Abstract
Background
People living in rural areas may be less likely to be up to date (UTD) with screening guidelines for colorectal cancer (CRC).
Objectives
To determine (1) rates of being UTD with screening or ever having had a test for CRC and (2) correlates for testing among patients living in a rural area who visit a provider.
Design
Cross-sectional survey.
Participants
Five hundred seventy patients aged 50 years and older who visited their health-care provider in High Plains Research Network (HPRN) practices.
Measurements
(1) Ever having had a CRC screening test, (2) being UTD with CRC screening, and (3) intention to get tested.
Results
The survey completion rate was 65%; 71% of patients had ever had any CRC screening test, while 52% of patients were UTD. Correlates of intending to get tested included having a family history of CRC, having a doctor recommend a test, knowing somebody who got tested, and believing that testing for CRC gives one a feeling of being in control of their health. Of those who had never had a CRC screening test, 12% planned on getting tested in the future, while 55% of those who were already up to date intended to be tested again (p < 0.001).
Conclusions
Prevalence of being UTD with CRC testing in the HPRN was on par with statewide CRC testing rates, but over three quarters of patients who had not yet been screened had no intention of getting tested for CRC, despite having a medical home.
KEY WORDS: colorectal cancer screening, rural health care, Practice-Based Research Network (PBRN)
BACKGROUND
Colorectal cancer (CRC) is a significant source of morbidity and mortality in the United States. Of all cancers in 2005, cancers of the colon and rectum were the third most common type diagnosed in men and women; of 141,405 total new cases diagnosed in the US, the mortality rates were 21.0 and 14.6 per 100,000, respectively.1
CRC is preventable, by detection and removal of precancerous polyps, and cancers of the rectum and colon found in their early stages are more likely to be curable. In the US, 5-year relative survival rates dropped dramatically if CRC was diagnosed at a later stage, from 90% survival for cancers diagnosed at localized stages to 10% survival for cancers diagnosed at the latest stages.2
Although early detection improves survival rates, barriers to CRC screening exist, including lower socioeconomic status, less than a high school education, lower number of visits to a physician in the preceding year, lack of health insurance, the inability to obtain proper screening easily and lack of patient motivation.3–5 Patients living in rural areas may experience many of these barriers in disproportionate numbers,3, 6–9 and this can adversely affect screening rates in these areas. For example, in a nationwide study using 1999 Behavioral Risk Factor Surveillance Survey (BRFSS) data, it was shown that compared with patients living in suburban or urban areas, patients living in rural areas were less likely to be up to date on CRC screening, defined as an FOBT in the last 1 year, or a sigmoidoscopy or colonoscopy in the last 5 years.10 Possibly as a result of the barriers mentioned above, rural residence has been described as a predictor of late stage of cancer at the time of initial diagnosis.3
Much of the scientific literature on CRC screening examines screening behavior from a population-based perspective. Less is known about screening behavior for patients who visit primary care practices. This is particularly true for rural primary care practices. A previous study reported on the CRC screening attitudes and behaviors of a random sample of community members in rural Colorado.11 Our study assessed the screening attitudes and behaviors of patients in those same communities attending primary care practices that are part of a practice-based research network (PBRN) in rural Colorado. Knowing what happens once patients access care can provide valuable information about patient and provider beliefs and behaviors that affect CRC screening. The ability to evaluate such behavior can help to direct and strengthen community-wide intervention efforts.
Specific objectives of this study were to determine (1) practice-based prevalence of being up to date with or ever having had a test for CRC and (2) correlates for testing among patients living in a rural area who visit a provider.
METHODS
This study was approved by the Colorado Multiple Institutional Review Board.
Study Setting
The High Plains Research Network (HPRN) is a practice-based research network in northeastern Colorado. It includes 24 primary care practices, both internal medicine and family medicine. All of the counties that make up the HPRN are rural, and many are designated either Medically Underserved Areas, primary care Health Professional Shortage Areas, or both. Compared to overall state levels, the percentage of residents who have at least a high school education is lower in HPRN counties, and poverty is more prevalent in this area. Hispanic ethnicity represents anywhere from 8 to 31% of the population; this percentage fluctuates during the year due to migration of seasonal agricultural employees. At the time of this study, the total population for the catchment area of the HPRN was approximately 90,000.
Survey Development
The survey instrument incorporated questions adapted from the Eastern Colorado Colon Cancer Questionnaire,11,12 which is a telephone scripted survey used to collect baseline colon cancer screening data from the HPRN catchment area. The Health Belief Model13 was used as the conceptual model for questions that addressed patient knowledge, attitudes, and beliefs regarding screening for CRC.
The survey was piloted with ten members of the Joint Planning Committee, a committee of local residents within the HPRN that had been informed as to the purpose of the study. The survey was modified based upon this feedback.
Data Collection
A clinic manager or charge nurse was responsible for handing out and facilitating the collection of surveys at each clinical site. All patients over the age of 50 were eligible to complete the survey unless they were unable to do so because clinic staff judged that they were physically or mentally incapacitated, or required immediate medical attention. Patients were invited to fill out a survey while waiting for their visit and were asked to return the survey to a secure box before leaving the clinic. Patients chose between Spanish and English language versions of the survey. The target number of surveys completed at each clinic site was 30. Reception staff made a notation at the top of each survey for patients who either chose not to participate or were judged to be ineligible. All surveys were returned to our primary research site via pre-addressed, postage-paid envelopes.
Variables
There were three primary outcome variables: (1) ever having had a test for colorectal cancer (CRC), (2) being up to date with CRC screening, and (3) intention to get screened for CRC. The dependent outcome variable of ever having had a test (fecal occult blood test-FOBT, flexible sigmoidoscopy, colonoscopy, or barium enema-BE) was categorical and was dichotomized as having had a test any time in the past or never having had any test. The dependent outcome variable of being up to date was categorical and was also dichotomized as being up to date or not being up to date for each testing modality. From this, a composite measure for the outcome of being up to date, defined as having had FOBT within 1 year, sigmoidoscopy or BE within 5 years, or colonoscopy within 10 years was created and similarly dichotomized. The dependent outcome variable of intention to get screened for colon cancer was categorical. There were five possible responses to this question in the survey, and these responses were dichotomized into two categories to simplify data analysis: (1) current plans or intention to get tested and (2) no current plans or intention to get tested. Two of the five possible responses included some uncertainty (“I am thinking about getting a test” and “I don’t know) and were included in the second category.
Co-variates, based upon findings in the literature and guided by the Health Belief Model for health-seeking behavior,13 included age, gender, race/ethnicity, education, not having insurance, previous inexperience with either colon cancer or colon polyps, family history of colon cancer or colon polyps, beliefs regarding perceived risk and perceived severity of colon cancer, influence of having a doctor recommend a test, knowing others who have been tested, and perception of whether or not getting screened allows one to feel in control of his or her health. Some of these co-variates described perceived barriers to getting tested; these co-variates were grouped into four domains: a “risk” domain (having no symptoms, no previous experience with either colon cancer or polyps, or not believing oneself to be at risk for CRC); a “logistic” domain (too busy, transportation problems, or having to travel too far to do the test); a “cost” domain (no insurance or too expensive); and a “test concerns” domain (concern about pain, being afraid or not wanting to know test results, tests being too unpleasant/messy, or being too embarrassed). Any of the co-variates within a domain could occur for a domain to be rated as present.
Data Analysis
Completion rates were determined based upon the number of surveys that were provided to each clinic site and upon the total number of completed/uncompleted/unused surveys that were returned. For those eligible patients who chose not to participate in the survey, or those who were unable to complete the survey, no demographic information was collected.
Unadjusted and adjusted mixed effects general logistic regression models were used to determine univariate and multivariate associations with being up to date with CRC testing and intention to be tested for CRC. A mixed effects model to account for clustering was used since variation among practices was evident (ICC: up to date: 4.0%, p = 0.07; intent to be tested: 3.3%, p = 0.09). Unadjusted models were first performed for each independent co-variate separately. Co-variates that were associated with the outcome at the univariate alpha level of 0.20 were adjusted for in the multivariate model. Thus, models for each outcome differed in terms of the independent co-variates selected, although each model did adjust for patient’s age, gender and education. For co-variates that were highly correlated, clinical expertise was used to determine which would be adjusted for in the final model.
For the outcome of intention of getting tested, we also compared frequencies by colon cancer testing status. A chi-squared analysis was performed to determine general associations between intention of being tested for CRC and up to date status (never been tested, tested but not up to date, tested and up to date).
All analyses were performed on complete data only using SAS v. 9.1. Mixed effects models were performed using the SAS GLIMMIX macro.
RESULTS
Twenty-one out of a total of 24 clinics in the HPRN participated in the study. Out of a total 917 surveys distributed to eligible patients, 30 patients were unable to complete the survey, 54 patients chose not to fill out the survey, 4 surveys were excluded on the basis of age, and 259 surveys were distributed but unaccounted for. A total of 570 completed surveys were received from eligible patients, yielding an overall survey completion rate of 65%. Fifty-five percent of the respondents were over the age of 65, 60% were female, 89% had at least a high school diploma, and 92% were classified as White/non-Hispanic (Table 1).
Table 1.
Sociodemographic Characteristics of Patients Visiting HPRN Practices
| Age | N (%)* |
| 50–59 | 167 (30.9) |
| 60–69 | 156 (28.6) |
| 70–79 | 143 (26.2) |
| 80–89 | 70 (12.8) |
| 90+ | 10 (1.8) |
| <65 years | 250 (45.8) |
| 65 years+ | 296 (55.2) |
| Gender | N (%)* |
| Male | 220 (40.2) |
| Female | 328 (59.8) |
| Education level | N (%)* |
| Less than HS diploma | 57 (10.6) |
| HS/GED | 211 (38.9) |
| More than HS diploma | 274 (50.6) |
| Race/ethnicity | N (%)* |
| White, NH | 501 (91.9) |
| All other | 44 (8.1) |
*Number of patients completing each response; % may not total 100 due to rounding
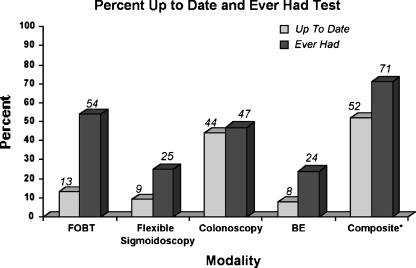
As shown in Figure 1, by screening modality, FOBT was the modality with the highest percentage of patients having ever had the test, while colonoscopy was the modality for which most patients were up to date. Using the composite outcome measures that were inclusive of all modalities, 71% of patients reported ever having had any test at any time in the past, while 52% of patients reported being up to date with any screening test for CRC (Fig. 1).
Figure 1.
*Includes FOBT within 1 year, flexible sigmoidoscopy within 5 years, colonoscopy within 10 years or barium enema (BE) within 5 years.
Table 2 shows results of patients intending to get tested for CRC by subgroups of up-to-date status. A statistically significant lower percentage of patients who had never been screened for CRC (12%) or had been screened for CRC but were not up to date (27%) reported that they intend to be tested in the future compared to the percent of patients who were up to date with CRC screening and reported their intention to get tested again in the future (55%) (p < 0.001). This relationship between up-to-date status and intention to get tested in the future was still statistically significant after taking into consideration a sensitivity analysis for the classification of those respondents who either didn’t know of their future testing plans or were thinking about getting tested (p = 0.016, table not shown).
Table 2.
Association of Current Testing Status with Intention to Get Tested in the Future
| Testing status | Intention to get tested | |
|---|---|---|
| Definitely planning | Not planning/don’t know/thinking about it | |
| Have been tested and am up to date with testing (n = 288) | 55% | 45% |
| Have been tested but not up to date with testing (n-117) | 27% | 73% |
| Have never been tested (n = 163) | 12% | 88% |
p ≤ 0.0001, comparing across all strata of testing status
As shown in Table 3, significant correlates in adjusted multivariate models for being up to date with CRC screening included patient age, having been told that one has had colon polyps, believing that CRC is preventable, thinking colon cancer is a severe problem, knowing someone who has been tested for CRC, believing they are at risk for colon cancer, and being concerned about the testing procedure. Patients 65 years of age or older, who have had a history of polyps, and who believe colon cancer is preventable and a severe problem had a higher odds of being up to date with CRC testing, whereas patients who knew someone who had been tested for CRC, who believe they are not at risk for colon cancer, or had concerns about the test procedure had lower odds of being up to date (Table 3). Positive correlates for intending to get tested for CRC in the future (Table 4) included having been tested for CRC in the past, having a family member with colon cancer, believing that testing for CRC gives one a feeling of being in control of their health, knowing someone who has been tested, and having a doctor recommend a test. Patients who have never been tested for CRC or have been tested but are not up to date report with lower frequency an intention to ever be tested for CRC than those who are already up to date (p < 0.001). Patients who believe they are not at risk for colon cancer also have lower odds of intending to be tested for CRC (p < 0.0001).
Table 3.
Factors Associated with Being Up to Date with Colon Cancer (CC) Testing
| Co-variates | Unadjusted OR (95% CI) | p | Adjusted OR (95% CI) | p |
|---|---|---|---|---|
| Age | ||||
| 50–64 years | 1.00 | <0.0001 | 1.00 | 0.02 |
| 65+ years | 2.20 (1.55–3.14) | 1.87 (1.16–3.02) | ||
| Gender | ||||
| Male | 0.84 (0.59–1.19) | 0.31 | 0.68 (0.44–1.06) | 0.09 |
| Female | 1.00 | 1.00 | ||
| Race/ethnicity | ||||
| White/non-Hispanic | 2.31 (1.14–4.66) | 0.02 | 2.29 (0.83–6.31) | 0.11 |
| All others | 1.00 | 1.00 | ||
| Education | ||||
| Less than HS diploma | 0.64 (0.35–1.17) | 0.276 | 0.55 (0.24–1.27) | 0.30 |
| High school diploma/GED equivalent | 1.06 (0.74–1.54) | 0.75 (0.46–1.22) | ||
| More than HS diploma | 1.00 | 1.00 | ||
| Knowledge of or experience with CC | ||||
| Personal history of colon polyps | 9.85 (5.14–18.88) | <0.001 | 11.05 (5.0–24.5) | <0.001 |
| Family history of CC | 2.01 (1.22–3.32) | 0.01 | 1.42 (0.76–2.68) | 0.27 |
| No experience with CC | 0.30 (0.20-0.43) | <0.001 | * | * |
| CC is preventable | 2.05 (1.37–3.08) | 0.01 | 1.88 (1.10–3.23) | 0.02 |
| Beliefs regarding CC | ||||
| At least somewhat likely at risk to get CC | 1.28 (0.83–1.97) | 0.26 | – | – |
| CC is a severe problem | 3.72 (1.94–7.13) | <0.0001 | 2.32 (0.99–5.41) | 0.05 |
| Attitudes regarding CC testing | ||||
| Getting tested would give me a feeling of being in control of my health | 1.59 (1.10–2.30) | 0.02 | 1.35 (0.84–2.17) | 0.21 |
| Having a doctor recommend a test would make me more likely to get tested | 1.09 (0.78–1.53) | 0.62 | – | – |
| Knowing somebody who got tested would make me more likely to get tested | 0.45 (0.23-0.88) | 0.02 | 0.33 (0.14-0.80) | 0.01 |
| Factors perceived to interfere with willingness to get tested | ||||
| Risk barriers: any of the following: not having any symptoms, no family history of CC, not at risk for CC | 0.34 (0.24-0.48) | <0.0001 | 0.34 (0.22-0.53) | <0.0001 |
| Logistic barriers: any of the following: too busy, transportation is a problem, have to travel too far | 0.55 (0.29–1.07) | 0.08 | 0.55 (0.23–1.32) | 0.18 |
| Cost barriers: any of the following: no insurance, testing too expensive | 0.38 (0.21-0.69) | 0.002 | 0.63 (0.29–1.36) | 0.24 |
| Test concerns barriers: any of the following: painful, don’t want to know the results, too unpleasant/messy, embarrassed | 0.54 (0.36-0.81) | 0.003 | 0.59 (0.35–1.00) | 0.05 |
+Variables with univariate p-values <0.20 were run in the final mulitvariate model with the exception of “Knowledge: no experience with CC”—which was highly correlated with “Knowledge: had polyps"
Table 4.
Factors Associated with Intention to Get Tested for Colon Cancer (CC)
| Co-variates | Unadjusted OR (95% CI) | p | Adjusted OR (95% CI) | p |
|---|---|---|---|---|
| Age | ||||
| 50–64 years | 1.00 | 0.96 | 1.00 | 0.12 |
| 65+ years | 1.01 (.71–1.44) | 0.64 (0.38–1.09) | ||
| Gender | ||||
| Male | 1.09 (0.77–1.55) | 0.63 | 1.53 (0.94–2.49) | 0.09 |
| Female | 1.00 | 1.00 | ||
| Race/ethnicity | ||||
| White/non-Hispanic | 1.76 (0.86–3.61) | 0.12 | 1.34 (0.41–4.43) | 0.63 |
| All others | 1.00 | 1.00 | ||
| Education | ||||
| Less than HS diploma | 0.55 (0.29–1.05) | 0.20 | 0.88 (0.33–2.33) | 0.90 |
| HS diploma/GED equivalent | 0.90 (0.62–1.30) | 0.89 (0.53–1.50) | ||
| More than HS diploma | 1.00 | 1.00 | ||
| Knowledge of or experience with CC | ||||
| Personal history of colon polyps | 3.42 (2.17–5.39) | <0.0001 | 1.89 (0.99–3.60) | 0.06 |
| Family history of CC | 3.29 (2.01–5.39) | <0.0001 | 2.84 (1.46–5.52) | 0.002 |
| No experience with CC | 0.42 (0.30-0.61) | <0.0001 | * | * |
| CC is preventable | 1.94 (1.27–2.98) | 0.002 | 1.28 (0.68–2.38) | 0.45 |
| Beliefs regarding CC | ||||
| I am at least somewhat likely at risk to get CC | 1.58 (1.04–2.42) | 0.03 | 0.80 (0.45–1.41) | 0.44 |
| CC is a severe problem | 5.09 (2.27–11.45) | <0.0001 | 2.66 (0.79–8.93) | 0.12 |
| Attitudes regarding CC testing | ||||
| Getting tested would give me a feeling of being in control of my health | 3.54 (2.44–5.15) | <0.0001 | 2.73 (1.65–4.53) | 0.0001 |
| Having a doctor recommend a test would make me more likely to get tested | 1.58 (1.12–2.23) | 0.01 | 1.69 (1.02–2.81) | 0.04 |
| Knowing somebody who got tested would make me more likely to get tested | 1.75 (0.93–3.32) | 0.08 | 2.68 (1.07–6.69) | 0.04 |
| Factors perceived to interfere with willingness to get tested | ||||
| Risk barriers: any of the following: not having any symptoms, no family history of CC, not at risk for CC | 0.32 (0.22-0.47) | <0.0001 | 0.32 (0.19-0.52) | <0.001 |
| Logistic barriers: any of the following: too busy, transportation is a problem, have to travel too far | 0.74 (0.37–1.46) | 0.38 | – | – |
| Cost barriers: any of the following: no insurance, testing too expensive | 0.51 (0.27-0.94) | 0.03 | 0.52 (0.22–1.25) | 0.15 |
| Test concerns barriers: any of the following: painful, don’t want to know the results, too unpleasant/messy, embarrassed | 0.84 (0.56-1.27) | 0.42 | – | – |
+Variables with univariate p-values <0.20 were run in the final mulitvariate model with the exception of “Knowledge: no experience with CC”—which was highly correlated with “Knowledge: had polyps"
DISCUSSION
Our study reveals that there are vulnerable patients among the population who visit rural primary care practices: those who aren’t already up to date with CRC screening do not intend to be screened in the future, even when they have access to the means to do so. Therefore, simply advising patients to go see their doctors for information regarding screening may not be enough to further increase CRC screening rates.
Many screening modalities for CRC exist. Guidelines for CRC screening include either FOBT, flexible sigmoidoscopy, colonoscopy, or barium enema, each at specific intervals.14–16 In this study colonoscopy is a common modality used for CRC screening, and most people who have had colonoscopy are up to date with it. Meanwhile, most patients who report having an FOBT are not up to date with it. This is telling, as FOBT is less invasive and theoretically more acceptable for patients.
An important finding in this study is that when patients feel like they are in control of their health, they are more likely to participate in recommended CRC screening practices. Patients are also more likely to be up to date with screening when they believe that CRC is a severe, preventable problem. Knowing that their provider recommends regular testing for CRC appears to be a motivating factor for intending to get tested. This should encourage providers to have targeted discussions with their patients regarding CRC screening. The physician-patient interaction that occurs at a primary care visit should be an ideal opportunity to reinforce such knowledge.
This study also confirms that a lack of symptoms can act as a barrier both for past screening behavior (being up to date) and future screening behavior (intending to get tested). Therefore, it is vital that patients be aware of the potential of lack of symptoms associated with CRC—even in advanced stages—or pre-cancerous colon polyps, and that they not wait until symptoms develop to begin testing.
One of the strengths of this study is that participation among the practices in the High Plains Research Network (HPRN)—the health-care providers for the rural communities in northeast Colorado—was very high. This extensive participation provides a fairly complete snapshot of how CRC screening is being utilized in primary care practices in rural northeastern Colorado.
We were limited in our ability to examine the effect of insurance or other financial factors on decisions to get CRC screening tests because of small sample sizes. Though a lack of having insurance showed a trend towards not being up to date with CRC testing, and towards a negative influence on intention to get tested for CRC, these trends were not statistically significant and could not be explored in further detail. Furthermore, patients self-reported their recollection of undergoing CRC tests. We did not conduct chart abstraction or medical record review to confirm the dates of having tests done. This may have influenced the categorization of patients as being up to date or not up to date; however, others have found that patient self-report of colon cancer screening behavior can be reliably used as an endpoint for intervention trials.17
This study was developed to provide information for a 4-year project in which community-based participatory methods were used to develop messages to promote colon cancer screening in rural communities. Through the formation of a Community Advisory Council (C.A.C.), community members with different backgrounds have had a voice in identifying pertinent research priorities and patient needs. Because the C.A.C. has recognized the need to learn more about CRC screening, the uptake of any health promotion efforts may be more likely to be supported. This could have influenced the observed outcomes. However, by being involved in the research process, community members can identify resources to deliver important health messages and aid in intervention efforts. In this instance, the goal is to further increase CRC screening rates.
Because CRC is a preventable cancer, it is critical that health-care providers strive to increase awareness of the disease and increase screening rates even further. A reasonable public health goal is to increase CRC screening rates to levels on par with breast, prostate, and cervical cancer screening rates. The clinic visit provides an ideal opportunity for such education to occur. Patients not tested or not up to date with CRC testing represent a particularly vulnerable population in this regard; only about one in ten patients in this study who have never been tested declared any intention to get tested in the future. This is significant because these are patients who already have a medical home. In a population-based survey study of members in the same HPRN counties as our clinic-based study,11 the prevalence of being up to date with any screening appeared to be slightly higher for those in the community than the population in our study who were seen in a clinic (58% vs. 52%, data not presented). This trend was true for every modality except for colonoscopy (37% vs. 44%). This comparison suggests that it may not be enough to simply get patients in to their health-care providers. If we are going to reduce the morbidity and mortality from colorectal cancer, we need to engage patients more actively.
Acknowledgements
Presented as a work in progress at the 2006 North American Primary Care Research Group Annual Meeting in Tuscon, Arizona. Funding provided by an SIP grant from the Centers for Disease Control and Prevention in Atlanta, Georgia.
This publication was supported by Cooperative Agreement no. 5 U48 DP000054-03 from the Centers for Disease Control and Prevention. Rocky Mountain Prevention Research Center SIP 20-04. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Conflict of Interest None
Footnotes
With special acknowledgement to the practices of the High Plains Research Network (HPRN) and the HPRN Joint Planning Committee.
References
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2005 Incidence and Mortality Web-based Report, Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2009. Available at: http://www.cdc.gov/uscs, accessed May 2009.
- 2.Ries LAG, Harkins D, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975–2004, National Cancer Institute. Bethesda, MD, Available at http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site 2007.
- 3.Parsons M, Askland K. Cancer of the colorectum in Maine, 1995–1998: determinants of stage at diagnosis in a rural state. J Rural Health. 2007;23(1):25–32. [DOI] [PubMed]
- 4.Cokkinides V, Chao A, Smith RA, Vernon SW, Thun MJ. Correlates of underutilization of colorectal cancer screening among US adults, age 50 years and older. Prev Med. 2003;36:85–91. [DOI] [PubMed]
- 5.Swan J, Breen N, Coates R, Rimer B, Lee N. Progress in cancer screening practices in the United States. Cancer. 2003;97(6):1528–40. [DOI] [PubMed]
- 6.Purkayastha S. Colorectal cancer and rectal bleeding in primary care: urban or rural myth? (Letter). BMJ. 2006;333:201–2. [DOI] [PMC free article] [PubMed]
- 7.Kinney AY, Harrell J, Slattery M, Martin C, Sandler RS. Rural-urban differences in colon cancer risk in blacks and whites: the North Carolina Colon Cancer Study. J Rural Health. 2006;22(2):124–30. [DOI] [PubMed]
- 8.Coughlin SS, Costanza ME, Fernandez ME, Glanz K, Lee JW, Smith SA, et al. CDC-funded intervention research aimed at promoting colorectal cancer screening in communities. Cancer. 2006;107(5 Suppl):1196–204. [DOI] [PubMed]
- 9.Eberhart M, Pamuk E. The importance of place of residence: examining health in rural and non-rural areas. Am J Pub Health. 2004;94(10):1682–6. [DOI] [PMC free article] [PubMed]
- 10.Coughlin SS, Thompson TD. Colorectal cancer screening practices among men and women in rural and nonrural areas of the United States, 1999. J Rural Health. 2004;20(2):118–24. [DOI] [PubMed]
- 11.Young WF, McGloin J, Zittleman L, West DR, Westfall JM. Predictors of colorectal screening in rural Colorado: testing to prevent colon cancer in the High Plains Research Network. J Rural Health. 2007;23(3):238–45. [DOI] [PubMed]
- 12.Eastern Colorado Colon Cancer Questionnaire, available at http://cudfm.org/highplains/ColonCancerQuestionnaireFinal.pdf, accessed June 2009.
- 13.Janz NK, Champion VL, Strecher VJ. The Health Belief Model, in Health Behavior and Health Education: Theory, Research and Practice, 3rd ed. San Francisco: John Wiley & Sons, Inc; 2002:45–66.
- 14.U.S. Preventive Services Task Force, 2008, available at http://www.ahrq.gov/clinic/uspstf/uspscolo.htm, accessed June 2009.
- 15.Smith RA, von Eschenbach AC, Wender R, Levin B, Byers T, Rothenberger D, et al. American Cancer Society Guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. CA Cancer J Clin. 2001;51(1):38–75. [DOI] [PubMed]
- 16.McCoughlin RN, O’Morain CA. Colorectal cancer screening. World J Gastroenterol. 2006;12(42):6747–50. [DOI] [PMC free article] [PubMed]
- 17.Baier M, Calonge N, Cutter G, McClatchey M, Schoentgen S, Hines S, et al. Validity of self-reported colorectal cancer screening behavior. Cancer Epidemiol Biomark Prev. 2000;9(2):229–32. [PubMed]
Further Reading
- 18.Hawley ST, Chang S, Risser D, Zhang Q. Colorectal cancer incidence and mortality in Texas 1990–1992: a comparison of rural classifications. J Rural Health. 2002;18(4):536–46. [DOI] [PubMed]
- 19.Launoy G, Le Coutour X, Gignoux M, Pottier D, Dugleux G. Influence of rural environment on diagnosis, treatment, and prognosis of colorectal cancer. J Epidemiol Commun Health. 1992;46(4):365–67. [DOI] [PMC free article] [PubMed]
- 20.Dodge JL, Mills PK, Riordan DJ. Cancer survival in California hispanic farmworkers, 1988–2001. J Rural Health. 2007;23(1):33–41. [DOI] [PubMed]
- 21.Stange KC, Flocke SA, Goodwin MA, Kelly RB, Zyzanski SJ. Direct observation of rates of preventive service delivery in community family practice. Prev Med. 2000;31(2 Pt. 1):167–76. [DOI] [PubMed]
- 22.Myers RE, Ross E, Jepson C, Wolf T, Balshem A, Millner L, et al. Modeling adherence to colorectal cancer screening. Prev Med. 1994;23(2):142–51. [DOI] [PubMed]
- 23.Pol LG, Rouse J, Zyzanski S, Rasmussen D, Crabtree B. Rural, urban and suburban comparisons of preventive services in family practice clinics. J Rural Health. 2001;17(2):114–21. [DOI] [PubMed]
- 24.Huang BS. Cancer death rates—Appalachia, 1994–1998. MMWR Weekly. 2002;51(24):527–29. [PubMed]
- 25.Larson SL, Fleishman JA. Rural-urban differences in usual source of care and ambulatory service use: analyses of national data using urban influence codes. Med Care. 2003;41(7 Suppl):III5–III74. [DOI] [PubMed]
- 26.Monroe AC, Ricketts TC, Savitz LA. Cancer in rural versus urban populations: a review. J Rural Health. 1992;8(3):212–20. [DOI] [PubMed]