Summary
The innate immune system of both plants and animals uses immune receptors to detect pathogens and trigger defence responses. Despite having distinct evolutionary origin, most plant and animal immune receptors have a leucine-rich repeat (LRR) domain. The LRR domain adopts a slender conformation that maximizes surface area and has been shown to be ideal for mediating protein-protein interactions. Although the LRR domain was expected to be a platform for pathogen recognition, the NB-LRR class of plant innate immune receptors uses its LRR domain to carry out many other roles. This review discusses the domain architecture of plant LRRs and the various roles ascribed to this motif.
Introduction
The innate immune system of both plants and animals employs cell-surface and intracellular receptors to detect pathogens and trigger downstream defences. Although of distinct evolutionary origin, animal and plant immune receptors share remarkable similarities (Ausubel, 2005). Most notable is the leucine-rich repeat (LRR) protein module, which is present in the majority of immune receptors. The LRR is a structural motif characterized by a conserved pattern of hydrophobic leucine residues. LRR domains display broad interaction surfaces that can tolerate high levels of variability. Therefore, LRRs provide diverse classes of immune receptors with a platform for mediating protein-protein interactions needed to perform the dual role of sentry and activator of defence.
The front line of the plant innate immune system is controlled by pattern recognition receptors (PRRs). PRRs detect microbe-associated molecular patterns (MAMPs) like flagellin and induce basal immune responses that impede pathogen ingress by diverse physical, chemical and molecular means (Zipfel, 2008). Plant PRRs identified to date are integral plasma membrane proteins featuring extracellular LRR domains involved in MAMP perception. PRRs with an intracellular kinase domain are classified as receptor-like kinases (RLKs), while those lacking an intracellular domain are known as receptor-like proteins (RLPs) (reviewed by Zipfel, 2008). Plant PRRs resemble mammalian Toll-like receptors (TLRs) (Palsson-McDermott and O’Neill, 2007) because both use extracellular LRRs to perceive MAMPs and activate downstream signalling using intracellular serine threonine kinases (Fig. 1A).
Fig. 1.
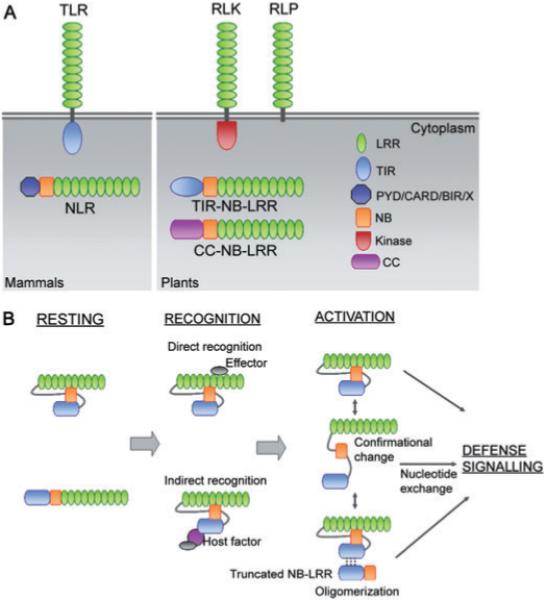
LRR-containing immune receptors, modes of pathogen perception and activation in plants.
A. Plant receptors that recognize microbe-associated molecular patterns (MAMPs) are characterized by an extracellular Leucine-rich repeat (LRR) domain and a variable C-terminus. Receptor-like kinases (RLK) have intracellular kinase domains, otherwise they are receptor-like proteins (RLP). Mammalian Toll-like membrane receptors (TLR) that recognize MAMPs possess similar extracellular LRRs but contain an intracellular Toll/interleukin-1 receptor homology (TIR) domain. Plant intracellular NB-LRR receptors that recognize specific pathogen effectors contain a centrally located nucleotide binding (NB) domain and a C-terminal LRR domain with either a TIR domain or Coiled-coil domain (CC) at the N-terminus. Mammalian intracellular NLRs that recognize MAMPs have structures similar to NB-LRRs; however, the N-terminal end is either a pyrin domain (PYR), caspase recruitment domain (CARD), a baculovirus inhibitor of apoptosis repeat (BIR) domain, or an undefined domain (X).
B. NB-LRRs are kept in a resting mode by intramolecular interactions involving the LRR domain or by absence of the effector. Pathogen recognition occurs either by direct association between the LRR domain and an effector protein or by indirect association mediated by a host protein associated with the N-terminus. Activation of the NB-LRR can occur through multiple processes. Simple binding to the effector could lead to activation. Alternatively, the effector could disrupt the intramolecular associations and/or induce new associations that shift the NB-LRR into a signalling phase. A third process may involve formation of oligomers via N-terminal associations between full-length NB-LRRs or truncated versions (lacking LRR) that may be translated from alternative transcripts. All of these scenarios are accompanied or followed by exchange of ADP for ATP at the NB domain leading to downstream signalling and initiation of defence.
To provide protection against pathogens that overcome or circumvent PRR-mediated basal defence, plants encode intracellular immune receptors called NB-LRRs (Jones and Dangl, 2006). Unlike PRRs, which recognize conserved MAMPs, NB-LRRs recognize specific pathogen-encoded effector proteins. Once activated, NB-LRRs typically induce the hypersensitive response, a form of localized programmed cell death (PCD) that is thought to contribute to resistance by physically isolating the infection (reviewed by Heath, 2000).
NB-LRRs contain a C-terminal LRR domain, a central nucleotide-binding domain (NB), and a variable N-terminal domain. CC-NB-LRRs have a predicted coiled-coil (CC) at their N-terminus, and TIR-NB-LRRs have an N-terminal domain with homology to Drosophila Toll and to the human interleukin-1 receptor (Ausubel, 2005; Jones and Dangl, 2006). Plant NB-LRRs thus appear to have combined structural motifs from mammalian TLRs and intracellular Nod-like receptors (NLRs) (Fig. 1A). Unlike animal innate immune receptors, however, plant NB-LRRs recognize specific pathogen effector proteins. Therefore, plant NB-LRRs perform a function analogous to the mammalian adaptive immune system. This implies that plant genomes must encode a large number NB-LRRs to confer resistance to many different pathogens. Indeed, the Arabidopsis genome encodes 149 predicted NB-LRRs (Meyers et al., 2003). While most NB-LRRs serve as pathogen receptors, a few have been shown to function in the downstream signalling pathways that lead to HR-PCD (Tameling and Joosten, 2007).
Early models predicted that the primary role of the LRR domain in NB-LRRs is pathogen recognition. Current evidence, however, supports a dynamic, versatile role for this supremely adaptable protein domain. This review discusses current understanding of plant NB-LRR recognition and signalling with emphasis placed on the diverse roles ascribed to the LRR domain.
LRR domain structure
Proteins with LRR domains exist in eukaryotes, prokaryotes and viruses; and dozens of these proteins have resolved crystal structures. While differences exist among the LRR structures resolved to date, all share some trademark features (Kobe and Kajava, 2001). LRR domains have a slender, arc-shaped structure with a high surface to volume ratio relative to globular proteins. This makes LRRs ideally suited for engaging in multiple physical interactions. In fact, repeat modules like the LRR are used to engineer proteins with novel interaction specificities (Kajander et al., 2006). Each LRR has a conserved core consensus of L-x-x-L-x-L-x-x-N that forms a β-strand followed by a more variable sequence. Each repeat makes a loop that reiterates to form a stack-like super-helix. The β-strands align on one side creating a continuous β-sheet along the arc’s concave surface. Regularly spaced leucine residues, which may be substituted by other hydrophobic residues, face inward to form a stable, hydrophobic core. The conserved asparagine and the variable domains make each repeat wedge-shaped, imposing the curve seen in all LRR structures resolved to date (Kobe and Kajava, 2001). Genome-wide analysis revealed that Arabidopsis NB-LRRs have a mean of 14 LRRs with a typical repeat length of 24 residues (Meyers et al., 2003).
As of yet, there are no plant NB-LRRs with determined structures. The only plant LRR protein with a resolved structure is the polygalacturonase inhibiting protein (PGIP), which has an extracellular LRR domain (eLRR) (Di Matteo et al., 2003). Plant eLRRs share a well-conserved L-t/s-g-x-I-P motif following the predicted β-strand, which led to their classification as a unique LRR subfamily (Jones and Jones, 1997; Kajava, 1998). The structure of PGIP revealed that the plant eLRR consensus forms a second β-sheet rarely seen in LRR structures (Di Matteo et al., 2003). This arrangement may be typical of plant eLRR domains.
The intracellular LRR (iLRR) domain of plant NB-LRRs is distinct from plant eLRRs, sharing features with the cysteine-containing LRR subfamily (Kajava, 1998). LRRs in this subfamily have a cysteine in the place of the conserved arginine. The cysteine-containing LRR subfamily protein human Skp2 has a resolved crystal structure (Schulman et al., 2000), but limited adherence to the subfamily consensus among NB-LRRs makes Skp2 a poor template for structural modeling. An attempt to model the structure of the LRR domain of the NB-LRR RPS5 based on the structure of bovine decorin revealed that the primary sequence of RPS5 is compatible with the expected LRR architecture (McHale et al., 2006). Further insights are limited, however, because these sequences share only ~14% identity. Furthermore, repeats in NB-LRRs are often imperfect, which likely results in structural irregularities that are difficult to predict (Kobe and Kajava, 2001). Determining the structure of NB-LRRs remains a priority for the field of plant innate immunity.
The variability in the LRR domains of NB-LRRs is evident from MEME analysis conducted with predicted NB-LRRs in Arabidopsis (Meyers et al., 2003). While some MEME motifs in the LRR domain are shared among closely related NB-LRRs, no broad patterns exist, except for the presence of the sequence VLDL in the third LRR. A mutation adjacent to this motif in RPS5 resulted in a loss-of-function, and a partial loss-of-function of other NB-LRRs (Warren et al., 1998). In the potato NB-LRR Rx, a VLDL to VLEL mutation resulted in constitutive activation of defence leading to PCD (Bendahmane et al., 2002). Therefore, the function of this motif in different NB-LRRs may vary, and its conservation is not absolute.
LRR domains are capable of tolerating duplications and deletions of entire repeats, making them well-suited to diversification and evolving new interaction specificities (reviewed by Ellis et al., 2000). Studies with the RGC2 gene cluster in lettuce showed that repeat numbers vary from 40 to 47, likely a result of unequal crossing over events (Kuang et al., 2004). Gene conversion between alleles and closely related genes is another driver of LRR divergence (Chin et al., 2001; Kuang et al., 2004).
It was noticed early on that the LRR domains of NB-LRRs undergo strong positive selection (reviewed by McDowell and Simon, 2008). Positive selection is usually strongest in the putative solvent-exposed residues occupying the concave β-sheet face. This inspired early models implicating the LRR domain in the direct recognition of effectors. Notably, however, positive selection was also reported beyond the putative concave face and on other domains (Luck et al., 2000). This foreshadowed later findings that interactions with LRR domains are not limited to the concave β-sheet (as with TLR3) and that other domains can specify recognition (Burch-Smith et al., 2007; Liu et al., 2008).
LRR domains and pathogen recognition
NB-LRRs recognize pathogen effectors either indirectly or by direct association (Fig. 1B) (Ellis et al., 2007; Caplan et al., 2008). In the case of direct recognition, the LRR domain interacts with the effector, much like TLR recognition of MAMPs. The first case of direct recognition was shown between the LRR domain of the rice NB-LRR Pi-ta and its cognate fungal effector Avr-Pita (Jia et al., 2000). A naturally occurring susceptible Pi-ta variant has a mutation at a predicted solvent-exposed residue in the LRR domain. The resistant Pi-ta variant interacts with Avr-Pita in a yeast two-hybrid assay, while the susceptible variant does not. Sequence analyses of susceptible Pi-ta alleles from wild rice suggest that the susceptible variants are ancestral. The unusually low number of polymorphisms in LRR domain of resistant alleles hints at a recent selective sweep favouring the advantageous mutation (Huang et al., 2008).
The most detailed studies on direct recognition were carried out in the flax-flax rust pathosystem. More than 30 closely linked TIR-NB-LRR genes clustered at five genetic loci (K, L, M, N and P) recognize approximately 30 flax rust effectors. The LRR domains in these proteins are under strong diversifying selection and serve as the major determinants of resistance specificity (Ellis et al., 1999). Comprehensive yeast two-hybrid analysis showed a strong correlation between the association of a flax NB-LRR with its corresponding effector and activation of PCD (Dodds et al., 2006). Evidence that polymorphisms within the LRR determine specificity comes from domain swap experiments, showing that changing just six amino acids in flax P2 can confer P1 specificity (Dodds et al., 2001). Similarly, L6 recognition of AvrL567 and L11 recognition of AvrL11 is exclusively controlled by the LRR domain (Ellis et al., 2007). These polymorphisms, which are predicted to lie primarily within the concave, solvent-exposed LRR surface, are spaced along the length of the LRR domain. It is predicted that specificity is dictated by cumulative effects of multiple amino acid associations between the LRR and effectors (Wang et al., 2007). In the future, showing these interactions in planta and confirming that interactions are direct using in vitro binding assays could strengthen this model. In studies on perception of flagellin by FLS2, an eLRR containing Arabidopsis RLK, Dunning and colleagues have outlined an effective approach towards identifying LRR functional sites. They used a combinatorial strategy that included an alanine scanning mutational survey within the solvent exposed regions and phylogenetic comparisons with orthologous proteins from related species (Dunning et al., 2007). Subsequent structural modeling and functional analysis helped to effectively narrow down the ligand binding region. A similar methodology could be used for NB-LRR structure-function analysis.
Although direct interactions between NB-LRRs and effectors have been shown in a few cases, most NB-LRRs studied to date recognize effectors indirectly by associating with host factors that are modified by a pathogen effector (reviewed in Caplan et al., 2008). Interestingly, in most of these cases, the N-terminal TIR or CC domain mediates indirect recognition.
LRR-mediated intramolecular associations
The LRR domain can regulate immune receptor activity by engaging in intramolecular interactions with other domains. Rx from potato and Bs2 from pepper are able to mediate effector-triggered PCD when the CC domain and NB-LRR domain are co-expressed as two separate peptides (Moffett et al., 2002; Leister et al., 2005). For Rx, the CC-NB domain and the LRR domain also function when co-expressed. Co-immunoprecipitation experiments confirmed that the domains of Rx and Bs2 that complement in trans also associate in vivo. Similarly, the LRR domain of the RPS5 immune receptor from Arabidopsis associates with the NB domain and with itself (Ade et al., 2007). The associations between Rx domains are thought to occur within the same receptor rather than between different molecules because full-length Rx does not self-associate. A potential role for these interactions came with the observation that associations between domains of Rx no longer occur in the presence of its cognate effector. This is consistent with data from the NB-LRR Mi-1, suggesting that intramolecular associations keep defence signalling off (Hwang et al., 2000) (Fig. 1B).
While a ‘closed’ conformation may inhibit defence signalling in Rx and in Mi-1, activation of Bs2 does not disrupt intramolecular associations. Therefore, abrogating intramolecular associations is not always necessary for NB-LRR-mediated defence signalling. Indeed, some constitutively active point mutants of Rx still maintain associations between domains (Rairdan and Moffett, 2006). While intramolecular interactions may be important for some immune receptors, the N immune receptor from tobacco fails to show domain interactions (Mestre and Baulcombe, 2006; S.P.D.-K., unpubl. data). In contrast to Rx, N undergoes oligomerization in the presence of its cognate effector mediated by the TIR domain. Self-association of the RPS5 LRR domains suggests that it too may form oligomers (Ade et al., 2007). Thus, disrupting intramolecular interactions and promoting oligomerization are two possible regulatory functions of the LRR domain in NB-LRRs.
The nucleotide binding domain
Current models for NB-LRR activation mechanistically link intramolecular interactions with the proposed role of the NB domain as a molecular switch (Tameling et al., 2006). NB-LRRs are predicted to be activated when bound to ATP, while intrinsic ATPase activity resets them to a resting state. Effector-induced changes in intramolecular interactions are proposed as a way of activating NB-LRRs by facilitating exchange of ADP for ATP. Consistent with this model, the purified NB domain of I-2, Mi-1 and N bind and hydrolyse ATP in vitro (Tameling et al., 2002; 2006; Ueda et al., 2006). Furthermore, two auto-activating NB domain point mutants in I-2 are impaired in their ability to hydrolyse ATP, suggesting that accumulation of ATP-bound I-2 promotes defence signalling (Tameling et al., 2006). Elaborating findings with I-2 and studying NB domain nucleotide binding and hydrolysis in other NB-LRRs remain important goals for the future. The NB domains of different NB-LRRs may work differently as sequence identity between them is rather low (~20% identity between I-2 and N). The animal apoptotic proteins APAF-1 and CED-4 carry out similar functions and have similar NB domains (14% identity), but only APAF-1hydrolyses ATP (Hu et al., 1999). In CED-4, ATP appears to play only a structural role (Seiffert et al., 2002).
The adenine nucleotide switch model for NB-LRRs proposes a cycle of nucleotide binding and hydrolysis composed of a series of defined steps (Fig. 2) (Tameling et al., 2006). In theory, rate constants for each of these steps are measurable using relatively simple, in vitro biochemical techniques. Given the recalcitrance of many NB-LRRs to overexpression, this certainly poses a challenge. Nevertheless, corroborating quantitative biochemical data with phenotypic observations is a promising means of testing the current model for NB-LRR activation.
Fig. 2.
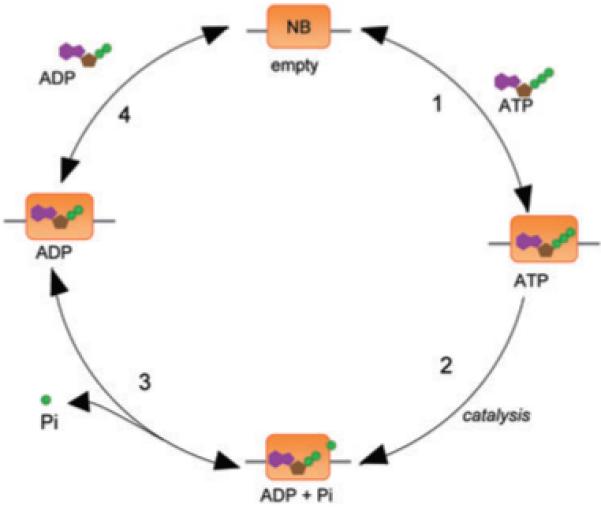
Proposed cycle of nucleotide binding and hydrolysis. Step 1: The empty NB domain binds to ATP. The NB-ATP complex may dissociate or undergo catalysis. 2: The NB domain catalyses the hydrolysis of ATP to ADP. Following hydrolysis the NB domain is bound to ADP and inorganic phosphate (Pi). 3: Pi dissociates from the NB-ADP complex. 4: ADP dissociates from the NB domain. The free domain may also bind to ADP.
Alternative transcripts encoding LRR variants
An interesting feature of some NB-LRR genes is the presence of alternative transcripts predicted to encode receptors with an absent or truncated LRR domain (Jordan et al., 2002; Schornack et al., 2004). Global expression analysis suggests that alternate splicing affects at least 30 Arabidopsis NB-LRRs (Tan et al., 2007). Multiple members of the mammalian TLR family also undergo alternate spicing (Wells et al., 2006), although these truncated TLRs lack their intracellular TIR domain. Most of the studied TLR truncations act in a dominant negative fashion to dampen immune signalling (Burns et al., 2003). The role of putative truncated receptors in plants seems to be less straightforward. For L6, the alternate transcripts appear to be dispensable (Ayliffe et al., 1999), while for N and RPS4, the presence of both full-length and alternative transcripts is essential for complete resistance (Dinesh-Kumar and Baker, 2000; Zhang and Gassmann, 2003). Interestingly, the alternative transcript of N becomes predominant for a brief period after infection. This suggests that a fine balance of full-length and truncated proteins is necessary for function. The two protein variants may interact via their TIR-NB domains to form an active signalling complex (Fig. 1B). This is consistent with the TIR-mediated oligomerization shown for N (Mestre and Baulcombe, 2006). Similarly, TLRs homo- and heterodimerize via their TIR domains during defence signalling (Palsson-McDermott and O’Neill, 2007). Complexes containing NB-LRR alternative products could also resemble TLRs complexed with TIR-containing adapters such as Myd88 and MAL. Finally, alternative transcript products may be relieved of LRR-mediated auto-regulation, which could modulate signalling. It must be noted that the existence and stability of the truncated proteins have not yet been demonstrated. Therefore, we cannot rule out that alternate transcripts serve to regulate mRNA stability and/or protein turnover from the full-length transcript.
Associations with host factors
Many NB-LRRs have been shown to require molecular chaperones like HSP90, SGT1 and RAR1 (reviewed by Shirasu and Schulze-Lefert, 2003; Schulze-Lefert, 2004) to keep them in a signalling-competent state. Impairing the function of these proteins has adverse effects on innate immunity ranging from reduced steady-state levels of certain NB-LRRs to loss of resistance. Coimmunoprecipitation assays reveal complex chaperone-chaperone and chaperone-NB-LRR interactions. In the latter, the LRR domain is often the site of binding. HSP90 binds to the LRR domain of tobacco N, tomato I-2 and barley MLA (Bieri et al., 2004; Liu et al., 2004; de la Fuente van Bentem et al., 2005). Protein phosphatase 5 (PP5), a co-chaperone that associates with HSP90, also binds to the LRR domain of multiple NB-LRRs (Liu et al., 2004; de la Fuente van Bentem et al., 2005). Although a biological function for PP5 in plant innate immunity has not been demonstrated, its role in animal systems suggests that it may assist HSP90 in fine-tuning the maturation of NB-LRRs.
The co-chaperone SGT1 associates with the LRR domain of barley MLA1 and pepper Bs2 (Bieri et al., 2004; Leister et al., 2005). SGT1 is also required for associations between the LRR and CC-NB domains of Bs2 (Leister et al., 2005). Detailed domain swap and mutational analysis of the MLA proteins shows a crucial role for the LRR-domain in determining engagement of the co-chaperone, RAR1 (Shen et al., 2003; Bieri et al., 2004). Remarkably, the RAR1-dependent MLAs (MLA6, MLA10, MLA12 and MLA13) share only two amino acids within the LRR that are different from the RAR1-independent MLA proteins (MLA1 and MLA7) (Halterman and Wise, 2004). Interestingly, substituting one of these amino acids in MLA6 is sufficient to make it RAR1-independent. This substitution is predicted to lie on the LRR domain’s convex surface, where it may impact domain stability. This substitution does not, however, affect effector recognition, which is also predicted to be mediated by the LRR domain (Shen et al., 2003). This uncoupling of recognition and chaperone engagement highlights the ability of the LRR domain to have multiple roles. RAR1-dependent MLA variants have greatly reduced levels in Rar-1 silenced plants, suggesting that they are more unstable than RAR1-independent MLAs. It has been proposed that LRR diversification compromises structural stability and underlies the need for molecular chaperones (Bieri et al., 2004).
Given the structural similarities between NB-LRRs and NLRs, it is not surprising that several mammalian NLRs also require chaperones for efficient signalling. SGT1 and HSP90 associate with the LRR domain of NOD1, NOD2, NALP3, NALP12 and IPAF and play a positive role in NLR signalling (reviewed in Ye and Ting, 2008). These studies confirmed that like plants, mammalian chaperones play roles in regulating immune receptor accumulation, preventing premature degradation and enabling downstream signalling.
In mammals, HSP90 and associated co-chaperones mediate the nucleocytoplasmic shuttling of hormone receptor complexes like the glucocorticoid receptor (reviewed by Pratt et al., 2004). Interestingly, a subset of NB-LRRs (RRS1-R, MLA1, MLA10, N and RPS4) has nuclear or nuclear-cytoplasmic localization (Deslandes et al., 2002; Burch-Smith et al., 2007; Shen et al., 2007; Wirthmueller et al., 2007). In plants, both HSP90 and SGT1 are localized to the nucleus and cytoplasm (Seguí-Simarro et al., 2003; Noel et al., 2007). Considering these findings, it would be interesting to test the role of chaperones in regulating the dynamics of NB-LRR nuclear transport.
Conclusions and perspectives
The LRR module serves as a highly adaptable structural platform onto which diverse binding specificities can be incorporated, and it appears that plant NB-LRRs have put the LRR domain to good use. From using it as a recognition motif, to regulating protein activation, to signal transduction, the LRR domain is an indispensable player in plant defence. However, the mechanistic details that enable these diverse functions are poorly understood. Determining the structure of LRR domains in the presence and absence of their interaction partners would greatly enhance our understanding of NB-LRRs. This would not only provide information on how they recognize pathogen effectors but also provide a clearer picture of the role of individual domains in modulating recognition and activation. While it is widely accepted that NB-LRRs function as multiprotein complexes, there are just a handful of identified partners. The dynamic nature of these interactions makes their identification a formidable task. A combinatorial strategy involving biochemical, genetic and bioinformatic approaches along with live cell imaging techniques will help unearth additional players.
Acknowledgements
We thank Jeffrey Caplan for critical reading of this review. Innate immunity research in S.P.D.-K. lab is supported by grants from NIH and NSF.
References
- Ade J, DeYoung BJ, Golstein C, Innes RW. Indirect activation of a plant nucleotide binding site-leucine-rich repeat protein by a bacterial protease. Proc Natl Acad Sci USA. 2007;104:2531–2536. doi: 10.1073/pnas.0608779104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM. Are innate immune signaling pathways in plants and animals conserved? Nat Immunol. 2005;6:973–979. doi: 10.1038/ni1253. [DOI] [PubMed] [Google Scholar]
- Ayliffe MA, Frost DV, Finnegan EJ, Lawrence GJ, Anderson PA, Ellis JG. Analysis of alternative transcripts of the flax L6 rust resistance gene. Plant J. 1999;17:287–292. doi: 10.1046/j.1365-313x.1999.00377.x. [DOI] [PubMed] [Google Scholar]
- Bendahmane A, Farnham G, Moffett P, Baulcombe DC. Constitutive gain-of-function mutants in a nucleotide binding site-leucine rich repeat protein encoded at the Rx locus of potato. Plant J. 2002;32:195–204. doi: 10.1046/j.1365-313x.2002.01413.x. [DOI] [PubMed] [Google Scholar]
- Bieri S, Mauch S, Shen QH, Peart J, Devoto A, Casais C, et al. RAR1 positively controls steady state levels of barley MLA resistance proteins and enables sufficient MLA6 accumulation for effective resistance. Plant Cell. 2004;16:3480–3495. doi: 10.1105/tpc.104.026682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Schiff M, Caplan JL, Tsao J, Czymmek K, Dinesh-Kumar SP. A novel role for the TIR domain in association with pathogen-derived elicitors. PLoS Biol. 2007;5:e68. doi: 10.1371/journal.pbio.0050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns K, Janssens S, Brissoni B, Olivos N, Beyaert R, Tschopp J. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med. 2003;197:263–268. doi: 10.1084/jem.20021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan J, Padmanabhan M, Dinesh-Kumar SP. Plant NB-LRR immune receptors: from recognition to transcriptional reprogramming. Cell Host Microbe. 2008;3:126–135. doi: 10.1016/j.chom.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Chin DB, Arroyo-Garcia R, Ochoa OE, Kesseli RV, Lavelle DO, Michelmore RW. Recombination and spontaneous mutation at the major cluster of resistance genes in lettuce (Lactuca sativa) Genetics. 2001;157:831–849. doi: 10.1093/genetics/157.2.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes L, Olivier J, Theulieres F, Hirsch J, Feng DX, Bittner-Eddy P, et al. Resistance to Ralstonia solanacearum in Arabidopsis thaliana is conferred by the recessive RRS1-R gene, a member of a novel family of resistance genes. Proc Natl Acad Sci USA. 2002;99:2404–2409. doi: 10.1073/pnas.032485099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Matteo A, Federici L, Mattei B, Salvi G, Johnson KA, Savino C, et al. The crystal structure of polygalacturonase-inhibiting protein (PGIP), a leucine-rich repeat protein involved in plant defense. Proc Natl Acad Sci USA. 2003;100:10124–10128. doi: 10.1073/pnas.1733690100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh-Kumar SP, Baker B. Alternatively spliced N resistance gene transcripts: their possible role in tobacco mosaic virus resistance. Proc Natl Acad Sci. 2000;97:1908–1913. doi: 10.1073/pnas.020367497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds PN, Lawrence GJ, Catanzariti AM, Teh T, Wang CI, Ayliffe MA, et al. Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc Natl Acad Sci USA. 2006;103:8888–8893. doi: 10.1073/pnas.0602577103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds PN, Lawrence GJ, Ellis JG. Six amino acid changes confined to the leucine-rich repeat beta-strand/beta-turn motif determine the difference between the P and P2 rust resistance specificities in flax. Plant Cell. 2001;13:163–178. doi: 10.1105/tpc.13.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning FM, Sun W, Jansen KL, Helft L, Bent AF. Identification and mutational analysis of Arabidopsis FLS2 leucine-rich repeat domain residues that contribute to flagellin perception. Plant Cell. 2007;19:3297–3313. doi: 10.1105/tpc.106.048801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JG, Dodds PN, Lawrence GJ. Flax rust resistance gene specificity is based on direct resistance-avirulence protein interactions. Annu Rev Phytopathol. 2007;45:289–306. doi: 10.1146/annurev.phyto.45.062806.094331. [DOI] [PubMed] [Google Scholar]
- Ellis JG, Lawrence GJ, Luck JE, Dodds PN. Identification of regions in alleles of the flax rust resistance gene L that determine differences in gene-forgene specificity. Plant Cell. 1999;11:495–506. doi: 10.1105/tpc.11.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J, Dodds P, Pryor T. The generation of plant disease resistance gene specificities. Trends Plant Sci. 2000;5:373–379. doi: 10.1016/s1360-1385(00)01694-0. [DOI] [PubMed] [Google Scholar]
- de la Fuente van Bentem S, Vossen JH, de Vries KJ, van Wees S, Tameling WI, Dekker HL, et al. Heat shock protein 90 and its co-chaperone protein phosphatase 5 interact with distinct regions of the tomato I-2 disease resistance protein. Plant J. 2005;43:284–298. doi: 10.1111/j.1365-313X.2005.02450.x. [DOI] [PubMed] [Google Scholar]
- Halterman DA, Wise RP. A single-amino acid substitution in the sixth leucine-rich repeat of barley MLA6 and MLA13 alleviates dependence on RAR1 for disease resistance signaling. Plant J. 2004;38:215–226. doi: 10.1111/j.1365-313X.2004.02032.x. [DOI] [PubMed] [Google Scholar]
- Heath MC. Hypersensitive response-related death. Plant Mol Biol. 2000;44:321–334. doi: 10.1023/a:1026592509060. [DOI] [PubMed] [Google Scholar]
- Hu Y, Benedict MA, Ding L, Nunez G. Role of cytochrome c and dATP/ATP hydrolysis in Apaf-1-mediated caspase-9 activation and apoptosis. EMBO J. 1999;18:3586–3595. doi: 10.1093/emboj/18.13.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Hwang SY, Chiang YC, Lin TP. Molecular evolution of the Pi-ta gene resistant to rice blast in wild rice (Oryza rufipogon) Genetics. 2008;179:1527–1538. doi: 10.1534/genetics.108.089805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CF, Bhakta AV, Truesdell GM, Pudlo WM, Williamson VM. Evidence for a role of the N terminus and leucine-rich repeat region of the Mi gene product in regulation of localized cell death. Plant Cell. 2000;12:1319–1329. doi: 10.1105/tpc.12.8.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, McAdams SA, Bryan GT, Hershey HP, Valent B. Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 2000;19:4004–4014. doi: 10.1093/emboj/19.15.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DA, Jones JDG. The role of leucinerich repeat proteins in plant defenses. Adv Bot Res. 1997;24:89–167. [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Jordan T, Schornack S, Lahaye T. Alternative splicing of transcripts encoding Toll-like plant resistance proteins - what’s the functional relevance to innate immunity? Trends Plant Sci. 2002;7:392–398. doi: 10.1016/s1360-1385(02)02311-7. [DOI] [PubMed] [Google Scholar]
- Kajander T, Cortajarena AL, Regan L. Consensus design as a tool for engineering repeat proteins. Methods Mol Biol. 2006;340:151–170. doi: 10.1385/1-59745-116-9:151. [DOI] [PubMed] [Google Scholar]
- Kajava AV. Structural diversity of leucine-rich repeat proteins. J Mol Biol. 1998;277:519–527. doi: 10.1006/jmbi.1998.1643. [DOI] [PubMed] [Google Scholar]
- Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol. 2001;11:725–732. doi: 10.1016/s0959-440x(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Kuang H, Woo SS, Meyers BC, Nevo E, Michelmore RW. Multiple genetic processes result in heterogeneous rates of evolution within the major cluster disease resistance genes in lettuce. Plant Cell. 2004;16:2870–2894. doi: 10.1105/tpc.104.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leister RT, Dahlbeck D, Day B, Li Y, Chesnokova O, Staskawicz BJ. Molecular genetic evidence for the role of SGT1 in the intramolecular complementation of Bs2 protein activity in Nicotiana benthamiana. Plant Cell. 2005;17:1268–1278. doi: 10.1105/tpc.104.029637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Botos I, Wang Y, Leonard JN, Shiloach J, Segal DM, Davies DR. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science. 2008;320:379–381. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Burch-Smith T, Schiff M, Feng S, Dinesh-Kumar SP. Molecular chaperone Hsp90 associates with resistance protein N and its signaling proteins SGT1 and Rar1 to modulate an innate immune response in plants. J Biol Chem. 2004;279:2101–2108. doi: 10.1074/jbc.M310029200. [DOI] [PubMed] [Google Scholar]
- Luck JE, Lawrence GJ, Dodds PN, Shepherd KW, Ellis JG. Regions outside of the leucine-rich repeats of flax rust resistance proteins play a role in specificity determination. Plant Cell. 2000;12:1367–1377. doi: 10.1105/tpc.12.8.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JM, Simon SA. Molecular diversity at the plant-pathogen interface. Dev Comp Immunol. 2008;32:736–744. doi: 10.1016/j.dci.2007.11.005. [DOI] [PubMed] [Google Scholar]
- McHale L, Tan X, Koehl P, Michelmore RW. Plant NBS-LRR proteins: adaptable guards. Genome Biol. 2006;7:212. doi: 10.1186/gb-2006-7-4-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre P, Baulcombe DC. Elicitor-mediated oligomerization of the tobacco N disease resistance protein. Plant Cell. 2006;18:491–501. doi: 10.1105/tpc.105.037234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell. 2003;15:809–834. doi: 10.1105/tpc.009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett P, Farnham G, Peart J, Baulcombe DC. Interaction between domains of a plant NBS-LRR protein in disease resistance-related cell death. EMBO J. 2002;21:4511–4519. doi: 10.1093/emboj/cdf453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel LD, Cagna G, Stuttmann J, Wirthmuller L, Betsuyaku S, Witte CP, et al. Interaction between SGT1 and cytosolic/nuclear HSC70 chaperones regulates Arabidopsis immune responses. Plant Cell. 2007;19:4061–4076. doi: 10.1105/tpc.107.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsson-McDermott EM, O’Neill LA. Building an immune system from nine domains. Biochem Soc Trans. 2007;35:1437–1444. doi: 10.1042/BST0351437. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Galigniana MD, Harrell JM, DeFranco DB. Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal. 2004;16:857–872. doi: 10.1016/j.cellsig.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Rairdan GJ, Moffett P. Distinct domains in the ARC region of the potato resistance protein Rx mediate LRR binding and inhibition of activation. Plant Cell. 2006;18:2082–2093. doi: 10.1105/tpc.106.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schornack S, Ballvora A, Gurlebeck D, Peart J, Baulcombe D, Ganal M, et al. The tomato resistance protein Bs4 is a predicted non-nuclear TIR-NB-LRR protein that mediates defense responses to severely truncated derivatives of AvrBs4 and overexpressed AvrBs3. Plant J. 2004;37:46–60. doi: 10.1046/j.1365-313x.2003.01937.x. [DOI] [PubMed] [Google Scholar]
- Schulman BA, Carrano AC, Jeffrey PD, Bowen Z, Kinnucan ER, Finnin MS, et al. Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature. 2000;408:381–386. doi: 10.1038/35042620. [DOI] [PubMed] [Google Scholar]
- Schulze-Lefert P. Plant immunity: the origami of receptor activation. Curr Biol. 2004;14:R22–R24. [PubMed] [Google Scholar]
- Seguí-Simarro JM, Testillano PS, Risueño MC. Hsp70 and Hsp90 change their expression and subcellular localization after microspore embryogenesis induction in Brassica napus L. J Struct Biol. 2003;142:379. doi: 10.1016/s1047-8477(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Seiffert BM, Vier J, Hacker G. Subcellular localization, oligomerization, and ATP-binding of Caenorhabditis elegans CED-4. Biochem Biophys Res Commun. 2002;290:359–365. doi: 10.1006/bbrc.2001.6211. [DOI] [PubMed] [Google Scholar]
- Shen QH, Zhou F, Bieri S, Haizel T, Shirasu K, Schulze-Lefert P. Recognition specificity and RAR1/SGT1 dependence in barley Mla disease resistance genes to the powdery mildew fungus. Plant Cell. 2003;15:732–744. doi: 10.1105/tpc.009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen QH, Saijo Y, Mauch S, Biskup C, Bieri S, Keller B, et al. Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science. 2007;315:1098–1103. doi: 10.1126/science.1136372. [DOI] [PubMed] [Google Scholar]
- Shirasu K, Schulze-Lefert P. Complex formation, promiscuity and multi-functionality: protein interactions in disease-resistance pathways. Trends Plant Sci. 2003;8:252–258. doi: 10.1016/S1360-1385(03)00104-3. [DOI] [PubMed] [Google Scholar]
- Tameling WI, Joosten MH. The diverse roles of NB-LRR proteins in plants. Physiol Mol Plant Pathol. 2007;71:126–134. [Google Scholar]
- Tameling WI, Elzinga SD, Darmin PS, Vossen JH, Takken FL, Haring MA, Cornelissen BJ. The tomato R gene products I-2 and MI-1 are functional ATP binding proteins with ATPase activity. Plant Cell. 2002;14:2929–2939. doi: 10.1105/tpc.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tameling WI, Vossen JH, Albrecht M, Lengauer T, Berden JA, Haring MA, et al. Mutations in the NB-ARC domain of I-2 that impair ATP hydrolysis cause autoactivation. Plant Physiol. 2006;140:1233–1245. doi: 10.1104/pp.105.073510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Meyers BC, Kozik A, West MA, Morgante M, St Clair DA, et al. Global expression analysis of nucleotide binding site-leucine rich repeat-encoding and related genes in Arabidopsis. BMC Plant Biol. 2007;7:56. doi: 10.1186/1471-2229-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Yamaguchi Y, Sano H. Direct interaction between the tobacco mosaic virus helicase domain and the ATP-bound resistance protein, N factor during the hypersensitive response in tobacco plants. Plant Mol Biol. 2006;61:31–45. doi: 10.1007/s11103-005-5817-8. [DOI] [PubMed] [Google Scholar]
- Wang CI, Guncar G, Forwood JK, Teh T, Catanzariti AM, Lawrence GJ, et al. Crystal structures of flax rust avirulence proteins AvrL567-A and -D reveal details of the structural basis for flax disease resistance specificity. Plant Cell. 2007;19:2898–2912. doi: 10.1105/tpc.107.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RF, Henk A, Mowery P, Holub E, Innes RW. A mutation within the leucine-rich repeat domain of the Arabidopsis disease resistance gene RPS5 partially suppresses multiple bacterial and downy mildew resistance genes. Plant Cell. 1998;10:1439–1452. doi: 10.1105/tpc.10.9.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CA, Chalk AM, Forrest A, Taylor D, Waddell N, Schroder K, et al. Alternate transcription of the Toll-like receptor signaling cascade. Genome Biol. 2006;7:R10. doi: 10.1186/gb-2006-7-2-r10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirthmueller L, Zhang Y, Jones JD, Parker JE. Nuclear accumulation of the Arabidopsis immune receptor RPS4 is necessary for triggering EDS1-dependent defense. Curr Biol. 2007;17:2023–2029. doi: 10.1016/j.cub.2007.10.042. [DOI] [PubMed] [Google Scholar]
- Ye Z, Ting JP. NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr Opin Immunol. 2008;20:3–9. doi: 10.1016/j.coi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zhang XC, Gassmann W. RPS4-mediated disease resistance requires the combined presence of RPS4 transcripts with full-length and truncated open reading frames. Plant Cell. 2003;15:2333–2342. doi: 10.1105/tpc.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C. Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol. 2008;20:10–16. doi: 10.1016/j.coi.2007.11.003. [DOI] [PubMed] [Google Scholar]


