Summary
We recently described a non-viral gene therapy paradigm offering long-term resolution of established neuropathic pain in several animal models. Here, the requirements for long term therapeutic effects are described, and evidence is provided for a mechanism of action based on immunological priming of the intrathecal space. Long-term pain reversal was achieved when two intrathecal injections of various naked plasmid DNA doses were separated by 5 hr to 3 days. We demonstrate that an initial DNA injection, regardless of whether a transgene is included, leads to an accumulation of phagocytic innate immune cells. This accumulation coincides with the time in which subsequent DNA injection efficacy is potentiated. We demonstrate the ability of non-coding DNA to induce short term pain reversal that is dependent on endogenous interleukin-10 (IL-10) signaling. Long term efficacy requires the inclusion of an IL-10F129S transgene in the second injection. Blockade of IL-10, via neutralizing antibody, either between the two injections or following the second injection induces therapeutic failure. These results demonstrate that this gene therapy paradigm utilizes an initial “priming” injection of DNA to induce accumulation of phagocytic immune cells, allowing for potentiated efficacy of a subsequent “therapeutic” DNA injection in a time and dose dependent manner.
Keywords: non-viral, interleukin-10, neuropathic pain, CNS
Introduction
Gene therapy is the delivery of nucleic acids (DNA or RNA) into host cells via viral or non-viral means in order to achieve chronic production or suppression of a protein with therapeutic significance. The main advantage of gene therapy lies in the potential for long-term expression of a therapeutic protein or in long term suppression of a deleterious protein. The potential for long-term efficacy is particularly important when treating a variety of chronic diseases.
Two major obstacles prevent the widespread use of gene therapy in the clinic. The first obstacle lies in the difficulty of delivering genes into host cells 1,2. The second obstacle lies in maintaining long-term efficacy, often due to a host immune response against either the therapeutic gene product or the delivery vector 3-6. The immune response can suppress gene expression as well as prevent successful vector reintroduction. Viral vectors are highly efficient at gene delivery, although these vectors tend to provoke an immune response that can terminate the therapy and can pose potential serious health risks to patients. Non-viral vectors are far less efficient at gene delivery, although they tend to be less immunogenic and therefore can lead to long-term gene expression, albeit at low levels. Thus, immunogenicity is generally viewed as a significant hindrance to the development of clinically viable gene therapies.
However, immunogenicity may powerfully benefit gene therapy when carefully utilized. We have developed a novel, two-injection non-viral gene therapy paradigm using naked plasmid DNA that suggests vector immunogenicity can be exploited to potentiate therapeutic efficacy.
The gene therapy described here makes use of naked plasmid DNA coding for a single amino acid variant of the anti-inflammatory cytokine interleukin-10 (IL-10F129S; see methods) to treat a model of neuropathic pain following peripheral nerve injury. Inflammation in the central nervous system (CNS) is a well documented phenomenon that occurs following a wide variety of insults including peripheral nerve injury 7-10. Neuropathic pain is one common symptom of CNS inflammation that has proven particularly difficult to treat in the clinic 11. A large body of evidence suggests that symptoms of inflammatory neuropathies, including chronic pain, may be controlled by targeting the activation of resident immune cells within the CNS (glia) 12-16. IL-10 has the well documented ability to suppress glial activation and the production of inflammatory mediators that contribute to the expression of pain 17-20. Intrathecal administration of either IL-10 protein or IL-10 viral vectors is able to reverse neuropathic pain 21,22. Consistent with a short half-life, the therapeutic effects of IL-10 protein for pain reversal normally lasts only a few hours, while viral vector IL-10 leads to pain reversal lasting a few weeks. Using a two successive injection approach, as will be described, reversal of pain can be achieved for a strikingly longer period of time, at least 3 months. The reversal of peripheral nerve injury induced pain is used as the primary measure of therapeutic efficacy in these studies.
Taken together, the data presented here are suggestive of a local innate immune response that is provoked by an intrathecal plasmid DNA injection and leads to the accumulation of activated immune cells which in turn allows for dramatically enhanced efficacy of a subsequent plasmid DNA treatment. This approach has potential applicability in a variety of other gene therapy contexts and may serve as a useful model in the development of clinically viable therapies.
Results
Long-term reversal of pain following 2 intrathecal pDNA-IL-10F129S injections
Initial work in our lab using intrathecal non-viral gene therapy for pain control demonstrated increasing efficacy of 100 ug intrathecal pDNA-IL-10F129S upon repeated injections such that the duration of pain suppression increased with each subsequent pDNA-IL-10F129S administration 23. When the time between two injections was shortened from 5 days to either 2 or 3 days, pain reversal was extended from less than a week (5 day interval) to over a month (2-3 day intervals). We now report a further extension of pain reversal to at least 3 months that is achieved by decreasing the dose of pDNA-IL-10F129S from 100 ug to 25 ug on the second intrathecal injection (Figure 1). While it is not certain why a lower dose of pDNA-IL-10F129S on a second injection should increase the therapeutic duration, we speculate that 100 ug of DNA on a second injection may induce a strong inflammatory response that ultimately shortens the duration of the therapy. The role of inflammatory signaling in both the induction and stability of this gene therapy will be described and discussed below.
Figure 1. Long term reversal of peripheral nerve injury induced neuropathic pain.
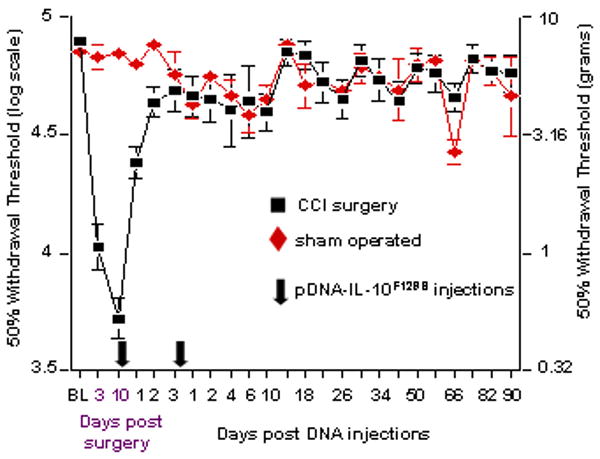
Baseline (BL) responsivity to calibrated low threshold mechanical stimuli (von Frey test) was recorded prior to either chronic constriction injury (CCI; black squares) of the left sciatic nerve or sham operation (red diamonds). Von Frey testing re-occurred through 100 days after surgery at time points indicated on the X-axis. Ten days after surgery, a time by which robust mechanical allodynia had developed, rats received an initial intrathecal injection of 100 ug pDNA-IL-10F129S (see first Arrow along the X-axis). This was followed 3 days later by a 25 ug pDNA-IL-10F129S injection (see second Arrow along the X-axis). This injection schedule led to at least 3 months of reversal of neuropathic pain. The lefthand y-axis represents 50% withdrawal threshold (i.e., absolute threshold) on the von Frey logarithmic scale. The righthand y-axis represents the 50% withdrawal threshold in gram force. Significant reversal of CCI induced allodynia observed on Day 10 rapidly developed within 24 hr following first injection, and lasted the duration of the experiment (p<0.01). The CCI operated and sham operated groups each include 6 rats across 2 replications. Error bars indicate SEM.
A minimum time delay between successive injections and innate immune cell accumulation is required for long term therapeutic effect
Given the dramatically extended therapeutic duration observed by decreasing the amount of time between injections, short inter-injection intervals were examined as well as intrathecal events in the hours following a pDNA injection. Figure 2a demonstrates a minimum inter-injection interval of approximately 5 hr is required to achieve long-term pain reversal. Separating the pDNA injections by 2 hr or administration of an equal total amount of pDNA-IL-10F129S (125 ug) as a single bolus, leads to pain reversal equivalent to a single 100 ug pDNA-IL-10F129S injection. While shorter inter-injection intervals (0-2 hr) lead to the same magnitude of pain reversal as do longer inter-injection intervals (5 days), the duration of pain reversal following a 0-2 hr inter-injection interval is approximately 7-10 days compared to a 5 hr inter-injection interval which produces pain reversal maintained for at least 90 days. Figure 2b demonstrates the time course of cellular accumulation in lumbosacral cerebrospinal fluid (CSF) in the hours and days after lumbosacral intrathecal injection. A significant increase in CSF cell count is observed at 6 hr post DNA injection, approximately the same time as the earliest inter-injection interval that leads to long term pain reversal. Cell counts in CSF peak at 24 hr, and return to near baseline levels at 6 days, corresponding to the window of time in which the efficacy of a second DNA injection is potentiated. The accumulation of cells within CSF only occurs following pDNA injection and not following equivolume vehicle injection. Figure 2c displays the cell phenotypes in CSF following DNA injection. At 6 hr following injection, a shift towards CD63+ cells (undifferentiated monocytes and mast cells) is observed. At all other time points, the overwhelming majority of cells were identified as macrophages (classically activated ED1+ or alternatively activated ED2+). Figure 3 includes representative photos of cells collected from lumbar CSF 24 hr following a 100 ug pDNA-Control (Figure 3a) or vehicle (Figure 3b) injection and plated for immunofluorescence analysis. Evidence that cell accumulation involves an inflammatory response to the pDNA injection is provided in Figure 3c. Intrathecal injections of anti-inflammatory IL-10 protein given 4 hr prior to and co-injected along with an intrathecal 100 ug pDNA injection prevents the accumulation of cells in CSF (Figure 3c) and prevents the long term reversal of pain normally observed following pDNA-IL-10F129S injections (Figure 3d).
Figure 2. Behavioral and intrathecal cellular events following a 100 ug DNA injection.
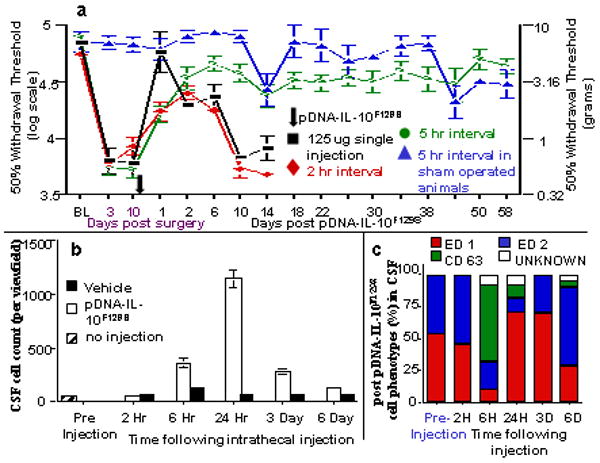
(a) A minimum of 5 hr lapse between successive pDNA-IL-10F129S injections is required to achieve long term efficacy in reversing neuropathic pain. Baseline (BL) responsivity to calibrated low threshold mechanical stimuli (von Frey test) was recorded prior to chronic constriction injury (CCI) of the left sciatic nerve or sham operation. Von Frey testing re-occurred through 68 days after surgery at time points indicated on the X-axis. Ten days after surgery, a time by which robust mechanical allodynia had developed, rats received either one (125 ug) or two (100 ug followed 2 or 5 hr later by 25 ug) intrathecal injections of pDNA-IL-10F129S totaling 125 ug for each group. The point in time where these injections occurred is indicated by an Arrow along the X-axis; a single arrow is shown given how compressed in time the 2 injections occur relative to the timescale of the X-axis. Long term therapeutic efficacy is not achieved if pDNA-IL-10F129S is given as a single 125 ug dose (black squares) or if a 25 ug second pDNA-IL-10F129S injection occurs 2 hr following an initial 100 ug pDNA-IL-10F129S injection (red diamonds). The reversal of allodynia as compared to pre-injection levels was observed until day 10 following pDNA-IL-10F129S injections for the 125 ug single injection as well as for the 2 hr interval (p<0.01). In contrast, 5 hr interval between the100 ug and 25 ug injections, was sufficient to reverse allodynia (green circles) throughout the duration of the experiment (58 days), as compared to pre-injection withdrawal thresholds recorded on days 3 and 10 post-CCI surgery (p<0.01). This same 5-hr dosing regimen delivered to sham operated controls (blue triangles) had no effect on responsivity to the von Frey test. Error bars indicate SEM. (b) Cell Influx into CSF occurs following intrathecal pDNA injection. Cell counts (y-axis label) in lumbar CSF per 20× magnification view field are displayed. Significant increases in cell counts compared to pre-injection are observed from 6 hr to 3 days following pDNA (white bars), but not vehicle (black bars) or no (diagonal stripes), injection. This 6 hr to 3 day temporal window corresponds to the time during which the efficacy of a second DNA injection for reversing neuropathic pain is potentiated (see Panel a of this figure). Error bars indicate SEM. (c) Cell phenotype percentages are displayed as percentage of total cells (y-axis labels) positively staining with ED1 (red bars), ED2 (blue bars), CD63 (green bars), or unlabeled (white bars) following vehicle pDNA-IL-10F129S injection. At the time of initial DNA injection (Pre-Injection; left-most bar), the local population of innate immune cells is relatively small. By 6 hr post DNA injection (6H Time After Injection; third bar), a dramatic shift in the number and activation state of immune cells is observed as compared to pre-injection cell counts (p<0.01). This same pattern of cell influx was observed following injection of pDNA without transgene (pDNA-Control), pDNA coding for Green Fluorescent Protein (GFP) as well as following a bicistronic plasmid construct containing IL-10 and GFP (data not shown). All time points reflect data collected from 4 animals per group. Error bars indicate SEM.
Figure 3. Long term therapeutic efficacy requires early intrathecal events.
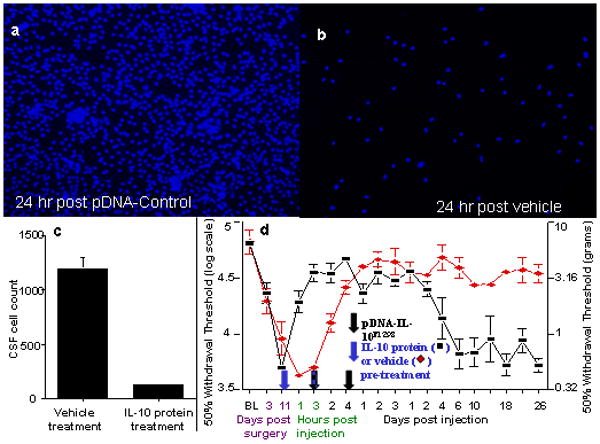
(a,b) Accumulation of innate immune cells is DNA dependent. Representative photos of the lumbar CSF 24 hr following 100 ug pDNA-Control (Panel a) or vehicle (Panel b) injection. Cell clusters were noteworthy in CSF following DNA injection. These aggregates were not observed following vehicle injection (compare Panel a vs. b). (c) Intrathecal injection of IL-10 protein prevents immune cell influx at 24 hr. Intrathecal treatment with IL-10 protein 4 hr (maximum IL-10 protein effects are observed from 2-4 hr following intrathecal injection) prior to pDNA-IL-10 and then IL-10 protein co-administered along with pDNA-IL-10 (protein and DNA; “IL-10 protein treatment”) of anti-inflammatory IL-10 protein (250 ng per injection) along with the initial 100 ug DNA injection blocks the accumulation of innate immune cells normally seen at 24 hr post DNA injection (compare to “vehicle treatment”) (N = 4 per group). Error bars indicate SEM. (d) IL-10 protein pre-treatment blocks long term gene therapy efficacy. IL-10 protein pre-treatment and co-injection (as in Panel c, above; black squares) induces a more rapid acute reversal of allodynia than vehicle treatment (red diamonds), but blocks long-term therapeutic efficacy following a second pDNA-IL-10F129S injection. Reversal of allodynia in the group receiving IL-10 protein pre-treatment was observed for only 6 days as compared to pre-injection withdrawal thresholds (p<0.01; N = 6 across 2 replications). Vehicle (equivolume PBS; red diamonds) pretreatment did not interfere with long-term therapeutic efficacy (N=6). Blue arrow indicates point of intrathecal IL-10 protein vs. vehicle pretreatment. Black+blue arrow indicates point of co-administration of IL-10 protein/vehicle co-administered with the first intrathecal injection of pDNA-IL-10 (100 ug). Black arrow indicates the point of the second injection of intrathecal pDNA-IL10 (25 ug). Error bars indicate SEM.
The immune response in CSF to DNA: cell influx and IL-10 induction
A large range of pDNA-IL-10F129S doses given as a second DNA injection produce long-term reversal of pain (Figure 4). 23 While the magnitude of pain reversal is equivalent between second injection doses ranging from 100 ug to 1 ug, the duration of pain reversal varies depending on dose. Two injections of 100 ug pDNA-IL-F129S lead to pain reversal lasting approximately 30-40 days 23 while a 25 ug second injection reverses pain for at least 3 months and a 1 ug second injection reverses pain for 82 days.
Figure 4. Dose dependent long-term efficacy of pDNA-IL-10 gene therapy.
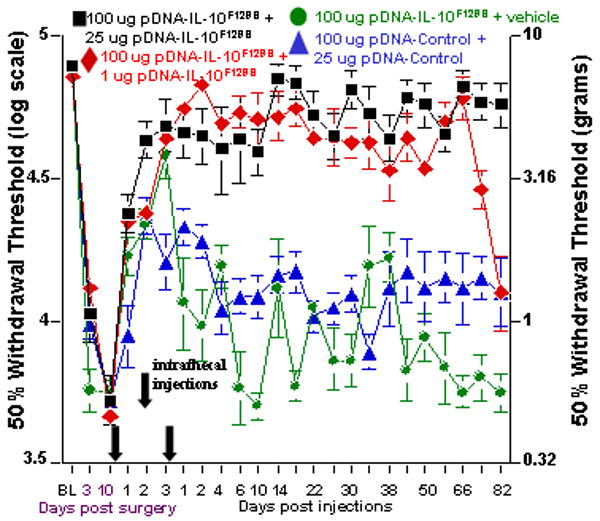
Long term behavioral reversal of allodynia can be achieved over a range of pDNA-IL-10F129S doses on a second intrathecal injection occurring 3 days after the 1st pDNA-IL-10F129S injection. Here, baseline (BL) responsivity to calibrated low threshold mechanical stimuli (von Frey test) was recorded prior to chronic constriction injury of the left sciatic nerve or sham operation. Von Frey testing re-occurred through 95 days after surgery at time points indicated on the X-axis. Ten days after surgery, a time by which robust mechanical allodynia had developed, rats (N=6 per group across 2 replications) received an initial 100 ug pDNA-IL-10F129S dose (at first Arrow along X-axis) followed by either 25 ug (black square) or 1 ug (red diamond) pDNA-IL-10F129S or vehicle (green circle) injection 3 days later (at second Arrow along X-axis); alternatively, a non-coding pDNA-Control was administered (at first Arrow) followed 3 days later by non-coding pDNA-Control (blue triangles) (at second Arrow). Reversal of allodynia, as compared to pre-injection withdrawal thresholds at days 3 and 10 post-surgery, was observed for the duration of the experiment in rats receiving an initial 100 ug pDNA-IL-10F129S dose followed by a second injection dose of 25 ug (p<0.01; black squares), until day 82 following a second injection dose of 1 ug (p<0.01; red diamonds), and until day 2 following a vehicle injection (p<0.01; green circles). Significant reversal of allodynia as compared to pre-injection withdrawal thresholds was noted following a 100 ug pDNA-Control injection followed 3 days later by 25 ug pDNA-Control (blue triangle symbol), starting 24 hr after the first injection and lasting until day 4 following the second injection (p<0.01; N=6). Error bars indicate SEM.
During the course of these experiments, we made the surprising observation that an intrathecal injection of pDNA that does not code for a gene (pDNA-Control), led to short-term reversal of pain. Reversal of pain 48 hr following injection of either pDNA-Control or pDNA-IL-10F129S is dependent on IL-10 signaling (Figure 5a). PCR analysis of plasmid derived IL-10 gene expression within CSF samples collected 48 hr following a single pDNA-IL-10F129S injection demonstrates significant gene expression as compared to a pDNA-Control injection (Figure 5b). There was no difference at this timepoint in endogenous IL-10 gene expression in CSF supporting the notion that the DNA injection itself leads to a temporary endogenous elevation of IL-10 protein (data not shown). Continuous IL-10 signaling is required between the two pDNA injections for long term pain reversal, as injection of IL-10 neutralizing antibody, but not control IgG, 24 hr (or 48 hr; data not shown) following the first pDNA-IL-10F129S injection caused an initial re-expression of pain behavior as well as the failure of the therapy following a properly timed second pDNA-IL-10F129S injection (Figure 5c).
Figure 5. IL-10 signaling mediates short and long term reversal of allodynia.
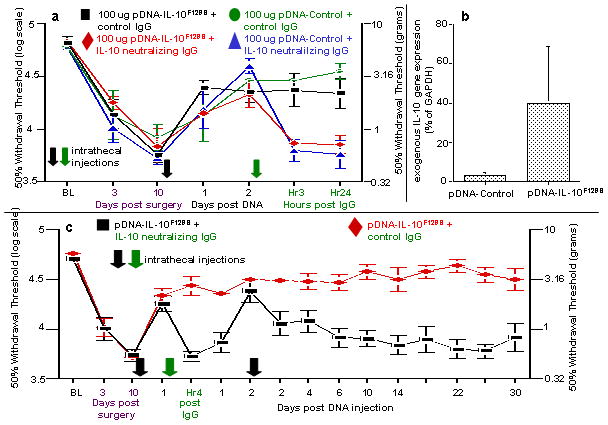
(a) Intrathecal pDNA injection induced reversal of allodynia. Reversal of neuropathic pain is dependent on IL-10 activity following an initial 100 ug pDNA-Control or pDNA-IL-10F129S injection. Here, baseline (BL) responsivity to calibrated low threshold mechanical stimuli (von Frey test) was recorded prior to chronic constriction injury of the left sciatic nerve. Von Frey testing re-occurred through 13 days after surgery at time points indicated on the X-axis. Ten days after surgery, a time by which robust mechanical allodynia had developed, all rats received a 100 ug intrathecal administration of either a non-coding pDNA (pDNA-Control) or pDNA-IL-10F129S (at black Arrow along X-axis). All rats received a second intrathecal injection 48 hr later, consisting of either IL-10 neutralizing IgG or an equal amount of control IgG (at green Arrow along the X-axis). Administration of IL-10 neutralizing antibody, but not control IgG at 48 hr following DNA injection (pDNA-Control or pDNA-IL-10F129S), leads to rapid re-expression of pain behavior within 3 hr (p<0.01; N=6 per group). This suggests a substantial endogenous IL-10 upregulation following pDNA-Control injection. Error bars indicate SEM. (b) Plasmid derived IL-10 gene expression in CSF. At 48 hr following the second DNA injection, plasmid derived IL-10 gene expression was detected in cells within CSF samples following pDNA-IL-10F129S injection (right bar) but not pDNA-Control injection (left bar) (p=0.05). (c) Long term efficacy of gene therapy is blocked by temporary blockade of IL-10 signaling. Long term therapeutic efficacy requires IL-10 signaling between the two DNA injections. Here, baseline (BL) responsivity to calibrated low threshold mechanical stimuli (von Frey test) was recorded prior to chronic constriction injury of the left sciatic nerve. Von Frey testing re-occurred through 40 days after surgery at time points indicated on the X-axis. Ten days after surgery, a time by which robust mechanical allodynia had developed, all rats received intrathecal administration of 100 ug pDNA-IL-10F129S (at first black Arrow along X-axis). A single administration of IL-10 neutralizing antibody (black squares), but not control IgG (red diamonds), 24 hr following pDNA-IL-10F129S injection (at green Arrow along X-axis) both acutely blocks (4 hr [Hr4]) short-term reversal of neuropathic pain as well as chronically blocking the sustained reversal of neuropathic pain induced by a second pDNA-IL-10F129S injection (N=6 per group across 2 replications). Re-expression of allodynia as compared to pre-injection withdrawal thresholds (days 3 and 10 post-surgery) was noted in IL-10 neutralizing antibody injected rats by day 2 following the second pDNA injection. Error bars indicate SEM.
Characterization of intrathecal sensitization following pDNA injection
Injection of 100 ug of pDNA-Control as an initial priming injection followed by 25 ug pDNA-IL-10F129S leads to the same reversal of pain as two injections of pDNA-IL-10F129S (Figure 6a). Importantly, injection of pDNA-IL-10F129S as the first injection followed by pDNA-Control on the second DNA injection fails to lead to long-term pain reversal (Figure 6b). Intrathecal sensitization is also observed following injection of stimulatory synthetic oligodeoxynucleotides (ODN), agonists of the innate immune receptor Toll-like receptor 9 (TLR 9), in a dose dependent manner (Figure S1). Disruption of IL-10 signaling via intrathecal injection of IL-10 neutralizing antibody (anti-IL-10) following the second pDNA injection leads to a rapid and sustained re-expression of pain behavior (Figure 7a). This induced failure of the therapy is remarkably persistent, lasting the duration of the experiment (30 days after neutralizing antibody administration). The persistent re-expression of neuropathic pain is not due to a lingering inability to respond to IL-10 protein as injection of IL-10 protein either 4 days (Figure S2) or 3 weeks (data not shown) following injection of anti-IL-10 is still highly effective at reversing pain. The strikingly long therapeutic duration of IL-10 protein observed here is addressed in the discussion below. Interestingly, the destabilizing effect of anti-IL-10 can be mimicked by intrathecal injection of the pro-inflammatory HIV coat protein, gp120, at a dose that is sufficient to cause the temporary expression of enhanced pain in naïve animals (Figure S3).
Figure 6. Intrathecal priming occurs following an initial pDNA injection and potentiates a subsequent therapeutic DNA injection.
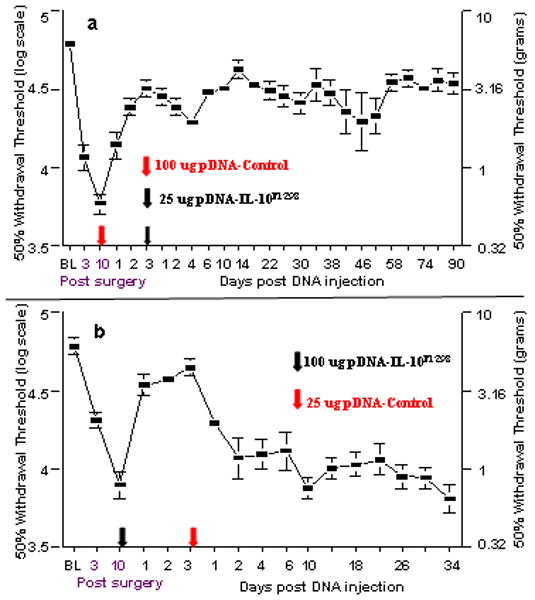
(a) Prior pDNA-Control injection potentiates a subsequent pDNA-IL-10F129S injection. Sensitization of the intrathecal environment occurs independent of the presence of a transgene on the 1st DNA injection. Here, baseline (BL) responsivity to calibrated low threshold mechanical stimuli (von Frey test) was recorded prior to chronic constriction injury of the left sciatic nerve. Von Frey testing re-occurred through 103 days after surgery at time points indicated on the X-axis. Ten days after surgery, a time by which robust mechanical allodynia had developed, one group of rats received an injection of 100 ug pDNA-Control (at red Arrow along X-axis) followed 3 days later by 25 ug pDNA-IL-10F129S (at black Arrow along X-axis). Reversal of allodynia as compared to pre-injection withdrawal thresholds was noted 24 hr following pDNA-Control injection and lasted the duration of the experiment (p<0.01; N=6). Error bars indicate SEM. (b) pDNA-IL-10F129S efficacy is not potentiated by a subsequent pDNA-Control injection. The first injection stimulus enhances only a subsequent injection. Rats were prepared and behaviorally tested as in Panel a. Ten days after surgery, rats received 100 ug pDNA-IL-10F129S intrathecally (at black Arrow along X-axis) followed 3 days later by 25 ug of non-coding pDNA-Control (at red Arrow along X-axis). Given the failure to attain long duration reversal of neuropathic pain, these data indicate that in order to achieve long term pain reversal, the second DNA injection must include the IL-10 gene. Efficacy of an initial 100 ug pDNA-IL-10F129S injection can not be enhanced by a subsequent 25 ug pDNA-Control injection. Allodynia is reversed until day 2 following second injection as compared to pre-injection withdrawal thresholds. Error bars indicate SEM.
Figure 7. Therapeutic stability and plasmid derived gene expression require continuous IL-10 signaling.
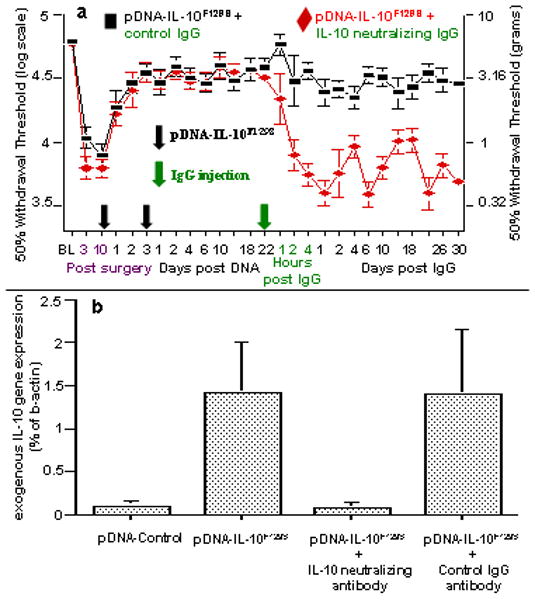
(a) Long term gene therapy stability requires IL-10 signaling. Long term therapeutic efficacy requires continuous IL-10 protein signaling following the second pDNA-IL-10F129S injection. Here, baseline (BL) responsivity to calibrated low threshold mechanical stimuli (von Frey test) was recorded prior to chronic constriction injury of the left sciatic nerve. Von Frey testing re-occurred through 62 days after surgery at time points indicated on the X-axis. Ten days after surgery, a time by which robust mechanical allodynia had developed, rats received 100 ug pDNA-IL-10F129S followed 3 days later by 25 ug pDNA-IL-10F129S (at time points indicated by two serial black Arrows on X-axis). A single injection (at green Arrow on X-axis) of IL-10 neutralizing antibody (0.2 ug; red diamonds; N=6, across 2 replications) led to the rapid and sustained re-expression of pain behavior as compared to pre-injection withdrawal thresholds (p<0.01 beginning at 2 hr following neutralizing antibody and lasting the remainder of the experiment). Control IgG injection (at green Arrow on X-axis) did not disrupt long term therapeutic efficacy (black squares; N=6, across 2 replications). Error bars indicate SEM. (b) Plasmid derived gene expression requires continuous IL-10 signaling. Rats were prepared as in Panel a. At 22 days following the second pDNA-IL-10F129S injection (100 ug followed by 25 ug), significant plasmid derived IL-10 expression is observed in cells contained in the lumbosacral CSF (second bar), compared to rats receiving 2 successive intrathecal injections (100 ug followed by 25 ug) of non-coding pDNA-Control (first bar) (p<0.05). The remaining rightward 2 bars represent data of separate groups of rats that also had received 2 successive intrathecal injections (100 ug followed by 25 ug) pDNA-IL-10F129S prior to challenge by intrathecal antibody. A single injection of IL-10 neutralizing antibody (0.2 ug; N=6, across 2 replications) given on day 22 post second pDNA-IL-10F129S injection (third bar) reduced plasmid derived IL-10 gene expression in lumbosacral CSF to background levels (equivalent to levels following pDNA-Control injections) within 4 days (the timepoint of CSF analysis shown here). Injection of an equivalent amount of control IgG antibody (N=6, across 2 replications) given under the same conditions has no effect on exogenous IL-10 gene expression in CSF as compared to the levels observed at day 22 post second pDNA-IL-10F129S injection (rightmost bar).
PCR analysis for plasmid derived IL-10 gene expression in CSF samples was conducted either prior to or following either neutralizing anti-IL10 or control antibody injection (Figure 7b). CSF samples collected 22 days following 2 successive pDNA-IL-10F129S injections (100 ug followed by 25 ug), but not 2 successive pDNA-Control injections (100 ug followed by 25 ug) demonstrated significant plasmid derived gene expression. Additional groups received intrathecal injection of either anti-IL-10 neutralizing antibody or control IgG antibody on day 22 after the second of 2 successive intrathecal injections of pDNA-IL-10F129S injections (100 ug followed by 25 ug), CSF samples were collected 4 days later. PCR analysis of these CSF samples demonstrated equivalent plasmid derived IL-10 gene expression following control IgG antibody injection as compared to 22 days following pDNA-IL-10F129S injection. Anti-IL-10 antibody injection, however, reduced plasmid derived IL-10 gene expression in CSF to background levels (equivalent to pDNA-Control) (Figure 7b).
Following the eventual re-expression of neuropathic pain behavior, a single pDNA-IL-10F129S injection re-instates long-term reversal of pain if delivered within days
An initial priming injection of 100 ug pDNA-IL-10F129S followed 3 days later by 25 ug pDNA-IL-10F129S leads to reversal of pain that outlasts the durability of the neuropathic pain model being tested. If, however, 2 successive 25 ug doses of DNA are administered, reversal of pain lasts for only 1 month (Figure 8a,b). Figure 8a demonstrates the ability of a single 25 ug pDNA-IL-10F129S injection to lead to at least 2 months of full pain reversal if delivered 4 days following the failure of the first two injections. If, instead, the third 25 ug injection is administered 3 weeks following the failure of the initial two injections, a partial reversal of pain is noted only at 24 hr following the third injection (Figure 8b).
Figure 8. Time dependent reinstatement of long term gene therapy efficacy.
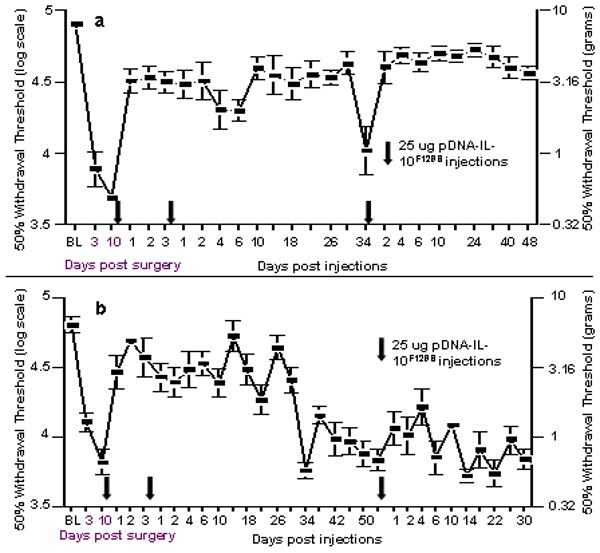
For both panels, baseline (BL) responsivity to calibrated low threshold mechanical stimuli (von Frey test) was recorded prior to chronic constriction injury of the left sciatic nerve. Von Frey testing re-occurred after surgery at time points indicated on the X-axis. Ten days after surgery, a time by which robust mechanical allodynia had developed, rats received 2 successive intrathecal injections of pDNA-IL-10F129S (at black Arrows along X-axis). (a) Sensitization of the intrathecal space to a pDNA-IL-10F129S injection is time dependent following failure of the therapy. A priming dose of 25 ug pDNA-IL-10F129S followed 3 days later by a second 25 ug pDNA-IL-10F129S dose leads to reversal of pain for approximately 1 mon (p<0.01; N=6, as 2 replications) as compared to pre-injection withdrawal thresholds. Here, a third 25 ug pDNA-IL-10F129S injection was administered within 4 days of the re-expression of pain, reinstating a long term therapeutic effect (> 2 month effect, p<0.01; N=6, as 2 replications). Error bars indicate SEM. (b) Here, the same 25 ug pDNA-IL-10F129S dose was administered approximately 3 weeks following re-expression of allodynia, leading to a small and variable therapeutic effect (peak therapeutic effect on day 4). Error bars indicate SEM.
Discussion
We here describe a non-viral gene therapy paradigm in which an interleukin-10 transgene containing a point mutation (pDNA-IL-10F129S) is injected into the intrathecal space around the spinal cord to treat a model of neuropathic pain. This point mutation retains in vivo anti-inflammatory activity as has been documented in several independent labs in models of chronic renal disease 24, aortic tissue transplantation 25, spinal nerve transection 26, experimental autoimmune encephalomyelitis27, and sciatic nerve ligation 22. Long-term efficacy of this therapy requires a pDNA-IL-10F129S injection anytime from 5hr to 3 days following an initial intrathecal injection of DNA. The initial DNA injection provokes the accumulation of phagocytic innate immune cells and the production of endogenous IL-10 whether or not a transgene is present in the plasmid vector. Long-term therapeutic stability requires an initial immune cell accumulation as well as continuous IL-10 signaling beginning at 24 hr following the first DNA injection. Upon failure of the therapy (measured by re-expression of pain behavior), a single injection of pDNA-IL-10F129S is able to re-instate long term pain relief if delivered within days of therapy failure.
The work presented here describes the timing and dose requirements of a non-viral gene therapy method by which long term suppression of neuropathic pain is achieved. The model of neuropathic pain used here to characterize therapeutic efficacy has been widely used to determine the mechanistic basis for the expression of neuropathic pain as well as to test potential therapeutics 28-31. This manuscript does not focus on the mechanistic basis of neuropathic pain expression and readers are referred to several articles on the contributions of cytokines to neuropathic pain and the therapeutic use of regulatory cytokines such as IL-10 22,32-35. A behavioral endpoint (von Frey test of allodynia) is used as the primary measure of therapeutic efficacy for several reasons. First, there is convincing pharmacological evidence indicating the crucial role of proinflammatory cytokines in the expression of neuropathic pain 12. For example, IL-1 receptor antagonist or IL-10 protein are both able to reverse neuropathic pain in this model of neuropathy 36. Because proinflammatory cytokines have rare and unstable mRNAs 37 and so few molecules need be released to exert biological actions, behavioral endpoints rather than protein or mRNA have proven to be the most sensitive assay. We demonstrate that long-term reversal of pain observed in this paradigm is dependent on the inclusion of an IL-10 transgene on the second injection. Pain reversal as well as plasmid derived gene expression following the second injection is mediated by IL-10 protein as is demonstrated by the re-expression of pain and a loss of gene expression following injection of IL-10 neutralizing antibody. The current studies indicate a requirement of innate immune cell influx prior to the “therapeutic” injection in order to achieve long term efficacy. Further study is necessary to determine if this influx facilitates gene uptake, subsequent gene expression, prevention of gene silencing, transfection of alternative cell types, or induction of specific immune cell phenotypes that perhaps exert bystander therapeutic benefits. The results of the present series of studies will form the basis for such mechanistic analyses now that the fundamental characterization of the phenomenon has been clarified.
Gene therapy vectors are inherently immunogenic
Elaborate and powerful immunologic mechanisms exist in an organism to prevent and contain infections. These mechanisms can result in recognition and destruction of therapeutic viral vectors, destruction of infected host cells, or in silencing of therapeutic gene expression 3. Gene therapy by design introduces exogenous RNA or DNA into host cells. It is not surprising then, that immunogenicity has been viewed as a major hurdle in the generation of viable gene therapy applications 2. Immune responses broadly fall into two categories, innate immunity and adaptive immunity. The innate immune response occurs locally at the site of infection, while an adaptive response is systemic and involves the generation of highly antigen specific effector cells (T cells and B cells). The innate response begins with the rapid production of a wide variety of cytokines and chemokines which occurs in the first few hours following detection of a “danger signal” 38,39, and promotes immune cell extravasation from circulation. The early response involves clearing of cellular debris and potential infectious agents by highly phagocytic cells recruited to the area which can be observed after approximately 4 hours 40. The innate immune response typically resolves 4 days following initiation when adaptive immunity becomes more prominent. The events occurring during an innate immune response (phagocytic cell influx from approx. 4 hr to 4 days) show strong parallels to events following the initial DNA injection in the current gene therapy paradigm (phagocytic cell accumulation and sensitized response to a subsequent plasmid DNA injection from approx. 5 hr to 3 days) and will be discussed below.
DNA based vectors trigger an innate immune response
A family of receptors known as the Toll Like Receptors (TLR) are found on many immune cells important for the innate immune response, including macrophages and mast cells. This family of receptors consists of more than 10 different receptor types, each of which differentially recognizes antigens that are associated with particular pathogens 41. TLR activation is a key event in immune response initiation and the downstream signaling of particular TLRs can have a profound impact on the phenotypes of cells that are ultimately localized to the site of infection 42. Of particular relevance to gene therapy applications are the TLRs that are able to recognize RNA (TLR 3 and 7) or specific unmethylated DNA sequences known as CpG motifs (TLR 9) that are common to bacteria and viruses as well as plasmid gene therapy vectors. TLR 9 stimulation in monocytic cell lines (which give rise to macrophages and mast cells) leads to the production of a panel of cytokines and chemokines that direct cell recruitment and differentiation into particular phenotypes 43. Cytokines are roughly categorized as either Th1 (T helper 1) or Th2 (T helper 2) types which are produced by, and direct the generation of, either Th1 or Th2 T cell subsets 40. TLR 9 activation in monocytes eventually results in a Th2 biased response including the production of IL-10 with little production of classic Th1 inflammatory mediators 43,44.
The gene therapy employed in the current studies is delivered intrathecally, into the cerebrospinal fluid (CSF) percolating through the fibrous network of cells that make up the meninges and surround the spinal cord. A large number of innate immune cells reside in the meninges including mast cells and macrophages (meningeal and perivascular macrophages) 45,46. These immune cells residing intrathecally express TLR 9 and tend to respond to immune challenges via production of Th2 cytokines, including the interleukins IL-4 and IL-10 47. It is demonstrated here that reversal of peripheral injury induced pain occurs in an IL-10 dependent manner by 48 hr following a single intrathecal DNA injection, regardless of the gene being delivered. Thus, it appears that resident innate immune cells within the meninges respond to DNA administration by producing Th2 cytokines including IL-10.
In addition to cytokine production, resident monocytic cells may produce a wide variety of chemokines following DNA administration that may induce cell extravasation from circulation. These chemokines include monocyte chemoattractant protein 1 (MCP-1) and monocyte inflammatory protein 1 beta (MIP-1b), both of which potently recruit undifferentiated monocytes. CD63 is a lysosomal protein expressed by undifferentiated monocytes 40. Monocytes differentiate into a mature phenotype once they are recruited from circulation into tissue following an immune challenge. It is likely a significant proportion of the CD63+ cells observed in CSF 6 hr following an intrathecal DNA injection are newly recruited monocytes that have not yet differentiated, and ultimately give rise to the macrophage population observed in CSF at later time points. IL-10 has the well documented ability to block the activation and production of multiple inflammatory cytokines and chemokines b y a variety of immune cells including macrophages and mast cells 48,49. We demonstrate here that intrathecal pre-treatment and co-injection of recombinant IL-10 protein along with a DNA injection, designed to block inflammatory signaling that leads to immune cell influx, in fact does prevent the accumulation of cells into CSF at the site of injection as well as block long term efficacy of a subsequent pDNA-IL-10F129S injection. This strongly suggests an initial inflammatory response to the DNA injection, with likely production of chemotactic factors, responsible for the extravasation of phagocytic immune cells. A required minimum inter-injection interval provides additional support for the crucial role played by cell accumulation in CSF.
Provoked accumulation of phagocytic cells into CSF facilitates enhanced gene delivery
Cells present in CSF at 24 hr are primarily macrophages that strongly express ED1, a marker of classic macrophage activation. Over the course of the following several days, a phenotype shift occurs with a larger percentage of macrophages expressing ED2 (also known as CD163), a marker of alternative activation associated with the resolution of an immune response50,51. Macrophages are best known for their role in the clearance of cellular debris through their highly phagocytic activity. An increase in the population of these cells would likely increase the chances of DNA uptake following injection. The ability of a very low dose second injection of pDNA-IL-10 to provide long term reversal of pain behavior, provides supporting evidence for increased gene uptake during the period of intrathecal cell accumulation. The focus of these studies is on the intrathecal response to DNA. It remains to be determined to what extent the immune response that enhances subsequent DNA injections is specific to DNA.
IL-10 protein stabilizes long term gene therapy efficacy and plasmid derived gene expression
Continuous IL-10 signaling appears to be required for long term reversal of pain beginning at 24 hr following the first DNA injection and lasting through the duration of the therapy. While the actions of IL-10 protein that provide long term therapeutic stability are unknown, it is possible that IL-10, via its anti-inflammatory activity, prevents the activation of immune cells that would otherwise lead to a migration of those accumulated immune cells away from the intrathecal space and to draining lymph nodes 40. There also appears to be a positive feedback loop between IL-10 signaling and ongoing plasmid derived gene expression as blockade of total IL-10 protein leads to a suppression of exogenous IL-10 gene expression, perhaps by transgene silencing. The surprising observation that a single injection of IL-10 protein, which normally leads to pain reversal lasting several hours, is able to lead to pain reversal lasting upwards of 30 days when administered following the induced failure of this gene therapy may lend credence to the notion of a positive feedback loop encouraging gene expression, perhaps by unmasking transgene expression and protecting it from further silencing by inducing the ongoing release of IL-10. Alternatively, it may be the case that local immune cell populations have become predisposed to the endogenous production of anti-inflammatory factors as is the case with alternatively activated macrophages which can be induced following prolonged exposure to IL-10 protein. Future studies are required to explore this question adequately. Plasmid derived IL-10 may contribute to a Th2 dominant cytokine profile that is permissive of continued plasmid gene expression. The fact that injection of the inflammatory protein gp120, a strong inducer of Th1 cytokines 52, mimics the long term destabilization of the gene therapy seen following administration of IL-10 neutralizing antibody suggests that the cytokine milieu is the key factor in stabilizing the gene therapy and not an effect specific to IL-10 receptor activity. The ability of a single pDNA-IL-10F129S injection to reinstate long term pain reversal if administered 4 days but not 3 weeks following a failure of gene therapy (re-expression of pain behavior) is strongly indicative of a parallel between the period of behavioral reversal (and Th2 dominant cytokine milieu) and the period of sensitization to DNA injection.
Harnessing innate immunity to enhance gene therapy is a model with potential widespread applicability
The paradigm presented here describes the effect of a “priming” intrathecal DNA injection followed by a “therapeutic” DNA injection. The priming injection serves the purpose of recruiting phagocytic cells from circulation to enhance uptake of gene therapy vector. The therapeutic injection contains the gene(s) of interest and is delivered into an environment that dramatically improves its efficacy. Further study is necessary to determine if this approach will enhance other gene delivery applications o r if the inclusion of Th2 type genes along with a desired therapeutic gene will broadly and consistently increase therapeutic stability and duration.
Materials and Methods
Subjects
Viral-free adult male Sprague-Dawley rats (350-450g; Harlan Labs) were used in all experiments. Rats were housed in temperature (23°C ± 3°C) and light (12:12 light:dark; lights on at 0700 hr) controlled rooms with standard rodent food and water available ad libitum. Behavioral testing was performed during the first half of the light cycle. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado at Boulder.
Surgery
CCI was created at mid-thigh level of the left hind leg as previously described 53. Briefly, under isoflurane anesthesia (Phonex Pharm, St Joseph, MO) the left sciatic nerve was exposed via blunt dissection of the overlying muscle and gently isolated with glass surgical tools. Four chromic gut sutures (cuticular 4-0, cutting FS-2; Ethicon, Somerville, NJ) were then tied loosely around the nerve. Sham operated animals had the sciatic nerve identically isolated but not ligated. The muscle was then sutured with silk suture (4.0, Ethicon) and the skin incision closed with wound clips. Animals were allowed to recover and were monitored daily for overall health.
von Frey Test
The von Frey test was performed on the hind paws as previously described in detail 22,27. Briefly, a logarithmic series of 10 calibrated Semmes-Weinstein monofilaments (von Frey hairs: Stoelting, Wood Dale, IL) was applied randomly to the left and right hind paws to determine the stimulus intensity threshold stiffness required to elicit a paw withdrawal response. Log stiffness of the hairs is determined by log10(milligrams × 10). The range of monofilaments used in these experiments (0.407-15.136gm) produces a logarithmically graded slope. Data are presented as interpolated 50% response threshold to stimulus intensity in log10(milligrams × 10). Behavioral measures were recorded prior to surgery, at days 3 and 10 following surgery, and every few days following injections for the remainder of each time course. Behavioral measures were recorded by multiple testers throughout the time course and were performed blind with respect to surgery and injection conditions.
Intrathecal Injections
Injections were acutely administered intrathecally (i.t.) as previously described 21. Briefly, animals were anesthetized with isoflurane and an 18-g hypodermic needle (Beckton Dickinson & CO, Franklin Lakes, NJ) was removed from its hub and percutaneously inserted between lumbar (L) vertebrae L5 and L6. A catheter of PE-10 tubing (Beckton Dickinson & CO, Sparks, MD) was then threaded through this 18-ga. guide 7.7-cm rostrally, allowing for the tip of the catheter to be placed at the level of the lumbosacral enlargement. Injections were administered over approximately 10-20 sec. The PE-10 catheter and 18-ga. needle were then removed and anesthesia discontinued. Animals were anesthetized for an average of 3-5 min during injection and no abnormal behavior was noted upon recovery from anesthesia.
Naked Plasmid DNA
All plasmid DNA injections were delivered at a concentration of 5-6 ug/uL. The plasmid construct coding for rat interleukin-10 (pDNA-IL-10F129S) was identical to that previously described 23. Briefly, this plasmid contains cDNA encoding rat IL-10 under the control of a hybrid cytomegalovirus (CMV) enhancer/chicken beta actin promoter. A point mutation resulting in an amino acid change (F129S) has been identified in the rat IL-10 gene expressed in these studies. This mutation lies outside of identified receptor binding regions. The effect of this point mutation on IL-10 bioactivity in vitro is currently under investigation. Preliminary results with macrophages and B cells suggest differences between wild type and variant (Busha and Chavez, personal communications). However, this construct has previously been documented to exert anti-inflammatory activity in vivo across diverse models of inflammation. Plasmid was grown up in SUREII cells (Stratagene, CA; cat #200152). Isolation and purification of the plasmid were performed using Qiagen Giga Endotoxin-Free kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The control plasmid (pDNA-Control) was constructed from the pDNA-IL-10F129S plasmid by enzymatic excision of the IL-10 gene and insertion of a poly-A sequence thereby keeping all other elements of the backbone identical. All purified plasmid was suspended in a vehicle of 0.2 micron filtered 3% sucrose solution in Dulbecco's Phosphate-Buffered Saline (DPBS; 0.1mM pore-filtered, pH 7.2; Gibco, Grand Island, NY) and stored at -20°C until thawed on ice prior to injection. Final concentration of plasmid solution was measured by optical density.
IL-10 neutralizing antibody and IgG control antibody
Lyophilized bulk ion exchange purified sheep anti-rat interleukin-10 (anti-IL-10) IgG neutralizing antibody as well as non-specific IgG control antibody (National Institute for Biological Standards and Control; Potters Bar, UK) were reconstituted to a concentration of 1.0 ug/ul, aliquoted, and stored at -20°C. Aliquots were slowly thawed on ice immediately before injection and diluted with DPBS to an injection concentration of 0.2 ug in 20 ul. Anti-IL-10 or control IgG were injected intrathecally as described.
Oligodeoxynucleotides (ODN)
Synthetic stimulatory K-type ODNs were provided by Dennis Klinman at the National Cancer Institute. A mix of ODN containing the stimulatory sequences TCGTA and TCGA were administered. Stock ODN was diluted in 20 ul PBS just prior to intrathecal injection.
glycoprotein120(gp120)
gp120 is a coat protein of the Human Immunodeficiency Virus that is a potent inflammatory agent, inducing the production of inflammatory cytokines from a variety of immune cells. gp120 was injected intrathecally at a dose of 3 ug (courtesy of Amgen, Thousand Oaks, CA).
interleukin-10 (IL-10) protein
Recombinant human IL-10 Fc chimera was purchased from Sigma. This construct is a human IL-10 chimera fused to IgG1 that has been mutated to prevent cytotoxicity. The Fc fusion extends the half-life of IL-10 allowing for prolonged activity.
Cerebrospinal fluid (CSF) collection
Lumbar CSF was collected using a similar protocol to that described for intrathecal injections. Under isoflurane anesthesia, an 18-g needle was inserted between L5 and L6 vertebrae for use as a guide cannula. PE-10 tubing was inserted into this guide and threaded rostrally to the lumbosacral enlargement as described for i.t. injections. A 3cc syringe with attached 30-g needle was connected to the free end of the PE-10 tubing and CSF gently withdrawn into the catheter and syringe. Approximately 100 ul of CSF was obtained from each rat and placed into an eppendorf tube on water ice for subsequent IHC analysis or frozen in liquid nitrogen for subsequent PCR analysis.
Immunohistochemistry on CSF samples
CSF samples were gently inverted to ensure a homogeneous suspension and then 40 ul drops from each sample were plated onto glass coverslips (Fisher Scientific, 12mm circular No.1) in the wells of a 24 well plate (Corning Inc; Corning, NY) immediately following collection. Cells within CSF were allowed to adhere to the coverslips for 40 min in an incubator (37°C, 5% CO2). Following incubation, immunohistochemical staining for cell phenotype markers was conducted according to standard procedures. All liquids used during the procedure were introduced to and removed from the wells via pippet at a volume of 500 ul/well. Samples were initially washed twice with PBS, then fixed with 4% paraformaldehyde for 30 min, and washed three times with PBS. Samples were permeabilized and cleared with a 5% H2O2 solution in PBS for 15 min, followed again by 3 PBS washes. Overnight incubation in primary detection antibody was done in an incubation buffer containing 5% normal goat serum, 0.1% Triton-X, and 0.1% sodium azide in PBS at 4°C on an agitator. Primary antibody specifications are as follows: ED1 (marker of classic macrophage activation; AbD Serotec; Raleigh, NC; 1:200 dilution for final concentration of 2.5 ug/ml), ED2 (marker of alternative macrophage activation; AbD Serotec; Raleigh, NC; 1:200 dilution for final concentration of 2.5 ug/ml), CD63 (marker of undifferentiated monocytes and mast cells; AbD Serotec; Raleigh, NC; 1:200 dilution for final concentration of 2.5 ug/ml). Primary antibody was removed with 3 PBS washes followed by incubation in fluorescence conjugated secondary antibody (Alexa Fluor 594; Invitrogen; Eugene, OR; 1:200 dilution for final concentration of 10 ug/ml) for 2 hr at room temperature. Unbound secondary antibody was removed with a final 3 washes with PBS. Following the IHC procedure, glass coverslips were mounted onto slides in Vectashield fluorescence mounting medium containing the nuclear stain DAPI (Vector Laboratories, Inc.; Burlingame, CA). Samples were analysed using a fluorescence microscope (Olympus BX61; Melville, NY) to capture multiple channel images (Olympus DP70 3.0 camera; Microsuite Biological Suite 2.6 software) to allow for analysis of cell density and phenotypes. Cell counts were conducted by hand using NIH ImageJ 1.37v (Bethesda, MD; http://rsb.info.nih.gov/ij/) image analysis software, and reflect data collected from 8 images per group. Phenotypes were also counted by hand from 4 images per marker per group and the percentage of positively labeled cells co-localizing with DAPI to the total DAPI count was calculated.
RT-PCR on CSF samples
CSF was collected as described, immediately frozen in liquid nitrogen, and stored at -80°C prior to processing. cDNA was synthesized using a Cells-Direct III cDNA synthesis kit (Invitrogen) according to manufacturer's instructions. Briefly, CSF samples were centrifuged (1000g for 10 min at 4 °C), the supernatant removed and the pellet washed with 100 ul ice cold DPBS, and re-centrifuged (1000g for 10 min at room temp) to pellet the cells within the sample and re-suspended in 11 ul lysis buffer and lysis solution for 10 min on ice. 10 ul of cell lysate was added to 1 ul RNAse inhibitor and incubated at 75°C for 10 min. First-strand cDNA was synthesized by adding 2 ul oligo (Dt), 1ul dNTP and 7.8 ul nuclease free water to the cell lysate and incubating at 70°C for 5 min. Following 2min on ice, 6ul 5× RT buffer, 1ul RNAse inhibitor, 1ul Superscript III, and 1ul DTT was added to the cell lysate and incubated at 50 °C for 50 min, 5min for 85 °C. Finally 1ul RNase H was added and incubated at 37 °C for 20 min. All cDNA was stored at -80 °C until RT-PCR was performed. Exogenous (plasmid derived) IL-10 gene expression was distinguished from endogenous IL-10 by utilizing a forward primer that crosses an exon/intron boundary within the IL-10 sequence and a reverse primer that crosses the boundary between an IL-10 exon and a unique plasmid sequence that forms the 3′ UTR. The primer sequences used are as follows: Forward: ACGCTGTCATCGATTTCT Reverse: CCGCTAGCTCAATTTTTCA.
Data Analysis
All statistical comparisons were computed using Statview 5.0.1 for the Macintosh. Data from the von Frey test were analyzed as the interpolated 50% threshold (absolute threshold) in log base 10 of stimulus intensity (monofilament stiffness in milligrams × 10). Behavioral tests of allodynia were analyzed by repeated measures ANOVA followed by Fisher's protected least significant difference posthoc comparisons, where appropriate. IHC data including cell counts from CSF was analyzed by one-way ANOVA followed by posthoc comparisons where appropriate.
Supplementary Material
K-type stimulatory synthetic oligodeoxynucleotides (ODN), agonists for TLR 9, were injected intrathecally at a 2 ug and 0.2ug dose as an initial priming injection 10 days following CCI surgery. This was followed 3 days later by 25 ug pDNA-IL-10F129S. Significant reversal of allodynia as compared to pre-injection withdrawal levels was noted in the 2 ug dose group (black square symbol) beginning at 24 hr following ODN injection and lasting the remainder of the time course (p<0.05; N=6). A partial reversal of allodynia was achieved using the lower 0.2 ug ODN dose (red diamond symbol; N=6). Error bars indicate SEM.
Efficacy of IL-10 protein injections is evident following pDNA-IL-10F129S injections. Here, two pDNA-IL-10F129S injections were spaced 3 days apart (100 ug followed by 25 ug dose; normally leads to >3 month pain reversal). 3 weeks following the second pDNA-IL-10F129S injection, IL-10 neutralizing antibody was injected to elicit a long term re-expression of pain behavior. If IL-10 protein is administered 4 days following re-expression of pain (at a dose that normally reverses pain in the CCI model for less than 24 hr), a 30 day reversal of pain is seen (N=6). Error bars indicate SEM.
The long term therapeutic failure induced by temporary blockade of IL-10 via neutralizing antibody is mimicked by a single intrathecal injection of the inflammatory HIV coat protein gp120 (3ug dose; sufficient to cause expression of pain behaviors for less than 24 hr when injected intrathecally in sham operated rats; N=6 per group). This figure indicates an ability of the intrathecal inflammatory milieu to influence therapeutic stability. Additionally, this figure provides evidence of the ability of the immune system to mount a response against immunogenic agents during the period of therapeutic efficacy. Error bars indicate SEM.
Acknowledgments
Financial support provided by Avigen and NIH grants DA018156, DA015642, DA015656, and HL5510.
Footnotes
This work was conducted at the University of Colorado, Boulder, CO USA.
Supplementary information is available at Gene Therapy's website
Literature Cited
- 1.Azzam T, Domb AJ. Current Developments in Gene Transfection Agents. Current Drug Delivery. 2004;1:165–193. doi: 10.2174/1567201043479902. [DOI] [PubMed] [Google Scholar]
- 2.Boulaiz H, et al. Non-viral and viral vectors for gene therapy. Cell Mol Biol. 2005;51:3–22. [PubMed] [Google Scholar]
- 3.Gronevik E, et al. Gene expression and immune response kinetics using electroporation-mediated DNA delivery to muscle. The Journal of Gene Medicine. 2005;7:218–227. doi: 10.1002/jgm.650. [DOI] [PubMed] [Google Scholar]
- 4.George JS. Gene therapy progress and prospects:adenoviral vectors. Gene Therapy. 2003;10:1135–1141. doi: 10.1038/sj.gt.3302071. [DOI] [PubMed] [Google Scholar]
- 5.Driesse M, et al. Intra-CSF administered recombinant adenovirus causes an immune response-mediated toxicity. Gene Therapy. 2000;7:1401–1409. doi: 10.1038/sj.gt.3301250. [DOI] [PubMed] [Google Scholar]
- 6.Zaiss A, Muruve D. Immune responses to adeno-associated virus vectors. Curr Gene Therapy. 2005;5:323–331. doi: 10.2174/1566523054065039. [DOI] [PubMed] [Google Scholar]
- 7.Hu P, Bembrick AL, Keay KA, McLachlan EM. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain, Behavior, and Immunity. 2007;21:599–616. doi: 10.1016/j.bbi.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Durrenberger PF, et al. Prostanoid receptor EP1 and Cox-2 in injured human nerves and a rat model of nerve injury: a time-course study. BMC Neurology. 2006;6 doi: 10.1186/1471-2377-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohtori S, Takahashi K, Moriya H, Myers R. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine. 2004;29:1082–1088. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- 10.Peters C, et al. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Experimental Neurology. 2005;193:85–100. doi: 10.1016/j.expneurol.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 11.McNicol E, et al. Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain. 2003;4:231–256. doi: 10.1016/s1526-5900(03)00556-x. [DOI] [PubMed] [Google Scholar]
- 12.Watkins L, Maier S. Glia: A Novel Drug Discovery Target For Clinical Pain. Nature Reviews Drug Discovery. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 13.Watkins L, Milligan E, Maier S. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- 14.Tsuda M, Inoue K, Salter M. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Milligan E, et al. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon S, Cafferty W, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Z, et al. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia. Gene Therapy. 2008;15:183–190. doi: 10.1038/sj.gt.3303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marques CP, et al. Interleukin-10 Attenuates Production of HSV-Induced Inflammatory Mediators by Human Microglia. Glia. 2004;47:358–366. doi: 10.1002/glia.20045. [DOI] [PubMed] [Google Scholar]
- 19.Plunkett J, et al. Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp Neurol. 2001;168:144–154. doi: 10.1006/exnr.2000.7604. [DOI] [PubMed] [Google Scholar]
- 20.Strle K, et al. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- 21.Milligan E, et al. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci. 2005;21:2136–2148. doi: 10.1111/j.1460-9568.2005.04057.x. [DOI] [PubMed] [Google Scholar]
- 22.Milligan E, et al. Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol Pain. 2005;25 doi: 10.1186/1744-8069-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milligan E, et al. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain. 2006;126:294–308. doi: 10.1016/j.pain.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Mu W, et al. IL-10 Suppresses Chemokines, Inflammation, and Fibrosis in a Model of Chronic Renal Disease. J Am Soc Nephrol. 2005;16:3651–3660. doi: 10.1681/ASN.2005030297. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, et al. Interleukin 10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. PNAS. 2005;102:7251–7256. doi: 10.1073/pnas.0502407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storek B, et al. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. PNAS. 2008;105:1055–1060. doi: 10.1073/pnas.0708003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sloane E, et al. Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental Multiple Sclerosis: MOG-EAE behavioral and anatomical symptom treatment with cytokine gene therapy. Brain Behavior Immunity. 2000;23:92–100. doi: 10.1016/j.bbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanella J, et al. Effect of etanercept, a tumor necrosis factor-alpha inhibitor, on neuropathic pain in the rat chronic constriction injury model. Spine. 2008;33:227–234. doi: 10.1097/BRS.0b013e318162340a. [DOI] [PubMed] [Google Scholar]
- 29.Dong XW, et al. Small interfering RNA-mediated selective knockdown of Nav1.8 tetrodotoxin-resistant sodium channel reverses mechanical allodynia in neuropathic rats. Neuroscience. 2007;146:812–821. doi: 10.1016/j.neuroscience.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 30.Sharp C, et al. Investigation into the role of P2X(3)/P2X(2/3) receptors in neuropathic pain following chronic constriction injury in the rat: an electrophysiological study. British Journal of Pharmacology. 2006;148:845–852. doi: 10.1038/sj.bjp.0706790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verge GM, et al. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. European Journal of Neuroscience. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee H, et al. Temporal expression of cytokines and their receptors mRNAs in a neuropathic pain model. Neuroreport. 2004;15:2807–2811. [PubMed] [Google Scholar]
- 33.Schafers M, et al. Selective increase of tumour necrosis factor-alpha ininjured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. European Journal of Neuroscience. 2003;17:791–804. doi: 10.1046/j.1460-9568.2003.02504.x. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto K, et al. Pro- and Anti-inflammatory Cytokine Gene Expression in Rat Sciatic Nerve Chronic Constriction Injury Model of Neuropathic Pain. Experimental Neurology. 2001;169:386–391. doi: 10.1006/exnr.2001.7677. [DOI] [PubMed] [Google Scholar]
- 35.Wagner R, Janjigian M, Myers RR. Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-α expression. Pain. 1998;74:35–42. doi: 10.1016/S0304-3959(97)00148-6. [DOI] [PubMed] [Google Scholar]
- 36.Milligan E, et al. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. European Journal of Neuroscience. 2005;21:2136–2148. doi: 10.1111/j.1460-9568.2005.04057.x. [DOI] [PubMed] [Google Scholar]
- 37.Watkins LR, et al. Dynamic Regulation of the Proinflammatory Cytokine, Interleukin-1β: Molecular Biology for Non-Molecular Biologists. Life Sciences. 1999;65:449–481. doi: 10.1016/s0024-3205(99)00095-8. [DOI] [PubMed] [Google Scholar]
- 38.Matzinger P. Essay 1: The Danger Model in Its Historical Context. Scand J Immunol. 2001;54:4–9. doi: 10.1046/j.1365-3083.2001.00974.x. [DOI] [PubMed] [Google Scholar]
- 39.Matzinger P. Tolerance, Danger, and the Extended Family. Annual Review of Immunology. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 40.Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology. Garland Science Publishing; 2004. [Google Scholar]
- 41.Akira S, Takeda K. Toll-like receptor signalling. Nature Reviews Immunology. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 42.Parker L, Prince L, Sabroe I. Translational Mini-Review Series on Toll-like Receptors: Networks regulated by Toll-like receptors mediate innate and adaptive immunity. Clinical and Experimental Immunology. 2007;147:199–207. doi: 10.1111/j.1365-2249.2006.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghosh TK, et al. Toll-like receptor (TLR) 2-9 agonists-induced cytokines and chemokines: I. Comparison with T cell receptor-induced responses. Cellular Immunology. 2006;243:48–57. doi: 10.1016/j.cellimm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Yasuda K, et al. Restricted cytokine production from mouse peritoneal macrophages in culture in spite of extensive uptake of plasmid DNA. Immunology. 2004;111:282–290. doi: 10.1111/j.1365-2567.2004.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braun J, Brigitte K, Hir ML, Zenker W. Cellular components of the immune barrier in the spinal meninges and dorsal root ganglia of the normal rat: immunohistochemical (MHC class II) and electron-microscopic observations. Cell Tissue Res. 1993;273:209–217. doi: 10.1007/BF00312822. [DOI] [PubMed] [Google Scholar]
- 46.McMenamin P, et al. Macrophages and dendritic cells in the rat meninges and choroid plexus: three-dimensional localization by environmental scanning electron microscopy and confocal microscopy. Cell Tissue Res. 2003;313:259–269. doi: 10.1007/s00441-003-0779-0. [DOI] [PubMed] [Google Scholar]
- 47.Masuda A, Yoshikai Y, Aiba K, Matsuquchi T. Th2 cytokine production from mast cells is directly induced by lipopolysaccharide and distincly regulated by c-Jun N-terminal kinase and p38 pathways. J Immunology. 2002;169:3801–3810. doi: 10.4049/jimmunol.169.7.3801. [DOI] [PubMed] [Google Scholar]
- 48.Ajuebor M, et al. Role of Resident Peritoneal Macrophages and Mast Cells in Chemokine Production and Neutrophil Migration in Acute Inflammation: Evidence for an Inhibitory Loop Involving Endogenous IL-10. The Journal of Immunology. 1999;162:1685–1691. [PubMed] [Google Scholar]
- 49.Fickenscher H, et al. The interleukin-10 family of cytokines. Trends Immunol. 2002;23:89–96. doi: 10.1016/s1471-4906(01)02149-4. [DOI] [PubMed] [Google Scholar]
- 50.Martinez F, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 51.Fabriek B, Dijkstra C, Berg Tvd. The macrophage scavenger receptor CD163. Immunobiology. 2005;210:153–160. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Milligan E, et al. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hartmann B, et al. The AMPA Receptor Subunits GluR-A and GluR-B Reciprocally Modulate Spinal Synaptic Plasticity and Inflammatory Pain. Neuron. 2004;44:637–650. doi: 10.1016/j.neuron.2004.10.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
K-type stimulatory synthetic oligodeoxynucleotides (ODN), agonists for TLR 9, were injected intrathecally at a 2 ug and 0.2ug dose as an initial priming injection 10 days following CCI surgery. This was followed 3 days later by 25 ug pDNA-IL-10F129S. Significant reversal of allodynia as compared to pre-injection withdrawal levels was noted in the 2 ug dose group (black square symbol) beginning at 24 hr following ODN injection and lasting the remainder of the time course (p<0.05; N=6). A partial reversal of allodynia was achieved using the lower 0.2 ug ODN dose (red diamond symbol; N=6). Error bars indicate SEM.
Efficacy of IL-10 protein injections is evident following pDNA-IL-10F129S injections. Here, two pDNA-IL-10F129S injections were spaced 3 days apart (100 ug followed by 25 ug dose; normally leads to >3 month pain reversal). 3 weeks following the second pDNA-IL-10F129S injection, IL-10 neutralizing antibody was injected to elicit a long term re-expression of pain behavior. If IL-10 protein is administered 4 days following re-expression of pain (at a dose that normally reverses pain in the CCI model for less than 24 hr), a 30 day reversal of pain is seen (N=6). Error bars indicate SEM.
The long term therapeutic failure induced by temporary blockade of IL-10 via neutralizing antibody is mimicked by a single intrathecal injection of the inflammatory HIV coat protein gp120 (3ug dose; sufficient to cause expression of pain behaviors for less than 24 hr when injected intrathecally in sham operated rats; N=6 per group). This figure indicates an ability of the intrathecal inflammatory milieu to influence therapeutic stability. Additionally, this figure provides evidence of the ability of the immune system to mount a response against immunogenic agents during the period of therapeutic efficacy. Error bars indicate SEM.


