Abstract
Background
Although necessary in developing a rationale for vaccination, the burden of severe respiratory syncytial virus (RSV) disease in children in the resource poor setting remains poorly defined.
Methods
We conducted prospective surveillance of severe and very severe pneumonia in children aged less than 5 years admitted between 2002 and 2007 to Kilifi district hospital, coastal Kenya. Nasal specimens were screened for RSV antigen by immunofluorescence. Incidence rates were estimated for the well-defined user population.
Results
Out of 25,149 admissions 7,359 (29%) had severe or very severe pneumonia, of whom 6,026 (82%) were enrolled. RSV prevalence was 15% (20% in infants) and 27% during epidemics (32% in infants). The proportion of cases >=3 months was 65% and >=6 months was 43%. Average annual hospitalization rates were 293 per 100,000 children under five years (95% CI 271-371) and 1,107 per 100,000 infants (1,012-1,211). Admission rates were double in the region close to the hospital. Few RSV cases had life-threatening clinical features or concurrent serious illnesses, and associated mortality was 2.2%.
Conclusions
In this low-income setting, rates of hospital admission with RSV-associated pneumonia are substantial, comparable to estimates from the US, yet considerably under-estimate the full community burden. An effective vaccine in children >2 months old, that is, outside the poor-responder age group, could prevent a large portion of RSV disease. Severity data suggest the basis for RSV vaccination will be the prevention of morbidity not mortality.
40 word summary
Six years surveillance of pediatric admissions to a rural Kenyan hospital provides well-defined incidence estimates and clinical characteristics of RSV pneumonia in a low-income setting. Data argue for vaccination based on morbidity, not mortality, targeted at age groups >2 months.
Keywords: respiratory syncytial virus, incidence, burden of disease, hospitalizations, vaccination, Kenya
Introduction
Respiratory syncytial virus (RSV) is a major cause of severe pneumonia and bronchiolitis in infants and children worldwide and is seen as an important target for a pediatric vaccine[1-3]. Much attention has focused on developing a live attenuated RSV vaccine targeting early infancy, with some encouraging results reported on the safety and immunogenicity of recent candidates [1, 4, 5]. In addition to research in vaccine design and development, a successful intervention program needs i) an accurate estimate of disease burden in the target population and ii) a comprehensive description of the epidemiology of infection and disease to optimise control strategies and estimate their potential impact. Both of these objectives can be reached through hospital-based surveillance for RSV disease within a well-defined user population. Furthermore, long-term disease monitoring establishes the necessary baseline of information by which to assess the impact and effectiveness of interventions. In the developing world hospital-based surveillance of RSV disease is infrequent, predominantly without an accurate population base and rarely sustained[6-8]. In this paper we present the results of prospective population-based surveillance for RSV disease spanning 6 years at a rural district hospital typical of tropical sub-Saharan Africa.
Methods
Study location and population
Surveillance was undertaken at Kilifi District Hospital (KDH) situated in the town of Kilifi, coastal Kenya, which serves as a primary care and referral facility for the predominantly rural farming population of around 500,000 of whom 18% are under 5 years of age [9]. The District has one of the lowest average per capita incomes in Kenya, and under 5 and infant (age <1 year) mortality rates are high (85 and 141, respectively[9]) although with considerable geographical variability. Malaria is transmitted throughout the year, with peaks in November-January and May-August following the rainy season but the incidence of malaria has declined sharply in recent years[10]. HIV-1 prevalence in women attending KDH antenatal care in 2004 was ~5%[11]. There are approximately 5,000 pediatric admissions (upper age of 12 years) each year. Each child is investigated with a standard computerized clinical history and examination and standard set of investigations on admission including a full haemogram, blood culture and, among febrile children, a Giemsa stained blood slide [12]. In 2000, a demographic surveillance study was established in an area of 891 km2 close to KDH monitoring births, deaths and migration events in a population of approximately 240,000 through 4 monthly enumeration rounds. From 16th April 2002, the residency status of each child presenting to KDH was established on admission through linkage to the population register of the Kilifi Epidemiological and Demographic Surveillance Study (EpiDSS)[12].
Patients
Surveillance for RSV was initiated on 1st January 2002. We report on all children aged 1 day to 59 months who presented with the clinical syndrome of pneumonia, either severe or very severe, excluding babies with neonatal tetanus. Clinical definitions, which follow, have been described previously[12, 13]. A history of cough or difficulty in breathing for less than 30 days, when accompanied by lower chest wall indrawing was defined as severe pneumonia, or when accompanied by any one of prostration, coma or hypoxemia was defined as very severe pneumonia. Prostration included the inability to feed or drink. Hypoxemia was defined by an oxygen saturation (pO2) of less than 90% determined by finger tip Oxymeter (Nelcor®). All children with hypoxaemia at admission are given supplemental oxygen. Malaria was defined by P. falciparum parasitemia on the blood slide. Severe malnutrition was defined as a small value (≤10th percentile by age group) for mid-upper arm circumference (MUAC), bipedal oedema or visible severe wasting, and shock by either a peripheral-central temperature gradient, a capillary refill time of >3sec or a weak peripheral pulse volume. A history of prematurity was elicited for all infant at admissions and heart disease established from diagnosis-on-discharge data. Bronchiolitis as a specific condition is not reported. In this setting it has little influence on patient management and, despite the long-term surveillance, remains poorly recognised by clinicians at admission (in the absence of laboratory confirmation). HIV diagnostic counseling and testing was not in place for most of the period of surveillance covered in this report and is not considered further here. Written informed consent was obtained for all eligible participants.
Nasal specimens were collected as soon as possible after admission by nasal washing; in children who were too unwell to undergo nasal washing a nasaopharyngeal aspirate was performed [14]. Sample collection was postponed to the following Monday for children admitted over the weekend (beginning Friday at 5pm). Specimens were processed as described previously[15] and screened for RSV antigen by using a Direct Immunofluorescent Antibody Test (Light Diagnostics RSV screen, Chemicon, Temecula, CA) following the manufacturer’s protocol.
Data analysis
Admission and demographic data were entered onto FileMaker 5.5, and cleaned and analysed using STATA 10.1 (StataCorp, Texas). Throughout, the term pneumonia refers to all cases with clinically severe or very severe pneumonia. The pattern of admissions to hospital with RSV-associated pneumonia was determined from January 2002 to December 2007 and the incidence of admission to hospital with RSV-associated pneumonia was determined between May 2002 and April 2007. The incidence and 95% confidence intervals were calculated as the mean annual number of resident children admitted with each case definition divided by the resident population size at the mid-point of the study period (29th October 2004), and adjusted for the estimated proportion of these cases with detectable RSV in their nasal specimens. This assumes that the proportion of children with pneumonia who had RSV in their nasal specimens was the same in both the tested and untested groups. Annual period incidence estimates (e.g. year May 2002 to April 2003) were calculated using the resident population denominator for the mid-point of each period (e.g. October 30th 2002). Incidence rate 95% confidence intervals were estimated using a normal approximation of the likelihood profile[16].
Geographical variation in the incidence of RSV-associated admissions was calculated for each administrative sub-location (of which there are 40 in Kilifi EpiDSS), and presented as a categorical variable using ArcView (ESRI ArcMap v9.2) and Adobe Illustrator (CS2 v12.1). The incidence was calculated separately for sub-locations close to the hospital meaning that at least part of the sub-location fell within a radius of 5km of KDH [12]. RSV epidemic periods were empirically defined to begin when at least 10% of tested samples were RSV positive or with the observation of at least two RSV cases per week in each of two consecutive weeks, and to continue while these conditions were satisfied, with the requirement that an epidemic must last for four or more weeks. Proportions were compared using Fisher exact or Chi-Squared tests (2 tailed) and equality of distributions evaluated using the Wilcoxon rank-sum test. Adjusted risk ratios were determined using binomial regression.
Results
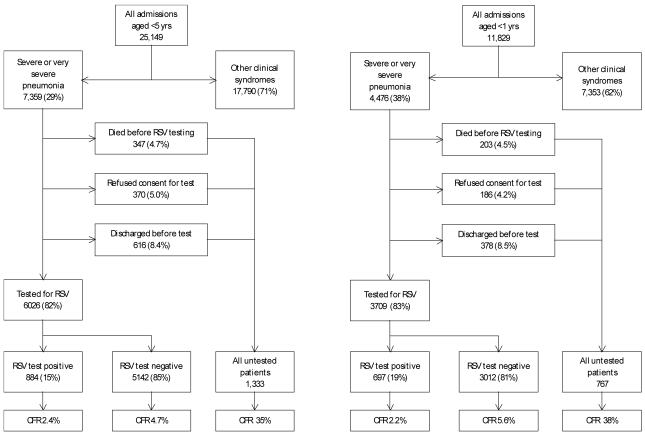
Between 1st January 2002 and 31st December 2007, 25,149 children aged <5 years, 11,829 infants, were admitted to KDH (Fig.1). Of the 7,359 eligible children 82% (6,026) were enrolled, of whom 15% (884) tested RSV positive (there was no difference by sex). The reasons for nonenrollment were early discharge, refusal and death in an approximate ratio of 2:1:1. Comparing children who were and were not enrolled, there was no difference in the median age (9m), or in the percentage male (56%), and the proportion of infants was higher in the enrolled group (59% versus 56%, P=0.037). Nonenrolment was higher outside of RSV epidemics than during (20% versus 16%, exact P<0.001). Among the enrolled 4.4% died in-hospital compared with 9.9% in all eligible children. Furthermore, for those enrolled, relative to the non-enrolled, at admission there was a lower proportion with malaria parasitemia (16% versus 21%, P <0.001), a pathogenic bacterial isolate (5.4% versus 11%, exact P <0.001), and very severe pneumonia (22% versus 46%, exact P<0.001), but there was no difference in the proportion with severe malnutrition (14% versus 16%, exact P=0.058).
Fig. 1.
Flow diagram of recruitment and sample testing of children aged under 5 years (left) and infants (right) admitted to Kilifi District Hospital from January 2002 to December 2007. ‘Tested’ children refers to those who were enrolled, had a sample collected and tested, with the remainder either not enrolled or enrolled and a sample not taken and tested. Percentages in brackets within a box refer to the proportion of individuals from the preceding box. Note that among those untested who refused participation 121 children aged less than 5 years and 90 infants subsequently died and contribute to the denominator of the case fatality ratio (CFR).
Epidemiology
Epidemics of RSV positive cases were periodic with a starting date between November and February, a peak month between January and May and an average duration of 18 weeks (range 13-27 weeks). Epidemics appear not to have a simple annual pattern, but rather the interepidemic period (from peak month to peak month) alternates between 9m and 15m (Fig.S1). Of all RSV-associated pneumonia admissions 815 (92%) occurred during an epidemic period. The number of RSV cases occurring in each epidemic ranged from 68 (17% of samples tested) in the epidemic of 2002/3 to 202 (30% of samples tested) in 2005/6, with an overall proportion of 27%. Seasonal variation in RSV admissions was roughly coincident with total pneumonia admissions. No temporal association with any meteorological measure or other disease syndrome was observed (Fig.S1).
The distribution of RSV-associated pneumonia admissions by age and severity is presented in Table 1. The proportion RSV positive within an age class was highest in children 0-5 months (around 21%), declined with increasing age, and was higher in children with severe compared with very severe pneumonia (16.5% versus 8.3%, P<0.001). The proportion of cases aged less than 3, 6, and 12 months were 35%, 57% and 79%, respectively. The age distribution of cases differed significantly by clinical category (P<0.001). In particular, the proportion of RSV-associated admissions that were aged 0-2 months was higher (51%) among the sub-set of children with very severe pneumonia.
Table 1.
Age-distribution of RSV-associated pneumonia admissions to Kilifi District Hospital 2002-2007 stratified by severity
| severe pneumonia | very severe pneumonia | all cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ageclass (months) | tested | positive | % positive in ageclass (row) | % of all positives (col) | tested | positive | % positive in ageclass (row) | % of all positives (col) | tested | positive | % positive in ageclass (row) | % of all positives (col) |
| 0-2 | 1210 | 255 | 21.1 | 33.0 | 311 | 57 | 18.3 | 50.9 | 1521 | 312 | 20.5 | 35.3 |
| 3-5 | 761 | 169 | 22.2 | 21.9 | 151 | 19 | 12.6 | 17.0 | 912 | 188 | 20.6 | 21.3 |
| 6-11 | 1023 | 183 | 17.9 | 23.7 | 253 | 14 | 5.5 | 12.5 | 1276 | 197 | 15.4 | 22.3 |
| 12-23 | 994 | 101 | 10.2 | 13.1 | 258 | 14 | 5.4 | 12.5 | 1252 | 115 | 9.2 | 13.0 |
| 24-35 | 388 | 47 | 12.1 | 6.1 | 198 | 4 | 2.0 | 3.6 | 586 | 51 | 8.7 | 5.8 |
| 36-59 | 307 | 17 | 5.5 | 2.2 | 172 | 4 | 2.3 | 3.6 | 479 | 21 | 4.4 | 2.4 |
| Total | 4683 | 772 | 16.5 | 100.0 | 1343 | 112 | 8.3 | 100.0 | 6026 | 884 | 14.7 | 100.0 |
Hospitalization rates
Over the 5 year period 1st May 2002 to 30th April 2007 the mean annual number of admissions from the community of Kilifi EpiDSS residents was 2,661 aged <5 years, and 1,188 aged <1 year. The rate of hospital admissions with severe or very severe pneumonia was 1,912 for children aged <5 years and 5,528 for infants. The incidence of admission with RSV-associated severe or very severe pneumonia was 293 (range between years 202-373) for children aged <5 years and 1,107 (range 692-1587) for infants (Table 2). Incidence estimates by year of study and finely stratified by age are provided as supplementary material (Table S1).
Table 2.
Estimated rates of hospitalization (per 100,000 per year) with RSV-associated pneumonia to Kilifi District Hospital over the period 1/5/2002 to 30/4/2007, by age and severity
| age class | pneumonia syndrome | cases | tested for RSV | RSV positive | % RSV positive | pyo | LRTI incidence | RSV incidence | lower 95% CI | upper 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| < 1 year | severe | 1889 | 1644 | 348 | 21.2 | 42960 | 4397 | 931 | 844 | 1027 |
| very severe | 486 | 344 | 50 | 14.5 | 42960 | 1131 | 164 | 130 | 208 | |
| severe or very severe | 2375 | 1988 | 398 | 20.0 | 42960 | 5528 | 1107 | 1012 | 1211 | |
| < 5 years | severe | 3037 | 2647 | 447 | 16.9 | 209075 | 1453 | 245 | 225 | 267 |
| very severe | 961 | 680 | 63 | 9.3 | 209075 | 460 | 43 | 35 | 52 | |
| severe or very severe | 3998 | 3327 | 510 | 15.3 | 209075 | 1912 | 293 | 271 | 317 |
Notes.
pyo - person years of observation. The estimated resident population for the specified age class at the mid-point of the study ie 29thOctober 2004, multiplied by 5 (ie the study period in years).
95% CI - 95% confidence intervals for RSV incidences.
Refer to Methods for the definitions used for pneumonia categories and incidence estimation procedure.
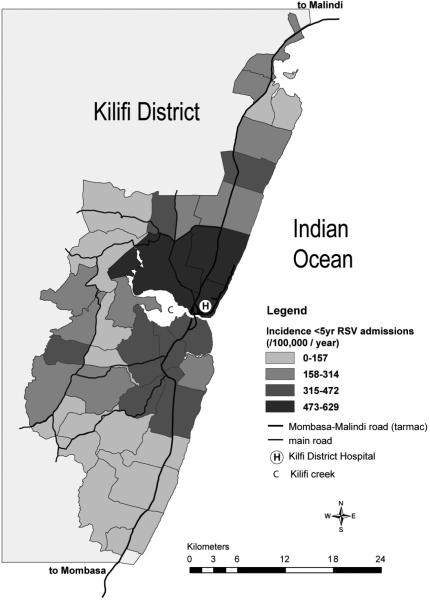
The geographical distribution of RSV associated severe and very severe pneumonia incidence in children under 5 years of age, presented in Fig.2, reveals considerable variation between administrative sub-locations, with incidence apparently declining with distance from the District hospital. For the population of children under 5 years of age living within the region defined as close to KDH, the hospitalization rate for severe or very severe pneumonia was 3,169 per 100,000 (95% CI 3,023-3,323) and for RSV-associated severe or very severe pneumonia 574 per 100,000 (95% CI 507-650). Among infants in the sub-locations closest to the hospital the incidence of severe or very severe pneumonia and RSV-associated severe or very severe pneumonia were 8,828 per 100,000 (8,297-9,393) and 2,112 per 100,000 (1,835-2,431), respectively.
Fig.2.
Geographical variation within the Kilifi Health and Demographic Surveillance System in the incidence of RSV associated severe or very severe pneumonia in under 5 year old admissions to Kilifi District Hospital (KDH). Represented is the rate of hospitalization (per 100,000 per year) for the 5 year period May 2002 to April 2007, categorised into 4 levels and stratified by administrative sub-location. Main roads, Kilifi Creek (C), and KDH (H), are identified. The zone marked by a white perimeter has a 5km radius from its central point, KDH.
Severity and concurrent illnesses
Among cases of severe or very severe pneumonia the clinical signs crackles, wheezing, nasal flaring and lower chest-wall indrawing were all associated with RSV whereas shock, hypoxemia, prostration and coma were inversely associated with RSV (Table 3). Concurrent malaria parasitemia, severe malnutrition and bacteraemia were all significantly less common among RSV-positive children with pneumonia. Furthermore, a history of premature birth in infants and a discharge diagnosis including heart disease were marginally less frequent in RSV-positive children. Cases of RSV-associated pneumonia had a significantly shorter in-patient stay and lower case fatality rate than cases of RSV-negative pneumonia. RSV positive cases were significantly younger than RSV negative cases (median age 4.9 vs 19.1 months, respectively; Wilcoxon rank-sum z=11.34, P<0.001). Adjusting the relative risk of each feature in Table 3 for age did not alter the pattern above. The proportion of RSV pneumonia cases in children under 5 years of age that were very severe did not differ by distance from KDH (near 11.2% versus far 13.5%, exact P=0.501).
Table 3.
Prevalence of features among RSV positive and negative children aged less than 5 years admitted with severe or very severe pneumonia to Kilifi District Hospital 2002-2007
| Characteristic | RSV positive n=884^ | RSV negative n=5,142^ | Adjusted RR* | 95% CI | |
|---|---|---|---|---|---|
| Clinical | |||||
| crackles | 66.7 | 44.1 | 1.43 | 1.34-1.52 | |
| wheezing | 15.2 | 11.2 | 1.43 | 1.18-1.73 | |
| nasal flaring | 61.8 | 48.2 | 1.16 | 1.09-1.24 | |
| indrawing | 98.3 | 90.2 | 1.04 | 1.03-1.05 | |
| shock% | 13.5 | 19.4 | 0.68 | 0.56-0.82 | |
| hypoxaemia | 10.3 | 13.7 | 0.73 | 0.59-0.91 | |
| prostrate/unconscious | 4.1 | 13.8 | 0.36 | 0.26-0.50 | |
| Concurrent illness | |||||
| slide positive malaria | 4.6 (864) | 18.3 (5,073) | 0.36 | 0.26-0.49 | |
| malnutrition@ | 6.6 | 15.4 | 0.47 | 0.36-0.61 | |
| bacteraemia | 2.3 (864) | 6.0 (5,071) | 0.4 | 0.25-0.63 | |
| Risk factors | |||||
| preamature (infants) | 4.6 (697) | 6.7 (3,005) | 0.67 | 0.45- 0.97 | |
| heart diseae | 1.0 | 2.1 | 0.49 | 0.24-1.00 | |
| Outcome | |||||
| in-patient duration > 12d | 3.8 | 8.8 | 0.46$ | 0.32-0.66 | |
| died | 2.4 | 4.7 | 0.41 | 0.26-0.64 | |
Notes:
Denominator unless otherwise specified in parentheses below.
Relative risk (RR) of feature in RSV-positive cases relative to RSV-negative cases, adjusted for age class (with 95% Confidence Intervals).
Temperature gradient, capillary refill>3sec or weak pulse volume
Small MUAC for age, bipedal oedema or visible severe wasting. Small MUAC was defined as ≤10th percentile calculated for all KDH admissions 2002-2007 generating the following cut-off values by age : 0-5m ≤7.8cm, 6-23m ≤11.0cm, 24-35m ≤11.6cm, 36-59m ≤12.2cm [12]
RR adjusted also for differential mortality between RSV positive and negative children.
Bacterial pathogens were identified from the blood of 20/864 (2%) RSV-positive cases of pneumonia. Isolates cultured were Group A β-haemolytic streptococci (5), Streptococcus pneumoniae (5), Acinetobacter sp (5), Haemophilus influenzae (4), Pseudomonas sp (1), and Escherischia coli (1). In one instance there was a dual co-infection with S. pneumoniae and H. influenzae. Three of the children with confirmed bacterial co-infection, subsequently died, two with H. influenzae and one with Acinetobacter sp. In comparison, 6% (302) of RSV negative cases were blood culture positive, with similar mortality (15%) and species distribution relative to RSV positive cases.
Discussion
Our surveillance at a district hospital in coastal Kenya spanning 6 years provides a highly detailed description of the burden of RSV severe disease in young children in a typical rural developing country setting. Over the period 2002 to 2007 we identified clinically severe or very severe pneumonia in roughly one third of under 5 year olds and 38% of infants admitted to KDH, of which an estimated 15% and 19%, respectively, were associated with RSV infection. The rate of hospital admissions with RSV-associated pneumonia within the demographically well-defined user population was 293 cases per 1000,000 per annum in children under 5 years and 1,107 cases per 100,000 per annum in infants. These data are similar in magnitude to those reported for an intensively monitored birth cohort within the same population (1,300 infant cases of RSV-associated severe pneumonia admitted to KDH per 100,000 child years of observation) [13], and for the small number of other denominator-based studies of hospital admissions in resource-poor countries[6, 7]. Furthermore, our reported rates mirror closely population-based hospitalization rates recently described for various locations in the US[17].
Thus our data indicate that an effective pediatric RSV vaccine could prevent a sizeable proportion of hospital pneumonia admissions. A useful reference point is afforded by comparison with severe rotavirus diarrhoea, targeted for vaccination[18], which exhibited an annual rate of hospitalization at KDH for the period 2002-04 of 478 per100,000 in the under 5 year olds and 1431 per 100,000 in infants[12]. Furthermore, the ‘distance decay’ in rates of hospitalization (Fig.2), previously reported in The Gambia [8], indicates a large (~two fold) additional burden of severe RSV present in the community but not recorded through in-patient surveillance. If we assume the estimates close to the hospital are more realistic of the community burden and representative of Kenya as a whole, the annual number of RSV-associated severe or very severe pneumonia cases in children under 5 and infants throughout the country are estimated at 37,000 and 29,500, respectively (based on population estimates for children <5 years and births in 2008 of 6.5m and 1.4m, respectively[19].)
We further point out an additional burden of RSV severe pneumonia in the community, missed by hospital surveillance, in the form of cases presenting to out-patient health facilities and not admitted, as was shown in our previous report of a birth cohort in the same setting as the present study[13]. A potential further source of under-estimation of RSV disease in our study arises from the immunofluorescence assay used to detect the virus, which has been shown to be less sensitive than molecular based detection methods [20, 21].
A clear impediment to vaccine intervention is the absence of an effective candidate for use in the key target age group of 0-2m [4, 22]. Nevertheless, studies consistently show good safety and immunogenicity of live attenuated RSV vaccines in older infants and young children[5, 23]. Data from the present study show that around 2/3 of hospitalised RSV-associated pneumonia occurs in children 3 months and over, and hence delayed vaccine delivery has the potential to prevent a significant fraction of RSV disease. Reduced transmission from older vaccinated infants to the vulnerable early infants may enhance the benefit of delayed vaccination.
Our data show that the incidence of RSV disease varies considerably year-by-year, particularly in infants, which varied by a factor in excess of two between the lowest and highest years, suggesting that short-term studies may present a biased picture of RSV disease burden. Interestingly the distribution of disease by age was roughly constant over time (in contrast to observations from The Gambia[24]).
In relation to pneumonia severity our study shows that non-life-threatening clinical features were more strongly associated with being RSV positive than negative, whereas the risks of very severe conditions, risk factors and concurrent illness were significantly higher in RSV negative cases. A similar clinical pattern was reported from an in-patient study in Mozambique[25]. The low case fatality rate of 2.2% for RSV positives (in line with most other reports[6, 26, 27]), compared with 4.7% in RSV negative cases, is therefore not surprising. These data support the rationale for a RSV vaccine based on prevention of morbidity rather than mortality. Nevertheless, as in previous studies there is some concern over bias in sampling of less severe cases[6], since 18% of eligible children were not tested, and the case fatality in the enrolled was less that half that found in all eligible children (4.4% versus 9.9%). In our setting, faced with a child undergoing emergency management, it was not a clear priority of the clinician to collect a nasal sample. Hence, it is possible that the true case fatality rate associated with RSV is significantly higher than observed, and consideration should be given to collecting a specimen post-mortem to address this unknown.
The prospect of widespread use of vaccines against H. influenza type b and S. pneumoniae across the developing world is set to alter substantially the landscape of pneumonia aetiology[3]. Surveillance for a broad aetiology of the remaining significant burden of childhood pneumonia is of increasing importance. Arguably, the most important remaining cause of childhood pneumonia potentially preventable by vaccine intervention is RSV. The present study reports a substantial burden of RSV associated severe pneumonia in a low-income setting that is equivalent to that identified in the high-income setting. This argues a need to investigate the potential use of vaccines for both developed and developing countries. This study also demonstrates the power of long-term surveillance in understanding the role of RSV in morbidity and mortality patterns in children in a low resource setting, and the requirement for population evidence in developing a rational strategy for future RSV vaccine intervention.
Supplementary Material
Acknowledgements
We would like to thank the participants involved in the study and their caregivers, and the staff of the RSV team (in the ward and the laboratory), pediatric wards, hospital administration and Kilifi EpiDSS team. The study is published with permission of the Director of KEMRI.
Funding: Financial support was provided by the Wellcome Trust (061584 and 076278). The funding agencies had no role in study design, data collection or preparation of this manuscript. The authors declare there to be no conflict of interest in relation to the publication of this work.
Footnotes
Ethical approval: The Kenyan National Research Ethical Committee and the Coventry Research Ethics Committee, UK, granted ethical approval for the study.
Referenecs
- 1.Collins PL, Murphy BR. Vaccines against hunam respiratory syncytial virus. In: Cane P, editor. Respiratory Syncytial Virus. Vol. 14. Elsevier; Amsterdam: 2007. pp. 233–78. [Google Scholar]
- 2.Mulholland K, Levine O, Nohynek H, Greenwood BM. Evaluation of vaccines for the prevention of pneumonia in children in developing countries. Epidemiol Rev. 1999;21(1):43–55. doi: 10.1093/oxfordjournals.epirev.a017987. [DOI] [PubMed] [Google Scholar]
- 3.Scott JAG, English M. What are the implications for childhood pneumonia of successfully introducing Hib and Pneumococcal vaccines in developing countries? PLoS Medicine. 2008;5(4):548–52. doi: 10.1371/journal.pmed.0050086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karron RA, Wright PF, Belshe RB, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005 Apr 1;191(7):1093–104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 5.Wright PF, Karron RA, Belshe RB, et al. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine. 2007 Oct 16;25(42):7372–8. doi: 10.1016/j.vaccine.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nokes DJ. Respiratory syncytial virus disease burden in the developing world. In: Cane PA, editor. Perspectives in Medical Virology: Respiratory Syncytial Virus. Vol. 14. Elsevier; Amsterdam: 2007. pp. 183–230. [Google Scholar]
- 7.Robertson SE, Roca A, Alonso P, et al. Respiratory syncytial virus infection: denominator-based studies in Indonesia, Mozambique, Nigeria and South Africa. Bull World Health Organ. 2004 Dec;82(12):914–22. [PMC free article] [PubMed] [Google Scholar]
- 8.Weber MW, Milligan P, Sanneh M, et al. An epidemiological study of RSV infection in the Gambia. Bull World Health Organ. 2002;80(7):562–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Ministry of Finance and Planning . Analytical Report on Population Projections. VII. Central Bureau of Statistics, Government of Kenya; Nairobi: 2002. [Google Scholar]
- 10.Okiro EA, Hay SI, Gikandi PW, et al. The decline in paediatric malaria admissions on the coast of Kenya. Malar J. 2007;6:151. doi: 10.1186/1475-2875-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okiro EA, Ngama M, Bett A, Cane PA, Medley GF, James Nokes D. Factors associated with increased risk of progression to respiratory syncytial virus-associated pneumonia in young Kenyan children. Trop Med Int Health. 2008 Jul;13(7):914–26. doi: 10.1111/j.1365-3156.2008.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nokes DJ, Abwao J, Pamba A, et al. Incidence and clinical characteristics of group A rotavirus infections among children admitted to hospital in Kilifi, Kenya. PLoS Med. 2008 Jul 22;5(7):e153. doi: 10.1371/journal.pmed.0050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nokes DJ, Okiro EA, Ngama M, et al. Respiratory syncytial virus infection and disease in infants and young children observed from birth in Kilifi District, Kenya. Clin Infect Dis. 2008 Jan 1;46(1):50–7. doi: 10.1086/524019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngama MJ, Ouma B, English ME, Nokes DJ. Comparison of three methods of collecting nasal specimens for respiratory virus analysis. East Afr Med J. 2004 Jun;81(6):313–7. doi: 10.4314/eamj.v81i6.9181. [DOI] [PubMed] [Google Scholar]
- 15.Nokes DJ, Okiro EA, Ngama M, et al. Respiratory Syncytial Virus Epidemiology in a Birth Cohort from Kilifi District, Kenya: Infection during the First Year of Life. J Infect Dis. 2004 Nov 15;190(10):1828–32. doi: 10.1086/425040. [DOI] [PubMed] [Google Scholar]
- 16.Clayton D, Hills M. Statistical models in epidemiology. Oxford University Press Inc.; New York: 1993. [Google Scholar]
- 17.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009 Feb 5;360(6):588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US CDC. WHO PATH Rotavirus vaccine programme: rotavirusUPDATE. 2007. http://wwwrotavirusvaccineorg/Rota_newsletter_Sept07htm. [cited; 7:[Available from:
- 19.Census Bureau US . IDB Summary Demographic Data for Kenya. Population Division/International Programs Center; 2008. Accessed October 25th 2008. at http://www.census.gov/ipc/www/idb/country/keportal.html) [cited; Available from: http://www.census.gov/cgi-bin/ipc/idbsum.pl?cty=KE. [Google Scholar]
- 20.Reis AD, Fink MC, Machado CM, et al. Comparison of direct immunofluorescence, conventional cell culture and polymerase chain reaction techniques for detecting respiratory syncytial virus in nasopharyngeal aspirates from infants. Rev Inst Med Trop Sao Paulo. 2008 Jan-Feb;50(1):37–40. doi: 10.1590/s0036-46652008000100008. [DOI] [PubMed] [Google Scholar]
- 21.Kuypers J, Wright N, Ferrenberg J, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006 Jul;44(7):2382–8. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Littel-van den Hurk SD, Mapletoft JW, Arsic N, Kovacs-Nolan J. Immunopathology of RSV infection: prospects for developing vaccines without this complication. Rev Med Virol. 2007 Jan-Feb;17(1):5–34. doi: 10.1002/rmv.518. [DOI] [PubMed] [Google Scholar]
- 23.Wright PF, Karron RA, Belshe RB, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182(5):1331–42. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 24.Weber M, Dackour R, Usen S, et al. The clinical spectrum of respiratory syncytial virus disease in The Gambia. Pediatric infectious disease journal. 1998;17(3):224–30. doi: 10.1097/00006454-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Loscertales MP, Roca A, Ventura PJ, et al. Epidemiology and clinical presentation of respiratory syncytial virus infection in a rural area of southern Mozambique. Pediatr Infect Dis J. 2002;21(2):148–55. doi: 10.1097/00006454-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Stensballe LG, Devasundaram JK, Simoes EA. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. Pediatr Infect Dis J. 2003;22(2 Suppl):S21–32. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- 27.Weber M, Mulholland E, Greenwood B. Respiratory syncytial virus infection in tropical and developing countries. Tropical Medicine and Internationl Health. 1998;3:268–80. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.