Abstract
Objective
To describe patterns and density of early tracheal colonization among intubated patients and correlate colonization status with levels of antimicrobial peptides and inflammatory cytokines.
Design
Prospective cohort study.
Setting
Medical and cardiovascular intensive care units (ICU) of a tertiary referral hospital.
Patients
74 adult patients admitted between March 2003 and May 2006.
Interventions
Tracheal aspirates were collected daily for the first four days of intubation using standardized, sterile technique and sent for quantitative culture and cytokines, lactoferrin and lysozyme measurements.
Measurements and main results
The mean APACHE II score in this cohort was 24 ± 7. Proportion of subjects colonized by any microorganism increased over the first four days of intubation (47%, 60%, 70%, 70%, p=0.08) but density of colonization for bacteria or yeast did not change significantly. No known risk factors predicted tracheal colonization on day 1 of intubation. Several patterns of colonization were observed (persistent, transient, new colonization and clearance of initial colonization).The most common organisms cultured were Candida albicans and coagulase negative staphylococcus. Levels of cytokines, lactoferrin or lysozyme did not change over time and were not correlated with tracheal colonization status. Four subjects (6%) had ventilator-associated pneumonia.
Conclusions
The density of tracheal colonization did not change significantly over the first four days of intubation in medical ICU patients. There was no correlation between tracheal colonization and the levels of antimicrobial peptides or cytokines. Several different patterns of colonization may have to be considered while planning interventions to reduce airway colonization.
Keywords: Tracheal aspirate, Quantitative culture, Cytokine, Intensive care unit, Lactoferrin, Lysozyme
Introduction
Tracheal colonization with pathogenic microorganisms has been shown to occur in a significant proportion of patients who are intubated and mechanically ventilated, often within the first 24 hours of intubation (1, 2). Tracheal colonization can be more prevalent than oropharyngeal colonization (3) and can occur in the absence of oropharyngeal or gastric colonization in ventilated patients (4). Literature also suggests an association between the occurrence of tracheal colonization and subsequent development of ventilator-associated pneumonia (VAP) (5–7).
In health, the airway protects itself from constant exposure to microorganisms without relying on inflammatory defenses by several mechanisms such as mucociliary clearance, cough reflex, phagocytosis by resident macrophages and the airway surface liquid (ASL). ASL is a thin layer of liquid covering the luminal surface of the airway and contains multiple antimicrobial factors including lysozyme, lactoferrin, and secretory leukoproteinase inhibitor (8–13). In a study of tracheal aspirates in newborn infants, pulmonary and systemic infections were associated with high concentrations of antimicrobial peptides (14). The concentrations of these peptides correlated positively with levels of inflammatory markers, interleukin-8 (IL-8) and tumor necrosis factor-α. Levels of lactoferrin and lysozyme have been found to be low in newborn infants who developed bronchopulmonary dysplasia (8). The concentrations of lactoferrin and lysozyme have been shown to be higher in bronchial lavage fluid in subjects with chronic bronchitis compared to normal subjects (15) suggesting upregulation with inflammation. However, in a study of ventilated newborns, lactoferrin levels in the tracheo-bronchial aspirates showed no correlation with culture results (16). Several studies have also shown increased levels of inflammatory markers in the airway secretions in conditions such as chronic bronchitis, asthma, ARDS and exacerbations of COPD due to bacterial infections (17, 18). There is little data on correlation between levels of inflammatory markers or antimicrobial peptides and tracheal colonization in adults.
In this prospective cohort study, we sought to describe the patterns and density of early tracheal colonization among patients intubated in the intensive care unit, examine the differences between those who are colonized on day 1 of intubation and those who are sterile, and correlate airway colonization with markers of inflammation. We hypothesized that colonization would increase with duration of intubation, and the density of tracheal colonization would be negatively correlated with the levels of antimicrobial peptides and positively correlated with the levels of inflammatory markers in the tracheal aspirates. To control for inflammation caused by the mere presence of endotracheal tube, we collected tracheal aspirates from 8 healthy subjects who were intubated in the operating room for elective surgery.
Materials and Methods
This was a prospective cohort study conducted from March of 2003 through May of 2006 in a university teaching hospital. The study was approved by the Institutional Review Board. Subjects from the medical (n=67) and cardiovascular (n=7) intensive care units who were intubated for less than 24 hours and expected to be intubated for at least 4 days were recruited after informed consent. Subjects were excluded if there was absence of a legally authorized representative for consent, if they had significant hemoptysis (to limit triggering while sample collection) or cystic fibrosis (to avoid outliers in the density data). Eight control subjects without major medical problems who were scheduled to undergo elective surgery were enrolled from the preanesthesia evaluation clinic.
Study Protocol
Once eligible for the study, written informed consent was obtained from a legally authorized representative or the subject in the case of controls. Tracheal aspirates were collected daily in a standardized fashion using a new in-line suction catheter through the existing ventilator circuit. Briefly, the suction catheter was advanced to its maximum distance quickly and 1 ml of normal saline flushed into the airways. Suction was then applied for 10 seconds as the catheter was withdrawn. The catheter was then rinsed with another 1ml of saline. Specimens was collected onto a sterile sputum trap and an aliquot transported to the microbiology lab within 15–30 minutes of collection. The remaining aspirate was stored at −70° C for batch analysis of cytokine and antimicrobial peptide levels. The procedure was repeated daily for the next 3 days of intubation for a maximum of 4 samples per subject. In the control group, tracheal aspirate was collected 30 minutes after intubation on the day of the elective surgery.
Data collected include demographics, APACHE II score upon study entry(19), admitting diagnosis, comorbid conditions, duration of ICU and hospital length of stay, duration of mechanical ventilation and presence of line sepsis and VAP, hospital mortality and disposition. Lines sepsis was diagnosed if a recognized pathogen was isolated from blood culture and the pathogen was not associated with infection at another site or common skin contaminant isolated from blood culture from patient with an intravascular access device and physician initiated appropriate therapy in the presence of fever, chills or hypotension (20). VAP was defined as the occurence of a new and persitent infiltrate 48 hours after endotracheal intubation associated with purulent tracheal secretions and any one of the following; 1. fever, leukocytosis and worsening oxygenation, 2. positive cultures from bronchoalveolar lavage or protected specimen brushing, 3. same organism isolated from tracheal aspirate and blood or pleural fluid, 4. histological diagnosis at post mortem or from an open lung biopsy or 5. rapidly cavitating infiltrate with positive fine needle aspiration (21).
Microbiology
Colonization was defined as presence of at least 103 CFU of organisms in the tracheal aspirate. The specimen was processed as follows: An equal amount of 2% N-acetyl cysteine was added to the specimen for a 1:2 dilution. It was vortexed lightly and 0.4 ml was added to 1.6 ml saline for 1:10 dilution. Using a 10−2 loop, blood agar, eosin methylene blue agar, chocolate agar and BCSA (Burkholderia cepacia agar) plates were inoculated with a sterile hockey stick which resulted in a 10−3 dilution. In a subset of aspirates, bacterial DNA was isolated using the BUGS’n BEADS bacterial DNA Isolation Kit (Genpoint, Oslo, Norway) and quantitated using PicoGreen Kit (Molecular Probes, Eugene, OR) as previously described (22). Quantitation of 16s rRNA gene copy number in experimental samples was determined by comparing Ct values to a standard curve generated by spiking mouse genomic DNA with serially diluted E. coli genomic DNA. Sensitivity of the assay was determined to be 100 copies of E. coli DNA per 2 ng sample.
Cytokines and antimicrobial peptides
The aspirate was homogenized using glassbeads, 100 µL aliquot removed and diluted with of 700 µL normal saline for a 1: 8 dilution, centrifuged at 40,000 rpm and the supernatant used for cytokine assays. Concentrations of Interleukin (IL)-6 (sensitivity of detection 0.70 pg/mL) and IL-8 (sensitivity: 3.5 pg/ mL) were measured using commercially available ELISA kits (R&D systems, MN). Lactoferrin level was measured using the ELISA kit by Calbiochem(sensitivity of detection 1 ng/mL) and lysozyme using Human Lysozyme EIA kit (Biomedical Technologies Inc. sensitivity: 0.78 ng/mL).
Statistical analysis
Mean and standard deviation was obtained using excel and SAS (version 9.1). Bivariate analyses was done using t-test, Wilcoxon rank-sum test and and Fisher’s exact test as indicated. Correlation analyses were done using Spearman nonparametric rank coefficient. Concentrations of inflammatory markers, antimicrobial peptides and tracheal colonization density over time were tested using nonparametric Friedman test. Log transformation was used to normalize data as needed. Logistic regression analysis was used with model fitted by generalized estimating equations to account for correlation of outcomes over time.
Results
Demographics and Descriptive Statistics
Of the 74 subjects recruited to the study, 66 subjects were intubated for at least 2 days and were included in the analyses. The mean age of the cohort was 58 (SD, 14) years and 55% were men. Admitting source was emergency room in 35% of the subjects, ward in 28% and another acute care hospital in 33%. Mean APACHE II score was 24 (SD, 7). Common admitting diagnoses were respiratory failure (12%), sepsis (12%), pneumonia (12%), liver failure (10%), pancreatitis (9%), gastrointestinal bleed (7%) and neuromuscular disease (6%). There was no difference in demographics between patients admitted to the medical and cardiovascular intensive care units.
Majority of subjects had received antibiotics prior to intubation (52%) and a higher proportion (93%), were started and maintained on antibiotics while intubated. The most common antibiotics used during the first 4 days of intubation were piperacillin/tazobactam (33%), vancomycin (29%), levofloxacin (24%), ceftriaxone (18%) and meropenem (8%). Antifungal agents were used in 18% of the subjects during the first 4 days of intubation. Almost all of the subjects (65/66) received stress ulcer prophylaxis. Digestive decontamination was not used throughout the study period.
There were no significant differences between the baseline characteristics of subjects who were colonized on day 1 of intubation compared to those who were sterile (Table 1) with the exception of the severity of illness score which was higher in the colonized cohort (p=0.046). There was notably, no difference in antibiotic use prior to intubation which can be expected to affect colonization status. There were no differences in outcomes including the overall mortality and length of stay between the two groups (Table 2). The incidence of VAP in this cohort was 6% (4/66) and all of which were late-onset (occurred after 5 days of intubation). One of the subjects with VAP had no growth in all four tracheal aspirates and grew Stenotrophomonas maltophilia in the BAL. The other three subjects did not undergo invasive microbiological diagnosis for VAP and had grown Candida albicans, Pseudomonas aeruginosa and coagulase negative Staphylococcus in their aspirates.
Table 1.
Baseline characteristics by colonization status on day 1 of intubation*
| Characteristics | Sterile (N=35) |
Colonized (N=31) |
|---|---|---|
|
Age, Mean (SD) Median (IQR) |
56 (15) 57 (45–72) |
58 (14) 57 (50–68) |
| Gender, Male (%) | 50 | 60 |
|
APACHE II, Mean (SD) Median (IQR) |
23 (8) 22 (18–27) |
26 (7) 25 (21–30) |
|
Hospital LOS Before Intubation, Mean (SD) Median (IQR) |
4.7 (8) 1.5 (0–4.5) |
4.4 (8) 1.5 (0–5) |
|
ICU LOS before Intubation, Mean (SD) Median (IQR) |
1 (1.5) 0 (0–1) |
1 (1.7) 0 (0–1.8) |
| Antibiotics Before Intubation (%) | 47 | 57 |
| COPD (%) | 3 | 4 |
| Diabetes (%) | 19 | 26 |
| Malignancy (%) | 17 | 17 |
| Levels in tracheal aspirates on Day 1 (Mean ± SD) | ||
| Lactoferrin, µg/ml | 154±69 | 124±57 |
| Lysozyme, ng/ml | 5.3±1.0 | 5.9±1.4 |
| Interleukin-6, ng/ml | 2.8±3.1 | 2.1±1.4 |
| Interleukin-8, ng/ml | 27±10 | 32±25 |
All P-values > 0.05 except for APACHE II (p=0.046)
Table 2.
Outcomes by colonization status on Day 1 of intubation
| Outcome | Sterile (N=35) | Colonized (N=31) |
|---|---|---|
| Hospital LOS, days Mean ± SD |
22±15 | 21±17 |
| ICU LOS, days Mean ± SD |
12±9 | 12±8 |
| Mortality, % | 33 | 52 |
| Tracheostomy, % | 14 | 10 |
| Line sepsis, % | 14 | 16 |
| VAP, % | 3 | 7 |
LOS: Length of Stay, SD: Standard Deviation. ICU: Intensive Care Unit. All p values >0.05.
Tracheal Colonization over time
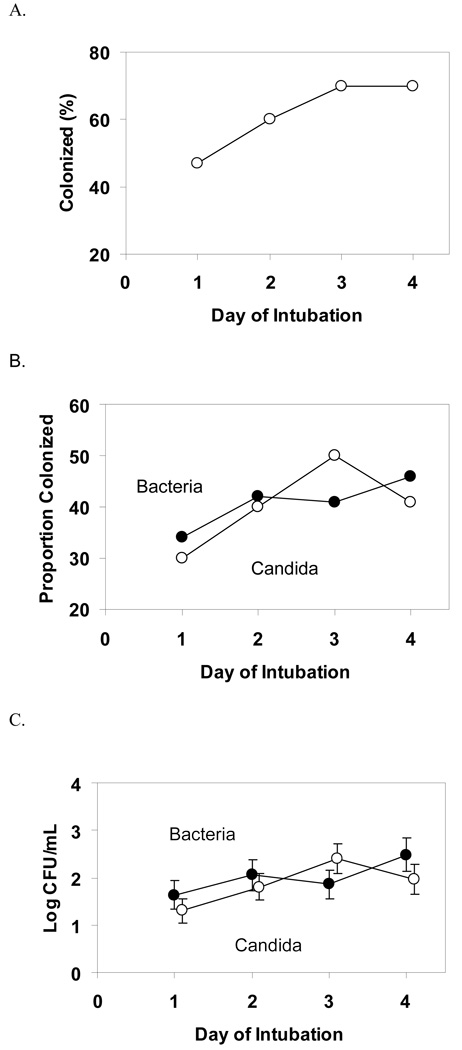
The increase in proportion of subjects colonized by any microorganism was not statistically significant over the 4 days of intubation (Day 1, 47%, Day 2, 60%, Day 3, 70%, and Day 4, 70%, P=0.08) Figure 1A. When broken down into colonization by bacteria or yeast, the proportion colonized did not increase significantly over time as seen in Figure 1B. Interestingly, the mean density of colonization of bacteria and yeast also did not change over time (Figure 1C). The most common organisms grown in the tracheal aspirates during the first 4 days of intubation were Candida albicans and coagulase negative Staphylococcus (Table E1).
Figure 1.
Tracheal colonization over time.
A. Proportion colonized with any organism over the first 4 days of intubation. P=0.08
B. Proportion colonized with bacteria and candida over the first 4 days of intubation. P-value for bacteria = 0.43, yeast = 0.24. Clear circles represent proportion colonized by yeast and solid circles represent bacteria.
C. Density of colonization of bacteria and candida over the first 4 days of intubation. CFU, colony forming units. P-value for bacteria = 0.52, yeast = 0.12. Clear circles represent mean density for yeast and solid circles for bacterial colonization.
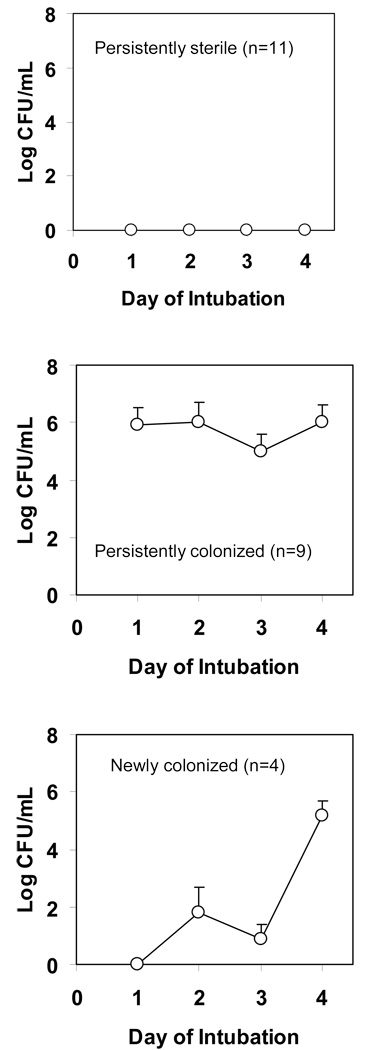
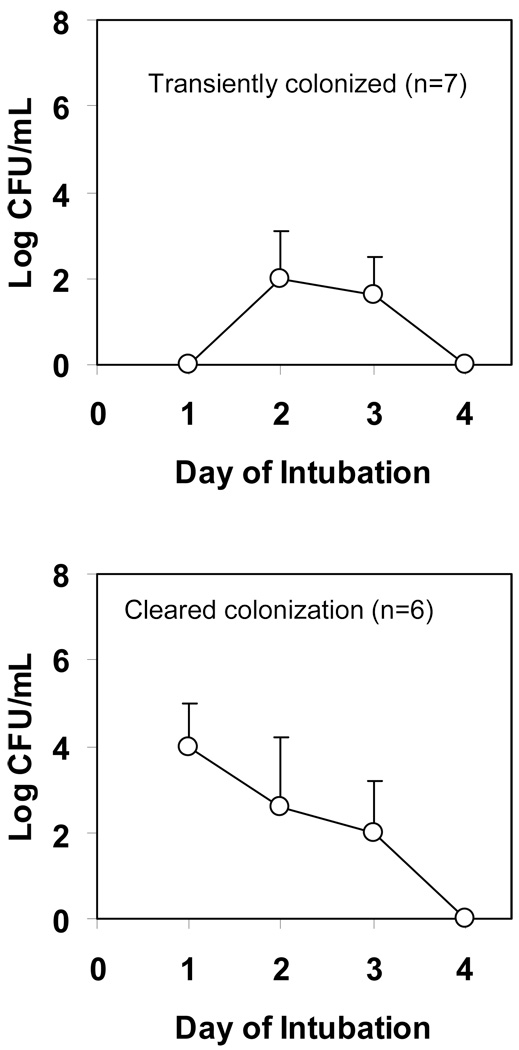
Patterns of colonization
Among the 37 subjects who were intubated for 4 days, we observed several patterns of colonization (Figure 2). Almost a third of the subjects had no bacterial growth in the tracheal aspirates during the 4 days. A significant proportion of subjects had bacterial colonization on all the 4 days. Some subjects were transiently colonized and some cleared their initial colonization. Four subjects who had sterile aspirates on day 1, developed persistent colonization. There were no significant differences among these 5 groups in baseline characteristics or outcomes examined (data not shown). Similar patterns were observed when colonization by yeast was examined. These data suggest a complex and dynamic process of early tracheal colonization that might explain the lack of significant increase in overall density when examined over time.
Figure 2.
Patterns of bacterial colonization among those intubated for 4 days (N=37)
Airway Inflammation
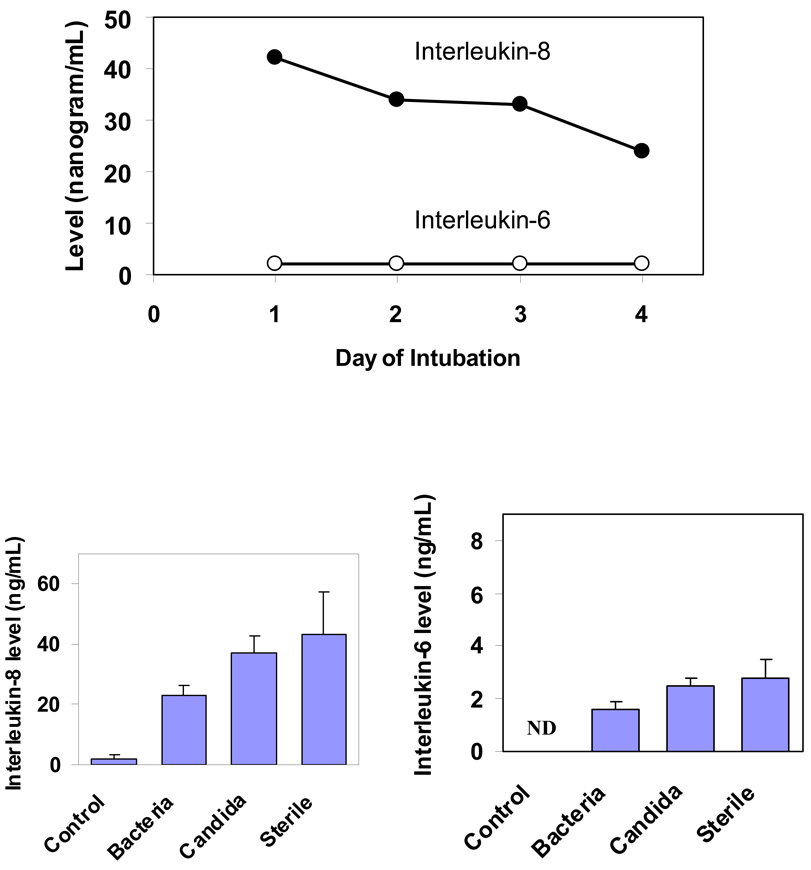
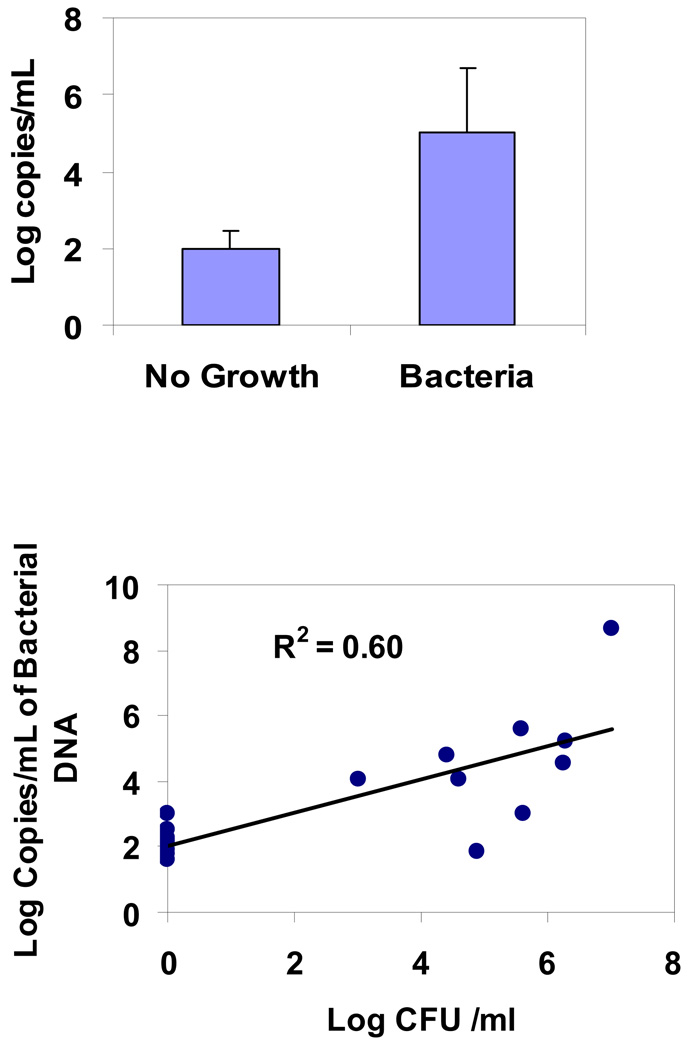
The levels of inflammatory cytokines did not change over the 4 days in the ICU subjects (Figure 3A). Interestingly, IL-8 levels were significantly higher in the sterile aspirates compared to aspirates with bacterial growth (p=0.005) and control aspirates from OR subjects (p=0.009) (Figure 3B). There was no significant difference in IL-6 levels between sterile and colonized aspirates. We were unable to adjust for confounders such as COPD due to very low prevalence in this cohort. To explore an alternative hypothesis of the presence of non-culturable bacteria in the sterile aspirates that may explain the high level of inflammatory markers, we did quantitative PCR which detects ribosomal RNA from 99.9% of known bacteria. As seen in Figure 4A, the copies of bacterial DNA were below detection limits in the sterile aspirates and significantly high in the colonized aspirates. There was also good correlation between bacterial density using PCR and that of the microbiological colony counts (Figure 4B). Tracheal aspirates from healthy controls had no microbial growth and no detectable IL-8 levels.
Figure 3.
3A. Cytokine levels in tracheal aspirates during intubation, p-value for change in IL-8 levels over time = 0.50, p-value for IL-6 = 0.44
3B. Comparison of cytokine levels by tracheal aspirate colonization status.
IL-8: p-value for sterile versus control aspirates = 0.009 and p-value for sterile versus aspirates colonized with bacteria = 0.005.
IL-6: ND: Levels were not done in the control aspirates. All p-values of comparsion among ICU aspirates were >0.05.
Figure 4.
4A. Bacterial density by quantitative PCR on sterile and colonized tracheal aspirates. P=0.005
4B. Correlation of bacterial density by PCR versus colony counts in quantitative cultures. P=0.005
Antimicrobial peptides
The levels of antimicrobial peptides did not change over time in the ICU subjects (Figure E1A). Levels of lactoferrin and lysozyme did not differ between sterile and colonized aspirates in the ICU (Figure E1B). Interestingly, lactoferrin levels were significantly higher in the aspirates from critically ill subjects compared to healthy controls (p-values of all ICU groups versus control <0.005). The pattern was reverse with lysozyme, where the levels were much higher in the healthy controls (p-values of comparison with all ICU groups <0.0001) but not different between sterile and colonized groups in the ICU.
Discussion
There are several interesting findings in this study of early patterns of tracheal colonization. Contrary to our initial hypothesis, microbial colonization did not progress significantly over the first four days of intubation. The proportion of subjects colonized by bacteria or yeast increased over the first 4 days of intubation but the increase was not statistically significant. The mean density of colonization remained stable over the 4 days. This was similar to the observation by Ewig et al in a group of patients with head injury (7). We observed several distinct patterns of colonization including those where the aspirates were persistently sterile or colonized for all 4 days, transiently colonized or cleared initial colonization. This might partially explain the overall stable density as seen in Figure 1C and as reported previously (7). When examined as a whole group, tracheal colonization has had mixed effects on outcomes. For instance, in a study by Ferrer et al, airway colonization by potentially pathogenic microorganisms on admission was associated with failure of non-invasive ventilation for exacerbation of COPD (23). In contrast, in a study of patients admitted to a respiratory intensive care unit, initial tracheal colonization was not associated with mortality or length of stay (24). In a prospective study of patients with community acquired pneumonia, Ortqvist et al found that respiratory tract colonization was associated with a significantly increased mortality and length of stay but was not a risk factor for nosocomial pneumonia (25). We did not find any baseline characteristics that predict these patterns of colonization, nor did we find association with outcomes. However, our study was not adequately powered. Future larger studies examining these patterns of colonization may identify subgroups that are at risk for poor outcomes and target those groups for interventions.
There was a high incidence of Candida colonization unlike reported in previous studies (1, 2, 7). Over 50% of the subjects were colonized with Candida species on any one of the four days. This may be a reflection of the high prevalence of antibiotic use in this cohort both prior to and during intubation as well as the case mix. Surprisingly, quite a few potentially resistant bacteria such as Pseudomonas aeruginosa and Acinetobacter baumannii were observed in this cohort of early colonization study. These organisms are typically identified as late colonizers in previous studies (6). With the exception of severity of illness, we were unable to identify independent risk factors for tracheal colonization on day 1 of intubation in this cohort. However, we did not examine certain risk factors such as upper airway colonization and presence of biofilms. Colonization on day 1 was particularly unrelated to hospital length of stay prior to intubation or prior antibiotic use. Tracheal colonization within 24 hours of intubation was shown to be an independent risk factor in a study of patients with head trauma (26). In our study, day 1 tracheal colonization was not an independent risk factor for VAP or other outcomes such as ICU LOS or mortality. This observation should be an important consideration when planning intervention studies to reduce the incidence of VAP through reduction of tracheal colonization.
There was no correlation between the levels of antimicrobial peptides or inflammatory cytokines and colonization status. In fact, aspirates with no growth had high levels of cytokines which raises several possibilities. There may be no association between colonization status and airway inflammation. Sterility of the aspirates may be the end result of successful bacterial killing. Finally, sterile aspirates may have non-culturable bacteria, which is the least likely of the 3 explanations since these aspirates also had undetectable bacterial DNA by PCR which is thought to detect 99% of bacteria known so far. Levels of inflammatory markers were high in the critically ill but were not associated with colonization status. It is possible therefore, that the ‘sterile aspirates’ were sterile because of successful killing resulting in inflammation. Levels of IL-8 were undetectable in the healthy controls as expected. We observed higher levels of lactoferrin in the ICU subjects compared to controls and an opposite trend with lysozyme levels which needs further investigation. Thompson et al found higher levels of lactoferrin and lysozyme in bronchoalveolar lavage samples of patients with chronic bronchitis compared to controls, suggesting a role for these peptides in airway inflammation (15).
Not surprisingly, the duration of ICU and hospital lengths of stay and mechanical ventilation were long in this cohort given the inclusion criteria of at least 3 days of intubation in this study as well as the higher severity of illness. However, incidence of VAP was surprisingly low compared to existing literature (27). This was unexpected given the inclusion of sensitive clinical criteria used for VAP diagnosis in this study. One of the possible explanations could be because of high antibiotic use in this cohort of medical ICU patients. We did not collect variables such as head of bed elevation and frequency of hand washing among healthcare providers which are known to reduce the incidence of this complication.
Several limitations must be acknowledged. First, we did not control for dilution of the tracheal aspirate. In spite of standardized collection method and minimal amount of saline used, it is possible that the quantitative data might not be generalizable given the nature of collection. Unfortunately, unlike with cystic fibrosis subjects who produce large amounts of sputum, ICU patients have variable amount of sputum production and saline irrigation is often necessary to retrieve a minimum amount. Furthermore, due to the blind nature of the collection process, retrieval of tracheal aspirate will vary depending on the location of the catheter during sample collection. Secondly, our sample size is too small to make definite conclusions about the predictive ability of the specific patterns of tracheal colonization for outcomes such as nosocomial infections and length of stay. However, we believe this data adds to existing literature significantly. To our knowledge this is the first study to measure and report correlation between inflammatory markers and antimicrobial peptides and airway colonization in adult ICU patients. We observed several patterns of colonization which should be characterized further and explored for association with outcomes in future studies of larger sample size. We report a lower incidence of VAP than what is commonly reported in the literature and a low incidence of colonization with potentially pathogenic microorganisms within the first 4 days of intubation.
Conclusion
We observed early tracheal colonization to be a complex and dynamic process in intubated medical patients. There was no significant change in density of tracheal colonization over the first four days of intubation. There was no correlation between colonization and the levels of antimicrobial peptides. Airway inflammation was independent of microbial colonization in this cohort of medical ICU patients. Incidence of VAP in this study was lower than what is typically reported in literature. Several different patterns of tracheal colonization may have to be considered if an intervention to reduce colonization is planned.
Supplementary Material
Acknowledgements
We extend our gratitude to the control subjects and the families of ICU subjects, respiratory therapists in the intensive care units for specimen collection, Abbey Fessler, Chris Janda, and Amanda Nymon for assistance with patient recruitment and specimen processing, pre-anesthesia clinic staff for help with control subjects’ recruitment, Bridget Zimmerman Ph.D. and Junting Zheng M.S. for statistical assistance, and Daniel Diekema, MD, James Torner PhD, and Michael Welsh, MD, for helpful comments and Ashley Small for secretarial assistance. The study was funded in part by grant support from the general clinical research center (RR00059), NIH K12 (RR017700), NIH K23 (HL075402), and NIH SCOR (HL61234).
References
- 1.Cardenosa Cendrero JA, Sole-Violan J, Bordes Benitez A, Noguera Catalan J, Arroyo Fernandez J, Saavedra Santana P, Rodriguez de Castro F. Role of different routes of tracheal colonization in the development of pneumonia in patients receiving mechanical ventilation. Chest. 1999;116:462–470. doi: 10.1378/chest.116.2.462. [DOI] [PubMed] [Google Scholar]
- 2.de Latorre FJ, Pont T, Ferrer A, Rossello J, Palomar M, Planas M. Pattern of tracheal colonization during mechanical ventilation. Am J Respir Crit Care Med. 1995;152:1028–1033. doi: 10.1164/ajrccm.152.3.7663779. [DOI] [PubMed] [Google Scholar]
- 3.Bonten MJ, Gaillard CA, van der Geest S, van Tiel FH, Beysens AJ, Smeets HG, Stobberingh EE. The role of intragastric acidity and stress ulcus prophylaxis on colonization and infection in mechanically ventilated ICU patients. A stratified, randomized, double-blind study fo sucralfate versus antacids. Am J Respir Crit Care Med. 1995;152:1825–1834. doi: 10.1164/ajrccm.152.6.8520743. [DOI] [PubMed] [Google Scholar]
- 4.Driks MR, Craven DE, Celli BR, Manning M, Burke RA, Grarvin GM, Kunches LM, Farber HW, Wedel SA, McCabe WR. Nosocomial pneumonia in intubated patients given sucralfate as compared with antacids or histamine type 2 blockers. The role of gastric colonization. N Engl J Med. 1987;317:1376–1382. doi: 10.1056/NEJM198711263172204. [DOI] [PubMed] [Google Scholar]
- 5.Leone M, Delliaux S, Bourgoin A, Albanese J, Garnier F, Boyadjiev I, Antonini F, Martin C. Risk factors for late-onset ventilator-associated pneumonia in trauma patients receiving selective digestive decontamination. Intensive Care Med. 2005;31:64–70. doi: 10.1007/s00134-004-2514-z. [DOI] [PubMed] [Google Scholar]
- 6.George DL, Falk PS, Wunderink RG, Leeper KVJ, Meduri GU, Steere EL, Corbett CE, Mayhall CG. Epidemiology of ventilator-acquired pneumonia based on protected bronchoscopic sampling. Am J Respir Crit Care Med. 1998;158:1839–1847. doi: 10.1164/ajrccm.158.6.9610069. [DOI] [PubMed] [Google Scholar]
- 7.Ewig S, Torres A, El-Ebiary M, Fabregas N, Hernandez C, Gonzalez J, Nicolas JM, Soto L. Bacterial colonization patterns in mechanically ventilated patients with traumatic and medical head injury. Incidence, risk factors,and association with ventilator-associated pneumonia. Am J Respir Crit Care Med. 1999;159:188–198. doi: 10.1164/ajrccm.159.1.9803097. [DOI] [PubMed] [Google Scholar]
- 8.Revenis ME, Kaliner MA. Lactoferrin and lysozyme deficiency in airway secretions: association with the development of bronchopulmonary dysplasia. J Pediatr. 1992;121:262–270. doi: 10.1016/s0022-3476(05)81201-6. [DOI] [PubMed] [Google Scholar]
- 9.Fleming A, Allison VD. Observations on a bacteriolytic substance ("lysozyme") found in secretions and tissues. Br J Exp Pathol. 1922;III:252–260. [Google Scholar]
- 10.Whitsett JA. Intrinsic and innate defenses in the lung: intersection of pathways regulationg lung morphogenesis, host defense, and repair. J Clin Invest. 2002;109:565–569. doi: 10.1172/JCI15209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole AM, Ganz T. Antimicrobial peptides and proteins in the CF airway. Methods Mol Med. 2002;70:447–464. doi: 10.1385/1-59259-187-6:447. [DOI] [PubMed] [Google Scholar]
- 12.Travis SM, Conway BA, Zabner J, Smith JJ, Anderson NN, Singh PK, Greenberg EP, Welsh MJ. Activity of abundant antimicrobials of the human airway. Am J Respir Cell Mol Biol. 1999;20:872–879. doi: 10.1165/ajrcmb.20.5.3572. [DOI] [PubMed] [Google Scholar]
- 13.Ganz T. Antimicrobial polypeptides in host defense of the respiratory tract. J Clin Invest. 2002;109:693–697. doi: 10.1172/JCI15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaller-Bals S, Schulze A, Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med. 2002;165:992–995. doi: 10.1164/ajrccm.165.7.200110-020. [DOI] [PubMed] [Google Scholar]
- 15.Thompson AB, Bohling T, Payvandi F, Rennard SI. Lower respiratory tract lactoferrin and lysozyme arise primarily in the airways and are elevated in association with chronic bronchitis. J Lab Clin Med. 1990;115:148–158. [PubMed] [Google Scholar]
- 16.Tegtmeyer FK, Maacks S, Wood WG, Wiebicke W. Elastase alpha 1-proteinase inhibitor and lactoferrin concentrations in endotracheal aspirates of ventilated newborns. Pediatr Pulmonol. 1992;13:90–94. doi: 10.1002/ppul.1950130206. [DOI] [PubMed] [Google Scholar]
- 17.Fahy JV, Wong H, Liu J, Boushey HA. Comparison of samples collected by sputum induction and bronchoscopy from asthmatic and healthy subjects. Am J Respir Crit Care Med. 1995;152:53–58. doi: 10.1164/ajrccm.152.1.7599862. [DOI] [PubMed] [Google Scholar]
- 18.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 19.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 20.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 21.Pingleton SK, Fagon JY, Leeper KVJ. Patient selection for clinical investigation of ventilator-associate pneumonia. Criteria for evaluating diagnostic techniques. Chest. 1992;102:553S–556S. doi: 10.1378/chest.102.5_supplement_1.553s. [DOI] [PubMed] [Google Scholar]
- 22.Ashare A, Powers LS, Butler NS, Doerschug KC, Monick MM, Hunninghake GW. Anti-inflammatory response is associated with mortality and severity of infection in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L633–L640. doi: 10.1152/ajplung.00231.2004. [DOI] [PubMed] [Google Scholar]
- 23.Ferrer M, Ioanas M, Arancibia F, Marco MA, de la Bellacasa JP, Torres A. Microbial airway colonization is associated with noninvasive ventilation failure in exacerbation of chronic obstructive pulmonary disease. Crit Care Med. 2005;33:2003–2009. doi: 10.1097/01.ccm.0000178185.50422.db. [DOI] [PubMed] [Google Scholar]
- 24.Drakulovic MB, Bauer S, Torres A, J G, MJ R, J A. Initial bacterial colonization in patients admitted to a respiratory intensive care unit: bacteriological pattern and risk factors. Respiration. 2001;68:58–66. doi: 10.1159/000050464. [DOI] [PubMed] [Google Scholar]
- 25.Ortqvist A, Hammers-Berggren S, Kalin M. Respiratory tract colonization and incidence of secondary infection during hospital treatment of community-acquired pneumonia. Eur J Clin Microbiol Infect Dis. 1990;9:725–731. doi: 10.1007/BF02184684. [DOI] [PubMed] [Google Scholar]
- 26.Sirvent JM, Torres A, Vidaur L, Armengol J, de Batlle J, Bonet A. Tracheal colonisation within 24 h of intubation in patients with head trauma: risk factor for developing early-onset ventilator-associated pneumonia. Intensive Care Med. 2000;26:1369–1372. doi: 10.1007/s001340000611. [DOI] [PubMed] [Google Scholar]
- 27.Guidelines for the management of adults with hospital-acquired, ventilator-assocaiated, and healthcare-assoicated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.