Abstract
We compared the concentration dependence of the ability of rats to identify odorants with the calcium signals in the nerve terminals of the olfactory receptor neurons. Although identification performance decreased with concentrations both above and below the training stimuli it remained well above random at all concentrations tested (between 0.0006% and 35% of saturated vapor). In contrast, the calcium signals in the same awake animals were much smaller than their maximum values at odorant concentrations less than 1% of saturated vapor. In addition, maps of activated glomeruli changed dramatically as odorant concentration was reduced. Thus perceptual stability exists in the face of dramatic changes in both the amplitude and the maps of the input to the olfactory bulb. The data for the concentration dependence of the response of the most sensitive glomeruli for each of five odorants was fitted with a Michaelis-Menten (Hill) equation. The fitted curves were extrapolated to odorant concentrations several orders of magnitude lower the smallest observed signals and suggest that the calcium response at low odorant concentrations is more than 1000 times smaller than the response at saturating odorant concentrations. We speculate that only a few spikes in olfactory sensory neurons may be sufficient for correct odorant identification.
Keywords: calcium imaging, odorant responses, awake recording, rats, behavior
Introduction
In the rodent olfactory system each glomerulus receives input from approximately 10,000 olfactory receptor neurons (Shepherd et al., 2004) and all of these sensory neurons express the same receptor protein (Mombaerts et al., 1996). The input from receptor neurons to glomeruli has been imaged by using calcium indicator dyes located in the receptor neuron nerve terminals (Friedrich and Korsching, 1997; Wachowiak and Cohen, 2001) or by monitoring the pH change measured with synapto-pHluorin during synaptic vesicle fusion (Bozza et al., 2004). Other imaging methods (intrinsic imaging, 2-deoxyglucose uptake, c-fos activation, and fMRI) report the combined pre-and post-synaptic activation of olfactory bulb glomeruli (e.g. Rubin and Katz, 1999; Johnson and Leon, 2000; Xu et al., 2000; Schaefer et al., 2001).
While these imaging methods are able to monitor the activity of a large number of glomeruli, it is not known how well these signals correlate with the animal’s ability to identify odorants over a wide range of odorant concentrations. We have compared the ability of rats to differentially identify (Youngentob et al., 1990) five odorants and in these same animals we monitored glomerular calcium signals in an awake/restrained preparation making both measurements as a function of odorant concentration. Previous studies in anesthetized rodents have shown that at higher odorant concentrations almost all monitored glomeruli are activated (Wachowiak and Cohen, 2001; Bozza et al., 2004) raising the possibility that animals might make errors in identification at high concentrations. Conversely, at odorant concentrations less than 0.1% of saturated vapor the nerve terminal optical signals are greatly reduced (and difficult to measure) suggesting that identification errors might occur at these concentrations as well.
Our behavioral measurements showed that the rats achieved greater than 75% correct odorant identification at all odorant concentrations we tested; from 0.0006% to 35% of saturated vapor. We then carried out measurements of the calcium signals in the same animals in an awake/restrained preparation. The rats were able to identify odors at concentrations where the calcium signals were too small to measure. And they made a high percentage of correct identifications at high odorant concentrations where a very large percentage of glomeruli are activated.
The concentration dependence of the calcium signals in the most sensitive dorsal glomeruli was fit by a Michaelis-Menten equation using a Hill coefficient of 1. When the fitted curves were extrapolated to an odorant concentration of 0.0006%, the amplitude was only 0.04% of the signal at saturating odorant concentrations. The correct identification when the calcium signal is more than 1000 times smaller than maximum suggests that relatively few spikes in olfactory receptor neurons are required for odorant identification.
Materials and methods
Six adult male Long-Evans rats (labeled LC1 through LC6) were used in the behavior measurements. Following the completion of behavioral testing we attempted to measure calcium signals in these same six animals in an awake/restrained preparation. Successful optical measurements were carried out in five of the six rats. At the time of the optical measurements the rats were eight months old and had an average weight of 400 g. We also made similar calcium measurements in an additional group four naive adult Long-Evans rats. The experimental protocols followed NIH guidelines and were approved by the appropriate institution’s Animal Care and Use Committee (SUNY Upstate Medical Center, Marine Biological Laboratory, and Yale University).
Choice of odorant set
As an initial screening we tested 17 odorants in rats. These odorants either had large dorsal signals in calcium measurements on mice (Wachowiak and Cohen, 2001, 2003) or had large dorsal signals in 2-deoxyglucose measurements (Glomerular Activity Response Archive, http://leonserver.bio.uci.edu). From the nine odorants with the largest signal in rats we chose five for the present experiments; propanal (Acros Organics, Geel, Belgium), hexanal (Acros Organics), octanal (Sigma-Aldrich, St. Louis, MO, USA), propyl acetate (Sigma-Aldrich) and isoamyl acetate (Sigma-Aldrich). Additional measurements of calcium signals were made with a sixth odorant, 2-hexanone (Sigma-Aldrich-Fluka, Hanover, Germany).
Behavior
Using previously established operant training procedures (Youngentob et al., 1990, 1991), the six rats were trained on a five-odorant identification confusion matrix task that can directly extract both odorant identification performance and perceptual quality relationships across a set of odorants (e.g., Youngentob et al., 1990, 2006; Kent et al., 2003). The rats were trained to break a photo-beam (trial initiating response) inside an odorant sampling port centrally located within a behavioral testing chamber and sample the odorant presented. Following a minimum sampling period (300 msec), the rats were trained to register a response choice in one of five alternative response tunnels that, by training, the animal had learned to associate with reinforcement for the odorant presented. Each animal had a different set of odorant/response-tunnel associations. A response choice was made when the animal licked the reinforcement cup at the end of the response tunnel. At the completion of training, the animals were capable of differentially reporting the random presentation of five different odorants. Criterion training was set at an overall performance level of >90% correct response over 200 training trials and no less than 80% identifiability for each individual odorant. With this standardly applied criterion the trained rats are near maximal performance.
The five odorant stimuli were generated and delivered according to previously established methods using flow dilution olfactometry and computer-controlled electronic mass flow controllers (e.g., Kent et al., 1995, 2003). Importantly, the design of the sampling port in conjunction with the delivery system obviated the potential for extraneous cues contributing to the odorant identification process (e.g., Youngentob et al., 1990, 1991, 1997; Youngentob and Schwob, 2006; see discussion). The training concentration for the odorants (expressed as percentage vapor saturation at 20°C) were 0.375%, 2.25%, 0.25%, 0.25%, and 0.375% for propyl acetate, octanal, amyl acetate, propanal, and hexanal, respectively.
Following criterion training, the animals were first tested stepwise on an ascending series of odorant concentrations (maximum concentration of each odorant was 35.3 % of saturated vapor). At the completion of this testing, the animals were then returned to the odorant concentrations used for training. This, in turn, was followed by a decreasing stepwise series of odorant concentrations (minimum concentration of each odorant 0.0006% (0.0012% for octanal)). In any one testing session only one odorant concentration of each odorant was used. Information about sampling time and sniff rates were not collected in the behavioral measurements. For analytic purposes, testing in any one session consisted of the randomized presentation of the odorants in pseudo-blocks of five trials with each pseudo-block consisting of the single presentation of each of the five different stimuli. Testing in any one session proceeded for 200 consecutive trials (40 trials per odorant).
Imaging
Staining and headpost implantation
Olfactory receptor neurons were loaded with Calcium Green-1 dextran (10 kD)(Molecular Probes/Invitrogen, Carlsbad, CA, USA) using methods similar to those described previously (Wachowiak and Cohen, 2001, 2003). The rats were anesthetized with Ketamine/Xylazine (“Ketaset”, Fort Dodge Animal Health, Fort Dodge, IA, USA; “Anased”, Lloyd Laboratories, Shenandoah, IA, USA) for dye perfusion into the nasal cavity. The dye-Triton solution was injected as a mixture with final concentrations of 4% dye and 0.15% Triton-X100. We used volumes between 20 and 30 μl. Two days after the dye injection the rats were reanesthetized with Ketamine/Xylazine. After application of a local anaesthetic, the skin covering the skull was retracted and the bone over the olfactory bulb was thinned and then covered with a layer of cyanoacrylate glue (Loctite) that protected the bone and prevented it from becoming opaque. A headpost was attached to the parietal and occipital bones using RelyX Luting cement (3M, ESPE; St. Paul, MN, USA). The optical recordings were carried out between 4 and 8 days following dye injection.
Awake/restrained animals
The rats were restrained first by placing them in a cylindrical cloth sack with their head protruding from one end but with all four legs inside the sack. This sack was then taped to restrict the animal’s movements and the headpost was fixed to a holder attached to the microscope stage. The rats seemed to accept the restraint without prior training to accommodate them to the experimental situation. These methods were demonstrated to us by Tomas Hromadka and Tony Zador (Hormadka et al, 2008). During most of the recording session the animals remained relaxed but even small movements such as whisking would introduce obvious noise into the optical recordings. For higher odorant concentrations we recorded two or four trials, for lower concentrations we recorded eight to 16 trials. All of the individual trials were saved and each trial inspected for obvious noise. Noisy trials (0% to 50% of each group of trials) were not included in the averaged response used for measuring signal amplitudes. The mean respiratory rate prior to odorant presentation during 37 trials from the six trained rats was 2.6 Hz; within the range of values reported for non-restrained rats (Walker et al, 1997). For high odorant concentrations an interval between trials of 60 seconds or greater was used; for low concentrations the interval was 30 seconds or greater. For each animal we attempted to measure the responses at four odorant concentrations to six odorants; one odorant concentration was used twice at the beginning and the end of the measurements using that odorant. The results of the repeated measurements were averaged and plotted in Figure 3. The above protocol required >150 trials over a period of two to three hours. By the end of the recording period the calcium signal response amplitudes had declined by more than 50%.
FIG. 3.
Normalized amplitude of the calcium responses (colored squares) for the most sensitive glomeruli and the mean percent correct behavior response (black squares) versus odorant concentration. The calcium signals are very small at the odorant concentration of 0.12% of saturated vapor. The smooth curves represent fits of the Michaelis-Menten equation using a Hill coefficient fixed at 1.0. The maximum value of the fitted curves was set to 1.0. The calculated curves fit the experimental points reasonably well. The data represent the response to five different odorants from one animal (LC1). For the 30 behavioral data points the range of the SEMs was between 0.4% and 3.9%.
Olfactometer used in imaging
To minimize the possibility of a mechanical response to the introduction of odorant into the airflow we used the olfactometer described earlier (Fig. 1 of Vucinic et al., 2006). This olfactometer was connected so that the odorant vapor, when introduced into the airflow, replaced the equivalent volume of diluent air so that the rate, humidity and direction of airflow over the nares was relatively constant throughout a measurement. The odorant flow rates were 37.5 ml/min for 11% of saturated odorant, 6.2 ml/min for 1.8%, 1.25 ml/min for 0.36%, and 0.4 ml/min for 0.12%. For the lower odorant concentrations the odorant syringe was turned on at least 50 seconds before the start of the trial to make sure that the odorant flow reached a steady state.
A two second odor pulse was delivered at a random time between 5 and 10 seconds after opening the incident light shutter. If a fixed two second interval between shutter and odor was used, it seemed that the animal would often move in anticipation of the odorant.
Optical recording
The dorsal olfactory bulb was imaged through a Wild 10× 0.4 NA objective on a Leitz Ortholux II upright microscope using Ploem epi-illumination; the actual magnification of the lens was 15×. Excitation light from a 100 W tungsten filament lamp was passed through a 488/50 nm band-pass filter and reflected by a 515 nm dichroic mirror. The fluorescence above 530 nm was recorded with a NeuroCCD-SM256 camera using NeuroPlex software (RedShirtImaging, Decatur, GA, USA) with a recorded frame rate of 125 frames per second and 128×128 pixel resolution. The data was temporally binned to 31.25 frames per second and saved to a computer disk. The trial duration was 6.4 seconds and the 2 second odorant pulse began 1 second after the beginning of the recording. The apparatus was mounted on a Nano-K Biscuit vibration isolation table (Minus-K Technologies, Inc., Inglewood, CA, USA).
Data analysis
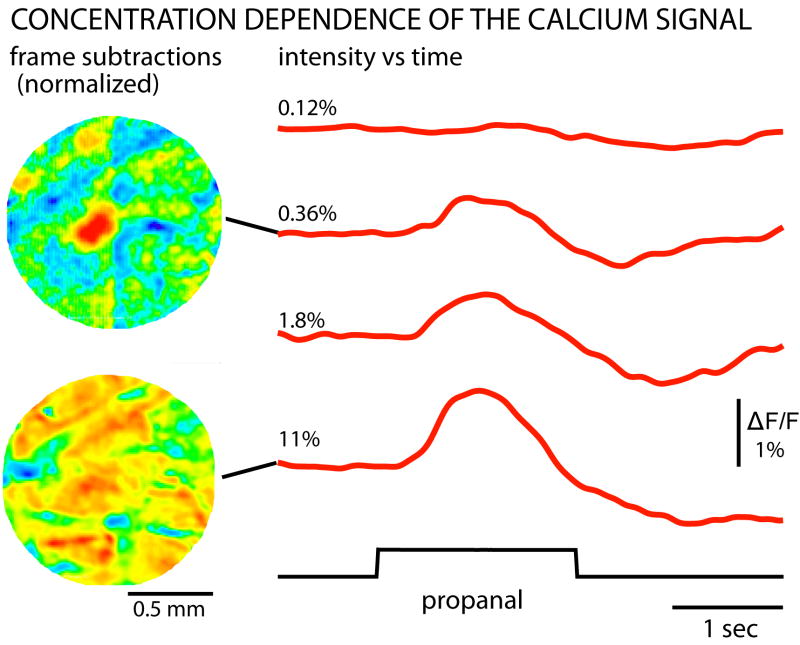
Less obvious remaining noise resulting from movement of the animal in the X-Y plane was reduced using a MATLAB (The MathWorks, Inc., Natick, MA, USA) program that maximized the correlation between frames by adjusting the X-Y position of one of the frames (Tay Netzog, Justus Verhagen, and Matt Wachowiak, personal communication). The results of analyses carried out before the movement correction were similar. The time course of the calcium signal was determined using NeuroPlex software (low pass Gaussian filter of 2 Hz; Fig. 2). We determined the location of the signal by subtracting the average of 16 frames prior to the odor presentation from 16 frames measured at the peak of the response. The resulting frame subtraction images (Fig. 2) were spatially smoothed by replacing each pixel value with the mean of 3×3 pixels centered on that pixel. These images were used to locate the most sensitive glomerulus; the glomerulus that had the largest signal at low odorant concentrations. We measured the signal amplitudes of the most sensitive glomeruli for each of the six odorants as the normalized fractional fluorescence change, ΔF/F, and plotted this value as a function of odorant concentration in Fig. 3. No correction was made for the background fluorescence of an unstained bulb; thus the fractional calcium dye fluorescence changes will be somewhat larger than the fractional changes shown in Fig. 2.
FIG. 2.
Calcium signals in the most sensitive dorsal glomerulus in response to four concentrations of propanal. Little or no signal could be detected at an odorant concentration of 0.12% of saturated vapor (top right trace). At 0.36% of saturation the largest signals were restricted to a localized region of the olfactory bulb (top left image). The image is the result of subtracting frames acquired prior to the odorant presentation from frames acquired during the response to odorant. At high odorant concentrations (11%) the signals were widespread (bottom left). The frame subtractions were normalized such that the largest signal in each frame subtraction is red and the absence of signal is blue. The traces are the average of 2 to 8 trials. They were temporally low-pass filtered with a 2 Hz Gaussian filter to reduce high frequency noise. The traces were also corrected for a sloping baseline by fitting the pre-stimulus period with an exponential and then subtracting that exponential curve from the entire time course. The images on the left were spatially smoothed by replacing each pixel value with the mean of 3×3 pixels centered on that pixel. Rat LC1.
The measured amplitude vs. concentration data points for the most sensitive glomerulus for each odorant were fit with a Michaelis-Menten equation with the Hill coefficient fixed at 1.0 using the Solver function in Excel 2003 (Microsoft, Bellevue, WA, USA).
Results
Behavior
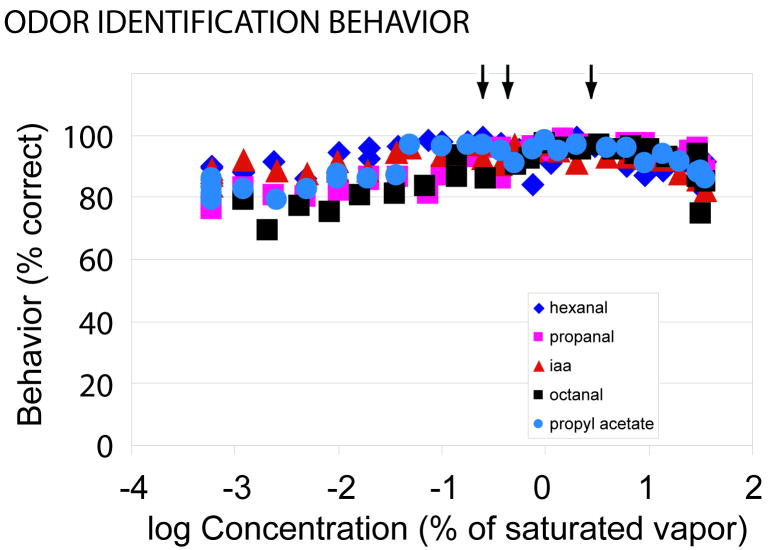
Behavior measurements were carried out on 6 Long-Evans rats. Fig. 1 is a plot of the mean % correct responses of the six animals for the five different odorants at concentrations between 0.0006% and 35% of saturated vapor (log10 of percentage of saturated vapor from -3.22 to 1.55). This range includes concentrations that are 50 times lower and 35 times higher than those used in previous odor identification measurements (Youngentob et al., 1990). The arrows indicate the odorant concentrations used for training. For all concentrations of all five odorants the percent correct is much higher than a random response (20%) indicating substantial performance stability. Nonetheless, overall identification performance did decrease for each of the odorants when concentrations were increased or decreased 1-2 log units from the training stimulus. On average, there was a 10.8% decrease in overall performance (t[5] = 3.42; P < 0.01) between the training stimuli and the lowest concentrations tested. Likewise, on average, there was 9.6% decrease (t[5] = 5.39; P < 0.002) between the training stimuli and the highest concentrations.
FIG. 1.
Percent correct odor identification as versus odorant concentration. At all tested concentrations the percent correct was far above random chance (20%). Five odorants were tested. Each data point is the average result from six animals. For the data points the range of the Standard Error of the Means (SEM) was between 0.5% and 13%; for most data points the SEM error bar would be smaller than the symbol. The left arrow indicates the training concentration for isoamyl acetate and propanal; the middle arrow indicates the training concentration for propyl acetate and hexanal; the right arrow indicates the training concentration for octanal.
An important question was whether, relative to the perceived odorant quality of the training stimuli, the animals’ retained perceptual stability at the high and low ends of the concentration range tested. To evaluate this hypothesis, the results from the testing sessions with the training stimuli as well as both the highest and lowest concentrations of odorant tested were each entered into a 5 × 5 odorant confusion matrix (e.g., Youngentob and Schwob, 2006; Youngentob et al., 1990, 1991, 2001, 2006). The rows represent the odorant presented, whereas the columns represent the same odorants as five stimulus response alternatives for each odorant presented. As such, the diagonal cells of the matrices contain the frequencies with which the rat responded with the correct response and the off-diagonal cells contain the frequencies of the four possible incorrect alternatives. One benefit of the confusion matrix method is that it permits the ability to directly extract perceptual quality relationships across a set of odorants (ibid). Briefly, the more two stimuli within the matrix have a similar smell, the greater is the probability they will be confused with each other. Consequently, for any two odorants of the identification task, the degree of similarity in their respective response distributions (both correct and incorrect responses) represents the degree to which any odorant pair is perceptually similar. Moreover, by extension, for any pair of rats, the degree of correspondence in the distributions of two entire matrices represents the extent to which the perceptual odor spaces of any two animals are similar.
For each of the six rats, the 5 × 5 odorant confusion matrices from the testing sessions with the training stimuli were compared to the matrices derived from testing with the highest and lowest concentrations both within and between rats yielding a dissimilarity matrix of odorant confusion matrices responses for all possible comparisons. The dissimilarity between any two matrices was an informational measure that quantified the pattern consistency of correct and incorrect responses across rats and conditions.
To quantitatively evaluate whether odorant quality perception was altered as a consequence of the extreme shifts in odorant concentration, we first applied the pairwise measures of rat dissimilarities to a multidimensional scaling analysis (MDS)(Kruskal scaling linear). For each of the two conditions (i.e., training vs. highest and training vs lowest) this analysis yielded a three-dimensional solution, with each rat occupying points in MDS space for both the training and concentration change conditions. The three-dimensional solutions accounted for 98% (stress = 0.05) and 96% (stress = 0.08) of the variance for training vs lowest concentration and training vs. highest concentration, respectively. The measure of variance expresses the degree of input/output variance accounted for by the three dimensional solution, i.e., how well does the solution accurately represent odorant dissimilarities as distances in MDS space. Stress is a goodness of fit statistic that MDS tries to minimize. Stress values vary between 0 and 1, with values near zero indicating a better fit.
To assess whether odorant quality perception was altered by the change in concentration we tested the null hypothesis that there was no difference in the training vs concentration change (either high or low) response patterns of the individual rats independent of performance on the task. To test the null hypothesis, a multivariate ANOVA was performed with each rat’s MDS coordinates, in three dimensions, as the dependent variables and concentration (as a categorical variable) as the independent variable. Performance at each concentration was used as a covariate in the model. The results of this analysis found no evidence for a shift in odorant quality perception with either the highest or lowest concentrations of odorant tested, F(3,7) = 0.35; P = 0.79 and F(3,7) = 0.48; P = 0.71, respectively.
Calcium signals
These same six animals were used for the optical measurements using a calcium dye that is loaded into the olfactory receptor neurons in the nose. Because it is conjugated to a high molecular weight dextran, it remains in the receptor neurons (Wachowiak and Cohen, 2001). In several days it travels to the receptor neuron nerve terminals in the olfactory bulb. Thus the glomerular calcium signals represent input from the nose to the olfactory bulb. Signals were obtained from only five of the six animals; the staining procedure was unsuccessful for the sixth. Fig. 2 illustrates the concentration dependence of the calcium signal for one of the five odorants (propanal) in one of the five rats (LC1). The two images on the left are frame subtractions of frames taken at the time of the signal peak minus frames prior to odorant presentation. The largest signal for the response to propanal at a concentration of 0.36% of saturation is localized near the center of the field of view. In contrast, as reported earlier in the mouse (Wachowiak and Cohen, 2001) the signals in response to 11% propanal are very widespread. The amplitude and time course of the signals at four concentrations from the dark red active region near the center in the top image is illustrated on the right. There was little or no detectable signal in response to 0.12% propanal; the signal then progressively increased as the propanal concentration increased. The bottom three traces in Figure 2 are a combination of a signal superimposed on noise. As a result of this noise, some amplitude measurements made in the absence of a signal will have a positive value and some a negative value.
In this animal we measured the calcium signal amplitude as a function of odorant concentration for five odorants. In Fig. 3 we have plotted (colored squares) the amplitudes as a function of the log10 of the percent odorant saturation. The figure shows the result for the most sensitive glomerulus for each of the five odorants. Four of the odorants were those used in the behavior experiments; 2-hexanone is an additional odorant. The mean of the behavioral results for the six animals and five odorants is also plotted (black squares). Clearly there is a poor correlation between the ability of the rat to identify odorants and the calcium signal amplitude. The calcium signals decline markedly over the concentration range of 0.1% -10% of saturated vapor while the ability of the rats to identify odors remains far above chance down to 0.0006%.
Fig. 3 shows fits to the data for the calcium responses using Michaelis-Menten kinetics (smooth curves). We used a Hill coefficient of 1.0; the k1/2 was the fitted parameter. The data points are reasonably well fit with this Hill coefficient; fits with a Hill coefficient of 0.67 or 2.0 were less good. This result is consistent with earlier fits where the Hill coefficient was a free parameter and its mean value was 1.2 (Wachowiak and Cohen, 2001).
The log10 of the k1/2 values from the fits in Fig. 3 are given in the first line in Table 1. The values obtained from the four additional trained animals where calcium signals were measured are also shown in the table. The mean log10 of the k1/2 for each odorant was determined and the mean of these means was 0.18 (1.5% of saturated vapor).
Table 1.
Sensitivity: k1/2 (in log10 of % of saturated vapor) of the most sensitive glomerulus.
| Trained Animals | ||||||
|---|---|---|---|---|---|---|
| propanal | hexanal | octanal | propyl acetate | isoamyl acetate | 2-hexanaone | |
| LC1 | -0.15 | 0.25 | 0.46 | 0.99 | -0.01 | |
| LC2 | 0.09 | 0.48 | 0.36 | 0.30 | ||
| LC3 | -0.58 | 0.06 | -0.09 | 0.36 | ||
| LC4 | -0.66 | 0.53 | 0.34 | -0.29 | -0.69 | |
| LC5 | -0.05 | -0.12 | -0.22 | -0.34 | ||
| Mean | -0.27 | 0.24 | 0.34 | 0.04 | 0.55 | -0.35 |
| Mean of means = 0.18 + 0.14 (SEM; N= 5) | ||||||
| Untrained Animals (N=3) | ||||||
| Mean | -0.56 | -0.43 | 0.24 | 0.01 | -0.07 | |
| Mean of means = -0.16 + 0.15 (SEM; N= 5) | ||||||
We made similar measurements on four untrained animals and obtained three measurements of k1/2 for each of the five odorants (Table 1). The mean of these means was log10 of -0.16. The means for trained and untrained animals are not significantly different; p>0.10. Although this result might be taken to indicate that behavioral training did not affect odorant sensitivity, it should be noted that the behavioral and calcium measurements were separated by more than one month.
Results for a sixth odorant, 2-hexanone, are also shown. On average the most sensitive 2-hexanone glomerulus was slightly more sensitive than the most sensitive propanal glomerulus.
To obtain an indication of the difference in sensitivity between the most sensitive glomerulus and other less sensitive glomeruli, for each odorant we measured the sensitivity of the most sensitive and the 6th most sensitive glomerulus. For both the trained and untrained animals the sixth most sensitive glomerulus had a k1/2 at a concentration that was on average 3 times higher than the most sensitive glomerulus.
Discussion
The results in Fig. 3 and Table 1 show that as a function of odorant concentration there is a poor correlation between olfactory receptor neuron calcium signals and the ability of the rat to correctly identify odorants. Reasonably accurate identification and stable odorant quality perception occurs at odorant concentrations where the calcium signals are much smaller than the maximum signal. Similarly, other imaging signals (synapto-pHluorin, intrinsic imaging, 2-deoxyglucose, and fMRI) also become much smaller than the maximum when the odorant concentration is reduced to 0.1% (e.g. Rubin and Katz, 1999; Johnson and Leon, 2000; Xu et al., 2000; Bozza et al., 2004). Thus this result is true for both the methods that monitor the activity of the receptor neuron terminals in the glomeruli (calcium-dextran dyes and synapto-pHluorin) and for methods (intrinsic imaging, 2-deoxyglucose, and fMRI) that measure a combination of pre-and postsynaptic glomerular activity.
Indirect evidence suggests that the odorant concentration dependence of the calcium signals in Figures 2-4 is not distorted by a threshold for the fluorescence change of Calcium Green 1. First, at low calcium concentrations the fluorescence of Calcium Green 1 is linearly related to calcium concentration (Invitrogen, Carlsbad, CA). Second, in in vitro preparations single olfactory nerve shocks lead to fluorescence changes in receptor nerve terminals and the size of this response decreased with repeated stimuli (Wachowiak et al, 2005, M. Wachowiak, personal communication) arguing against the possibility that there is a facilitation which results in a reduced calcium signal a low odorant concentrations which evoke modest receptor neuron activity. Other indirect evidence suggests that the fluorescence signals are likely to reflect the action potential activity in the presynaptic terminals. Measurements of somatic Ca2+ signals and action potential activity from in vivo mammalian somata have shown that the somatic signals directly reflect spiking. (Kerr et al., 2005).
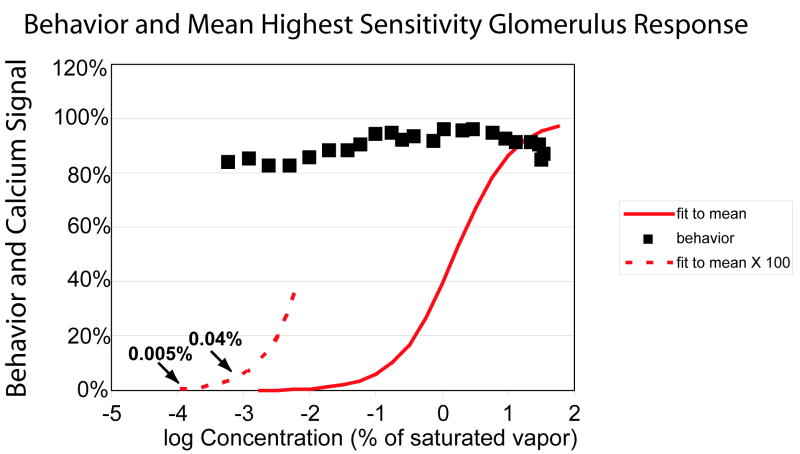
FIG. 4.
The mean percent correct behavior response (black squares) and the Michaelis-Menten equation (red curves) as a function of odorant concentration. On the left the Michaelis-Menten equation is shown in the dashed curve with a Y-axis expansion of a factor of 100. At the lowest odorant concentration tested in the behavior experiment the calcium signal is 0.04% of the maximum. The k1/2 of for the Michaelis-Menten equation was the mean of the means of the most sensitive glomeruli from Table 1 (log10 of k1/2 = 0.18); the Hill coefficient was 1.0.
The results in Figure 3 and Table 1 show that majority of the olfactory sensory neuron response range lies between 0.1% and 10% of saturated vapor. The fact that most of the dynamic range of the receptor input is devoted to higher odorant concentrations may suggest that identification becomes more difficult as large numbers of glomeruli are activated and thus more of the dynamic range is needed to achieve correct identification at high concentrations. A related hypothesis is that correct identification of odors at high concentrations could be more important. The decision about food ingestion is often made when the food is just under the nose and odorant concentration is relatively high. A mistake of ingesting a toxic substance can be life threatening.
In addition to the lack of correlation between calcium signal amplitude and odorant quality identification, the reported input maps and glomerular maps in mammalian preparations also change qualitatively as odorant concentration increases. At higher odorant concentrations more glomeruli are activated (Fig. 3; Wachowiak and Cohen, 2001, 2003; Rubin and Katz, 1999; Johnson and Leon, 2000; Xu et al., 2000; Bozza et al., 2004). Thus all of the maps are a confound of odor quality and odorant concentration.
It is perhaps not surprising therefore that the input to the bulb may not be directly related to odorant identification and quality perception over the very large range of odorant concentrations that we have tested. Presumably sensory processing in the bulb itself or in higher olfactory centers is important for odorant identification and maintaining perceptual quality invariance.
One concern about the optical imaging measurements (calcium, synapto-pHluorin, and intrinsic imaging) is that they sample only the dorsal glomeruli (about 15% of the total) and thus could miss glomeruli that might be more sensitive but were located in other regions of the bulb. However, this concern does not apply to 2-deoxyglucose and fMRI techniques that measure activity everywhere in the bulb; they also yield greatly reduced responses as odorant concentration is reduced. Furthermore, 2-hexanone, which has large dorsal signals in 2-deoxyglucose measurements (Johnson and Leon, 2000), has a k1/2 only slightly lower than the other odorants (Table 1). Finally, many odorants elicit a widespread 2-deoxyglucose uptake (Woo et al., 2007). Thus it is unlikely that our calcium measurements missed a hidden glomerulus with orders of magnitude more sensitivity.
In contrast to the poor correlation between identification performance, perception and imaging signals as a function of odorant concentration, strong relationships between behavior and imaging signals have been seen in other settings at relatively high odorant concentrations (1% 12% of saturated vapor). Using z-scored [14C]-2-deoxyglucose glomerular uptake, Youngentob et al. (2006) demonstrated that glomerular activity patterns predict perceptual quality relationships for odorants. Similarly, patterns of 2-deoxyglucose glomerular uptake predict the differential ability of rats to perceive two chemically similar stimuli as different in an olfactory habituation/dishabituation task (Linster et al., 2001; Cleland et al., 2002; Ho et al., 2006).
A potential concern regarding the poor correlation between the behavioral assessments and imaging signals in our measurements is the possibility that the rats were using extraneous cues to perform the task with changes in concentration. Several lines of evidence, however, argue against this possible caveat: (1) Air only trials have confirmed that the design of the odorant sampling port and delivery system obviate valve or flow cues (Youngentob et al., 1990, 1991). (2) this system was successfully used to assess threshold sensitivity in both mice (Youngentob and Margolis, 1999) and rats (Youngentob et al., 1997). (3) Using the present behavioral task animals with massive lesions to the olfactory epithelium are profoundly compromised in odorant quality identification and perception (Youngentob and Schwob, 2006). (4) Although there was a significant decline in identification performance with both increasing and decreasing concentration, we found no evidence for a shift in odorant quality perception at the two ends of the concentration range. It is difficult to envision how the animal’s responses, if driven by non-olfactory cues, would yield patterns of identification and misidentification identical to those using the training concentrations of stimuli. Our behavioral results are the first to demonstrate perceptual stability across such a large range of odorant concentration.
A speculation about the number of olfactory receptor neuron spikes needed for correct odorant identification
Fig. 4 illustrates a comparison of the mean behavior score (black squares) and the Michaelis-Menten equation with a k1/2 equal to the mean of means of the most sensitive glomeruli (Table 1, log10 = 0.19) and a Hill coefficient of 1.0. On the lower left this same fit is shown with the Y-axis expanded by a factor of 100 (dashed curve). At the lowest odorant concentration tested in the behavioral experiments the fit value is 0.04% of its maximum. With 10,000 axons innervating each glomerulus, 0.04% of the maximum signal would correspond to four axons with the same spike activity as they had at high odorant concentrations.
There are several critical assumptions in this estimation. One is the use of a Hill coefficient of 1.0. A smaller value would result in a larger predicted activity at the lowest odorant concentration. A second is that the animals are in different behavioral contexts for the optical measurements and the behavioral measurements. In the optical measurements the rat is passively responding to the odorant while in the behavioral measurements the rat is motivated to sample the odorant carefully in order to obtain a reward and may use a different odorant sampling activity. In the only other measurements of receptor neuron responses in awake mammals that we are aware of, carried out on behaving animals in a go/no-go task, reducing the odorant concentration to 0.1% also often resulted in a large reduction in the fluorescence response (M. Wachowiak, personal communication). Other assumptions are that the calcium signal is a linear reflection of the spike activity in the nerve terminals (see earlier discussion) and that the Michaelis-Menten equation will hold at concentrations that are several orders of magnitude lower than the concentrations where we can measure a signal. These assumptions remain to be tested. In the meantime it seems likely that the rat can correctly identify odorants with relatively few action potentials in relatively few olfactory receptor neurons.
We don’t know how much lower the odorant concentration has to be before the rat’s ability to identify odorants approaches random chance (20%). If, for example, the ability to identify odorants remains high at log10 = -4, the Michaelis-Menten curve used in Figure 4 predicts an activity of 0.005% of the maximum which corresponds to only a single active receptor neuron with half of the spike activity that it had at high odorant concentrations.
The above estimate leads to two predictions. The first is that monitoring many hundreds of olfactory sensory neurons would reveal a small number of cells that respond to odorant concentrations of <0.01%. The same might hold true for juxtaglomerular neurons. For juxtaglomerular neurons the prediction could be tested by measuring the odorant responses of a large number of cells using the bolus loading method and 2-photon microscopy (Stosiek et al., 2003). The second prediction is that a reduction in odorant concentrations by two orders of magnitude from those used in the present experiments should lead to substantially reduced odorant identification performance.
We found that the k1/2 for the sixth most sensitive glomerulus was 3 times more concentrated. These less sensitive glomeruli might also make a contribution at odorant concentrations of 0.0006% of saturated vapor. However, this contribution will not be easy to estimate. In rodents the number of activated glomeruli increases with increasing odorant concentration and some of the newly recruited glomeruli have larger signals than the most sensitive glomerulus (Fig. 2; Fig. 7B in Wachowiak and Cohen, 2001) implying that their Hill coefficient is greater than 1.0. Coefficients larger than 1.0 result in reduced responses at low concentrations.
Our finding that rats successfully identify odorants even at concentrations where the number of activated receptor neurons is small is especially striking considering that in awake rodents the spontaneous activity of mitral cells is greatly increased (Kay and Laurent, 1999; Rinberg et al., 2006; Davison and Katz, 2007) and odorant responses are detectable in only a small fraction of the mitral cells.
Acknowledgments
We thank Tomas Hromadka and Tony Zador for demonstrating the method for recording from awake rats, Robert Sachdev for helpful suggestions about the surgical and restraint procedures, and Matt Wachowiak for advice about staining. We thank Tay Netzog, Justus Verhagen, and Matt Wachowiak for the MATLAB program for correcting for movements in the X-Y plane. Brad Baker, Barbara Ehrlich, Matt Wachowiak, and Dejan Zecevic kindly reviewed an earlier version of the paper. Supported by NIH grants DC05259 and AA014871 (SLY).
References
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42:9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behavioral Neuroscience. 2002;116:222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Davison IG, Katz LC. Sparse and selective odor coding by mitral/tufted neurons in the main olfactory bulb. J Neuroscience. 2007;27:2091–2101. doi: 10.1523/JNEUROSCI.3779-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Combinatorial, and chemotropic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Ho SL, Johnson BA, Chen AL, Leon M. Differential responses to branched and unsaturated aliphatic hydrocarbons in the rat olfactory system. J Comp Neurol. 2006;499:519–532. doi: 10.1002/cne.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormadka T, DeWeese MR, Zador AM. Sparse Representation of Sounds in the Unanesthetized Auditory Cortex. PLoS Biol. 2008;6(1):e16. doi: 10.1371/journal.pbio.0060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Modular glomerular representations of odorants in the rat olfactory bulb and effects of stimulus concentration. J Comp Neurol. 2000;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Kay LM, Laurent G. Odor-and context dependent modulation of mitral cell activity in behaving rats. Nature Neuroscience. 1999;2:1003–1009. doi: 10.1038/14801. [DOI] [PubMed] [Google Scholar]
- Kent PF, Youngentob SL, Sheehe PR. Odorant-specific spatial patterns in mucosal activity predict perceptual differences among odorants. J Neurophysiol. 1995;74:1777–1781. doi: 10.1152/jn.1995.74.4.1777. [DOI] [PubMed] [Google Scholar]
- Kent PF, Mozell MM, Youngentob SL, Yurco P. Mucosal activity patterns as a basis for olfactory discrimination: comparing behavior and optical recordings. Brain Research. 2003;981:1–11. doi: 10.1016/s0006-8993(03)02512-5. [DOI] [PubMed] [Google Scholar]
- Kerr JND, Greenberg D, Helmchen F. Imaging input and output of neocortical networks in vivo. Proc Natl Acad Sci USA. 2005;39:14063–14068. doi: 10.1073/pnas.0506029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Yue E, Morse A, Xu Z, Hingco EE, Choi Y, Choi M, Messiha A, Leon M. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neuroscience. 2001;15:9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Sparse Odor Coding in Awake Behaving Mice. J Neurosci. 2006;26:8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B, Katz L. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Young DA, Restrepo D. Olfactory fingerprints for major histocompatibility complex-determined body odors. J Neurosci. 2001;21:2481–2487. doi: 10.1523/JNEUROSCI.21-07-02481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Chen W, Greer CA. Olfactory bulb. In: Shepherd GM, editor. Synaptic Organization of the Brain. Oxford University Press; New York: 2004. p. 165. [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucinic D, Cohen LB, Kosmidis EK. Presynaptic centre-surround inhibition shapes odorant evoked input to the mouse olfactory bulb in vivo. J Neurophysiology. 2006;95:1881–1887. doi: 10.1152/jn.00918.2005. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Correspondence between odorant-evoked patterns of receptor neuron input and intrinsic optical signals in the mouse olfactory bulb. J Neurophysiology. 2003;89:1623–1639. doi: 10.1152/jn.00747.2002. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, McGann JG, Heyward PM, Shao Z, Puche AC, Shipley MT. Inhibition of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx. J Neurophysiol. 2005;94:2700–2712. doi: 10.1152/jn.00286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JKL, Lawson BL, Jennings DB. Breath timing, volume and drive to breathe in conscious rats: comparative aspects. Respiratory Physiology. 1997;107:241–250. doi: 10.1016/s0034-5687(96)02520-0. [DOI] [PubMed] [Google Scholar]
- Woo CC, Hingco EE, Johnson BA, Leon M. Broad activation of the glomerular layer enhances subsequent olfactory responses. Chemical Senses. 2007;32:51–55. doi: 10.1093/chemse/bjl035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Kida I, Hyder F, Shulman R. Assessment and discrimination of odor stimuli in rat olfactory bulb by dynamic functional MRI. Proc Natl Acad Sci USA. 2000;97:10601–10606. doi: 10.1073/pnas.180321397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Margolis FL. OMP gene deletion causes an elevation in behavioral threshold sensitivity. NeuroReport. 1999;10:15–19. doi: 10.1097/00001756-199901180-00003. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Markert LM, Hill TW, Matyas EP, Mozell MM. Odor identification in rats: an update. Physiology and Behavior. 1991;49:1293–1296. doi: 10.1016/0031-9384(91)90366-v. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Markert LM, Mozell MM, Hornung DE. A method for establishing a five odorant confusion matrix task in rats. Physiology and Behavior. 1990;4:1053–1059. doi: 10.1016/0031-9384(90)90352-5. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Schwob JE, Sheehe PR, Youngentob LM. Odorant threshold following methyl bromide-induced lesions of the olfactory epithelium. Physioloy and Behavior. 1997;62:1241–1252. doi: 10.1016/s0031-9384(97)00301-6. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Johnson BA, Leon M, Sheehe PR, Kent PF. Predicting odorant quality perceptions for multidimensional scaling of olfactory bulb activity patterns. Behavioral Neuroscience. 2006;120:1337–1345. doi: 10.1037/0735-7044.120.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Schwob JE. Odorant identification following acute methyl bromide-induced lesions of the olfactory epithelium. Behavioral Neuroscience. 2006;120:1346–1355. doi: 10.1037/0735-7044.120.6.1346. [DOI] [PubMed] [Google Scholar]