Abstract
OBJECTIVES
We sought to determine which transthoracic echocardiographic (TTE) measurements most strongly predict heart failure (HF) and to develop an index for risk stratification in outpatients with coronary artery disease (CAD).
BACKGROUND
Many TTE measurements have been shown to be predictive of HF, and they might be useful if aggregated into a risk-prediction index.
METHODS
We performed TTE in 1,024 outpatients with stable CAD enrolled in the Heart and Soul study and followed them for 4.4 years. With Cox proportional hazard models, we evaluated the association of 15 TTE measurements with subsequent HF hospital stay. Those measurements that independently predicted HF were combined into an index. Variables were defined as normal or abnormal on the basis of dichotomous cutoffs determined from the American Society of Echocardiography. Abnormal variables in each measurement were assigned points on the basis of strength of association with HF.
RESULTS
Of the 15 variables, 5 measurements were independent predictors of HF: left ventricular mass index (LVMI), left atrial volume index (LAVI), mitral regurgitation (MR), left ventricular outflow tract velocity-time integral (VTILVOT), and diastolic dysfunction (DD). In multivariate analysis, each of the 5 measurements independently predicted HF: LVMI >90 g/m2 (hazard ratio [HR]: 4.1; 95% confidence interval [CI]: 2.3 to 7.2, p < 0.0001); pseudo-normal or restrictive DD (HR: 2.9; 95% CI: 1.8 to 4.5, p < 0.0001); VTILVOT <22 mm (HR: 2.2; 95% CI: 1.4 to 3.5, p = 0.0004); mild, moderate, or severe MR (HR: 1.8; 95% CI: 1.2 to 2.8, p = 0.009); and LAVI >29 ml/m2 (HR: 1.6; 95% CI: 1.0 to 2.5, p = 0.06). Combining these measurements, the Heart Failure Index ranged from 0 to 8, representing risk as follows: 3 points for LVMI, 2 points for DD, and 1 point for VTILVOT, MR, and LAVI. Among participants with 0 to 2 points: 4% had HF hospital stays (reference); 3 to 4 points: 10% (HR: 2.4; 95% CI: 1.3 to 4.4, p = 0.003); 5 to 6 points: 24% (HR: 6.2; 95% CI: 3.6 to 10.6, p < 0.0001); 7 to 8 points: 48% (HR: 13.7; 95% CI: 7.2 to 25.9, p < 0.0001).
CONCLUSIONS
We identified 5 TTE measurements that independently predict HF in patients with stable CAD and combined them as an index that might be useful for risk stratification and serial observations.
Keywords: coronary artery disease, echocardiography, heart failure, prognostic index, risk stratification
Transthoracica 2-dimensional echocardiogram and Doppler flow examination (TTE) generate plethora of high-quality anatomic and physiologic data. However, the abundance and variety of these data can complicate acquisition and analysis, limiting effective integration into clinical settings. To relevantly and efficiently aggregate this information, it is desirable to identify the most potent predictors of dysfunction and adverse outcomes among them.
Left ventricular (LV) ejection fraction (EF) is commonly used to predict heart failure (HF) in patients with coronary artery disease (CAD) (1) but is an overtaxed descriptor of systolic function. Numerous TTE-derived measurements can detect subtle changes in myocardial structure and function that offer prognostic information beyond EF. For example, left ventricular mass index (LVMI) predicts mortality independent of other cardiovascular risk factors and electrocardiogram-derived LV hypertrophy (2). Likewise, left atrial enlargement, mitral regurgitation (MR), and diastolic dysfunction (DD) have been shown to predict cardiovascular events, HF, and mortality (3– 6). Other techniques such as Doppler-derived stroke distance (i.e., left ventricular outflow tract velocity–time integral [VTILVOT]) have potential applications in describing global function that might carry unique prognostic relevance (7).
The Heart and Soul Study has completed 4.4 years of follow-up on a population of 1,024 subjects with CAD. A comprehensive quantitative echocardiogram and Doppler examination was administered to each participant at enrollment. This study, therefore, provided a suitable vehicle from which to stratify and aggregate TTE data and to determine the most effective combination of noninvasive parameters for the prediction of congestive HF hospital stay.
We hypothesized that an array of TTE-derived measurements shown to independently predict congestive HF hospital stay can be combined into a risk-stratification index predictive of HF (the TTE Heart Failure Index). We intend for this index to be readily accessible and to streamline communication among providers.
METHODS
The Heart and Soul Study is a prospective cohort study investigating the influence of psychosocial factors on cardiovascular events. The enrollment process for the Heart and Soul Study has been previously described (8). Patients were enrolled from 2 Departments of Veterans Affairs (San Francisco and Palo Alto, California), the University of California-San Francisco, and 9 public health clinics from the Community Health Network of San Francisco. Criteria for enrollment were: 1) history of myocardial infarction; 2) angiographic evidence of at least 50% stenosis by area in at least 1 coronary vessel; 3) evidence of exercise-induced ischemia by treadmill electrocardiogram or stress nuclear perfusion imaging; 4) history of coronary revascularization; or 5) a prior diagnosis of coronary disease by an internist or cardiologist. Individuals were excluded if they had a myocardial infarction within the prior 6 months, deemed themselves unable to walk 1 block, or were planning to move out of the local area within 3 years. A total of 1,024 study participants provided informed consent and completed baseline echocardiographic and laboratory testing, including 549 (54%) with a history of myocardial infarction, 237 (23%) with a history of revascularization but not myocardial infarction, and 238 (23%) with diagnosis of coronary disease that was documented by their physician (on the basis of a positive angiogram or treadmill test in >98% of cases). The institutional review board at each of the enrolling centers approved the study protocol.
Echocardiographic measurements
A complete resting 2-dimensional echocardiogram with an Acuson Sequoia ultrasound system (Siemens Medical Solutions, Mountain View, California) with a 3.5-MHz transducer and Doppler ultrasound examination was performed in all patients. Standard 2-dimensional parasternal short-axis and apical 2- and 4-chamber views during quiet respiration or held expiration were obtained. Two highly experienced sonographers made all sonographic measurements, and a single cardiologist reader (N.B.S.), who was blinded to clinical and laboratory information, evaluated, confirmed, and—when needed—corrected each measurement.
Fifteen candidate echocardiographic variables were chosen a priori from the data base by the investigators:
LV end-systolic volume index
LV end-diastolic volume index
LVEF
left atrial volume index (LAVI)
right atrial volume index
LVMI
pulmonary artery peak systolic pressure
right ventricular outflow tract velocity-time integral (VTIRVOT)
VTILVOT
aortic valve area
right atrial pressure
DD
MR severity
tricuspid regurgitation severity
resting wall motion score index
Standard apical 2- and 4-chamber views were obtained. LV end-systolic and end-diastolic volumes were obtained by planimetry with the biplane method of discs as described (9). The LVEF was calculated as (end-diastolic volume – end-systolic volume)/end-diastolic volume.
Left and right atrial volumes were obtained at end-ventricular systole by manual planimetry with the biplane method of discs for the left atrium and single plane method of discs for the right atrium, as previously described and validated (10). All chamber volumes were subsequently indexed to body surface area.
LV mass was calculated with a truncated ellipsoid equation and indexed to body surface area as previously described and validated (9,11).
The tricuspid regurgitation jet was visualized with color flow mapping, and continuous wave Doppler was used to capture the flow signal from measurement of peak tricuspid regurgitant velocity. The peak tricuspid regurgitant velocity for the current study was the highest measurement obtainable by Doppler imaging among the parasternal, apical, and subcostal views. The right ventricular systolic pressure was estimated with the modified Bernoulli equation (p = 4v2) and added to the estimated right atrial pressure to obtain the pulmonary artery systolic pressure (12).
The VTIRVOT was obtained by placing a pulsed wave Doppler sample volume in the proximal right ventricular outflow tract at the level of the pulmonic valve, in the parasternal short-axis view and tracing the outer boundaries of the spectral Doppler signal to obtain the VTI. The sample volume was placed such that the opening valve Doppler signal was greater than or equal to the closing signal.
The VTILVOT was obtained by placing a pulsed wave Doppler sample volume in the left ventricular outflow tract (LVOT) immediately proximal to the aortic valve in the anteriorly angulated apical 4-chamber view and tracing the outer boundaries of the peak spectral Doppler signal to obtain the VTILVOT. Proper location in the LVOT was confirmed by visualization of the aortic valve closure signal (7). The LVOT diameter was measured at the level of the aortic annulus from the parasternal long-axis view in midsystole. The aortic valve area was derived from the velocity-time integrals of the aortic valve and the LVOT tract with the continuity equation (13).
Diastolic dysfunction was defined as the presence of at least 1 of the following: impaired relaxation defined as a ratio of peak mitral early diastolic to atrial contraction velocity (E/A) of ≤0.75 with systolic dominant pulmonary vein flow; pseudonormal defined as 0.75 < E/A <1.5 with diastolic dominant pulmonary vein flow; restrictive filling defined as an E/A ≥1.5 with diastolic dominant pulmonary vein flow (14). Diastolic dysfunction was only determined if both pulmonary vein flow and E/A were both recorded. Pulmonary vein flow was recorded in 1,011 patients, and E/A was recorded in 971 patients.
The severity of mitral and tricuspid regurgitation was determined according to American Society of Echocardiography guidelines (15). The right atrial pressure was obtained by inspection of the inferior vena cava during respiration as previously described (16).
Regional LV function was assessed with a standard 16-segment model (10). Segmental scores were assigned as follows: normal or hyperkinesis = 1; hypokinesis = 2; akinesis = 3; dyskinesis = 4; and aneurismal = 5. The wall motion score index was derived as the sum of all scores divided by the number of segments visualized.
Cardiovascular outcomes
We conducted annual telephone follow-up interviews and questioned participants or their proxies regarding recent emergency room visits and hospital stays. Medical records, death certificates, and coroner’s reports were retrieved. Participants were censored at point of HF admission, when lost to follow-up, or upon death. Two blinded adjudicators reviewed each event, and if there was agreement, the outcome classification was binding. If there was disagreement, a third blinded adjudicator reviewed the event and determined the outcome classification.
Hospital stay for HF was defined for a clinical syndrome with a minimum 1-night hospital stay and involving at least 2 of the following: paroxysmal nocturnal dyspnea, orthopnea, elevated jugular venous pressure, pulmonary rales, a third heart sound, cardiomegaly on chest radiography, or pulmonary edema on chest radiography. These clinical signs and symptoms must have represented a clear change from the normal clinical state of the patient and must have been accompanied by either failing cardiac output as determined by peripheral hypoperfusion (in the absence of other causes such as sepsis or dehydration) or peripheral or pulmonary edema treated with intravenous diuretics, inotropes, or vasodilators.
Other participant characteristics
Each patient completed a detailed questionnaire that included age, gender, race, medical history (including history of HF), level of physical activity, current smoking, and level of alcohol consumption. Study personnel recorded all current medications and measured height, weight, and blood pressure. Medication was recorded by having subjects bring medication bottles to the baseline interview and categorize the medications according to Epocrates Rx (San Mateo, California). Total, low-density lipoprotein, and high-density lipoprotein cholesterol were measured from fasting serum samples. Creatinine clearance was determined with 24-h urine sample (17).
Development of TTE Heart Failure Index
We first calculated correlation coefficients among all of the 15 candidate variables and eliminated variables that were highly correlated (r > 0.4) with one another. When 2 variables were highly correlated with one another, the 1 that was a stronger predictor in univariate unadjusted analysis was chosen and the other eliminated. We then evaluated the strength of association between each candidate variable and subsequent hospital stay for HF, with age-adjusted Cox proportional hazards models. To determine which of these candidate variables were independent predictors of HF, we simultaneously entered all variables into a single proportional hazards model.
From this multivariable model, we selected all variables that independently predicted HF (at p < 0.05) to be included in the Heart Failure Index. To develop the Heart Failure Index, we first chose cut-points to determine an abnormal value for each of the 5 variables with reference ranges established by the American Society of Echocardiography and other studies: LVMI ≥90 g/m2; LAVI >29 ml/m2; VTILVOT <22 mm; DD as pseudonormal or restrictive; and MR as mild, moderate, or severe (4,7,9,10,18). We then entered all of these index variables into a single multivariable Cox proportional hazards model and assigned points corresponding to the hazard ratios for abnormal values for each index variable. Each subject’s index score was calculated as the sum of points on this index. Of note, if a measurement was missing, “0” points was recorded for that single measurement, but the patient’s other measurements would be tallied to calculate the index score.
To evaluate the prognostic value of the Heart Failure Index, we calculated the relative hazard of HF hospital stay according to score on the Heart Failure Index, adjusted for age, gender, history of HF, hypertension, myocardial infarction, diabetes, smoking, renal insufficiency, and body mass index. Another analysis was done, adjusting for N-terminal part of the pro-B-type natriuretic peptide (NT-proBNP); EF; and size and treatment of myocardial infarction via wall motion score, revascularization, and medication use (beta-blocker, angiotensin-converting enzyme inhibitor, statin, and aspirin) (19). To evaluate whether this index predicts incident HF, we repeated this analysis in patients without a self-reported history of HF. We verified the proportionality assumptions of all models. All analyses were performed with Statistical Analysis Software (version 8, SAS Institute Inc., Cary, North Carolina).
RESULTS
Of the 1,024 participants, 1,015 (>99%) provided an average 4.4 years of follow-up. Baseline characteristics of the participants are described in Table 1. Of the 15 candidate variables, 2 were initially eliminated because they were highly correlated (r > 0.4) with other candidate variables: LV end-diastolic volume index (highly correlated with end-systolic volume index) and VTIRVOT (highly correlated with VTILVOT). All of the remaining 13 candidate variables were significant univariate predictors of HF, 5 were independent predictors of HF: MR, LAVI, LVMI, VTILVOT, and DD (Table 2).
Table 1.
Baseline Characteristics of 1,024 Participants With Coronary Heart Disease
| Age, yrs | 67 ± 11 |
| Male | 840 (82) |
| Race | |
| White | 615 (60) |
| Black | 168 (16) |
| Asian | 118 (12) |
| Other | 122 (12) |
| Medical history | |
| Hypertension | 723 (71) |
| MI | 547 (54) |
| Stroke | 148 (15) |
| Diabetes | 265 (26) |
| Revascularization | 602 (59) |
| Asthma/COPD | 163 (16) |
| CHF | 179 (18) |
| Renal insufficiency (CrCl <60) | 236 (24) |
| Measured characteristics | |
| LVEF | 0.62 ± 0.10 (0.13–0.83) |
| SBP | 133 ± 21 (90–235) |
| DBP | 75 ± 11 |
| LDL | 104 ± 34 |
| HDL | 46 ± 14 |
| BMI | 28.4 ± 5.3 |
| BSA | 1.95 ± 0.22 |
| Use of medications | |
| Beta-blocker | 593 (58) |
| ACE inhibitor | 524 (51) |
| Statin | 657 (64) |
| Aspirin | 792 (77) |
| Behavioral risk factors | |
| Physically inactive | 371 (36) |
| Current smoking | 201 (20) |
| Regular alcohol use | 293 (29) |
Values given as n (%) or mean ± SD with values in parentheses as ranges. ACE = angiotensin-converting enzyme; BMI = body mass index; BSA = body surface area; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; CrCl = creatinine clearance; DBP = diastolic blood pressure; HDL = high-density lipoprotein (cholesterol); LDL = low-density lipoprotein (cholesterol); LVEF = left ventricular ejection fraction; MI = myocardial infarction; SBP = systolic blood pressure.
Table 2.
Association of 13 Baseline TTE Variables With Hospital Stays for HF During 4.4 Years of Follow-Up
| Variable* | Age-Adjusted HR (95% CI) | p Value | Adjusted HR (95% CI)† | p Value |
|---|---|---|---|---|
| LVESVI | 1.6 (1.5–1.8) | <0.0001 | 1.2 (0.8–1.7) | 0.44 |
| Aortic valve area* | 1.4 (1.2–1.6) | 0.0003 | 1.2 (0.9–1.5) | 0.15 |
| MR | ||||
| None to trace | reference | |||
| Mild | 2.4 (1.7–3.5) | <0.0001 | 1.7 (1.0–2.9) | 0.05 |
| Moderate or severe | 10.2 (4.4–23.5) | <0.0001 | 2.2 (0.4–13.1) | 0.38 |
| Tricuspid regurgitation | ||||
| None to trace | reference | |||
| Mild | 1.5 (1.1–2.3) | 0.02 | 0.9 (0.5–1.6) | 0.70 |
| Moderate or severe | 6.4 (2.6–15.8) | <0.0001 | 0.3 (0.04–2.5) | 0.28 |
| Right atrial pressure | 1.3 (1.2–1.4) | <0.0001 | 1.2 (1.0–1.5) | 0.11 |
| Left atrial volume index | 1.8 (1.6–2.0) | <0.0001 | 1.4 (1.1–1.8) | 0.01 |
| LVMI | 1.8 (1.6–2.0) | <0.0001 | 1.5 (1.2–1.8) | 0.0001 |
| Wall motion score index | 1.5 (1.3–1.6) | <0.0001 | 0.9 (0.7–1.2) | 0.57 |
| Pulmonary artery systolic pressure | 1.5 (1.2–1.8) | <0.0001 | 0.9 (0.7–1.5) | 0.57 |
| LVEF* | 1.7 (1.5–2.0) | <0.0001 | 1.0 (0.7–1.4) | 0.99 |
| VTILVOT* | 1.9 (1.5–2.3) | <0.0001 | 1.3 (1.0–1.8) | 0.05 |
| Right atrial volume index | 1.6 (1.4–1.9) | <0.0001 | 1.1 (0.9–1.4) | 0.46 |
| Diastolic dysfunction | ||||
| Normal | reference | reference | ||
| Impaired relaxation | 2.2 (1.4–3.5) | 0.0003 | 1.4 (0.8–2.3) | 0.22 |
| Pseudonormal or restrictive | 5.5 (3.1–9.7) | <0.0001 | 2.3 (1.1–5.0) | 0.04 |
Entered per standard deviation decrease; all other continuous variables entered per standard deviation increase.
Adjusted for age plus all other candidate variables.
CI = confidence interval; HF = heart failure; HR = hazard ratio; LVEF = left ventricular ejection fraction; LVESVI = left ventricle end-systolic volume index; LVMI = left ventricular mass index; MR = mitral regurgitation; TTE = transthoracic echocardiographic; VTILVOT = left ventricular outflow tract velocity–time integral.
We entered these 5 variables into a single multivariable model and calculated TTE Heart Failure Index points by dividing each variable’s hazard ratio by 1.6, which was the lowest HR. The resulting TTE Heart Failure Index ranged from 0 to 8 points, with 1 point for MR (mild, moderate, or severe), LAVI (>29 ml/m2), and VTILVOT (<22 mm); 2 points for DD (pseudonormal or restrictive); and 3 points for LVMI (>90 g/m2) (Table 3).
Table 3.
Heart Failure Index Score Definitions: 5 Variable Model; Primary Outcome Is HF
| TTE HF Index Measurements | Abnormalities/Number With Measurement | Adjusted HR (95% CI)* | p Value | Points Assigned to Index |
|---|---|---|---|---|
| Mild, moderate, or severe MR | 196/1,015 | 1.8 (1.2–2.8) | 0.009 | 1 |
| LAVI >29 ml/m2 | 580/1,010 | 1.6 (1.0–2.5) | 0.06 | 1 |
| LVMI >90 g/m2 | 565/1,005 | 4.1 (2.3–7.2) | <0.0001 | 3 |
| VTILVOT <22 mm | 547/985 | 2.2 (1.4–3.5) | 0.0004 | 1 |
| Pseudonormal or restrictive diastolic function | 116/903 | 2.9 (1.8–4.5) | <0.0001 | 2 |
Adjusted for the other 5 TTE variables as well as age.
LAVI = left atrial volume index; other abbreviations as in Table 2.
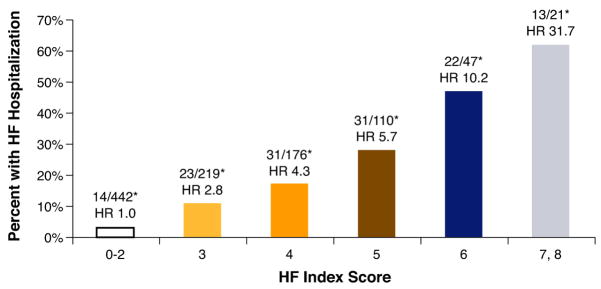
Among participants with 0 to 2 points, 4% had HF hospital stays (reference); among those with 3 to 4 points: 10% had HF hospital stays (hazard ratio [HR]: 2.4; 95% confidence interval [CI]: 1.3 to 4.4, p = 0.003); among those with 5 to 6 points, 24% had HF hospital stays (HR: 6.2; 95% CI: 3.6 to 10.6, p < 0.0001); and among those with 7 to 8 points, 48% had HF hospital stays (HR: 13.7; 95% CI: 7.2 to 25.9, p < 0.0001) (Fig. 1). Associations were somewhat attenuated but remained strong after further adjustment for gender, history of HF, smoking, renal insufficiency, history of hypertension, history of myocardial infarction, history of diabetes, and body mass index. We performed a separate subgroup analysis to adjust for EF, size and treatment of myocardial infarction (wall motion score, revascularization, and medication use with beta blockers, angiotensin-converting enzyme inhibitors, statins, and aspirin), and NT-proBNP (Table 4).
Figure 1. TTE Heart Failure Index: Risk Stratification of HF Hospital Stay by Score Increase in All 1,024 Patients.
Transthoracic echocardiographic (TTE) Heart Failure Index predicts heart failure (HF) hospital stays in patients with stable coronary artery disease (CAD). The Heart Failure Index ranged from 0 to 8 points, representing risk as follows: 3 points for left ventricular mass index, 2 points for diastolic dysfunction, and 1 point for left ventricular outflow tract velocity-time integral, mitral regurgitation, and left atrial volume index. The graph shows risk stratification for HF hospital stay per increasing score on the index. This can be used to predict HF in patients with stable CAD. p < 0.05, adjusted for age, gender, body mass index, ejection fraction, renal insufficiency, history of heart failure, myocardial infarction, hypertension, and diabetes. *Number of participants hospitalized for HF/number with given TTE Heart Failure Index score. HR = hazard ratio.
Table 4.
Association of Heart Failure Index Score With Hospital Stay for HF During 4.4 Years of Follow-Up Among 1,015 Participants With Coronary Artery Disease
| Index Score |
% With Events |
Age-Adjusted HR (95% CI) |
p Value | MV-Adjusted HR (95% CI)* |
p Value | MI Size/Treatment- Adjusted HR (95% CI)† |
p Value | EF-Adjusted HR (95% CI)‡ |
p Value | NT-proBNP–adjusted HR (95% CI)§ |
p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–2 | 4% (17/397) | reference | — | reference | — | reference | — | reference | — | reference | — |
| 3–4 | 10% (32/313) | 2.4 (1.3–4.4) | 0.003 | 1.9 (0.9–3.9) | 0.08 | 2.0 (1.0–4.2) | 0.06 | 2.0 (0.9–4.1) | 0.07 | 1.9 (0.9–4.1) | 0.09 |
| 5–6 | 24% (63/259) | 6.2 (3.6–10.6) | <0.0001 | 4.1 (2.1–8.1) | <0.0001 | 4.5 (2.2–9.2) | <0.0001 | 3.9 (1.9–8.1) | 0.0003 | 3.0 (1.4–6.5) | 0.006 |
| 7–8 | 48% (22/46) | 13.7 (7.2–25.9) | <0.0001 | 9.1 (4.1–20.1) | <0.0001 | 10.1 (4.4–23.4) | <0.0001 | 9.6 (4.2–22.0) | <0.0001 | 4.3 (1.7–11.0) | 0.002 |
Adjusted for age, gender, history of HF, smoking, renal insufficiency, history of hypertension, history of myocardial infarction, history of diabetes, and body mass index.
Adjusted for above variables plus: 1) resting wall motion score; 2) prior revascularization; and 3) medication use (beta-blocker, ACE, aspirin, statin).
Adjusted for above variables plus EF.
Adjusted for above variables plus log N-terminal part of the pro-B-type natriuretic peptide (NT-proBNP).
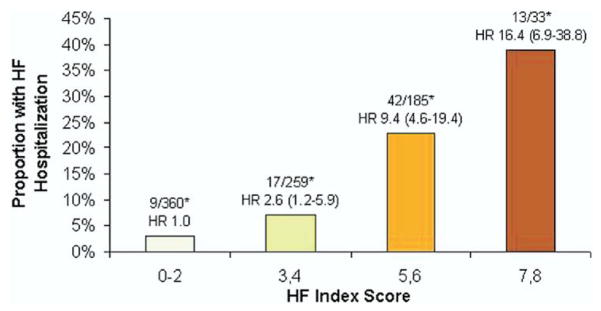
Of the 831 participants without a baseline history of HF, among those with 0 to 2 points, 3% had HF hospital stays (reference); among those with 3 to 4 points, 7% had HF hospital stays (HR: 2.6; 95% CI: 1.2 to 5.9, p = 0.02); among those with 5 to 6 points, 23% had HF hospital stays (HR: 9.4; 95% CI: 4.6 to 19.4, p < 0.0001); among those with 7 to 8 points, 39% had HF hospital stays (HR: 16.4; 95% CI: 6.9 to 38.8, p < 0.0001) (Fig. 2, Table 5).
Figure 2. TTE Heart Failure Index: Risk Stratification of HF Hospital Stay by Score Increase in 831 Patients Without History of HF at Baseline.
The TTE Heart Failure Index predicts HF hospital stays in subgroup analysis of 831 patients with stable CAD and no history of HF. The Heart Failure Index ranged from 0 to 8 points, representing risk as follows: 3 points for LVMI; 2 points for diastolic dysfunction; and 1 point for left ventricular outflow tract velocity-time integral, mitral regurgitation, and left atrial volume index. The graph shows risk stratification for HF hospital stay per increasing score on the index. This can be used to predict incident HF in patients with stable CAD and no history of HF. p < 0.05. *Number of participants hospitalized for HF/number with given TTE Heart Failure Index score. Abbreviations as in Figure 1.
Table 5.
Association of Heart Failure Index Score With Hospital Stay for HF During 4.4 Years of Follow-Up Among 831 Participants Without a History of HF
| Index Score |
% With Events |
Age-Adjusted HR (95% CI) |
p Value | MV-Adjusted HR (95% CI)* |
p Value | MI Size/Treatment- Adjusted HR (95% CI)† |
p Value | EF-Adjusted HR (95% CI)‡ |
p Value | NT-proBNP–Adjusted HR (95% CI)§ |
p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–2 | 3% (9/360) | reference | — | reference | — | reference | — | reference | — | reference | — |
| 3–4 | 7% (17/259) | 2.6 (1.2–5.9) | 0.02 | 2.0 (0.8–5.3) | 0.15 | 1.8 (0.7–4.9) | 0.22 | 1.7 (0.6–4.5) | 0.29 | 2.0 (0.7–5.6) | 0.18 |
| 5–6 | 23% (42/185) | 9.4 (4.6–19.4) | <0.0001 | 6.9 (2.9–16.6) | <0.0001 | 6.8 (2.7–16.6) | <0.0001 | 5.3 (2.1–13.4) | 0.0004 | 4.2 (1.5–11.5) | 0.006 |
| 7–8 | 39% (13/33) | 16.4 (6.9–38.8) | <0.0001 | 11.0 (3.9–30.9) | <0.0001 | 9.5 (3.3–27.4) | <0.0001 | 8.4 (2.9–23.9) | <0.0001 | 5.7 (1.8–17.8) | 0.003 |
Adjusted for age, gender, smoking, renal insufficiency, history of hypertension, history of myocardial infarction, history of diabetes, and body mass index.
Adjusted for above variables plus: 1) resting wall motion score; 2) prior revascularization; and 3) medication use (beta-blocker, ACE, aspirin, statin).
Adjusted for above variables plus EF.
Adjusted for above variables plus log N-terminal part of the pro-B-type natriuretic peptide (NT-proBNP).
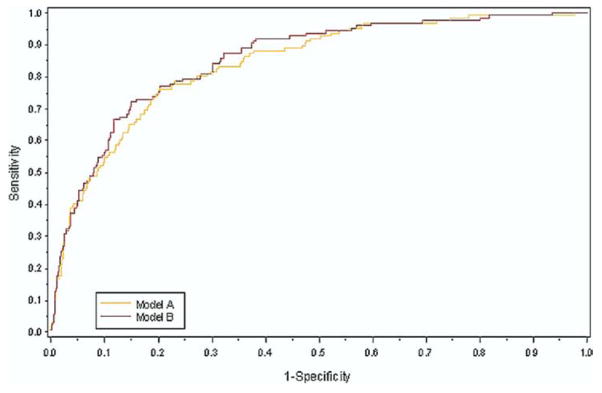
The TTE Heart Failure Index plus age, LVEF, and NT-proBNP was compared in a receiver-operating characteristic curve (c = 0.86) with age, LVEF, and NT-proBNP alone (c = 0.84, p = 0.09) (Fig. 3).
Figure 3. ROC for Congestive HF Hospital Stay Comparing the TTE Heart Failure Index Plus EF, NT-proBNP, and Age With EF and NT-proBNP Alone.
The TTE Heart Failure Index can be an adjunct to age, left ventricular ejection fraction (LVEF), and N-terminal part of the pro-B-type natriuretic peptide (NT-proBNP) to predict HF, with the receiver operating characteristic (ROC) curve shown in the figure (c = 0.86). This was compared with the ROC curve of age, LVEF, and NT-proBNP (Model A, c = 0.84, p = 0.09). Abbreviations as in Figure 1.
DISCUSSION
There is an abundance of transthoracic echocardiographic measurements with predictive value. Thus far, there has not been a prior effort to sort through them and identify those with independent and incremental value in predicting HF. In an ambulatory cohort of patients with stable CAD, we evaluated 15 candidate TTE measurements to see which were independent predictors of HF hospital stay. The 5 measurements that were independent predictors were combined into an index that incrementally stratifies patients according to their risk of HF. Furthermore, we did a subgroup analysis among the 831 patients without a history of HF and showed that the index predicts incident HF.
In creating this index, we initially studied a group of 15 historically and intuitively promising echocardiographic measurements. By choosing the most predictive among them, we eliminated those that were statistically redundant, leaving those that were independent predictors of HF: LVMI, LAVI, MR, VTILVOT, and DD (Table 3). Accordingly, the Heart Failure Index, derived from these 5 measurements, presents compelling evidence that these measurements be routinely performed in most clinical settings. Additionally, combining the aggregate information of these measurements allows the clinician or researcher to simplify the wealth of information into a single number. Routine calculation of this value might streamline communication among health care providers and scientists.
Each of the 5 parameters included in the index have data to support their prognostic value (2–7). Yet, standing alone as individual measurements, each examines a relatively narrow window of pathophysiology. Hence, as might be anticipated, each is limited as a stand-alone gauge of function and risk. When used in combination, however, the index recruits the potency of echocardiography for defining clinical risk and potentially influencing clinical management (Fig. 1).
It is noteworthy that EF was not included in the index. Two reasons justify excluding EF from the index: first, there was not a large proportion of patients in this study with reduced EF (92.7% with EF >45%, mean 62 ± 10%), making it a relative weak predictor in our cohort; and second, we addressed its potential effect on the index by adjusting for it in the multivariate analysis. We also would like to draw attention to the TTE Heart Failure Index’s unique role in identifying diastolic HF, given the relatively preserved EF in this patient population and that DD, LV mass, and left atrial volume make up 6 of the index’s 8 points.
Some candidate variables were not included in the index because they were not independent predictors of HF but notably have a rich history supporting their role in predicting poor cardiovascular outcomes, mortality, and HF. For example, volumetric assessment of the LV (end-systolic volume index and end-diastolic volume index) is known to be strongly predictive of HF and mortality (20,21). Meanwhile, pulmonary artery systolic pressure is a surrogate for right HF, often the end stage of a decompensating heart (22). In advanced HF these measurements certainly are imperative to assessment but might become abnormal in later stages of HF than that seen in the Heart and Soul patient population. Therefore, the TTE Heart Failure Index might be most suitable for predicting HF in a patient population with relatively preserved systolic function. Also, many of the measurements were much stronger univariate predictors than independent predictors, suggesting they might have been eliminated merely because they were correlated to the other echocardiographic measurements (Table 2).
Study limitations
Several limitations must be considered when interpreting the results of our study. First, the Veterans Affairs hospital has a unique and homogenous patient population proportionally dominated by Caucasian (60%) men (82%) with a mean age of 67 ± 11 years. Therefore, to validate the TTE Heart Failure Index and to prove generalizability, the index must be applied to another population of patients with stable CAD. Second, the primary outcome is HF hospital stay, which might be prone to error despite rigorous adjudication, depending on clinical assessment, coding, and accuracy of chart review. Third, the TTE Heart Failure Index was limited to TTE measurements available at the time of the initial echocardiographic examination. Therefore we were unable to incorporate newer techniques in echocardiography, such as tissue Doppler imaging, which have proven prognostic value (23). Fourth, we also included variables that might not be measured routinely in common echocardiography practice, such as VTI. However, to maximize the prognostic value of the index and perhaps to stimulate expanded use in clinical practice, we included VTILVOT in the final index, because it was 1 of the 5 independent predictors of HF hospital stay in the 13-variable model. Fifth, creating an index from multiple echocardiographic variables is limited by the correlation of the various measurements to each other. We accounted for this by eliminating those variables highly correlated to each other (r > 0.4). For example, VTIRVOT was excluded because of its relationship to VTILVOT.
Finally, the index uses dichotomous cut-offs for each measurement. However, the severity of each abnormality beyond that cut-off is not taken into account, thereby potentially losing incremental prognostic information.
CONCLUSIONS
The TTE Heart Failure Index is a unique combination of 5 measurements that independently predict HF when compared in a multivariate analysis. These 5 measurements are: LVMI, LAVI, MR, VTILVOT, and DD (Table 3). The index effectively stratifies patients with stable CAD according to their risk of HF hospital stay in a manner more useful than any 1 of the aforementioned measurements alone. Further studies are needed to elucidate the value of this index in clinical decision-making in patients with CAD as well as other forms of cardiomyopathy.
Acknowledgments
This study was supported by the Department of Veterans Affairs (Epidemiology Merit Review Program), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), the Nancy Kirwan Heart Research Fund, and a loan of equipment from Siemens Corporation (Mountain View, California).
ABBREVIATIONS AND ACRONYMS
- CAD
coronary artery disease
- CI
confidence interval
- DD
diastolic dysfunction
- E/A
ratio of peak mitral early diastolic to atrial contraction velocity
- EF
ejection fraction
- HF
heart failure
- HR
hazard ratio
- LAVI
left atrial volume index
- LV
left ventricle/ventricular
- LVMI
left ventricular mass index
- LVOT
left ventricular outflow tract
- MR
mitral regurgitation
- NT-proBNP
N-terminal part of the pro-B-type natriuretic peptide
- TTE
transthoracic echocardiography
- VTILVOT
left ventricular outflow tract velocity–time integral
- VTIRVOT
right ventricular outflow tract velocity–time integral
References
- 1.Cheitlin MD, Alpert JS, Armstrong WF, et al. ACC/AHA guidelines for the clinical application of echocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography). Developed in collaboration with the American Society of Echocardiography. J Am Coll Cardiol. 1997;29:862–79. doi: 10.1016/s0735-1097(96)90000-5. [DOI] [PubMed] [Google Scholar]
- 2.Sundstrom J, Lind L, Arnlov J, Zethelius B, Andren B, Lithell HO. Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001;103:2346–51. doi: 10.1161/01.cir.103.19.2346. [DOI] [PubMed] [Google Scholar]
- 3.Moller JE, Hillis GS, Oh JK, et al. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–12. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 4.Ren X, Ristow B, Na B, Ali S, Schiller NB, Whooley MA. Prevalence and prognosis of asymptomatic left ventricular diastolic dysfunction in ambulatory patients with coronary heart disease. Am J Cardiol. 2007;99:1643–7. doi: 10.1016/j.amjcard.2007.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beinart R, Boyko V, Schwammenthal E, et al. Long-term prognostic significance of left atrial volume in acute myocardial infarction. J Am Coll Cardiol. 2004;44:327–34. doi: 10.1016/j.jacc.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 6.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–64. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 7.Goldman JH, Schiller NB, Lim DC, Redberg RF, Foster E. Usefulness of stroke distance by echocardiography as a surrogate marker of cardiac output that is independent of gender and size in a normal population. Am J Cardiol. 2001;87:499–502. doi: 10.1016/s0002-9149(00)01417-x. [DOI] [PubMed] [Google Scholar]
- 8.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–21. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Schiller NB, Skioldebrand CG, Schiller EJ, et al. Canine left ventricular mass estimation by two-dimensional echocardiography. Circulation. 1983;68:210–6. doi: 10.1161/01.cir.68.1.210. [DOI] [PubMed] [Google Scholar]
- 12.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–62. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 13.Otto CM, Pearlman AS, Comess KA, Reamer RP, Janko CL, Huntsman LL. Determination of the stenotic aortic valve area in adults using Doppler echocardiography. J Am Coll Cardiol. 1986;7:509–17. doi: 10.1016/s0735-1097(86)80460-0. [DOI] [PubMed] [Google Scholar]
- 14.Ren X, Ristow B, Na B, Ali S, Schiller NB, Whooley MA. Prevalence and prognosis of asymptomatic left ventricular diastolic dysfunction in ambulatory patients with coronary heart disease. Am J Cardiol. 2007;99:1643–7. doi: 10.1016/j.amjcard.2007.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 16.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493–6. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 17.Ix JH, Shlipak MG, Liu HH, Schiller NB, Whooley MA. Association between renal insufficiency and inducible ischemia in patients with coronary artery disease: the Heart and Soul Study. J Am Soc Nephrol. 2003;14:3233–8. doi: 10.1097/01.asn.0000095642.25603.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–84. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 19.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB, Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA. 2007;297:169–76. doi: 10.1001/jama.297.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 1997;336:1350–5. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 21.McManus DD, Shah SJ, Fabi MR, Rosen A, Whooley MA, Schiller NB. Prognostic value of end-systolic left ventricular volume index as a predictor of Heart Failure Hospitalization in Stable Coronary Artery Disease: data from the Heart and Soul Study. J Am Soc Echocardiogr. 2008 doi: 10.1016/j.echo.2008.11.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ristow B, Ali S, Ren X, Whooley MA, Schiller NB. Elevated pulmonary artery pressure by Doppler echocardiography predicts hospitalization for heart failure and mortality in ambulatory stable coronary artery disease: the Heart and Soul Study. J Am Coll Cardiol. 2007;49:43–9. doi: 10.1016/j.jacc.2006.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol. 2007;49:1903–14. doi: 10.1016/j.jacc.2007.01.078. [DOI] [PubMed] [Google Scholar]