Summary
Actigraphy is applicable for studying sleep in populations who are unable to tolerate traditional sleep-recording techniques, such as nursing-home patients who are infirm and demented. This study examined whether actigraphy can accurately reflect sleep/wake activity in this population by testing the reliability of a wrist-activity monitor, the Actillume, against traditional sleep measurements and against observations of nursing-home patients. Data from the Actillume are presented as two variables, the sum (total of all activity movements within the prescribed epoch) and the maximum activity (the largest or maximum movement recorded during the prescribed epoch), and by electroencephalogram (EEG). One difficulty in making comparisons was that the EEG records showed diffuse slowing, making it extremely difficult to score sleep/wake activity and making it difficult to use the EEG as a “gold standard”. Nevertheless, the correlation for total sleep time from EEG and Actillume was r = 0.91 (p < 0.001) for sum activity and r = 0.81 for maximum activity (p < 0.005). Correlations for percent sleep were r = 0.78 (p < 0.01) for maximum activity and r = 0.61 for sum activity. The comparison of sleep/wake determined by the Actillume vs. observations resulted in a sensitivity of 87% and specificity of 90%. We conclude that the Actillume is the most feasible technique for studying sleep and wake activity in demented nursing-home patients.
Keywords: Actigraphy, EEG, Validity, Nursing-home patients, Observations
The traditional “gold standard” for recording sleep has been the polysomnogram (PSG) which involves wiring the patients with electroencephalogram (EEG) electrodes, electrooculogram (EOG) electrodes, and chin electromyogram (EMG) electrodes. However, certain populations, such as infirmed, demented nursing-home patients, do not tolerate this procedure. In the last 15 years, a new technology, wrist activity, has evolved allowing less intrusive monitoring of sleep and wake.
Wrist activity provides a way to quantify inactivity vs. activity, or sleep vs. wake for consecutive 24-hour periods. In the past, sleep/wake activity of nursing-home patients was often determined via observations that were made either at prescribed intervals, in which case large blocks of time would go unobserved and data were lost, or observations were made for continuous periods, in which case they were very labor intensive and allowed the patient no privacy (1). Observations have also been made by videotaping; however, patients wander from room to room and would be required to be sitting facing the camera i.e. not a naturalistic situation.
Wrist actigraphy allows continuous measurement of movement for consecutive days or weeks. The use of wrist activity for distinguishing wake from sleep has been validated in younger adults and in healthy elderly (2). In general, correlations for total sleep time were consistently higher than for wake time.
Activity monitors have been used in studies with other populations including Alzheimer's Disease, Parkinson's patients, patients with complaints of insomnia, sleep-disorders-clinic patients, patients with rheumatoid arthritis, and in normal aging individuals (2). However, few validation studies have been carried out in these diverse populations. Cole et al. developed and validated automatic scoring methodology of sleep/wake activity based on wrist activity and found that compared to PSG, in normal adults as well as psychiatric and sleep disorders patients, sleep percentage correlated at 0.82 (3). Sadeh et al. examined the validity of actigraphy in normal adults, sleep apnea patients, insomniacs, and children and concluded that scoring of these data allowed for discrimination between clinical groups with 65% accuracy (4). Aharon-Peretz et al. used actigraphy to study ambulatory patients with mild or moderate dementia and were able to determine differences in sleep/wake activity patterns (5). Continuing the examination of demented patients, we conducted a validation study of a wrist-activity device, the Actillume, compared to traditional PSG recordings and to observations in severely demented nursing-home patients.
METHODS
Subjects
Ten nursing-home patients (18% men and 82% women), aged 74–96 years (mean age = 86.4, SD = 6.0 years) participated. All 10 patients were wheelchair bound, and all were severely demented [Mini-Mental Status Examination Score (MMSE) < 20].
Apparatus
Sleep/wake activity was measured with an Actillume recorder (Ambulatory Monitoring, Inc., Ardsley, NY) that measures both activity and illumination exposure. The Actillume is a 1 × 3 × 6 cm device that is worn on the wrist. It contains a piezoelectric linear accelerometer (sensitive to 0.003 g and above) that records both intensity and frequency of movement. This results in two variables, the sum (total of all activity movements within the prescribed epoch) and the maximum activity (the largest or maximum movement recorded during the prescribed epoch). The Actillume also has a log-linear photometric transducer (sensitive from <0.01 lux to >100,000 lux) that measures light once per second in log lux. There is also a microprocessor, 32K RAM memory, and associated circuitry. The Actillume operates on batteries and will run for up to 21 days. The microprocessor is uploaded and downloaded into a portable computer. The orientation and sensitivity of the accelerometer are optimized for highly effective sleep–wake inference from wrist activity, which has been previously validated (3,6). The illumination measurements were roughly log-linear from a range below moonlight to the brightest summer day at noon. Records were scored automatically, based on the algorithm developed by Cole et al. (3).
The Oxford 9000 8-channel portable Medilog recorder was used to record a more traditional polysomnogram. Records were played back onto a Grass model 78 polygraph.
Procedure
Patients were studied at the nursing homes and recorded in their own beds. Patients were simultaneously recorded with Actillume and portable PSG. The following PSG channels were recorded: EEG (FP1-A2, FP2-A1), EOG, chin EMG, EKG, and thoracic and abdominal respiration. Although the frontal-parietal area is not traditionally recorded in the study of sleep, use of this site in this population increased both patient comfort and compliance. All recordings were begun near bedtime with times chosen to include known wake and sleep periods. Actillume data were collected for 24 hours, being sure that data collection completely overlapped with the EEG recordings. For this protocol, activity data were collected in 1-minute epochs.
All Medilog records were played back onto paper via a Grass model 78 polygraph. Two certified polysomnographic technologists hand-scored five records each for wake and sleep according to traditional scoring criteria. However, since the Actillume data were collected in in 1-minute epochs, EEG also had to be scored in 1-minute epochs.
Observations were made by trained research staff members every 30 minutes to determine sleep/wake activity. An observation consisted of locating the patient in the facility, observing them for 30 seconds, and noting the time and whether they seemed to be asleep or awake. Approximately 17 observations, between the hours of 1000 hours and 1930 hours were made per person. Actillumes were worn throughout the observation periods.
RESULTS
Spearman rank order correlations were computed between determinations of sleep and wake on EEG recordings vs. sum activity recordings and EEG vs. maximum activity. Sensitivity and specificity were computed for sleep/wake activity as determined by Actillume vs. observations. Total EEG recording periods ranged from 108 minutes to 933 minutes (mean = 637, SD = 287.6 minutes).
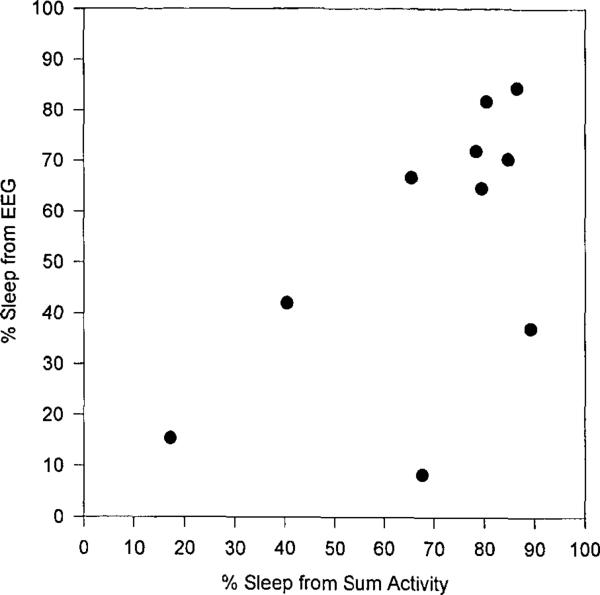
Since there was a wide range of both recording times and total sleep periods, analyses were done on total sleep time (TST), total wake time (TWT), and percent sleep. Correlations, shown in Table 1, were slightly higher for sleep and wake determined from summary activity than maximum activity, but both were significantly related to EEG. A scatterplot for percent sleep for the summary activity is presented in Fig. 1.
TABLE 1.
Correlations between EEG and Actillume
| EEG vs. maximum activity | EEG vs. sum activity | |
|---|---|---|
| TST | 0.81a | 0.91b |
| Percent sleep | 0.78c | 0.61 |
| TWT | 0.67d | 0.75d |
p < 0.005.
p < 0.001.
p < 0.01.
p < 0.05.
FIG. 1.
Scatterplot of percent sleep from EEG and from summary activity.
Actillume recordings were also compared to observations. The sensitivity (the ability to detect wake) of the actillume, compared to observations, was 87%. The specificity (ability to detect sleep) was 90%.
DISCUSSION
Recording EEG in this demented, institutionalized population was extremely difficult, and only certain patients could tolerate the equipment for any length of time. In addition, the EEG records showed much diffuse slowing, which made it difficult to score sleep or wake. Vitiello et al. suggest that in Alzheimer's Disease, impaired sleep may be secondary to degenerative changes in the brainstem regions and pathways that regulate sleep as well as in the cortical tissues that generate EEG slow-wave activity during sleep (7). It is possible that the recordings from the frontal/parietal areas exaggerated the amount of diffuse slowing. Nevertheless, it has been well documented that there is a high prevalence of EEG abnormalities among older patients with dementia, delirium, and other cognitive impairments (8). The waking EEG of demented patients shows abundant diffuse slow activity, similar to that seen in sleep (8). Bliwise, in his review of sleep and dementia (8), concluded that the slowing of the EEG activity makes discriminations between sleep and wake extremely difficult. Therefore, in older, demented patients, the EEG cannot be used as a “gold standard”.
In fact, in his review of the use of activity in psychology and medicine, Tryon presented evidence that questioned whether EEG measurement is a reliable and valid gold standard (9). He continued to review validity studies that have shown that with actigraphy, one can record microarousals not normally detected by EEG. Since the EEG was originally validated against behavioral criteria, Tryon concludes that one cannot fault actigraphy for disagreeing with EEG any more than one can fault EEG for disagreeing with behavior (9). Disagreement between behavioral observations and EEG most often occur during the transition from wake to sleep, i.e. sleep onset. Most disagreements between actigraphy and EEG also occur on the definition of sleep onset.
For this reason, minute-by-minute rates of agreement were not presented for these data. Agreement rates ranged from 0.33 to 0.85 for maximum activity and from 0.29 to 0.95 for sum activity, indicating that there was no consistently acceptable level of agreement between the two methods when evaluation was made minute-by-minute. While estimations of total sleep time and wake time can be reliably made, given the problems with scoring EEG, it is unclear how reliable minute-by-minute data actually are.
The estimations of total-sleep time and wake time from EEG and from Actillume were significantly correlated. In order to more fully examine the problems with scoring EEG, the two experienced sleep technologists, each with over 20 years’ experience, both scored a sub-set of the EEG records. The inter-rater correlation for total sleep on these difficult records was 0.87, very similar to the correlations between EEG and Actillume. Sleep/wake activity based on the Actillume was also compared with observations of sleep/wake. The sensitivity and specificity of the Actillume was well within acceptable limits when compared to observation. The Actillume is clearly superior to observation since it will unobtrusively record 24-hour periods without staff intervention.
Given the difficulties in both recording and interpreting EEG in demented nursing-home populations, the Actillume seems to be a viable, reliable alternative for studying sleep/wake activity. If any studies of etiology or treatment are to be done, the Actillume appears to be one of the most feasible techniques for doing so in this population.
Acknowledgements
The project could not have been completed without the help and support of the administration, staff, families, and patients of the San Diego Hebrew Home and Seacrest Village. Supported by NIA AG02711 NIA AG08415, NIMH 49671, NHLBI HL44915, the Research Service of the Veterans Affairs Medical Center, and the Sam and Rose Stein Institute for Research on Aging.
REFERENCES
- 1.Bliwise DL, Bevier WC, Bliwise NG, Edgar DM, Dement WC. Systemic 24-hr behavior observations of sleep and wakefulness in a skilled-care nursing facility. Psychol Aging. 1990;15:16–24. doi: 10.1037//0882-7974.5.1.16. [DOI] [PubMed] [Google Scholar]
- 2.Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- 3.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–9. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 4.Sadeh A, Alster J, Urbach D, Lavie P. Actigraphically based automatic bedtime sleep–wake scoring: validity and clinical applications. J Ambulatory Monitoring. 1989;2(3):209–16. [Google Scholar]
- 5.Aharon-Peretz J, Masiah A, Pillar T, Epstein R, Tzischinsky O, Lavie P. Sleep–wake cycles in multi-infarct dementia and dementia of the Alzheimer type. Neurology. 1991;41(10):1616–9. doi: 10.1212/wnl.41.10.1616. [DOI] [PubMed] [Google Scholar]
- 6.Mullaney DJ, Kripke DF, Messin S. Wrist-actigraphic estimation of sleep time. Sleep. 1980;3:83–92. doi: 10.1093/sleep/3.1.83. [DOI] [PubMed] [Google Scholar]
- 7.Vitiello MV, Bliwise DL, Prinz PN. Sleep in Alzheimer's Disease and the sundown syndrome. Neurology. 1992;42(7 Suppl 6):83–93. [PubMed] [Google Scholar]
- 8.Bliwise DL. Review: sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 9.Tryon WW, editor. Activity measurement in psychology and medicine. Plenum Press; New York: 1991. [Google Scholar]