Abstract
Background
Upper airway patency may be compromised during sleep and anesthesia due to either anatomical alterations (mechanical properties) or disturbances in the neural control (compensatory neuromuscular responses). The pathophysiology of upper airway obstruction during anesthesia may differ between men and women. Recently, we reported that the upper airway mechanical properties were comparable to those found during natural non-rapid eye movement sleep, as evaluated by measurements of passive critical closing pressure (PCRIT) and upstream resistance (RUS) during midazolam sedation. In this study, we compared the effects of gender on compensatory neuromuscular responses to upper airway obstruction during midazolam general anesthesia.
Method
Thirty-two subjects (14 men, 18 women) were studied. We constructed pressure-flow relationships to evaluate PCRIT and RUS during midazolam anesthesia. The midazolam anesthesia was induced with an initial dose of midazolam (0.07 to 0.08 mg/kg bolus) and maintained by midazolam infusion (0.3 to 0.4 µg/kg/min), and the level of anesthesia was assessed by Ramsay score (level 5) and Observer’s Assessment of Alertness/Sedation score (level 2). Polysomnographic and hemodynamic variables were monitored while nasal pressure (via mask), inspiratory air flow (via pneumotachograph) and genioglossal electromyograph (EMGGG) were recorded. PCRIT was obtained in both the passive condition, under conditions of decreased EMGGG (passive PCRIT), and in an active condition, whereas EMGGG was increased (active PCRIT). The difference between the active PCRIT and passive PCRIT (ΔPCRIT P-A) was calculated in each subject to determine the compensatory neuromuscular response.
Results
The difference between the active PCRIT and passive PCRIT (ΔPCRIT A-P) was significantly greater in women than in men (4.6 ± 2.8 cmH2O and 2.2 ± 1.7 cmH2O, respectively; p<0.01), suggesting greater compensatory neuromuscular response to upper airway obstruction independent of arousal.
Conclusion
We demonstrate that the arousal-independent compensatory neuromuscular responses to upper airway obstruction during midazolam anesthesia were partially maintained in women, and that gender may be a major determinant of the strength of compensatory responses during anesthesia.
INTRODUCTION
Maintenance of upper airway patency is critical for spontaneous breathing during sedation and general anesthesia, whereby upper airway dilator muscle activity becomes significantly compromised in the absence of arousal responses.1–3 It is well recognized clinically that there is an increased risk of apnea and hypopnea during deep sedation due to additional anesthesia administered in order to mitigate surgical stress or pain during procedures.4 These transient changes from deep sedation to general anesthesia may cause further sustained upper airway obstruction, similar to the sleep-disordered breathing events that occur in patients with obstructive sleep apnea hypopnea syndrome.5
In the unconscious or sleep states, upper airway patency may be compromised due to anatomical alterations (i.e., mechanical properties) or disturbances in upper airway neural control (i.e., compensatory neuromuscular responses).6 In previous studies, we have indicated that the hypotonic upper airway mechanical properties, evaluated by critical closing pressure (PCRIT) and up-stream resistance (RUS), during natural non-rapid eye movement (REM) sleep are comparable to those measured during moderate midazolam sedation.7,8 Moreover, we have shown9 that defects in both upper airway mechanical properties and the compensatory neuromuscular responses to upper airway obstruction are necessary for the development of obstructive sleep apnea. The difference between PCRIT under hypotonic conditions (passive PCRIT) and following progressive increases in neuromuscular activity (active PCRIT) is a measurement of the strength of dynamic compensatory neuromuscular responses to upper airway obstruction (ΔPCRIT A-P).9
The major risk factors for compromised upper airway patency during sleep (i.e., obesity, age, sex) may also play a role in collapse of the upper airway during general anesthesia. In healthy subjects and sleep apnea patients, we have recently shown differences in upper airway mechanical properties between men and women during sleep, independent of age and Body Mass Index (BMI).10 During moderate sedation, men also appear to have greater dynamic negative airway pressure-dependent decreases in upper airway patency than women,11 suggesting that there are gender differences in hypotonic upper airway mechanics. Nevertheless, it remains unclear whether men and women have differences in compensatory neuromuscular responses to upper airway obstruction during midazolam general anesthesia.
The aim of the present study was to quantify the magnitude of the effect of gender on the compensatory neuromuscular responses by assessing ΔPCRIT A-P during midazolam general anesthesia. We hypothesized that women have greater compensatory neuromuscular responses to upper airway collapse than men during midazolam anesthesia.
MATERIALS AND METHODS
Subjects
Thirty-two healthy subjects (14 men , 18 women; Table 1) were recruited, and a detailed clinical history was obtained. Subjects were excluded if they were overweight or obese (BMI>25 kg/m2); were frequent or excessive snorers (more than 3 times/wk); had abnormal sleep patterns or reported excessive daytime sleepiness (Epworth Sleepiness Score >10); had significant medical disease (cardiopulmonary pathology) or other clinical history (allergy to anesthesia); or reported tobacco use or chronic alcohol or drug use. Subjects were also excluded if they had an anatomical deformation of the upper airway, such as retrognathia (assessed by lateral view and occlusial condition), as a normal range of overbite (2–3 mm) and overjet (2–3 mm). All subjects had to have a Mallampati score of I or II and a thyromental distance longer than 60 mm. The experimental protocol was approved by the Human Investigation Committee of the Nagasaki University School of Dentistry and written informed consent was obtained from all subjects.
Table 1.
Subject Demographics
| Men | Women | P value | |
|---|---|---|---|
| Age (years) | 24.2±3.7 | 23.5±2.1 | n.s. |
| Height (m) | 64.4±6.4 | 49.9±5.5 | <0.001 |
| Weight (kg) | 1.7±0.1 | 1.6±0.1 | <0.001 |
| BMI (kg/m2) | 21.6±2.1 | 20.3±1.3 | n.s. |
| Completed Time (minutes) | 53.4±7.4 | 55.2±5.9 | n.s. |
| Initial midazolam dose (mg) | 4.8±0.5 | 3.9±0.4 | <0.001 |
| Total midazolam dose (mg) | 6.2±0.7 | 5.0±0.5 | <0.001 |
| Mean midazolam dose (µg/kg/min) | 1.83±0.23 | 1.82±0.17 | n.s. |
| Passive BIS | 53.4±4.3 | 51.5±4.6 | n.s. |
| Active BIS | 55.9±2.9 | 54.2±4.6 | n.s. |
BMI = Body Mass Index, BIS = Bispectral Index, n.s. = not significant
Experimental Techniques
Polysomnographic Measurements
All subjects were placed supine in a dark and quiet room and the head, neck and mandible were kept in a constant neutral position throughout the study using a molded pillow, as previously described.12 All subjects underwent routine hemodynamic monitoring (systolic and diastolic blood pressure, heart rate) and electrocardiogram, polysomnographic monitoring of sleep state, with bilateral electrooculograms, electroencephalograms (EEGs) and submental electromyograms (EMGs) to confirm steady-state anesthetic level. EEG signals were processed by the Bispectral Index (BIS) monitor (Aspect Medical Systems Inc., Natick, MA) in order to quantify the depth of anesthesia (Table 1). Oxygen saturation (SpO2) was measured by finger pulse oximetry, and transcutaneous CO2 (TcCO2, Tina, TCM4, Radiometer, Tokyo, Japan) was monitored continuously. Both thoracic and abdominal movements were recorded by inductance plethysmography.
Respiratory Effort, Pressure and Flow
Respiratory effort was monitored via a Hyatt-type esophageal balloon (Ackrad Laboratories, Cranford, NJ) placed pernasally for monitoring esophageal pressure. The tip of the catheter was positioned 45 to 50 cm from the nares. Airflow was measured by a pneumo-tachometer (#3830, Hans Rudolph, Inc., Kansas City, MO, USA), and nasal pressure (PN) was measured by a differential pressure transducer (#1100, Hans Rudolph, Inc., Kansas City, MO, USA), both connected to the mask. A variable pressure device (MAP/ResMed, Martinsreid, Germany) was used to deliver constant pressure in the nasal mask (PN) over the range from −15 to +15 cm H2O. These measurements were displayed and stored simultaneously on a computer using a data acquisition device (Embla A-10 with Somnologica, Medcare, Broomfield, CO, USA). Air leaks were prevented by applying surgical tape to the subject’s mouth after the bio-calibrations and immediately before the commencement of data acquisition.
Experimental protocols
Anesthesia
Midazolam anesthesia was maintained by the same infusion method previously described.8,13 Briefly, midazolam (~0.07 to 0.08 mg/kg) was initially injected at a rate of 0.5 mg/min until a steady-state of general anesthesia was attained. The behavioral responses as classified using the Ramsay scale (i.e., score 5 = sluggish response to a light glabellar tap or loud auditory stimulus) and Observer’s Assessment of Alertness/Sedation (i.e., score 2 = Responsiveness: responds only after mild prodding; Speech: Not recognizable) were used to titrate the maintenance infusion rate of midazolam (range: 0.2 to 0.4 µg/kg/min; Table 1) for the assessment of upper airway patency and compensatory neuromuscular responses. At the conclusion of the measurements, the total time needed for the completion of the study was recorded. The midazolam infusion was then withdrawn and all subjects remained supine until spontaneous emergence.
Measurement of upper airway collapsibility
Assessment of passive PCRIT
After reaching the maintenance level of anesthesia, PN was initially set at atmospheric pressure, then gradually increased to a holding pressure (range: 0–8 cm H2O) sufficient to abolish inspiratory airflow limitation, as previously described.14,15 Thereafter, the PN was rapidly changed from the holding pressure to a lower pressure for 5 successive breaths before being returned to the holding PN (“Passive state”; Figure 1 and Figure 2 – top panels).
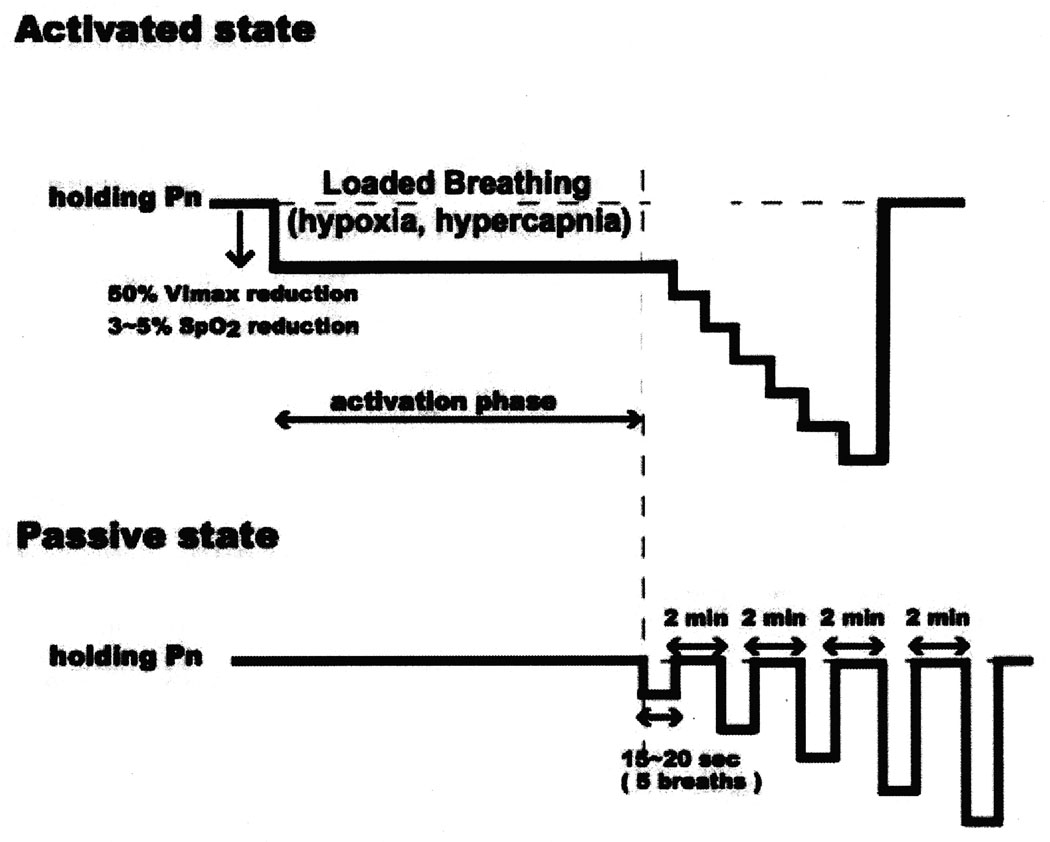
Figure 1.
Schematic diagram of the experimental protocol. The nasal pressure (PN) is modulated in a stepwise fashion to produce either the “passive state” for evaluating the mechanical properties of the upper airway during reduced genioglossal electromyograph (EMGGG) or the “active state” for evaluating the upper airway in the presence of increased tonic and phasic EMGGG activity.
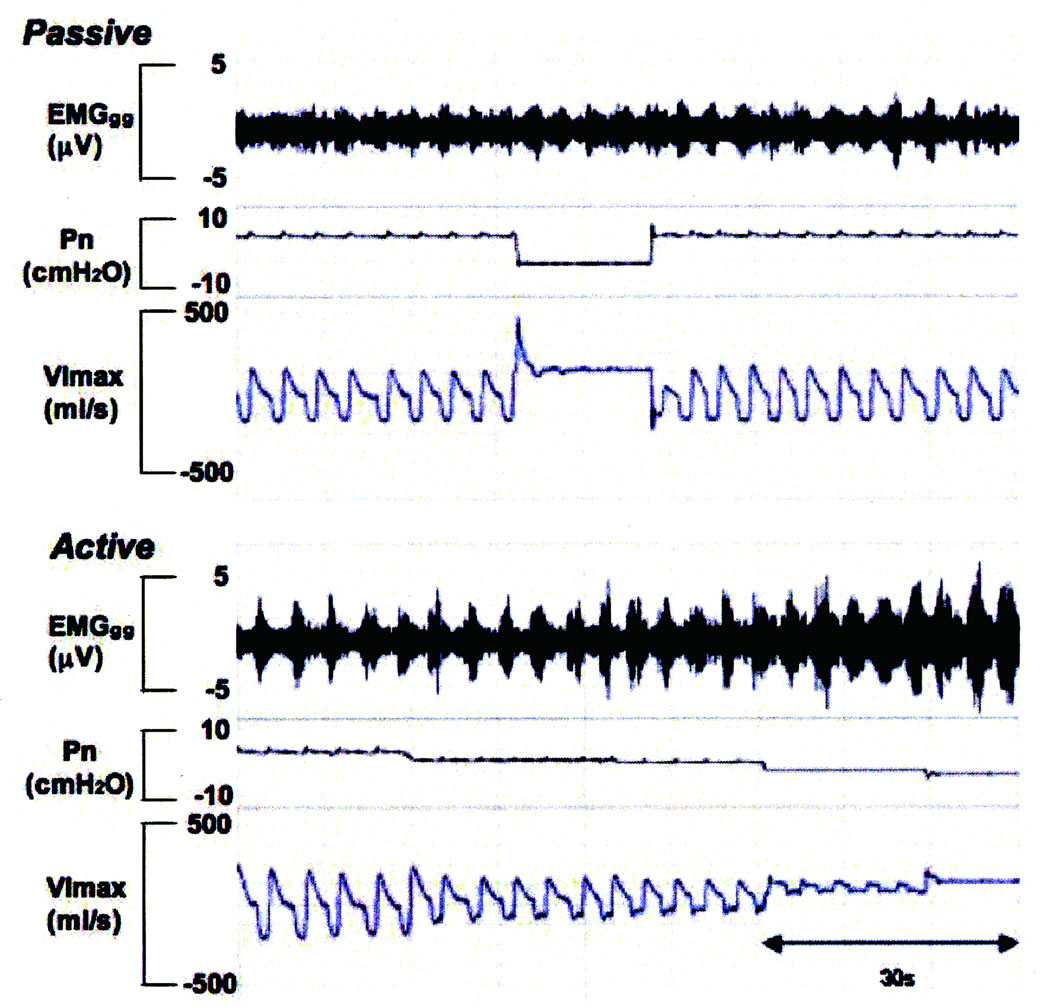
Figure 2.
Raw data obtained from the protocol in passive and active states. In the passive state, nasal pressure (PN) was reduced in a stepwise fashion from holding pressure (non-flow-limited breathing) to zero flow. In the activated state, after obtaining a stable unobstructed breathing pattern at a positive holding pressure, nasal pressure was then decreased by 2 cmH2O (associated with 40 ~50% reduction in maximum inspiratory airflow) from holding pressure to cause hypercapnia and resistive loads with partial flow limitation (“activation state”). Once the activity of the genioglossal electromyogram (EMGGG) started to increase, associated with a reduction in oxygen saturation of between 3% and 5% as well as an increase in transcutaneous PCO2 of between 5 and 10 mmHg above baseline value, then PN was held constant for 1 minute. Thereafter, PN was decreased again by 2 cmH2O in a stepwise fashion and maintained for 5 breaths until either obtaining zero flow or a lower limit of oxygen saturation of 88%. nasal pressure (Pn), maximum inspiratory airflow (VImax), genioglossal EMG activity (EMGGG)
Assessment of active PCRIT
We used a successive continuous pressure decrease method to produce transient hypoxia and hypercapnia under loaded breathing, similar to the method described in our previous study by Patil et al.9 After obtaining a stable unobstructed breathing pattern at a positive holding pressure, PN was then decreased by approximately 2 to 4 cmH2O (producing a 40% to 50% reduction in VImax) from holding pressure to produce flow-limited breaths associated with increased genioglossus EMG (EMGGG) activity (“Activated state”; Figure 1 and Figure 2 – bottom panels). Once the activity of genioglossus EMG started to increase, associated with moderate SpO2 desaturation (3–5%) as well as an increase in TcCO2 (5–10 mmHg above baseline value), PN was maintained for 5 minutes. Thereafter, PN was decreased again by 2 cm H2O in a stepwise fashion and maintained for 5 breaths at each PN level until either obtaining zero flow or reaching a lower SpO2 limit of 88% to 90%.
EMGGG monitoring
EMGGG was monitored (men, n = 10/14; women, n = 14/18), and the tonic and phasic EMGGG activity was analyzed using the method described by Eastwood et al.4,16 EMGGG was measured using a pair of unipolar intramuscular electrodes referenced to a single ground, thus producing a bipolar recording. Two 25-gauge needles containing 0.05 mm diameter, teflon-coated, stainless steel wires were inserted perorally ~1.5–2.0 cm into the body of the genioglossus muscle at points 3 mm lateral to the frenulum and midway between the first mandibular incisor and the sublingual fold. The needles were removed, leaving the wires in place. The raw EMGGG signal was amplified, bandpass filtered, and recorded at 200 Hz on the polysomnographic unit. Post hoc, the data were exported to analysis software (Chart 5.4.2, ADinstrument Pty Ltd, Bella Vista NSW, Australia), full-wave rectified, and integrated with a time constant of 100 ms.
Tonic activity of integrated EMGGG activity (minimum EMGGG value during expiration) was defined as the difference between electrical zero and end-expiratory activity during passive state and active state for each breath. Phasic activity of integrated EMGGG was defined as the difference between end-expiratory and peak-inspiratory activity when PN was decreased to near PCRIT level (i.e., presence of marked flow limitation without complete airway collapse). Measurements were expressed as a percent change from the maximal value obtained during voluntary tongue protrusion against the upper incisors and forced swallows.
Data analysis
Upper airway pressure-flow relationship
Peak inspiratory airflow and corresponding mask pressure for each breath were analyzed at all levels of PN and evaluated for the presence of inspiratory airflow limitation, as previously described.15,17 Briefly, inspiratory flow limitation was defined as the presence of a plateau in inspiratory airflow in association with a continued decrease in esophageal pressure by at least 1 cm H2O beyond the onset of the plateau. Flow limitation in the presence of an abdominal strain gauge was determined using the criterion of Hosselet et al.18 Breaths associated with arousal were excluded from analyses. As reported previously,19 the inspiratory pressure-flow relationship was analyzed by Least-squares linear regression and fitted by the following equation: VImax = (PN − PCRIT) / RUS, where PCRIT is the critical closing pressure (PN at zero flow) and RUS is the resistance of the portion of the tube upstream from the site of collapse (Figure 3).
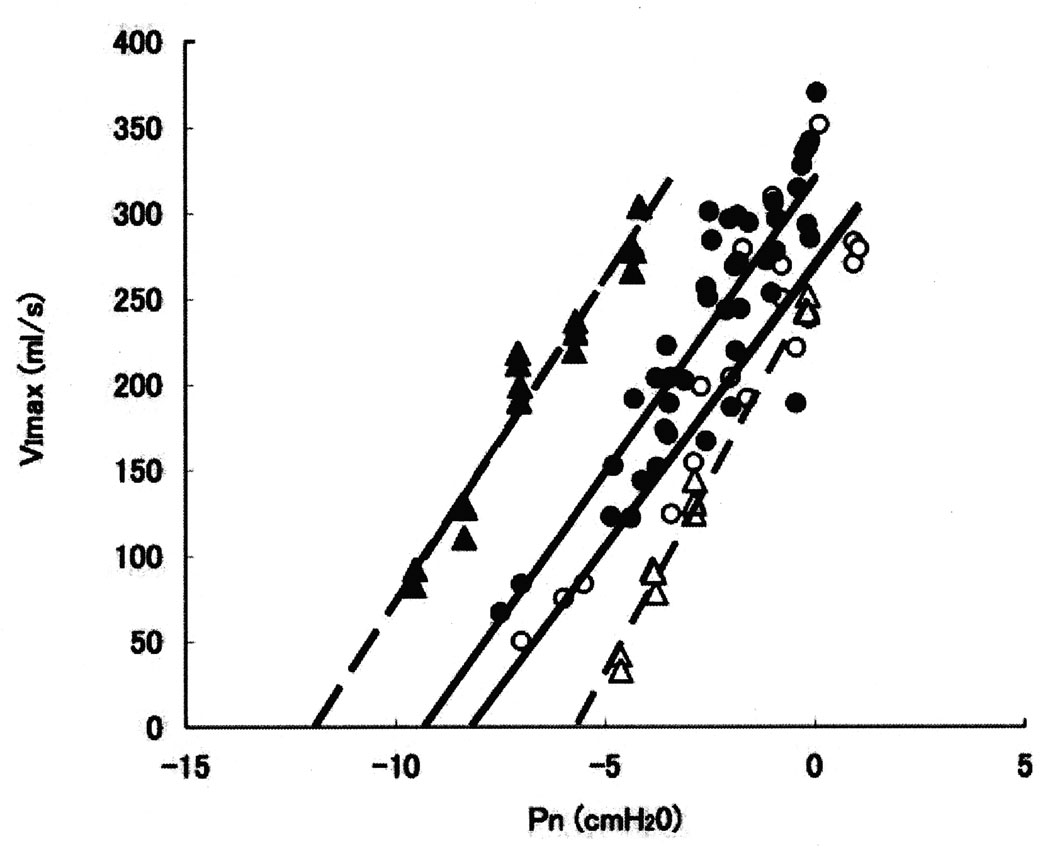
Figure 3.
An example of the nasal pressure (Pn) vs. maximum inspiratory airflow (VImax) relationships in one female subject (ID = p) and one male subject (ID = M) during passive and active conditions. Upstream resistance (RUS) was defined as the reciprocal of slope of the relationship between VImax and PN, and critical closing pressure (PCRIT) as the x intercept of the regression line. In the passive state for subject - p, PCRIT = −5.7 cmH2O (open triangle) and RUS = 22.2 cmH2O/L/sec. In the active state, PCRIT = −12.0 cmH2O (solid triangle) and RUS = 24.9 cmH2O/L/sec. Similarly for subject M, In the passive state, PCRIT = −7.9 cmH2O (open circle) and RUS = 31.4 cmH2O/L/sec, whereas in the active state, PCRIT = −9.7 cmH2O and RUS = 34.3 cmH2O/L/sec (solid circle). There was a larger increase in difference between the passive and active PCRIT values in subject p, representing a greater improvement in collapsibility in the activated state, compared to subject M.
Magnitude of the Compensatory Neuromuscular Response (ΔPCRIT A-P)
The compensatory neuromuscular response to upper airway obstruction (ΔPCRIT A-P) is determined by the difference between passive PCRIT and active PCRIT in the following equation:
where passive PCRIT represents the hypotonic mechanical properties of the upper airway and active PCRIT is the arithmetic sum of the mechanical properties and the compensatory neuromuscular responses.9
Statistical Analysis
All statistical analyses were performed using SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). The primary outcomes in the study including the passive PCRIT and active PCRIT were analyzed using General Linear Model (GLM) for repeated measures, with a post hoc protected Bonferroni adjustment test after confirmation of normal data distribution (test for skewness and kurtosis). Secondary outcomes included difference in RUS and difference between the active and passive PCRIT (ΔPCRIT A-P), and tonic and peak phasic EMGGG were statistically analyzed using the Mann-Whitney test because data were not normally distributed. Statistical significance was assumed for P < 0.05. The data are presented as mean ± standard deviation (SD) with 95% confidence intervals (95% CI), unless otherwise noted.
Due to technical failure of the intramuscular electrode placement, we were unable to obtain EMGGG measurements in 4 female and 4 male subjects. Data from these subjects were included in the primary analysis of the gender differences in passive PCRIT, active PCRIT and ΔPCRIT A-P measurements and in secondary analysis of RUS. There was no effect on the reported gender differences in the upper airway properties when these subjects were excluded from the analysis.
RESULTS
In 32 subjects (14 men, 18 women; Table 1) there was a significant difference in height and body weight between men and women but no difference in BMI values. In men the initial dose of midazolam administered was 4.8 ± 0.5 mg and the total dose was 6.2 ± 0.7 mg over 53.4 ± 7.4 minutes for the completed study (1.83 ± 0.23 µg/kg/min). In women the initial dose of midazolam was 3.9 ± 0.4 mg and the total dose was 5.0 ± 0.5 mg over 55.2 ± 5.9 minutes (1.82 ± 0.17 µg/kg/min). Although the initial and total doses of midazolam were larger in men than in women, the weight-adjusted doses were the same (Table 1).
There was no significant difference in BIS values between men (53.4 ± 4.3) and women (51.5 ± 4.6%) or between the passive (51.8 ± 6.3) and active (54.2 ± 9.3%) states (P=0.16). During steady-state anesthesia >85% of EEG power spectrum had frequencies in the <4Hz range (delta waves) for all subjects, i.e., comparable to EEG power spectrum of non-REM stage 3 to 4 sleep. During the passive state there was no significant increase of TcCO2 from baseline (39.6 ± 6.4 mmHg) to passive level (41.7 ± 4.9 mmHg, P=0.13), whereas during the active state TcCO2 increased from baseline (40.8 ± 5.1 mmHg) to the activated level (48.1 ± 4.9 mmHg; p<0.05). There were no changes in the SpO2 during measurements in the passive state, whereas during the active state, the lowest value of SpO2 decrease from a baseline of 97.2 ± 0.9% to 91.6 ± 1.8% (p<0.05).
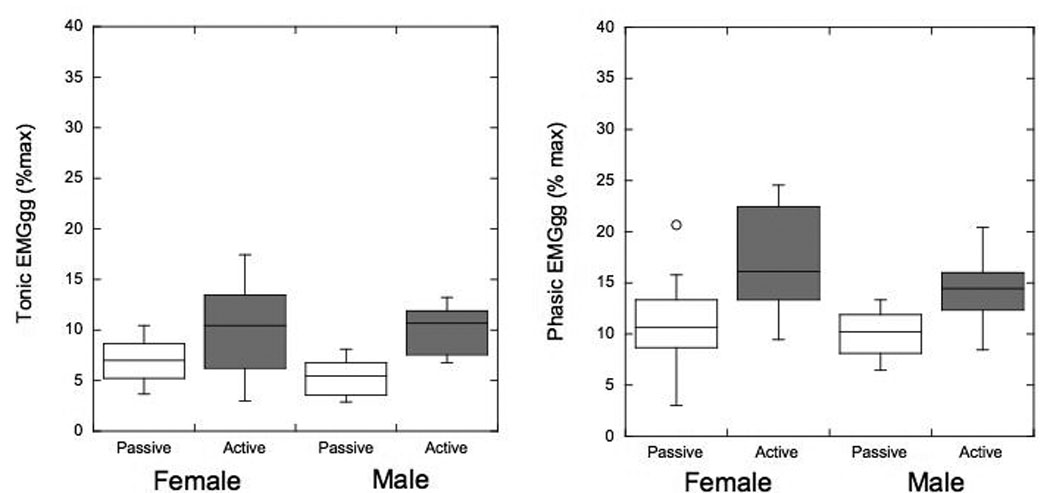
The tonic EMGGG activity significantly increased from 5.4 ± 1.8% in the passive state to 10.2 ± 2.3% in the active state (p<0.01) in men and from 7.2 ± 2.1% in the passive state to 10.2 ± 4.5% in the active state (p<0.05) in women. Similarly, peak phasic EMGGG activity increased from the passive state (10.1 ± 2.5%) to the active state (14.4 ± 3.7 %; P<0.01) in men; and from the passive state (11.1 ± 4.4%) to the active state (16.9 ± 4.8%; P<0.01) in women. There was no significant difference in the EMGGG in any state between men and women (all P>0.3). (Fig 4)
Figure 4.
Box plots showing the tonic and phasic genioglossus electromyograph (EMG) (EMGGG) activity as a percent of max in male and female subgroups. Line = median, box = 25th–50th percentiles, whiskers and cap = 95th percentile, diamond-shaped = 95% confidence interval; *=p<0.05. Note that there is an increase in the tonic and phasic EMGGG in the activated state; however, there were no significant differences between men and women.
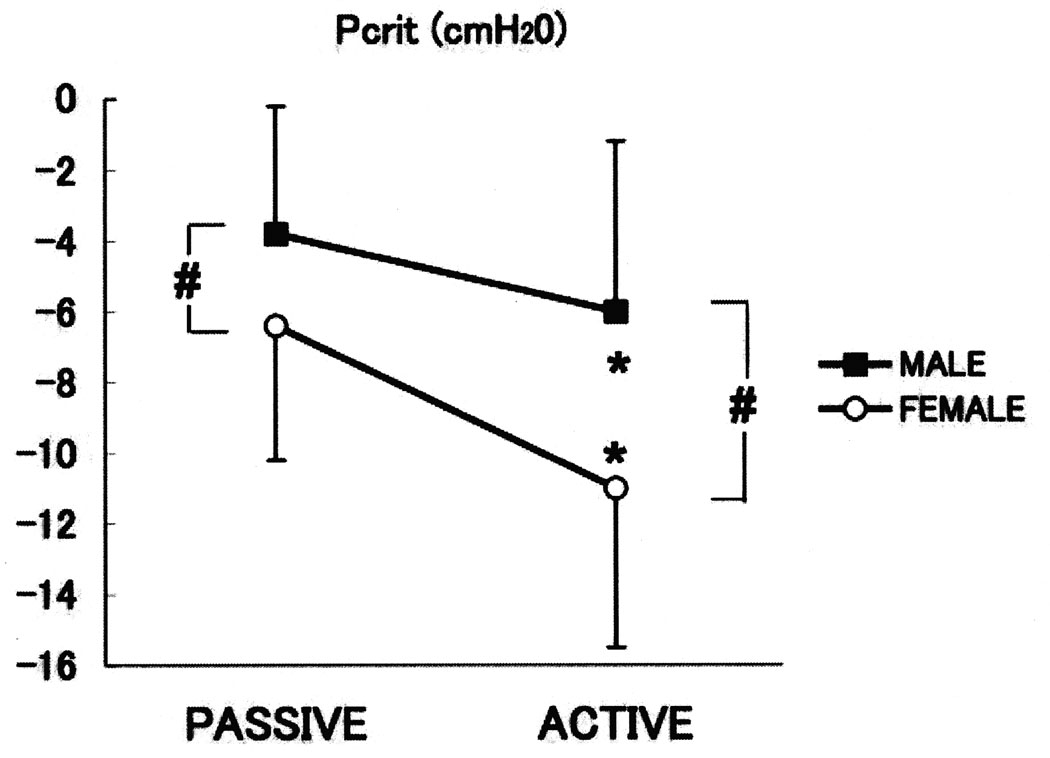
The individual subjects’ passive PCRIT values are shown for both men and women (Tables 2A and 2B). The mean passive PCRIT in women was significantly lower (more negative) than in men (−6.4 ± 3.8 cmH2O and −3.8 ± 3.8 cmH2O, respectively; P<0.05) (Figure 5); however, there was no difference in the RUS in the passive state (30.7 ± 19.2 cmH2O and 36.0 ± 20.7 cmH2O, respectively). Similarly, active PCRIT was significantly decreased in women compared to men (−11.0 ± 4.5 cmH2O and −6.0 ± 4.6 cmH2O, respectively; p<0.05); however, there was no difference in the active RUS (32.6 ± 20.1 cmH2O/ml/s and 38.5 ± 21.6 cmH2O/ml/s, respectively). The ΔPCRIT A-P was significantly larger in women than men (4.6 ± 2.8 cmH2O and 2.2 ± 1.7 cmH2O, respectively; p=0.007; Tables 2A and 2B).
Table 2.
| Table 2 A. Individual Upper Airway Collapsibility Values in Men (n=14) | |||||
|---|---|---|---|---|---|
| Passive | Active | ||||
| Subject ID |
PCRIT (cm H2O) |
RUS (cm H2O/L/s) |
ΔPCRIT A-P (cm H2O) |
PCRIT (cm H2O) |
RUS (cm H2O/L/s) |
| A | −7.2 | 38.5 | 2.6 | −9.8 | 54.3 |
| B | −5.7 | 24.2 | 1.8 | −7.5 | 32.9 |
| C | 1.2 | 21.7 | 4.1 | −2.9 | 19.5 |
| D | 1.0 | 25.0 | 0.1 | 0.9 | 22.0 |
| E | −6.3 | 21.4 | 2.3 | −8.6 | 36.9 |
| F | −0.6 | 68.5 | 1.5 | −2.1 | 54.6 |
| G | −2.7 | 15.9 | 0.3 | −3.0 | 10.8 |
| H | −10.6 | 21.2 | 5.0 | −15.6 | 47.6 |
| I | 1.9 | 76.1 | 0.2 | 1.7 | 74.4 |
| J | −7.9 | 31.4 | 1.8 | −9.7 | 34.3 |
| K | −6.0 | 25.7 | 0.8 | −6.8 | 36.1 |
| L | −3.8 | 12.3 | 1.9 | −5.7 | 2.3 |
| M | −2.0 | 34.9 | 5.7 | −7.7 | 12.3 |
| O | −4.7 | 13.4 | 2.6 | −7.3 | 18.9 |
| Mean (95%CI) |
−3.8 (−5.9, −1.8) |
30.7 (20.5, 41.0) |
2.2 (1.2, 3.2) |
−6.0 (−8.5, −3.5) |
32.6 (21.2, 44.1) |
| SD | 3.8 | 19.2 | 1.7 | 4.6 | 20.1 |
| Table 2 B. Individual Upper Airway Collapsibility Values in Women (n=18) | |||||
|---|---|---|---|---|---|
| Passive | Active | ||||
| Subject ID |
PCRIT (cm H2O) |
RUS (cm H2O/L/s) |
ΔPCRIT A-P (cm H2O) |
PCRIT (cm H2O) |
RUS (cm H2O/L/s) |
| a | −3.8 | 21.5 | 4.1 | −7.8 | 17.8 |
| b | −4.6 | 25.6 | 2.3 | −6.9 | 19.5 |
| c | −4.0 | 15.0 | 2.1 | −6.11 | 16.0 |
| d | −8.0 | 40.2 | 3.1 | −11.1 | 38.5 |
| e | −2.8 | 46.5 | 11.0 | −13.8 | 80.6 |
| f | −14.5 | 81.0 | 4.5 | −19.0 | 90.0 |
| g | −4.7 | 32.7 | 5.2 | −9.9 | 64.8 |
| h | −13.5 | 67.2 | 2.0 | −15.5 | 58.2 |
| i | −8.0 | 30.5 | 2.0 | −10.0 | 28.0 |
| j | −5.5 | 33.3 | 8.6 | −14.1 | 28.7 |
| k | −4.3 | 21.4 | 1.6 | −5.9 | 19.8 |
| l | −1.4 | 26.9 | 1.6 | −3.0 | 40.7 |
| m | −8.8 | 33.4 | 3.6 | −12.4 | 36.9 |
| n | −2.2 | 44.3 | 3.1 | −5.3 | 36.4 |
| o | −3.9 | 16.1 | 8.5 | −12.4 | 28.0 |
| p | −5.7 | 22.2 | 6.3 | −12.0 | 24.9 |
| q | −11.2 | 25.9 | 7.0 | −18.3 | 22.3 |
| r | −8.3 | 64.4 | 6.6 | −14.9 | 41.1 |
| Mean (95% CI) |
−6.4 (−8.2 −4.6) |
36.0 (27.0 45.0) |
4.6 (3.2 6.0) |
−11.0 (−13.2 −8.8) |
38.5 (28.4 48.5) |
| SD | 3.8 | 18.5 | 2.8 | 4.5 | 21.6 |
PCRIT = critical closing pressure; RUS = upstream resistance; ΔPCRIT A-P = difference between the active and passive PCRIT; 95% CI= confidence interval, SD = standard deviation
Figure 5.
The mean data in passive and active critical closing pressure (PCRIT) in men (squares) and women (circles) subjects are shown in Figure 5. There is a significant difference between active PCRIT and passive PCRIT values (p<0.05).
DISCUSSION
The main findings of this study were that under midazolam anesthesia: 1) the mechanical upper airway properties were more collapsible in female subjects than in men, and 2) the strength of the compensatory neuromuscular responses (ΔPCRIT A-P) to upper airway obstruction was greater in women than in men.
Effect of Gender on Upper Airway Collapsibility
Mechanical Properties
We found a significant gender effect on the mechanical properties of the upper airway, as evaluated by measurements of the passive PCRIT. The passive PCRIT was lower in women (−6.4 ± 3.8 cmH2O) than in men (−3.8 ± 3.8 cmH2O) under midazolam anesthesia, and approximated the differences found by Norton et al.11 during midazolam sedation and the passive PCRIT values (2.6 cmH2O) reported in our previous study10 and in a smaller study cohort by Jordan et al.20 during sleep. Norton et al. investigated the hypotonic upper airway mechanics during a moderate level of midazolam sedation and reported that men have increased dynamic pressures required to induce upper airway obstruction (a method similar yet not as sensitive as the passive PCRIT method). In the current study men and women had approximately the same BMI and age, therefore the difference in upper airway mechanical properties may be related to other factors, i.e., pharyngeal size, changes in resting lung volume, surrounding soft tissue structures and fat distribution,21,22 or female sex hormones. It has been shown that the pharynx is more elongated in men than in women,23,24 leaving a larger region exposed to upper airway collapse. An elongated upper airway may be more vulnerable, especially with the depressed level of neuromuscular activity of dilator muscles under midazolam anesthesia. Deposition of truncal or central adiposity may be associated with decreased lung volumes and diminish sternomedial caudal traction on upper airway structures, and in turn compromise upper airway patency and increase PCRIT.25–29 It has also been suggested that the resting activity of the genioglossus muscle is higher in healthy women than in men during wakefulness21 and higher in women in the luteal than the follicular menstrual phase.23 Therefore, female sex hormones may play a role in determining upper airway mechanical properties, although it is still uncertain how the resting level of the genioglossus muscle alters upper airway collapsibility during sleep. Future research should examine the effect of menstrual hormones on upper airway patency in normal women during general anesthesia and sleep.
Compensatory Neuromuscular Responses to Upper Airway Collapse
We are not aware of any other study that examined the compensatory neuromuscular responses to upper airway collapse during anesthesia with midazolam or any other sedative hypnotic drugs. The magnitude of ΔPCRIT A-P represents the contribution of the upper airway muscles to counterbalance intra- and extraluminal collapsing pressures. For example, a 5cm H2O ΔPCRIT A-P, due to increased neuromuscular activity, has the same stabilizing effect as applying 5 cm H2O of continuous positive airway pressure to the upper airway. Previously, we have shown that a change in PCRIT of ~5cm H2O due to neuromuscular activity is clinically relevant,9 because this represents the magnitude of the response required to convert either obstructive apneic events to the less severe hypopneic events or hypopneic events to stable breathing. In the current study, the component of upper airway collapsibility attributed to increased neuromuscular activity in response to obstruction in men ranged from almost no response (0.1 cm H2O) to a maximum of 5.7 cm H2O, with only 2 of the 14 men having a ΔPCRIT A-P more than or equal to 5 cm H2O. Therefore, less than 15% of men have compensatory neuromuscular responses that provide sufficient airway support during upper airway collapse with midazolam anesthesia. In contrast, the compensatory neuromuscular response in women ranged from 1.6 cm H2O to 11.0 cm H2O, and more than one-third of the women had a ΔPCRIT A-P of more than 5 cm H2O. More than twice as many women maintained adequate neuromuscular activity, suggesting that women preserve upper airway compensatory neuromuscular responses to a greater extent than men during midazolam anesthesia.
The gender effect on compensatory neuromuscular responses is not due to differences in the tonic activity of upper airway dilator muscles, although we cannot exclude the possibility of a small increase in the phasic EMGGG in response to obstruction. Furthermore, the ΔPCRIT is not simply a measure of upper airway dilator muscle activity, but is the composite of the alterations in muscle activity and any structural changes that result from increased activity. Gender-related differences in the hypoxic and hypercapnic ventilation response may partly determine the threshold for recruiting upper airway dilator muscles. It has also been suggested that the arousal response and compensatory neuromuscular response (arousal-independent airway opening) are the 2 most important factors that promote upper airway reopening in the face of sustained obstruction.30 Previous studies have reported that the arousal response is triggered when a critical level of chemo-mechanoreceptor input is reached31 and that the arousal-independent recruitment of upper airway dilators is sensitive to the same inputs.32 Younes30 indicated that many older subjects are protected from further hypoxia by a relatively high arousal threshold from sleep. Therefore, if men have a relatively high threshold for chemo-mechanoreceptor inputs (hypoxia and hypercapnia) compared to women, men may require the arousal response to compensate for upper airway collapse. Women, on the other hand, are able to activate their compensatory neuromuscular response to upper airway obstruction via an arousal-independent mechanism. We speculate that male subjects are unable to elicit a compensatory neuromuscular response (arousal-dependent system) because midazolam significantly alters the threshold for chemo-mechanoreceptor input and arousal response.
Resistance Upstream from the Site of Collapse (RUS)
RUS reflects the degree of upper airway narrowing upstream from the site of collapse. The lack of change in RUS reported in our subjects may reflect a relatively constant airway size between the passive and active states at a given VImax despite changes in upper airway PCRIT in response to increased EMGGG.14,33 The most likely explanation for this finding is that the upper airway segment upstream from the site of collapse was not involved in the compensatory neuromuscular response at the depressed level of dilator muscle activity associated with midazolam anesthesia. The velopharyngeal segment of the upper airway is particularly prone to collapse and has been found to be the predominant flow-limiting site during sleep,34 sedation,35–37 and anesthesia.38 In the current study, we did not find any difference in the upstream resistance between men and women in either the passive or active states, which suggests that the site of upper airway collapse is most likely the same.
Possible Limitations of the Current Study
Several anatomic, demographic and anthropometric factors related to the study population may have influenced our findings. Older individuals,39 those with increased BMI,10 and postmenopausal women23,40 have more collapsible upper airways. Older individuals also seem to be more susceptible to midazolam anesthesia.41 The current study, however, was performed in a homogenous population of normal, young healthy Japanese men and women. Although it is difficult to generalize our findings to all patients undergoing midazolam anesthesia, the current study is well suited to examine the effect of gender on upper airway neuromuscular responses to upper airway obstruction. Moreover, it has previously been shown that progesterone decreases tonic neuromuscular activity of dilator muscles during the luteal phase of the menstrual cycle.23 The phase of menstruation was not recorded in the present study, thus we cannot exclude the potential for menopause or variations in sex hormones to influence either upper airway dilator muscle activity or upper airway collapsibility. Additionally, there are a variety of anatomical features of the maxilla and mandible in Japanese subjects that contribute to the patency of the upper airway42 but which do not necessarily apply to other ethnic populations.
A number of methodological factors were also considered as potential confounders of this study. First, there is not always a direct correlation between the Ramsay Score and BIS values. In the current study, however, steady-state anesthesia was established using the behavioral indicators of the Ramsay Score both before and after measurement in the passive and active states. BIS values were recorded as a secondary quantitative measure, as commonly adopted in clinical practice. Second, we applied a number of constraints to ensure the safety of the healthy participants in our study. The SpO2 was maintained above 90% during the application of negative collapsing pressure to the airway. In the current study, we have not determined whether more severe oxyhemoglobin desaturation and concomitant hypercapnia alter the neuromuscular responses to obstruction. Third, the absence of supraglottic pressure measurements limits our ability to determine changes in airway resistance during non-flow-limited breathing from wakefulness to anesthesia. Since spontaneous breathing during general anesthesia in the absence of secure airway management procedures is likely to be associated with airflow limitation, the relevant comparison for the assessment of compensatory neuromuscular responses to airway obstruction is between the baseline passive state and the heightened EMG neuromuscular activity in the active state. Additional investigations are required to assess the maintenance of neuromuscular responses as patients transition from wakefulness to general anesthesia. Lastly, in the current study only a single pair of bipolar intramuscular electrodes was used to directly record the genioglossus, one of many dilator muscles involved in patency of the upper airway. Thus, we acknowledge that we are only able to determine the contribution of the small increase in EMGGG that is associated with the total increased neuromuscular activity of the numerous upper airway muscles and may not have fully represented the overall neuromuscular responses to airflow obstruction. Also in the current study, EMGGG activity was monitored in approximately 75% of our subjects. Thus we acknowledge the possibility of incorrectly reporting no gender difference in the magnitude of the phasic EMGGG during the active state, as this study does not have adequate power to discern a group difference. If women truly have greater phasic EMGGG responses to upper airway collapse, group sample sizes of 26 would be required to achieve 95% power to detect a difference of 5% of maximum EMGGG with group standard deviations of less than 5% of maximum at a significance level of 0.05 using a two-sided two-sample t-test. Furthermore, in the current study the compensatory neuromuscular responses were not examined during wakefulness, therefore future studies should investigate the magnitude of these responses preserved with general anesthesia in both men and women.
Clinical Implications
The major clinical finding of this study is that men have depressed compensatory neuromuscular responses to upper airway obstruction (ΔPCRIT A-P) during midazolam anesthesia compared to women. It appears that healthy women are able to preserve their capacity to respond to upper airway obstruction in the absence of an intact means of arousal, to a greater extent than men. In addition to a mechanical disadvantage, healthy men have a reduced ability to respond to upper airway obstruction without an intact arousal response, further predisposing them to upper airway obstruction during sleep or deepened anesthesia.
There are still many clinical advantages and considerable demand for the use of midazolam sedation and anesthesia during surgical procedures in intensive care units,43 emergency departments,44 and out-patient surgical units. We would recommend that the use of midazolam general anesthesia or deep sedation with spontaneous breathing be accompanied by additional secure airway management, i.e., continuous positive airway pressure treatment,45 and progressive mandible advancement,46 especially in men, since they are particularly vulnerable to reduced compensatory neuromuscular responses to sustained periods of upper airway obstruction. Furthermore, the implementation of quantitative measurement of airflow allows spontaneous assessment of ventilatory variables, such as tidal volume and maximum inspiratory airflow, on a continuous breath-by-breath basis. Moreover, the consciousness of anesthetized patients should be assessed if they display any signs of reduced airflow, snoring, reduced tidal volume, or paradoxic breathing. Inadequate neuromuscular responses may be exaggerated in other populations susceptible to upper collapse, such as the elderly or people with craniofacial abnormalities such as retrognathia.
Sleeping infants have stronger negative pressure reflex and responses to chemical stimuli than adults, which does not require full wakening for the reversal of pharyngeal airway obstruction.47,48 It is possible that some airway-protective mechanisms that are present in infants are preserved to a greater extent in women than men. We further speculate that the reduced compensatory neuromuscular response to upper airway obstruction is a major contributor to upper airway obstruction in both anesthesia and sleep, and thus may explain the increased severity of sleep apnea disease in men.
In conclusion, our findings demonstrate that women have greater compensatory neuromuscular responses to upper airway obstruction during midazolam general anesthesia than men. Furthermore, we speculate that gender-related differences in the strength of compensatory responses to sustained upper airway obstruction may explain the increased prevalence of upper airway collapse during sleep in sleep apnea.
Acknowledgements
This study was supported by institutional funding and Grants-in-Aid for scientific research no.18592189 from the Japanese Ministry of Education, Science, Sports and Culture to Terumi Ayuse, and by NIH HL50381 and HL37379 and NHLBI HL077137 as well as NHMRC 353705. The authors would like to acknowledge Mr. Joseph J. Maly for assistance in the analysis of data.
Footnotes
Conflict of Interest: None
References
- 1.Montravers P, Dureuil B, Desmonts JM. Effects of i.v. midazolam on upper airway resistance. Br J Anaesth. 1992;68:27–31. doi: 10.1093/bja/68.1.27. [DOI] [PubMed] [Google Scholar]
- 2.Drummond GB. Comparison of sedation with midazolam and ketamine: effects on airway muscle activity. Br J Anaesth. 1996;76:663–667. doi: 10.1093/bja/76.5.663. [DOI] [PubMed] [Google Scholar]
- 3.Oshima T, Masaki Y, Toyooka H. Flumazenil antagonizes midazolam-induced airway narrowing during nasal breathing in humans. Br J Anaesth. 1999;82:698–702. doi: 10.1093/bja/82.5.698. [DOI] [PubMed] [Google Scholar]
- 4.Eastwood PR, Platt PR, Shepherd K, Maddison K, Hillman DR. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology. 2005;103:470–477. doi: 10.1097/00000542-200509000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anaesthesia and sleep. THE LANCET. 2002;359:1207–1209. doi: 10.1016/S0140-6736(02)08224-7. [DOI] [PubMed] [Google Scholar]
- 6.Pillar G, Malhotra A, Fogel R, Beauregard J, Schnall R, White DP. Airway mechanics and ventilation in response to resistive loading during sleep: influence of gender. Am J Respir Crit Care Med. 2000;162:1627–1632. doi: 10.1164/ajrccm.162.5.2003131. [DOI] [PubMed] [Google Scholar]
- 7.Ayuse T, Inazawa T, Kurata S, Okayasu I, Sakamoto E, Oi K, Schneider H, Schwartz AR. Mouth-opening increases upper-airway collapsibility without changing resistance during midazolam sedation. J Dent Res. 2004;83:718–722. doi: 10.1177/154405910408300912. [DOI] [PubMed] [Google Scholar]
- 8.Inazawa T, Ayuse T, Kurata S, Okayasu I, Sakamoto E, Oi K, Schneider H, Schwartz AR. Effect of mandibular position on upper airway collapsibility and resistance. J Dent Res. 2005;84:554–558. doi: 10.1177/154405910508400613. [DOI] [PubMed] [Google Scholar]
- 9.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102:547–556. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 10.Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, McGinley BM, Magnuson T, Schweitzer M, Smith PL, Patil SP. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol. 2008;104:1618–1624. doi: 10.1152/japplphysiol.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norton JR, Ward DS, Karan S, Voter WA, Palmer L, Varlese A, Rackovsky O, Bailey P. Differences between midazolam and propofol sedation on upper airway collapsibility using dynamic negative airway pressure. Anesthesiology. 2006;104:1155–1164. doi: 10.1097/00000542-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda H, Ayuse T, Oi K. The effects of head and body positioning on upper airway collapsibility in normal subjects who received midazolam sedation. J Clin Anesth. 2006;18:185–193. doi: 10.1016/j.jclinane.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Litman RS, Hayes JL, Basco MG, Schwartz AR, Bailey PL, Ward DS. Use of dynamic negative airway pressure (DNAP) to assess sedative-induced upper airway obstruction. Anesthesiology. 2002;96:342–345. doi: 10.1097/00000542-200202000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz AR, O'Donnell CP, Baron J, Schubert N, Alam D, Samadi SD, Smith PL. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med. 1998;157:1051–1057. doi: 10.1164/ajrccm.157.4.9706067. [DOI] [PubMed] [Google Scholar]
- 15.Boudewyns A, Punjabi N, Van de Heyning PH, De Backer WA, O'Donnell CP, Schneider H, Smith PL, Schwartz AR. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest. 2000;118:1031–1041. doi: 10.1378/chest.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 16.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64:535–542. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 18.Hosselet JJ, Ayappa I, Norman RG, Kreiger AC, Rapoport DM. Classification of sleep-disordered breathing. Am J Respir Crit Care Med. 2001;163:398–405. doi: 10.1164/ajrccm.163.2.9808132. [DOI] [PubMed] [Google Scholar]
- 19.Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest. 1996;110:1077–1088. doi: 10.1378/chest.110.4.1077. [DOI] [PubMed] [Google Scholar]
- 20.Jordan AS, Wellman A, Heinzer RC, Lo YL, Schory K, Dover L, Gautam S, Malhotra A, White DP. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax. 2007;62:861–867. doi: 10.1136/thx.2006.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popovic RM, White DP. Influence of gender on waking genioglossal electromyogram and upper airway resistance. Am J Respir Crit Care Med. 1995;152:725–731. doi: 10.1164/ajrccm.152.2.7633734. [DOI] [PubMed] [Google Scholar]
- 22.Kairaitis K, Parikh R, Stavrinou R, Garlick S, Kirkness JP, Wheatley JR, Amis TC. Upper airway extraluminal tissue pressure fluctuations during breathing in rabbits. J Appl Physiol. 2003;95:1560–1566. doi: 10.1152/japplphysiol.00432.2003. [DOI] [PubMed] [Google Scholar]
- 23.Popovic RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol. 1998;84:1055–1062. doi: 10.1152/jappl.1998.84.3.1055. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra A, Huang Y, Fogel RB, Pillar G, Edwards JK, Kikinis R, Loring SH, White DP. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166:1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 25.Rowley JA, Sanders CS, Zahn BR, Badr MS. Gender differences in upper airway compliance during NREM sleep: role of neck circumference. J Appl Physiol. 2002;92:2535–2541. doi: 10.1152/japplphysiol.00553.2001. [DOI] [PubMed] [Google Scholar]
- 26.Rowley JA, Zhou X, Vergine I, Shkoukani MA, Badr MS. Influence of gender on upper airway mechanics: upeer airway resistance and Pcrit. J Appl Physiol. 2001;91:2248–2254. doi: 10.1152/jappl.2001.91.5.2248. [DOI] [PubMed] [Google Scholar]
- 27.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–1326. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 28.Thut DC, Schwartz AR, Roach D, Wise RA, Permutt S, Smith PL. Tracheal and neck position influence upper airway airflow dynamics by altering airway length. J Appl Physiol. 1993;75:2084–2090. doi: 10.1152/jappl.1993.75.5.2084. [DOI] [PubMed] [Google Scholar]
- 29.Series F, Cormier Y, Desmeules M. Influence of passive changes of lung volume on upper airways. J Appl Physiol. 1990;68:2159–2164. doi: 10.1152/jappl.1990.68.5.2159. [DOI] [PubMed] [Google Scholar]
- 30.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 31.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20:654–675. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- 32.Wheatley JR, Mezzanotte WS, Tangel DJ, White DP. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis. 1993;148:597–605. doi: 10.1164/ajrccm/148.3.597. [DOI] [PubMed] [Google Scholar]
- 33.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol. 1988;64:789–795. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 34.Shepard JW, Jr, Thawley SE. Localization of upper airway collapse during sleep in patients with obstructive sleep apnea. Am Rev Respir Dis. 1990;141:1350–1355. doi: 10.1164/ajrccm/141.5_Pt_1.1350. [DOI] [PubMed] [Google Scholar]
- 35.Mathru M, Esch O, Lang J, Herbert ME, Chaljub G, Goodacre B, vanSonnenberg E. Magnetic resonance imaging of the upper airway. Effects of propofol anesthesia and nasal continuous positive airway pressure in humans. Anesthesiology. 1996;84:273–279. doi: 10.1097/00000542-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Eastwood PR, Szollosi I, Platt PR, Hillman DR. Collapsibility of the upper airway during anesthesia with isoflurane. Anesthesiology. 2002;97:786–793. doi: 10.1097/00000542-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Litman RS, Weissend EE, Shrier DA, Ward DS. Morphologic changes in the upper airway of children during awakening from propofol administration. Anesthesiology. 2002;96:607–611. doi: 10.1097/00000542-200203000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Isono S, Tanaka A, Tagaito Y, Sho Y, Nishino T. Pharyngeal patency in response to advancement of the mandible in obese anesthetized persons. Anesthesiology. 1997;87:1055–1062. doi: 10.1097/00000542-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Eikermann M, Jordan AS, Chamberlin NL, Gautam S, Wellman A, Lo YL, White DP, Malhotra A. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131:1702–1709. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gislason T, Benediktsdottir B, Bjornsson JK, Kjartansson G, Kjeld M, Kristbjarnarson H. Snoring, hypertension, and the sleep apnea syndrome. An epidemiologic survey of middle-aged women. Chest. 1993;103:1147–1151. doi: 10.1378/chest.103.4.1147. [DOI] [PubMed] [Google Scholar]
- 41.Sun GC, Hsu MC, Chia YY, Chen PY, Shaw FZ. Effects of age and gender on intravenous midazolam premedication: a randomized double-blind study. Br J Anaesth. 2008;101:632–639. doi: 10.1093/bja/aen251. [DOI] [PubMed] [Google Scholar]
- 42.Lam B, Ip MS, Tench E, Ryan CF. Craniofacial profile in Asian and white subjects with obstructive sleep apnoea. Thorax. 2005;60:504–510. doi: 10.1136/thx.2004.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huey-Ling L, Chun-Che S, Jen-Jen T, Shau-Ting L, Hsing IC. Comparison of the effect of protocol-directed sedation with propofol vs. midazolam by nurses in intensive care: efficacy, haemodynamic stability and patient satisfaction. J Clin Nurs. 2008;17:1510–1517. doi: 10.1111/j.1365-2702.2007.02128.x. [DOI] [PubMed] [Google Scholar]
- 44.Hohl CM, Nosyk B, Sadatsafavi M, Anis AH. A cost-effectiveness analysis of propofol versus midazolam for procedural sedation in the emergency department. Acad Emerg Med. 2008;15:32–39. doi: 10.1111/j.1553-2712.2007.00023.x. [DOI] [PubMed] [Google Scholar]
- 45.Nozaki-Taguchi N, Isono S, Nishino T, Numai T, Taguchi N. Upper airway obstruction during midazolam sedation: modification by nasal CPAP. Can J Anaesth. 1995;42:685–690. doi: 10.1007/BF03012665. [DOI] [PubMed] [Google Scholar]
- 46.Kuna ST, Woodson LC, Solanki DR, Esch O, Frantz DE, Mathru M. Effect of progressive mandibular advancement on pharyngeal airway size in anesthetized adults. Anesthesiology. 2008;109:605–612. doi: 10.1097/ALN.0b013e31818709fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McNamara F, Wulbrand H, Thach BT. Characteristics of the infant arousal response. J Appl Physiol. 1998;85:2314–2321. doi: 10.1152/jappl.1998.85.6.2314. [DOI] [PubMed] [Google Scholar]
- 48.Isono S. Developmental changes of pharyngeal airway patency: implication for pediatric anesthesia. Pediatr Anesth. 2006;16:109–122. doi: 10.1111/j.1460-9592.2005.01769.x. [DOI] [PubMed] [Google Scholar]