Abstract
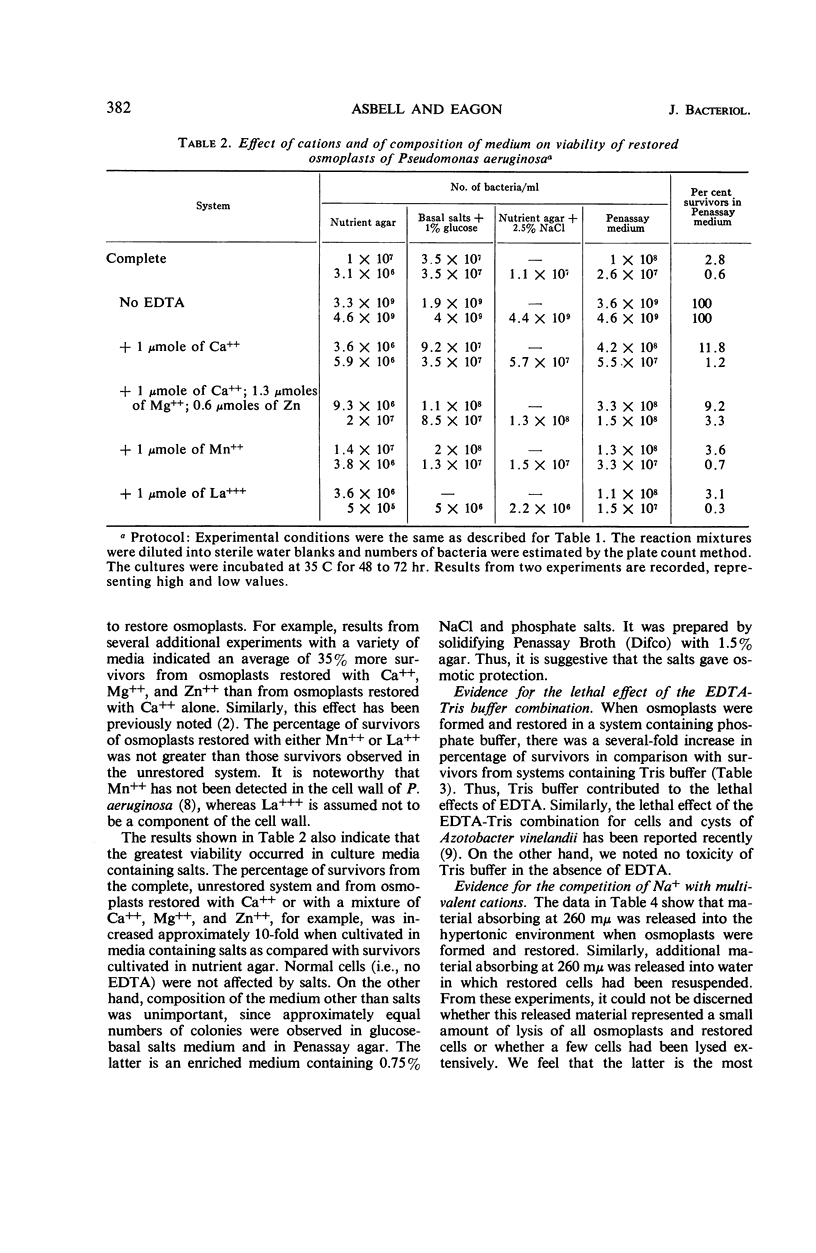
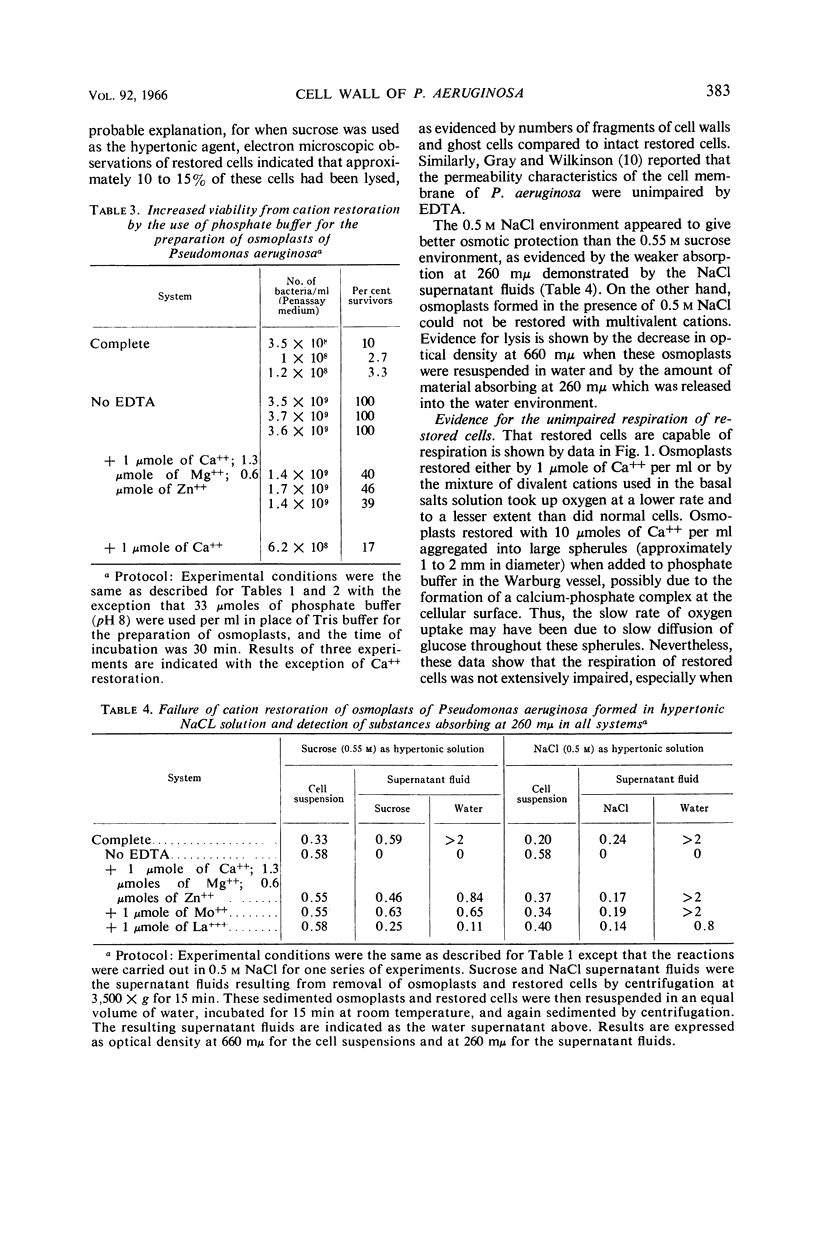
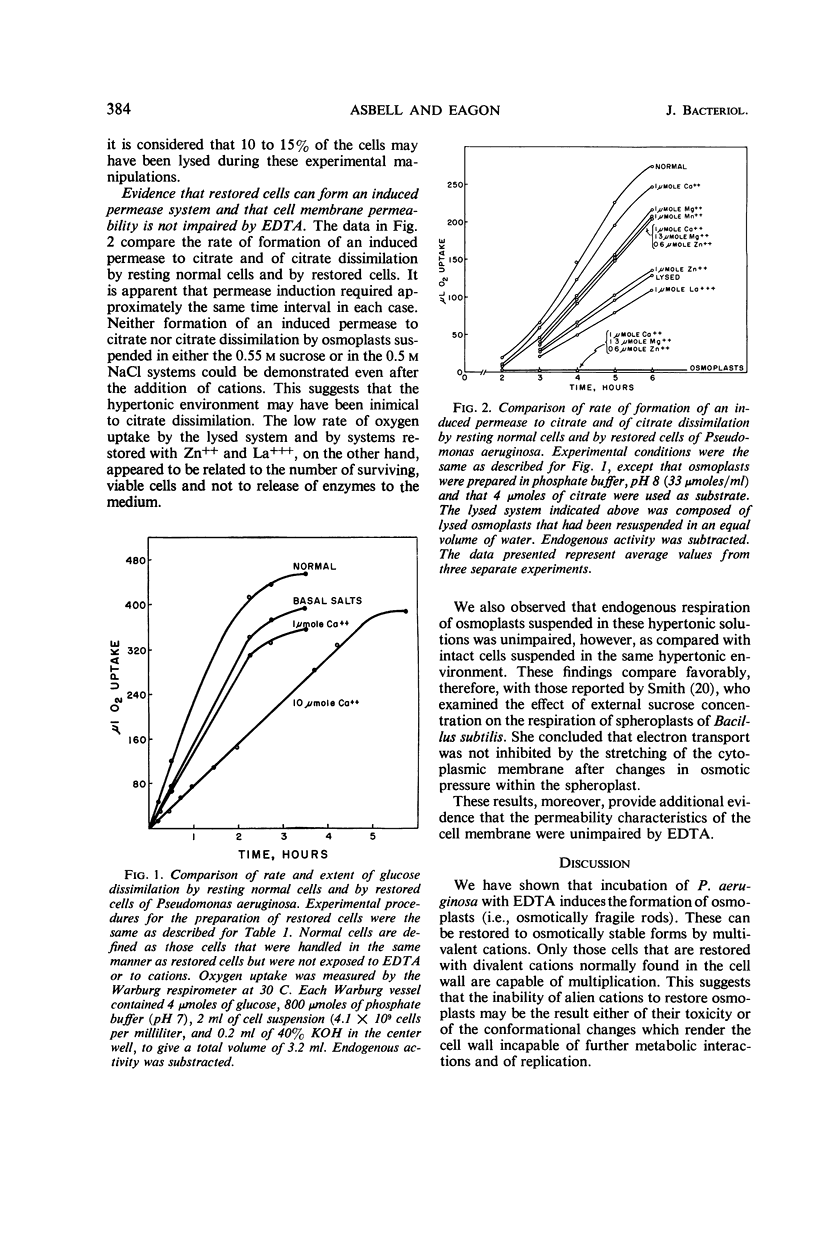
Asbell, Mary A. (University of Georgia, Athens), and R. G. Eagon. Role of multivalent cations in the organization, structure, and assembly of the cell wall of Pseudomonas aeruginosa. J. Bacteriol. 92:380–387. 1966. —Incubation of Pseudomonas aeruginosa with ethylenediaminetetraacetate induced the formation of osmotically fragile rods termed osmoplasts. These could be restored to osmotically stable forms by multivalent cations. Only those cells restored by divalent cations normally found in the cell wall were capable of multiplication. The respiration of restored cells, however, was unimpaired, irrespective of whether they were capable of multiplication. Moreover, the permeability characteristics of osmoplasts and restored cells were unimpaired. When multivalent cations were chelated from the cell wall and replaced by sodium, a weakened cell wall and an osmotically fragile cell resulted. This was apparently caused by the absence of cross-linkages in the cell wall via multivalent cations. Tris(hydroxymethyl)aminomethane buffer compounded the lethal effects of ethylenediaminetetraacetate. The lipopolysaccharide component was inferred to be the site of attack by ethylenediaminetetraacetate. A mechanism for the synthesis of the lipopolysaccharide sacculus was proposed whereby negatively charged subunits are “trapped” by forming ionic and coordinate bonds intermediated by multivalent cations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D., GIBBONS N. E. The effect of chlorides of monovalent cations, urea, detergents, and heat on morphology and the turbidity of suspensions of red halophilic bacteria. Can J Microbiol. 1961 Oct;7:741–750. doi: 10.1139/m61-088. [DOI] [PubMed] [Google Scholar]
- Asbell M. A., Eagon R. G. The role of multivalent cations in the organization and structure of bacterial cell walls. Biochem Biophys Res Commun. 1966 Mar 22;22(6):664–671. doi: 10.1016/0006-291x(66)90198-7. [DOI] [PubMed] [Google Scholar]
- BROWN A. D. ASPECTS OF BACTERIAL RESPONSE TO THE IONIC ENVIRONMENT. Bacteriol Rev. 1964 Sep;28:296–329. doi: 10.1128/br.28.3.296-329.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN J. W. EVIDENCE FOR A MAGNESIUM-DEPENDENT DISSOCIATION OF BACTERIAL CYTOPLASTIC MEMBRANE PARTICLES. Biochim Biophys Acta. 1965 Jan 25;94:97–101. doi: 10.1016/0926-6585(65)90012-9. [DOI] [PubMed] [Google Scholar]
- BURTON A. J., CARTER H. E. PURIFICATION AND CHARACTERIZATION OF THE LIPID A COMPONENT OF THE LIPOPOLYSACCHARIDES FROM ESCHERICHIA COLI. Biochemistry. 1964 Mar;3:411–418. doi: 10.1021/bi00891a018. [DOI] [PubMed] [Google Scholar]
- Carson K. J., Eagon R. G. Lysozyme sensitivity of the cell wall of Pseudomonas aeruginosa. Further evidence for the role of the non-peptidoglycan components in cell wall rigidity. Can J Microbiol. 1966 Feb;12(1):105–108. doi: 10.1139/m66-015. [DOI] [PubMed] [Google Scholar]
- EAGON R. G., CARSON K. J. LYSIS OF CELL WALLS AND INTACT CELLS OF PSEUDOMONAS AERUGINOSA BY ETHYLENEDIAMINE TETRAACETIC ACID AND BY LYSOZYME. Can J Microbiol. 1965 Apr;11:193–201. doi: 10.1139/m65-025. [DOI] [PubMed] [Google Scholar]
- Eagon R. G., Simmons G. P., Carson K. J. Evidence for the presence of ash and fivalent metals in the cell wall of Pseudomonas aeruginosa. Can J Microbiol. 1965 Dec;11(6):1041–1042. doi: 10.1139/m65-144. [DOI] [PubMed] [Google Scholar]
- Goldschmidt M. C., Wyss O. Chelation effects on Azotobacter cells and cysts. J Bacteriol. 1966 Jan;91(1):120–124. doi: 10.1128/jb.91.1.120-124.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. W., Wilkinson S. G. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J Gen Microbiol. 1965 Jun;39(3):385–399. doi: 10.1099/00221287-39-3-385. [DOI] [PubMed] [Google Scholar]
- Kushner D. J., Onishi H. Contribution of protein and lipid components to the salt response of envelopes of an extremely halophilic bacterium. J Bacteriol. 1966 Feb;91(2):653–660. doi: 10.1128/jb.91.2.653-660.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Onishi H., Kushner D. J. Mechanism of dissolution of envelopes of the extreme halophile Halobacterium cutirubrum. J Bacteriol. 1966 Feb;91(2):646–652. doi: 10.1128/jb.91.2.646-652.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITZURRA M., SZYBALSKI W. Formation and multiplication of spheroplasts of Escherichia coli in the presence of lithium chloride. J Bacteriol. 1959 May;77(5):614–620. doi: 10.1128/jb.77.5.614-620.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. G. Acids and bases. Science. 1966 Jan 14;151(3707):172–177. doi: 10.1126/science.151.3707.172. [DOI] [PubMed] [Google Scholar]
- Razin S., Morowitz H. J., Terry T. M. Membrane subunits of Mycoplasma laidlawii and their assembly to membranelike structures. Proc Natl Acad Sci U S A. 1965 Jul;54(1):219–225. doi: 10.1073/pnas.54.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAH D. O., SCHULMAN J. H. BINDING OF METAL IONS TO MONOLAYERS OF LECITHINS, PLASMALOGEN, CARDIOLIPIN, AND DICETYL PHOSPHATE. J Lipid Res. 1965 Jul;6:341–349. [PubMed] [Google Scholar]
- SMITH L. Structure of the bacterial respiratory-chain system. Respiration of Bacillus subtilis spheroplasts as a function of the osmotic pressure of the medium. Biochim Biophys Acta. 1962 Jul 30;62:145–152. doi: 10.1016/0006-3002(62)90499-7. [DOI] [PubMed] [Google Scholar]
- VOSS J. G. LYSOZYME LYSIS OF GRAM-NEGATIVE BACTERIA WITHOUT PRODUCTION OF SPHEROPLASTS. J Gen Microbiol. 1964 May;35:313–317. doi: 10.1099/00221287-35-2-313. [DOI] [PubMed] [Google Scholar]



