem cells also express nucleostemin that is diminished in response to commitment to a differentiated phenotype. Overexpression of nucleostemin in cultured cardiac stem cells increases proliferation while preserving telomere length, providing a mechanistic basis for potential actions of nucleostemin in promotion of cell survival and proliferation as seen in other cell types.
Keywords: Nucleostemin, cardioprotection, cardiomyocytes, stem cells, Pim-1, telomerase
Introduction
Cellular based myocardial regeneration depends upon tightly regulated signaling cascades that control survival and proliferation. In the case of stem cell populations, these signaling pathways have been predominantly defined by decades of study in hematopoietic1–4 and developmental contexts.5–7 The relatively recent advent of myocardial adult stem cells and their distinctive characteristics has prompted re-examination of the operational definition of “stem cells” and “stemness”.8, 9 The traditional view of stem cell behavior as derived from classic lineage studies may not appropriately reflect the biology of stem cells in tissues characterized by slow cellular turnover such as the myocardium. For example, activation of signaling typically associated with regulation of proliferation and survival in stem cells is also observed in combination with partial or fully committed cellular phenotypes following tissue injury.10–12 These revelations have prompted dissolution of long standing assertions related to “stem cell-associated” signaling, now viewed as regulation of tissue repair and regeneration, or in some cases oncogenic transformation.13–16
Nucleostemin is found at high levels in various stem cells and human cancers17 where it has been associated with maintenance of proliferation.17–20 Expression of nucleostemin drops precipitously during differentiation21, 22 and genetic deletion of nucleostemin results in embryonic lethality around day 4 post coitum with blastocysts comprised of cells that fail to enter S phase.23 Similar arrest in G0/G1 phase of cell cycle was observed in HeLa cells if nucleostemin was eliminated by RNAi.20 Nucleostemin has been purported to mediate cellular dedifferentiation and regenerative processes in newts.24 Although the molecular basis of nucleostemin-mediated actions remains controversial, evidence supports mechanisms related to inhibition of p5317 or telomeric repeat-binding factor 1 (TRF1) that negatively regulates telomere length.25 Collectively, these characteristics point to a pivotal role for nucleostemin in maintenance of cell survival, antagonizing senescence, and promotion of regenerative potential.
Participation of nucleostemin in myocardial repair and regeneration has no precedent in the literature. Our findings establish a role for nucleostemin in response to pathologic injury and demonstrate biological properties of nucleostemin expression in cardiac stem cells, postnatal development, and response to paracrine fibroblast growth factor (FGF) treatment as well as induction by Pim-1 kinase activity. Beneficial action depends upon enhanced cell proliferation coupled with maintainance of telomeric length, which is preserved in c-kit+ cardiac stem cells by nucleostemin overexpression. Therefore, nucleostemin is a novel marker of protective signaling in the myocardium that, together with established links to stem cells, point to a role in myocardial repair and regeneration.
Materials and Methods
Nucleostemin cDNA, adenovirus, fibroblast growth factor (FGF) treatments, antibodies and immunoblotting
Details are provided in the supplement.
Immunohistochemistry and confocal microscopy including cell proliferation and telomere length measurements
Performed as previously described26, 27 with details in the supplement.
Stem cell and adult cardiomyocyte cultures
Cultured as previously described28,29 with details in the supplement.
Murine surgical procedures
Performed as previously described30 with details provided in the supplement.
Pim-1 studies
Pim-1 overexpressing transgenic mice were created as previously described.31
Data analyses
All data expressed as mean ± SEM. Differences in variables examined by Student’s t-test. p< 0.05 was considered significant. Statistical data analyzed using Microsoft Excel software.
Results
Nucleostemin expression declines rapidly after birth
Nucleostemin is detectable within nuclei of cardiomyocytes in sections of neonatal mouse myocardium as well as cultured neonatal rat cells (Fig. 1). Nucleostemin expression diminishes rapidly within weeks after birth evidenced by fewer positive nuclei with lower intensity immunofluorescence in sections of older hearts relative to postnatal sections (Fig. 1A). Nucleoli of cultured cardiomyocytes are labeled consistent with nucleostemin localization (Fig. 1B).32 Progressive loss of nucleostemin correlated to increased age in myocardial sections (Fig. 1A) and lysates showing significant (p<0.01) decreases in nucleostemin protein (Fig 1C). These results indicate nucleostemin association with young myocytes possessing proliferative potential during early postnatal growth.33–36 Exposure of neonatal rat cardiomyocytes to doxorubicin significantly decreases nucleostemin protein levels (Online Figure I-A), indicating cardiotoxic effects of doxorubicin may impair proliferation of young myocytes through antagonizing nucleostemin. However, nucleostemin overexpression is ineffective at antagonizing p53 protein in this system (Online Figure I-B).
Figure 1. Postnatal nucleostemin expression declines upon maturation.
(A) Confocal microscopy of myocardial sections 2 days after birth show widespread nucleostemin (green, at arrows) immunoreactivity relative to sections from hearts at 2 weeks or 2 months after birth. Tropomyosin (red) labels sarcomeric structure and nuclei are labeled with Topro-3 stain (blue). Single channel scans used for creation of color overlays are shown to the left of each panel. (B) Confocal microscopy and immunoblot (inset, lower right) of nucleostemin expression in cultured neonatal rat cardiomyocytes. Nucleostemin is predominately nucleolar. Immunloblot shows nucleostemin expression relative to Hela cell lysate positive control. (C) Decline in nucleostemin expression after birth assessed by quantitative immunoblot analyses. A statistically significant (p<0.01) decrease in nucleostemin expression occurs between 2 days and 2 weeks after birth and continues to decline from 2 weeks and 2 months after birth. Heart lysates signals are normalized to GAPDH to control for minor variation in protein loading.
Nucleostemin is induced following pathological challenge
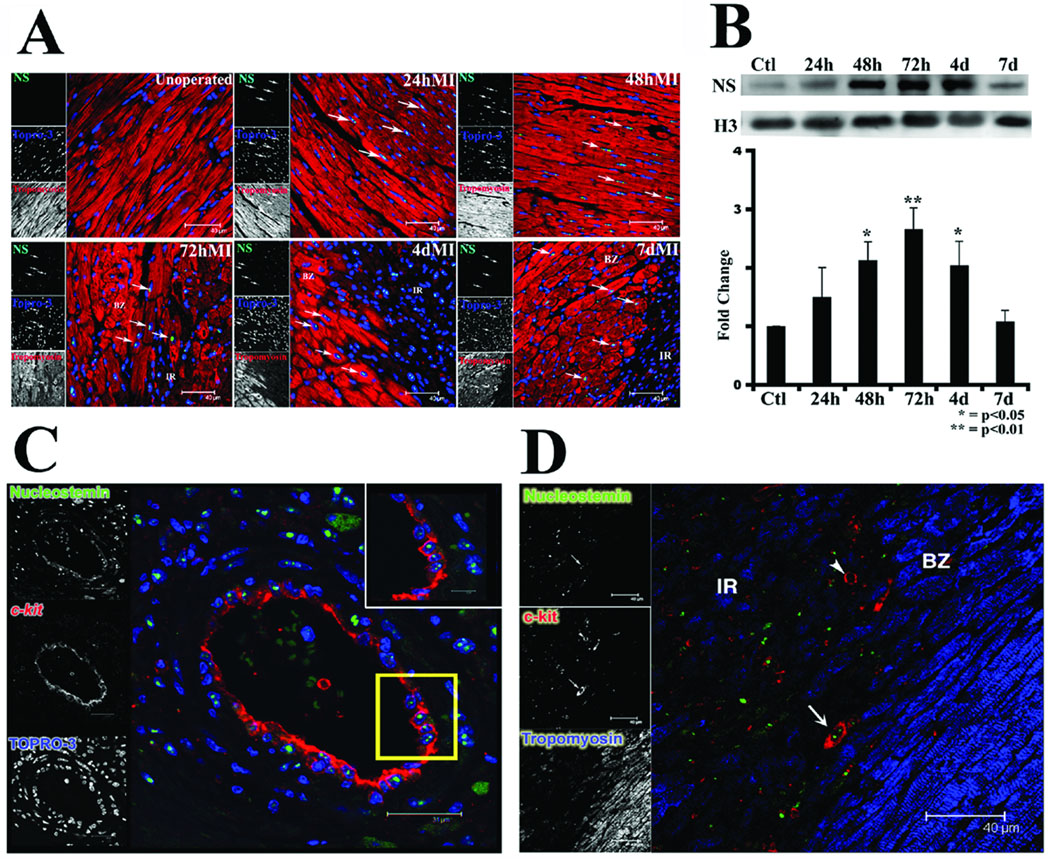
Relatively low level nucleostemin expression in adult myocardium is markedly increased by acute pathological challenge or chronic heart failure (Fig. 2). Myocardial infarction prompts nucleostemin expression in nuclei of cardiomyocytes primarily localized to the border zone adjacent to the ischemic region (Fig. 2A). Immunoblot analyses of excised border zone / infarct regions reveal nucleostemin is increased at 24 hours after induction of myocardial infarction, with significant elevation of protein level within 48 hours that peaks at 72 hours. After 96 hours, expression of nucleostemin decreases from peak levels and returns to basal levels within one week (Fig. 2B). In addition to cardiomyocyte expression, nucleostemin is also expressed in c-kit+ cells observed four days post-infarction. Two areas of enrichment for these c-kit+/nucleostemin+ cells were the endothelial layer of healthy vessels in proximity to the infarct (Fig. 2C) and individual cells in proximity to the border zone of damaged tissue (Fig. 2D, at arrow).
Figure 2. Nucleostemin expression is induced by myocardial infarction.
(A) Confocal microscopy of myocardial sections at various time points following myocardial infarction. Nucleostemin expression (green, at arrows) is observed in surviving cardiomyocytes within the border zone surrounding the infarct. Tropomyosin (red) labels sarcomeric structure and nuclei are labeled with Topro-3 stain (blue). (B) Immunoblot shows time course of nucleostemin expression after myocardial infarction peaking at 72 hours post-induction. (C) Confocal microscopy showing coincidence of nucleostemin (green) and c-kit (red) expression in cells lining a vessel proximal to the region of injury at four days post-infarction. Inset at upper right shows the boxed region (yellow) at higher magnification. Nuclei are labeled with Topro-3 stain (blue) (D) Confocal microscopy showing nucleostemin (green) and c-kit (red) expression coincident in a small cell (arrow) at the interface between the border zone (BZ) and infarct region (IR). A cell expressing c-kit but lacking nucleostemin is also shown (arrowhead). Single channel scans that were used for creation of the color overlays are shown on the left of each panel.
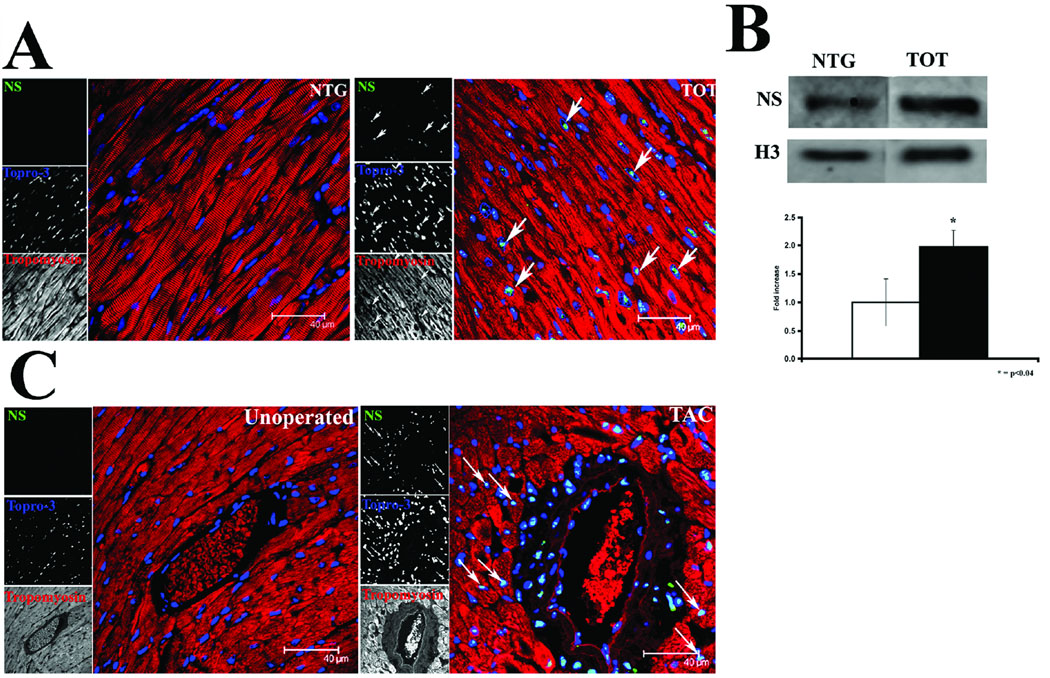
Observations of nucleostemin expression in pathologically challenged myocardium were extended to include additional models of cardiac stress characterized by heart failure or pressure overload hypertrophy. The tropomodulin overexpressing transgenic (TOT) mouse model is a well characterized model of chronic dilated cardiomyopathy developed by our group.37–39 TOTs show nucleostemin expression throughout the myocardium by confocal microscopy (Fig. 3A) and elevated protein level by immunoblot (Fig. 3B). In comparison, pressure overload-induced hypertrophy also induced increased nucleostemin immunoreactivity in sections prepared from mice subjected to trans-aortic constriction. Areas of nucleostemin reactivity are restricted to cells neighboring and comprising large vessels such as endothelium lining the interior as well as cardiomyocytes surrounding vessels (Fig. 3C). Quantitative immunoblot analysis of TAC-induced nucleostemin expression in the vasculature is not practical because of comparatively restricted regionalization of protein expression around large vessels relative to the whole heart.
Figure 3. Nucleostemin expression is increased by pathological stress.
(A) Confocal microscopy showing increased nucleostemin immunoreactivity (green, at arrows) in myocardial sections from tropomodulin overexpressing transgenic (TOT) mice suffering from chronic dilated cardiomyopathy. Tropomyosin (red) labels sarcomeric structure and nuclei are labeled with Topro-3 stain (blue). Single channel scans that were used for creation of the color overlays are shown on the left of each panel. (B) Immunoblot and quantitation of nucleostemin protein expression in lysates prepared from nontransgenic (NTG) or TOT hearts show a significant increase in protein associated with the heart failure phenotype. (C) Confocal microscopy showing increased nucleostemin immunoreactivity (green, at arrows) proximal to a large vessel in myocardial sections from mice subjected to pressure overload hypertrophy by trans-aortic constriction at four days after banding.
Nucleostemin expression is induced by fibroblast growth factor (FGF)
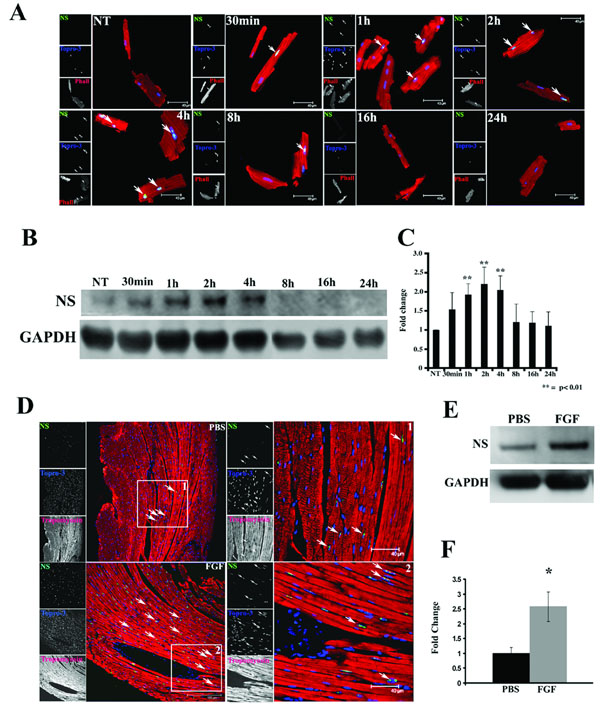
At present, relatively little is known about inductive signals that mediate nucleostemin expression, but FGF-2 increases nucleostemin in adult bone marrow stem cells.21 Similarly, treatment of cultured adult mouse cardiomyocytes with FGF-2 prompts induction of nucleostemin immunoreactivity (Fig. 4A). Immunofluorescence microscopy of FGF-2 treated cells show relatively preserved rod-shaped morphology of the FGF-treated cultures compared to vehicle-treated cells (Fig. 4A). Immunoblot analyses demonstrate significant elevation of nucleostemin protein expression that peaks within two hours post treatment but returns to basal levels after 8 hours (Fig. 4B and C). In vitro findings were validated in vivo using systemic FGF-2 delivery by osmotic pump. Myocardial sections show increased nucleostemin immunoreactivity in cardiomyocytes of mice receiving osmotic pumps with FGF-2 compared to control samples (Fig. 4A). This increase in myocardial nucleostemin is significant as assessed by quantitative immunoblots (Fig. 4E and F).
Figure 4. Nucleostemin expression is up regulated by FGF in vitro and in vivo.
(A) Adult cardiomyocytes at various time points following treatment with FGF-2. Nucleostemin expression (green, at arrows) in treated cardiomyocytes within 30 minutes after exposure. Phalloidin (red) labels sarcomeric structure and nuclei are labeled with Topro-3 (blue). Single channel scans used for creation of the color overlays are shown on the left of each panel. (B) Immunoblot demonstrates a 2–4 hour peak in nucleostemin expression following FGF treatment. (C) Quantitation from adult cardiomyocyte cultures shows a significant increase in nucleostemin expression between 2–4 hours after FGF treatment. (D) Myocardial sections from mice implanted with osmotic pumps filled with vehicle (PBS) or FGF-2 (FGF) shows immunoreactivity for nucleostemin (green, at arrows) intensified by FGF-2 exposure. (E) Immunoblot quantitation shows significant increase in myocardial nucleostemin protein level accompanies after three days of FGF-2 exposure.
Nucleostemin is expressed in cardiac stem cells and declines upon differentiation
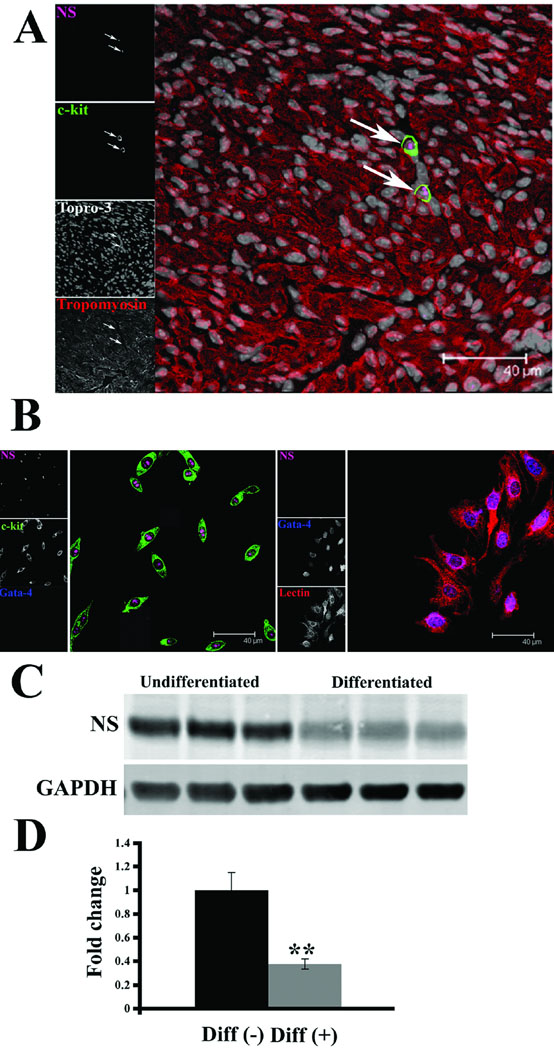
Established association of nucleostemin with stem cells17, 21 and c-kit+ cells in the myocardium (Fig. 2C and D) prompted further assessment of nucleostemin expression in cardiac stem cells (CSC). Neonatal mouse myocardium, which is enriched for c-kit+ cells26, shows colocalization between c-kit and nucleostemin immunoreactivity (Fig. 5A). Furthermore, cultured CSCs express high levels of nucleostemin as observed by immunohistochemistry (Fig. 5B) as well as immunoblot (Fig. 5C). Expression of nucleostemin in CSCs is associated with maintenance of an undifferentiated phenotype. When induced to lineage commitment by exposure to dexamethasone40, CSCs show a precipitous decline in nucleostemin expression that is statistically significant (Fig. 5D) along with increased labeling for GATA-4 (Fig. 5B) and loss of c-kit expression (not shown).
Figure 5. Nucleostemin expression in cardiac stem cells declines upon differentiation.
(A) Myocardial section from a mouse at two days after birth shows c-kit+ cells (green) with coincident expression of nucleostemin (magenta) at arrows. Sarcomeres are labeled with tropomyosin (red), nuclei detected with Topro-3 (white). Single channel scans used for creation of overlays are shown on the left of each panel. (B) Cultured cardiac c-kit+ cells (green) express high levels of nucleostemin (magenta). Cardiac stem cell cultures induced to differentiate by dexamethasone treatment show decreased nucleostemin expression and increased labeling for GATA-4 (blue). Lectin (right panel) is used a cytoplasmic marker due to loss of c-kit expression. (C) Decreased nucleostemin expression in cardiac stem cell culture following dexamethasone treatment. (D) Quantitation demonstrates a significant decrease in expression of nucleostemin in cardiac stem cell cultures following dexamethasone treatment. Whole cell lysates are normalized to GAPDH to correct for minor variation in protein loading.
Nucleostemin expression is associated with Pim-1 kinase activity
Recent studies from our group have identified Pim-1 kinase as an essential regulator of cell survival downstream of Akt.31 Pim-1 is associated with cell proliferation and survival in the hematopoetic system41, so experiments were performed to assess the relationship between Pim-1 activity and nucleostemin expression in myocardium. Normal mice show minimal levels of Pim-1 or nucleostemin expression (Fig. 6A), whereas sections from transgenic mice created to overexpress Pim-1 kinase show accumulation of nucleostemin in cardiomyocyte nuclei (Fig. 6B, at arrows). Induction of nucleostemin expression is also demonstrable by immunoblot analyses of lysates created from Pim-1 overexpressing transgenics relative to nontransgenic controls (Fig. 6C). Furthermore, colocalization is observable in myocardial sections from mice at four days after infarction challenge where surviving myocytes in the border zone co-express both Pim-1 as well as nucleostemin (Fig. 6D, at arrows).
Figure 6. Pim-1 kinase activity induces nucleostemin expression.
Myocardial sections from a nontransgenic (A) or transgenic mouse created with cardiac-specific expression of Pim-1 kinase (B). Single channel scans shown along left of each micrograph correspond to color overlays representing merged images of nucleostemin (red), Pim-1 kinase (green), or trpopmyosin (blue) scans. Nucleostemin is evident in nuclei of cardiomyocytes as indicated (arrows in B). (C) Immunoblot of lysates created from a nontransgenic or transgenic mouse created with cardiac-specific expression of Pim-1 kinase show increased nucleostemin with histone bands shown to demonstrate comparable loading of protein samples. (D) Myocardial section from infarcted mouse heart showing colocalization of Pim-1 and nucleostemin (at arrows) in surviving myocytes. Single channel scans shown along left of micrograph corresponding to color overlays representing merged images of nucleostemin (magenta), Pim-1 kinase (green), or trpopmyosin (red) scans.
Nucleostemin increases TERT and telomere regulatory protein expression
The molecular basis for nucleostemin effects upon telomere regulatory components was assessed by immunoblot analyses of CSC culture lysates. Nucleostemin overexpression prompted concomitant increases in levels of telomere-associated regulatory proteins TERT, TRF1, and TRF2 (Online Figure II). These results are consistent with preservation of telomeric length mediated by nucleostemin overexpression in cultured CSCs.
Nucleostemin increases cardiac stem cell proliferation while preserving telomere length
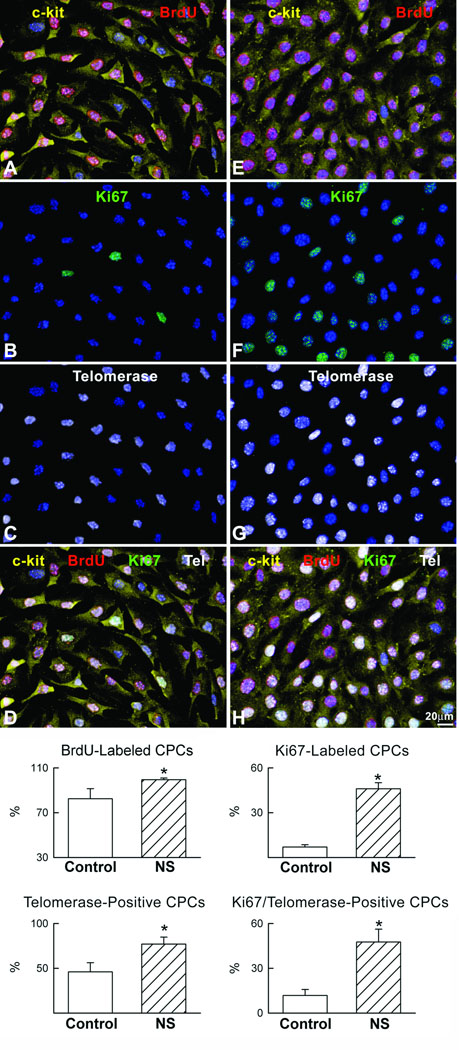
Effects of nucleostemin overexpression upon c-kit+ cultured CSCs were studied to assess consequences for cell proliferation and preservation of telomeric length. Increased nucleostemin expression was readily detected in the CSC cultures following infection with the adenoviral vector by immunoblot (Online Figure III). Nucleostemin overexpression in CSC promotes increased BrdU labeling of nuclei indicative of DNA synthesis as well as increased cell cycle progression as demonstrated by a greater percentage of cells labeled by Ki67 (Fig. 7). The number of CSCs with telomerase detectable by immunolocalization was significantly increased following nucleostemin overexpression, corresponding with a higher percentage of proliferative cells within the telomerase positive CSC population when nucleostemin is overexpressed. Despite enhanced CSC proliferation resulting from nucleostemin overexpression, average telomeric length in the CSC population was preserved and remains unchanged relative to normal control CSCs that undergo proliferative growth at a lower rate (Online Figure III).
Figure 7. Cardiac progenitor cell proliferation is increased by nucleostemin.
(A–H) Left and right panels correspond to control and nucleostemin-overexpressing CPCs, respectively. CPCs (A, E: c-kit, yellow) incorporate BrdU (A, E: red) and express the cell cycle protein Ki67 (B, F: green) and the catalytic subunit of telomerase (C, G: white). Merge of stainings (D, H). (I) Results are mean ± SD. *Indicates p<0.05 versus control.
Discussion
Traditional categorizations of “stem cell-associated” molecules such as nucleostemin are being redefined as these signaling cascades are discovered in partially committed or fully differentiated cells and tissues.30 As nucleostemin is associated with cellular proliferation it is not surprising that this pathway is activated in response to postnatal growth or pathologic injury. Initially our intent was to demonstrate expression of nucleostemin in regenerative processes associated with cardiac stem and progenitor cell populations. However, in addition to observing associations between nucleostemin and c-kit+ cells, we noted profound increases in nucleostemin activation in neonatal as well as pathologically challenged myocardium within cardiomyocytes, prompting additional studies to understand the role of nucleostemin signaling in response to myocardial injury and survival signaling. Since the discovery of nucleostemin five years ago17 subsequent literature has focused predominantly upon aspects of cancer18–20, 42, 43, stem cells21, 22, 44, 45, and developmental biology.23
Previous studies of nucleostemin establish the connection between nucleostemin and proliferative populations, either in the form of stem cells21, 46 or cancer20. In this context nucleostemin appears to be a consistent marker for maintenance of a proliferative state, as expression is rapidly lost upon commitment to a differentiated post-mitotic phenotype22 and depletion of nucleostemin leads to cell cycle arrest47. Conversely, nucleostemin expression is rapidly induced in response to regenerative growth in the newt48 and is required for embryonic development as nucleostemin null mice die in blastocyst stage approximately four days after fertilization.23 Loss of nucleostemin apparently renders cells incapable of DNA synthesis completion in S phase for HeLa cell cultures20. In the context of myocardium, nucleostemin is enriched in postnatal myocytes as well as cultured neonatal rat cardiomyocytes and is down-regulated in adult heart (Fig. 1) or in cardiac stem cells induced to differentiation (Fig. 5). These findings are consistent with expression of nucleostemin in a pro-proliferative state as neonatal myocytes are capable of limited mitotic activity. While nucleostemin overexpression does not stimulate proliferation or hypertrophy in cultured adult cardiomyocytes (not shown), induction of nucleostemin expression may be useful for antagonizing telomeric shortening associated with cardiomyocyte senescence and death.49, 50 In addition, increased TERT expression following nucleostemin expression in CSCs may promote cell proliferation similar to that observed for hair follicle stem cells.51 Thus, in CSCs the presence of nucleostemin may serve important roles in maintaining proliferative potential as well as antagonizing telomeric shortening associated with enhanced mitotic activity (Fig. 7 and Online Figure II).
Functionally competent telomerase is restricted to a few cells in adult organism, germ cells and stem/progenitor cells.52 In telomerase-competent cycling cells, detection of TERT in combination with markers of the cell cycle indicates that telomerase is active and prevents telomeric shortening. TERT expression is higher in nucleostemin-overexpressing CPCs than in control CPCs (Fig. 7 and Online Figure II). Additionally, TERT and Ki67 co-localize in CPCs (Fig. 7). Cycling CPCs that express TERT represent morphological counterparts of telomerase activity detectable with PCR-based methods. Importantly, the fraction of telomerase-competent cycling CPCs was higher in NS-infected CPCs indicating that nucleostemin promotes CPC proliferation without affecting telomere length. In fact, by upregulating TERT expression, nuleostemin allows CPCs to undergo multiple divisions opposing telomere attrition. It is not surprising that length of telomeres did not differ in non-infected and nucleostemin expressing CPCs. Although nucleostemin overexpressing CPCs showed higher levels of TERT (Fig. 7 and Online Figure II), control CPCs also possess telomerase. In physiological conditions, the function of telomerase is not to elongate telomeres beyond their physiological length but to prevent telomeric shortening. Finally, 3–5 days in culture is a very short time-interval for the control cells that would not be expected to show detectable erosion of telomeres due to rounds of replication.
TRF1 and TRF2 are two telomere-related protein components of a multiple protein complex, shelterin, that control homeostasis of telomeres by modulating access of telomerase to telomeres.53 In this regard, decreased TRF1 binding to telomeres reduce the affinity of telomerase to telomeres.54 TRF1 and TRF2 promote formation of T loops in which the telomere terminus is concealed to prevent its recognition as DNA strand break by DNA damage-repair machinery.55 This particular conformation of telomeres is non-accessible to telomerase, thereby blocking telomere elongation. TRF1 and TRF2 are abundant in long telomeres but are absent in short telomeres allowing telomerase to act only upon short telomeres to prevent further erosion.56 Increases in TRF1 and TRF2 protein resulting from nucleostemin overexpression (Online Figure II) may indicate that 3’ telomere termini are sequestered within the T loops opposing telomerase-dependent elongation of telomeres of normal length. TRF1 and TRF2 are critical to T-loop formation and maintenance of this specific telomere-associated molecular structure is essential for continued cellular proliferation and prevention of senescence.
Since telomerase activity is critical for maintenance of cardiac structure and function57, nucleostemin may act to fine tune endogenous telomerase activity and promote maintenance of telomere length as well as inhibit p53-associated signaling resulting from shortened telomeres. These postulates would be consistent with dimunution of nucleostemin following exposure of cultured cardiomyocytes to doxorubicin (Online Figure I) and lack of nucleostemin overexpression affecting p53 levels in this context may be explained by inhibition of MDM2 resulting from aberrant nucleostemin levels.58 Under normal circumstances where telomeric shortening is linked to senescence and possibly apoptosis57, 59, 60, nucleostemin accumulation may serve to antagonize these processes in injured or aging myocardium as implicated by increases in nucleostemin resulting from cardiomyopathic injury (Fig. 2 and Fig. 3). In the case of stem cells, nucleostemin may enable cell cycling as would be desirable in regenerative processes resulting from tissue injury or stress as supported by association of nucleostemin with c-kit+ cells in the myocardium and cultured cardiac stem cells under proliferative conditions (Fig. 5).
Signal transduction controlling nucleostemin expression is not well documented, but FGF-2 induces nucleostemin expression in bone marrow stem cells21. Interestingly, FGF-2 exerts pro-survival effects in myocardium, is a potent angiogenic molecule, and a crucial factor for proliferation and maintenance of several cell types including stem cell populations.61 Interestingly, FGF-2 promotes differentiation of resident cardiac precursors into functional cardiomyocytes62, which would seem at odds with maintenance of a proliferative state unless the action of FGF-2 occurs at a early stage of commitment when limited mitotic activity occurs in concert with lineage specification. FGF-2 stimulates Akt activity that could account for pro-survival and pro-proliferative effects63–65, and Akt activation lies upstream from Pim-1 induction in cardiomyocytes31. Induction of nucleostemin expression by Pim-1 activity (Fig. 6) is without precedent in the literature and reveals an important mechanistic basis for Pim-1 mediated promotion of proliferation in the myocardium that will require further investigation.
The expression of nucleostemin in proliferative neonatal cardiomyocytes and cardiac stem cells together with re-emergence of this protein in damaged myocardium opens up a new facet of our understanding of reparative and regenerative signaling in the heart. Nucleostemin may be useful as a molecular interventional tool for antagonizing cellular senescence as well as maintaining proliferation. Alternatively, nucleostemin in mature post-mitotic cells such as cardiomyocytes may represent part of the reversion to a fetal or embryonic gene expression profile associated with cardiomyopathic challenge or stress. For the emerging field of cardiac stem cells, nucleostemin could be useful as a marker for identification of activated stem cells in the heart and provide a valuable marker of cellular proliferative state similar to Ki6726, 37, 66. Future studies need to expand upon these intriguing seminal observations and define relationships between nucleostemin and cell status as well as functional effects in myocardial cells of both multipotent and lineage committed cell types.
Acknowledgments
Sources of Funding
The authors wish to thank all members of the Sussman laboratory for helpful discussion and comments. Dr. Sussman is supported by NIH grants 5R01HL067245, 1R01HL091102, 1P01HL085577, and 1P01AG023071 (Anversa P.I.). Natalie Gude and John Muraski are Fellows of the Rees-Stealy Research Foundation and the San Diego State University Heart Institute.”
Footnotes
Disclosures: None.
References
- 1.Suda T, Arai F, Hirao A. Hematopoietic stem cells and their niche. Trends Immunol. 2005;26:426–433. doi: 10.1016/j.it.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Larsson J, Karlsson S. The role of Smad signaling in hematopoiesis. Oncogene. 2005;24:5676–5692. doi: 10.1038/sj.onc.1208920. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T, Chiba S. Notch signaling in hematopoietic stem cells. Int J Hematol. 2005;82:285–294. doi: 10.1532/IJH97.05115. [DOI] [PubMed] [Google Scholar]
- 4.Dumortier A, Wilson A, MacDonald HR, Radtke F. Paradigms of notch signaling in mammals. Int J Hematol. 2005;82:277–284. doi: 10.1532/IJH97.05099. [DOI] [PubMed] [Google Scholar]
- 5.Luo D, Renault VM, Rando TA. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin Cell Dev Biol. 2005;16:612–622. doi: 10.1016/j.semcdb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita YM, Fuller MT, Jones DL. Signaling in stem cell niches: lessons from the Drosophila germline. J Cell Sci. 2005;118:665–672. doi: 10.1242/jcs.01680. [DOI] [PubMed] [Google Scholar]
- 7.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nature Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 8.Parker GC, Anastassova-Kristeva M, Broxmeyer HE, Dodge WH, Eisenberg LM, Gehling UM, Guenin LM, Huss R, Moldovan NI, Rao M, Srour EF, Yoder MC. Stem cells: shibboleths of development. Stem Cells Dev. 2004;13:579–584. doi: 10.1089/scd.2004.13.579. [DOI] [PubMed] [Google Scholar]
- 9.Parker GC, Anastassova-Kristeva M, Eisenberg LM, Rao MS, Williams MA, Sanberg PR, English D. Stem cells: shibboleths of development, part II: Toward a functional definition. Stem Cells Dev. 2005;14:463–469. doi: 10.1089/scd.2005.14.463. [DOI] [PubMed] [Google Scholar]
- 10.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 11.Givogri MI, de Planell M, Galbiati F, Superchi D, Gritti A, Vescovi A, de Vellis J, Bongarzone ER. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev Neurosci. 2006;28:81–91. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- 12.Mitsiadis TA, Fried K, Goridis C. Reactivation of Delta-Notch signaling after injury: complementary expression patterns of ligand and receptor in dental pulp. Exp Cell Res. 1999;246:312–318. doi: 10.1006/excr.1998.4285. [DOI] [PubMed] [Google Scholar]
- 13.Jurynczyk M, Jurewicz A, Bielecki B, Raine CS, Selmaj K. Inhibition of Notch signaling enhances tissue repair in an animal model of multiple sclerosis. J Neuroimmunol. 2005;170:3–10. doi: 10.1016/j.jneuroim.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Kohler C, Bell AW, Bowen WC, Monga SP, Fleig W, Michalopoulos GK. Expression of Notch-1 and its ligand Jagged-1 in rat liver during liver regeneration. Hepatology. 2004;39:1056–1065. doi: 10.1002/hep.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjolund J, Manetopoulos C, Stockhausen MT, Axelson H. The Notch pathway in cancer: differentiation gone awry. Eur J Cancer. 2005;41:2620–2629. doi: 10.1016/j.ejca.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Thelu J, Rossio P, Favier B. Notch signalling is linked to epidermal cell differentiation level in basal cell carcinoma, psoriasis and wound healing. BMC Dermatol. 2002;2:7. doi: 10.1186/1471-5945-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai RY, McKay RD. A nucleolar mechanism controlling cell proliferation in stem cells and cancer cells. Genes Dev. 2002;16:2991–3003. doi: 10.1101/gad.55671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan Y, Liu Z, Zhao S, Lou F, Nilsson S, Ekman P, Xu D, Fang X. Nucleostemin mRNA is expressed in both normal and malignant renal tissues. Br J Cancer. 2006;94:1658–1662. doi: 10.1038/sj.bjc.6603145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu SJ, Cai ZW, Liu YJ, Dong MY, Sun LQ, Hu GF, Wei YY, Lao WD. Role of nucleostemin in growth regulation of gastric cancer, liver cancer and other malignancies. World J Gastroenterol. 2004;10:1246–1249. doi: 10.3748/wjg.v10.i9.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sijin L, Ziwei C, Yajun L, Meiyu D, Hongwei Z, Guofa H, Siguo L, Hong G, Zhihong Z, Xiaolei L, Yingyun W, Yan X, Weide L. The effect of knocking-down nucleostemin gene expression on the in vitro proliferation and in vivo tumorigenesis of HeLa cells. J Exp Clin Cancer Res. 2004;23:529–538. [PubMed] [Google Scholar]
- 21.Kafienah W, Mistry S, Williams C, Hollander AP. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells. 2006;24:1113–1120. doi: 10.1634/stemcells.2005-0416. [DOI] [PubMed] [Google Scholar]
- 22.Yaghoobi MM, Mowla SJ, Tiraihi T. Nucleostemin, a coordinator of self-renewal, is expressed in rat marrow stromal cells and turns off after induction of neural differentiation. Neurosci Lett. 2005;390:81–86. doi: 10.1016/j.neulet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Beekman C, Nichane M, De Clercq S, Maetens M, Floss T, Wurst W, Bellefroid E, Marine JC. Evolutionarily Conserved Role of Nucleostemin (NS): Controlling Proliferation of Stem/Progenitor Cells during Early Vertebrate Development. Mol Cell Biol. 2006 doi: 10.1128/MCB.01183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maki N, Takechi K, Sano S, Tarui H, Sasai Y, Agata K. Rapid accumulation of nucleostemin in nucleolus during newt regeneration. Dev Dyn. 2006 doi: 10.1002/dvdy.21027. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Q, Yasumoto H, Tsai RY. Nucleostemin Delays Cellular Senescence and Negatively Regulates TRF1 Protein Stability. Mol Cell Biol. 2006 doi: 10.1128/MCB.00724-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gude N, Muraski J, Rubio M, Kajstura J, Schaefer E, Anversa P, Sussman MA. Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ Res. 2006;99:381–388. doi: 10.1161/01.RES.0000236754.21499.1c. [DOI] [PubMed] [Google Scholar]
- 27.Kato T, Muraski J, Chen Y, Tsujita Y, Wall J, Glembotski CC, Schaefer E, Beckerle M, Sussman MA. Atrial natriuretic peptide promotes cardiomyocyte survival by cGMP-dependent nuclear accumulation of zyxin and Akt. J Clin Invest. 2005;115:2716–2730. doi: 10.1172/JCI24280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao RP. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol. 2000;279:H429–H436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 30.Gude NA, Emmanuel G, Wu W, Cottage CT, Fischer K, Quijada P, Muraski JA, Alvarez R, Rubio M, Schaefer E, Sussman MA. Activation of Notch-Mediated Protective Signaling in the Myocardium. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.107.164749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmanuel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, Houser SR, Schaefer EM, Anversa P, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nature Med. 2007 doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 32.Bernardi R, Pandolfi PP. The nucleolus: at the stem of immortality. Nature Med. 2003;9:24–25. doi: 10.1038/nm0103-24. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhry HW, Dashoush NH, Tang H, Zhang L, Wang X, Wu EX, Wolgemuth DJ. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J Biol Chem. 2004;279:35858–35866. doi: 10.1074/jbc.M404975200. [DOI] [PubMed] [Google Scholar]
- 34.Hudlicka O, Brown MD. Postnatal growth of the heart and its blood vessels. J Vasc Res. 1996;33:266–287. doi: 10.1159/000159155. [DOI] [PubMed] [Google Scholar]
- 35.Tseng YT, Kopel R, Stabila JP, McGonnigal BG, Nguyen TT, Gruppuso PA, Padbury JF. Beta-adrenergic receptors (betaAR) regulate cardiomyocyte proliferation during early postnatal life. FASEB J. 2001;15:1921–1926. doi: 10.1096/fj.01-0151com. [DOI] [PubMed] [Google Scholar]
- 36.Wulfsohn D, Nyengaard JR, Tang Y. Postnatal growth of cardiomyocytes in the left ventricle of the rat. Anat Rec A Discov Mol Cell Evol Biol. 2004;277:236–247. doi: 10.1002/ar.a.20009. [DOI] [PubMed] [Google Scholar]
- 37.Welch S, Plank D, Witt S, Glascock B, Schaefer E, Chimenti S, Andreoli AM, Limana F, Leri A, Kajstura J, Anversa P, Sussman MA. Cardiac-specific IGF-1 expression attenuates dilated cardiomyopathy in tropomodulin-overexpressing transgenic mice. Circ Res. 2002;90:641–648. doi: 10.1161/01.res.0000013780.77774.75. [DOI] [PubMed] [Google Scholar]
- 38.Sussman MA, Welch S, Gude N, Khoury PR, Daniels SR, Kirkpatrick D, Walsh RA, Price RL, Lim HW, Molkentin JD. Pathogenesis of dilated cardiomyopathy: molecular, structural, and population analyses in tropomodulin-overexpressing transgenic mice. Am J Pathol. 1999;155:2101–2113. doi: 10.1016/S0002-9440(10)65528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sussman MA, Welch S, Cambon N, Klevitsky R, Hewett TE, Price R, Witt SA, Kimball TR. Myofibril degeneration caused by tropomodulin overexpression leads to dilated cardiomyopathy in juvenile mice. J Clin Invest. 1998;101:51–61. doi: 10.1172/JCI1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fransioli J, Bailey B, Gude NA, Cottage CT, Muraski JA, Emmanuel G, Wu W, Alvarez R, Rubio M, Ottolenghi S, Schaefer E, Sussman MA. Evolution of The c-kit Positive Cell Response to Pathological Challenge in the Myocardium. Stem Cells. 2008 doi: 10.1634/stemcells.2007-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammerman PS, Fox CJ, Birnbaum MJ, Thompson CB. Pim and Akt oncogenes are independent regulators of hematopoietic cell growth and survival. Blood. 2005;105:4477–4483. doi: 10.1182/blood-2004-09-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han C, Zhang X, Xu W, Wang W, Qian H, Chen Y. Cloning of the nucleostemin gene and its function in transforming human embryonic bone marrow mesenchymal stem cells into F6 tumor cells. Int J Mol Med. 2005;16:205–213. [PubMed] [Google Scholar]
- 43.Cada Z, Boucek J, Dvorankova B, Chovanec M, Plzak J, Kodets R, Betka J, Pinot GL, Gabius HJ, Smetana K., Jr Nucleostemin expression in squamous cell carcinoma of the head and neck. Anticancer Res. 2007;27:3279–3284. [PubMed] [Google Scholar]
- 44.Lacina L, Smetana K, Jr, Dvorankova B, Stork J, Plzakova Z, Gabius HJ. Immunocyto- and histochemical profiling of nucleostemin expression: Marker of epidermal stem cells? J Dermatol Sci. 2006;44:73–80. doi: 10.1016/j.jdermsci.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz PH, Bryant PJ, Fuja TJ, Su H, O'Dowd DK, Klassen H. Isolation and characterization of neural progenitor cells from post-mortem human cortex. J Neurosci Res. 2003;74:838–851. doi: 10.1002/jnr.10854. [DOI] [PubMed] [Google Scholar]
- 46.Hoshi N, Kusakabe T, Taylor BJ, Kimura S. Side population cells in the mouse thyroid exhibit stem/progenitor cell-like characteristics. Endocrinology. 2007;148:4251–4258. doi: 10.1210/en.2006-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma H, Pederson T. Depletion of the nucleolar protein nucleostemin causes G1 cell cycle arrest via the p53 pathway. Mol Biol Cell. 2007;18:2630–2635. doi: 10.1091/mbc.E07-03-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maki N, Takechi K, Sano S, Tarui H, Sasai Y, Agata K. Rapid accumulation of nucleostemin in nucleolus during newt regeneration. Dev Dyn. 2007;236:941–950. doi: 10.1002/dvdy.21027. [DOI] [PubMed] [Google Scholar]
- 49.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 50.Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, Anversa P. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res. 2003;93:604–613. doi: 10.1161/01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- 51.Sarin KY, Cheung P, Gilison D, Lee E, Tennen RI, Wang E, Artandi MK, Oro AE, Artandi SE. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores I, Benetti R, Blasco MA. Telomerase regulation and stem cell behaviour. Curr Opin Cell Biol. 2006;18:254–260. doi: 10.1016/j.ceb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, de Lange T. Control of human telomere length by TRF1 and TRF2. Mol Cell Biol. 2000;20:1659–1668. doi: 10.1128/mcb.20.5.1659-1668.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seimiya H, Muramatsu Y, Ohishi T, Tsuruo T. Tankyrase 1 as a target for telomere-directed molecular cancer therapeutics. Cancer Cell. 2005;7:25–37. doi: 10.1016/j.ccr.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 55.Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 56.Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 57.Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, Limana F, Nadal-Ginard B, Kajstura J, Anversa P, Blasco MA. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 2003;22:131–139. doi: 10.1093/emboj/cdg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai MS, Sun XX, Lu H. Aberrant expression of nucleostemin activates p53 and induces cell cycle arrest via inhibition of MDM2. Mol Cell Biol. 2008 doi: 10.1128/MCB.01662-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oh H, Taffet GE, Youker KA, Entman ML, Overbeek PA, Michael LH, Schneider MD. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc Natl Acad Sci U S A. 2001;98:10308–10313. doi: 10.1073/pnas.191169098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heist EK, Huq F, Hajjar R. Telomerase and the aging heart. Sci Aging Knowledge Environ. 2003;2003:PE11. doi: 10.1126/sageke.2003.19.pe11. [DOI] [PubMed] [Google Scholar]
- 61.Kardami E, Detillieux K, Ma X, Jiang Z, Santiago JJ, Jimenez SK, Cattini PA. Fibroblast growth factor-2 and cardioprotection. Heart Fail Rev. 2007;12:267–277. doi: 10.1007/s10741-007-9027-0. [DOI] [PubMed] [Google Scholar]
- 62.Rosenblatt-Velin N, Lepore MG, Cartoni C, Beermann F, Pedrazzini T. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Invest. 2005;115:1724–1733. doi: 10.1172/JCI23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson-Farley NN, Patel K, Kim D, Cowen DS. Interaction of FGF-2 with IGF-1 and BDNF in stimulating Akt, ERK, and neuronal survival in hippocampal cultures. Brain Res. 2007;1154:40–49. doi: 10.1016/j.brainres.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rose K, Kriha D, Pallast S, Junker V, Klumpp S, Krieglstein J. Basic fibroblast growth factor: lysine 134 is essential for its neuroprotective activity. Neurochem Int. 2007;51:25–31. doi: 10.1016/j.neuint.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 65.Kalluri HS, Vemuganti R, Dempsey RJ. Mechanism of insulin-like growth factor I-mediated proliferation of adult neural progenitor cells: role of Akt. Eur J Neurosci. 2007;25:1041–1048. doi: 10.1111/j.1460-9568.2007.05336.x. [DOI] [PubMed] [Google Scholar]
- 66.Lacina L, Smetana K, Jr, Dvorankova B, Pytlik R, Kideryova L, Kucerova L, Plzakova Z, Stork J, Gabius HJ, Andre S. Stromal fibroblasts from basal cell carcinoma affect phenotype of normal keratinocytes. Br J Dermatol. 2007;156:819–829. doi: 10.1111/j.1365-2133.2006.07728.x. [DOI] [PubMed] [Google Scholar]