Abstract
Adult age (24 years to 91 years) was examined as a potential moderator of the relations among cognitive abilities in an aggregate dataset based on studies conducted at the Cognitive Aging Lab at the University of Virginia (N = 2,227). A novel approach was applied by which the manifestations of latent ability factors were free to differ across age groups, and age trends in the interrelations among the factors were tested. Contrary to the dedifferentiation hypothesis, there was no evidence for systematic increases in the magnitudes of relations among cognitive abilities. Conventional analytic procedures replicated these findings.
Keywords: aging, dedifferentiation, cognitive abilities, intelligence, measurement invariance
Spearman (1904) was the first to establish that all cognitive variables are positively related to one another. He was also among the first to propose moderators of the magnitudes of these relations (Spearman, 1927). In particular, Spearman (1927) hypothesized that ability level modifies the magnitude of ability covariation such that ability interrelations are weaker at higher ability levels. He reasoned that at lower ability levels, a scarcity of domain-general resources constrains performance across a wide range of behaviors, whereas at higher ability levels, domain-general resources are profuse, and behavior is instead limited by the quality of domain-specific structures.
Garrett (1938, 1946) applied Spearman's hypothesis to childhood development by arguing that “abstract or symbol intelligence changes in its organization as age increases from a fairly unified and general ability to a loosely organized group of abilities or factors” (Garrett, 1946, p. 373). He termed this conjecture the differentiation hypothesis. Balinsky (1941) similarly examined Spearman's hypothesis in both development and aging, and on the basis of his cross-sectional analyses of the Wechsler–Bellevue standardization sample, Balinsky observed that “less of the variance can be accounted for by a single factor through the age group 25 to 29, while more and more of the variance can be so accounted for as the higher age groups are reached” (p. 227). Balinsky concluded that “there is a greater specialization up to a certain point, followed by a later reintegration of the various abilities into a flexible whole” (p. 227). Hence, the hypothesis that abilities become more highly related with adult age has come to be termed reintegration or dedifferentiation (see also Baltes, Cornelius, Spiro, Nesselroade, & Willis, 1980).
McHugh and Owens (1954) supported the dedifferentiation hypothesis in a 31-year longitudinal sample of adults (the mean age at their first testing was 19 years). Lienert and Crott (1964) found cross-sectional support for differentiation followed by dedifferentiation in samples of children, adolescents, and adults. They proposed that, because cognitive abilities decline with adult age, their age-based dedifferentiation could be explained by Spearman's (1927) hypothesis that abilities are more closely related at lower levels. Comprehensively surveying the extant body of research at the time, Reinert (1970) acknowledged a predominance of findings in favor of age differentiation followed by dedifferentiation but raised concerns about methodological shortcomings and uncertainties. He thus concluded “the available data do not allow for a clear-cut description of life-span changes in factor structure of intelligence” (p. 479).
In more recent years, comprehensive developmental theories have specifically posited mechanisms that are responsible for differentiation–dedifferentiation. Cattell's (1971, 1987) investment theory postulates that in early childhood “a single, general, relation-perceiving ability” (Cattell, 1987, p. 138) is invested in the development of a number of knowledge-based capacities, but with maturation and experience, new investment patterns arise. These new investment patterns result in “correlational disturbances” (p. 142) whereby individual differences in cognitive abilities become less related to one another as children mature to adulthood. A number of researchers (Baltes & Lindenberger, 1997; Li et al., 2004; Lovden, Ghisletta, & Lindenberger, 2004) have elaborated on this hypothesis. They have suggested that during child development, learning supports ability proliferation, whereas during aging, common constraints limit the expression of these diverse abilities. Li's neurocomputational model of cognitive aging (Li & Lindenberger, 1999; Li, Lindenberger, & Sikström, 2001) proposes that age-related increases in proportion of random variability in the central nervous system, which result from decreased efficiency of neuromodulation, may be the basis for these common constraints on functioning. Hofer and Sliwinski (2001) have analytically demonstrated that if common constraints were manifest in correlated rates of age-associated cognitive changes, increased ability interrelations with age (i.e., dedifferentiation) could result.
Recent advances in adult developmental theory have also been accompanied by advances in statistical methodology and larger, more diverse multivariate cross-sectional and longitudinal datasets that together have produced mixed support for the dedifferentiation hypothesis. Cross-sectional patterns consistent with dedifferentiation have been found by Baltes and Lindenberger (1997); Deary, Whiteman, Starr, Whalley, and Fox (2004);1 de Frias, Lovden, Lindenberger, and Nilsson (2007);2 and Li et al. (2004), but little or no evidence for a shift in the magnitudes of the correlations has been found by Bickley, Keith, and Wolf (1995); Juan-Espinosa et al. (2002); Lindenberger and Baltes (1997); and Park et al. (2002). Moreover, inspection of the correlations reported in the technical manuals for the nationally representative standardization samples of several popular intelligence batteries— for example, the Wechsler Adult Intelligence Scale (3rd ed.; Wechsler, 1997a), the Wechsler Adult Intelligence Scale (Rev.; Wechsler, 1981), the Wechsler Abbreviated Scale of Intelligence (The Psychological Corporation, 1999), and the Kaufman Adolescent and Adult Intelligence Test (Kaufman & Kaufman, 1993)— reveals little evidence of systematic increases in correlation magnitudes with adult age.
Longitudinal studies have also produced mixed findings. Dedifferentiation in the form of increasing ability interrelations was not supported by Anstey, Hofer, and Luszcz (2003); Schaie, Maitland, Willis, and Intrieri (1998); and Zelinski and Lewis (2003; see for a review of key studies). However, in a series of longitudinal studies, Ghisletta and colleagues (Ghisletta & de Ribaupierre, 2005; Ghisletta & Lindenberger, 2003, 2004) have found support for their hypothesis that declining process aspects of cognition constrain the culture-based aspects of cognition with advancing adult age through demonstrating that levels of process abilities predict changes in culture-based abilities, more so than the converse. On the basis of extant literature, Reinert's (1970) statement therefore seems to remain true that “optimistic conclusions, however, that the question should not be any longer oriented toward ‘whether’ or ‘whether not’ but rather ‘why’ … are not yet justified” (p. 482).
Given the mixed support for the dedifferentiation hypothesis, the goal of this study was to investigate cross-sectional age trends in the magnitudes of correlations among cognitive abilities of participants in a large dataset with a diversity of cognitive variables that were representative of ability constructs already well established within the empirical literature (Carroll, 1993; Salthouse, 2004). Whereas most previous studies have included only a minimal number of age cohorts from a segment of the adult age range, we examined dedifferentiation across seven contiguous age cohorts spanning close to the entirety of the adult age range. Moreover, we took an analytic approach that allows for changing manifestations of abilities with age, and we tested for noninvariance (dedifferentiation) at the construct level. If dedifferentiation hypotheses are correct, we would have expected to find increases in the relations among abilities, or indicators of abilities, with increases in adult age.
Conceptual Approach
In 1970, referring to the work of Coan (1966) and much of his own work, Nesselroade made the observation that “the universe of behaviors is not constant for different age levels and therefore the manifest nature of the factor in behavioral measures will change” (pp. 199–200). Building upon this observation, Nesselroade argued that the invariance of factor loading patterns with age and the stability of factor scores with age should be regarded as independent empirical questions. Nesselroade's assertion is akin to Spearman's (1927) theorem of the indifference of the indicator (Jensen, 1992), which states that the latent ability (in Spearman's case, general intelligence) remains invariant regardless of the test used to measure it. Whereas Spearman's theorem explained that the same ability can be manifest in different forms depending on the testing material, Nesselroade's proposition was that the same ability can be manifest in different forms depending on the individual, group, or stage of development.
Nesselroade (2007) and his colleagues (Nesselroade, Gerstorf, Hardy, & Ram, 2007) have recently formalized a method for the idiographic measurement of constructs among which the relations are invariant across individuals. In short, using multiple observations per person (time series or P-technique data), they demonstrated how affective states that fluctuate around fixed points in the short term can be manifest in different ways (i.e., by different factor loadings patterns) for different individuals but that the patterns of interrelations among these states (the latent variable intercorrelations) remain invariant across individuals. Here we applied a similar approach to age groups, rather than individuals, to address Nesselroade's (1970) proposition.3
Method
Participants
The dataset was aggregated from seven different studies conducted since 2001 at the Cognitive Aging Lab at the University of Virginia (Salthouse, 2007; Salthouse, Atkinson, & Berish, 2003; Salthouse, Berish, & Siedlecki, 2004; Salthouse & Ferrer-Caja, 2003; Salthouse, Nesselroade, & Berish, 2006; Salthouse, Pink, & Tucker-Drob, in press; Salthouse & Siedlecki, 2007; Salthouse, Siedlecki, & Krueger, 2006). Participants were recruited with newspaper advertisements, flyers, and referrals from other participants. Participants ranged in age from 24 years old to 92 years old (N = 2,227). Participants were divided into seven approximate 10-year age groups. Descriptive statistics of participants are presented in Table 1.
Table 1. Descriptive Statistics by Age Group.
| Age group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 24–29 (N = 181) | 30–39 (N = 253) | 40–49 (N = 430) | 50–59 (N = 534) | 60–69 (N = 382) | 70–79 (N = 312) | 80–91 (N = 135) | All (N = 2227) | Age r |
| Age, M (SD) | 26.25 (1.72) | 34.21 (2.82) | 44.86 (2.97) | 54.34 (2.83) | 64.30 (2.82) | 74.15 (2.78) | 83.21 (2.84) | 54.17 (16.06) | |
| WAIS-III vocab ss, M (SD) | 13.47 (2.95) | 12.17 (3.41) | 11.83 (2.94) | 12.82 (2.82) | 13.33 (2.41) | 13.12 (3.03) | 13.64 (2.84) | 12.79 (2.95) | .10 |
| WAIS-III digit sym ss, M (SD) | 11.80 (2.66) | 11.82 (3.05) | 11.03 (2.89) | 11.68 (2.95) | 11.43 (2.54) | 11.83 (2.87) | 11.70 (3.01) | 11.56 (2.86) | .02 |
| WMS-III log mem ss, M (SD) | 11.87 (2.81) | 11.72 (2.71) | 11.46 (2.73) | 12.05 (2.83) | 12.47 (2.75) | 12.44 (2.82) | 12.26 (3.13) | 12.02 (2.82) | .10 |
| WMS-III recall ss, M (SD) | 12.72 (3.48) | 12.18 (3.17) | 12.28 (3.30) | 12.69 (3.32) | 13.09 (3.10) | 12.37 (3.19) | 11.77 (3.48) | 12.52 (3.28) | .01 |
| Education, M (SD) | 15.88 (2.25) | 16.05 (2.79) | 15.44 (2.56) | 16.06 (2.58) | 16.38 (2.72) | 15.79 (2.96) | 16.08 (3.09) | 15.94 (2.70) | .02 |
| Male (0) vs. female (1) | 0.65 | 0.72 | 0.76 | 0.69 | 0.64 | 0.58 | 0.50 | 0.67 | −.11 |
| Score reliability, rOE, (SD) | |||||||||
| Matrix reasoning Gf | 0.75 (15.00) | 0.83 (16.32) | 0.76 (14.29) | 0.74 (14.18) | 0.75 (14.31) | 0.74 (13.89) | 0.76 (13.06) | 0.81 (16.59) | −.49 |
| Shipley abstraction Gf | 0.81 (15.00) | 0.85 (16.68) | 0.88 (18.11) | 0.84 (16.71) | 0.80 (16.31) | 0.84 (18.77) | 0.90 (19.92) | 0.86 (18.67) | −.38 |
| Letter sets Gf | 0.74 (15.00) | 0.79 (15.27) | 0.74 (16.37) | 0.71 (15.86) | 0.72 (16.39) | 0.71 (17.49) | 0.79 (20.67) | 0.77 (17.61) | −.33 |
| Form boards Gv | 0.89 (15.00) | 0.89 (14.58) | 0.86 (11.41) | 0.85 (12.49) | 0.82 (11.01) | 0.83 (10.48) | 0.80 (8.83) | 0.87 (12.98) | −.36 |
| Paper folding Gv | 0.77 (15.00) | 0.84 (17.01) | 0.74 (15.29) | 0.69 (14.47) | 0.71 (14.37) | 0.59 (12.87) | 0.60 (12.50) | 0.75 (15.71) | −.37 |
| Spatial relations Gv | 0.93 (15.00) | 0.93 (15.67) | 0.89 (13.43) | 0.89 (12.97) | 0.86 (12.01) | 0.82 (10.42) | 0.73 (9.11) | 0.90 (13.71) | −.34 |
| WAIS vocab Gc | 0.92 (15.00) | 0.95 (18.39) | 0.92 (16.31) | 0.91 (14.74) | 0.92 (13.01) | 0.92 (15.72) | 0.92 (14.29) | 0.92 (15.54) | .05 |
| WJ picture vocab Gc | 0.90 (15.00) | 0.90 (15.39) | 0.90 (15.22) | 0.87 (12.64) | 0.83 (11.93) | 0.84 (12.56) | 0.85 (14.81) | 0.88 (14.27) | .19 |
| Synonym vocab Gc | 0.79 (15.00) | 0.82 (16.59) | 0.79 (15.00) | 0.83 (14.07) | 0.80 (12.66) | 0.80 (13.36) | 0.82 (13.34) | 0.82 (14.81) | .24 |
| Antonym vocab Gc | 0.72 (15.00) | 0.81 (17.40) | 0.84 (17.01) | 0.87 (16.58) | 0.81 (14.95) | 0.84 (16.60) | 0.90 (16.63) | 0.84 (16.66) | .13 |
| Recall Gm | 0.90 (15.00) | 0.85 (14.36) | 0.88 (14.73) | 0.90 (15.21) | 0.91 (15.40) | 0.92 (16.88) | 0.94 (19.08) | 0.91 (17.31) | −.42 |
| Log mem Gm | 0.77 (15.00) | 0.74 (15.00) | 0.77 (14.63) | 0.75 (14.20) | 0.72 (13.19) | 0.76 (13.57) | 0.85 (17.79) | 0.77 (14.93) | −.24 |
| Paired associates Gm | 0.74 (15.00) | 0.84 (16.50) | 0.75 (15.47) | 0.80 (15.59) | 0.75 (15.28) | 0.76 (13.92) | 0.65 (11.00) | 0.80 (16.21) | −.36 |
| Pattern comparison Gs | 0.75 (15.00) | 0.80 (15.77) | 0.83 (15.18) | 0.81 (13.48) | 0.78 (12.36) | 0.86 (11.92) | 0.88 (12.85) | 0.87 (16.28) | −.53 |
| Letter comparison Gs | 0.91 (15.00) | 0.91 (13.98) | 0.93 (13.79) | 0.93 (13.78) | 0.93 (12.51) | 0.92 (12.83) | 0.94 (13.80) | 0.93 (15.70) | −.50 |
| Digit sym Gs | — (15.00) | — (17.44) | — (16.37) | — (15.77) | — (14.56) | — (15.00) | — (16.85) | — (19.53) | −.59 |
Note. Descriptive statistics are based on raw data with pairwise deletion to handle missing data. Age and education are reported in years. Correlations that are significantly different from 0 at p < .01 are in bold. Dashes show that the reliabilities for the digit symbol test could not be computed because the test does not contain distinct items. WAIS-III = Wechsler Adult Intelligence Scale—Third Edition; vocab = vocabulary; ss = scaled score; log mem = logical memory; sym = symbol; WMS-III = Wechsler Memory Scale—Third Edition; Gf = fluid reasoning; Gv = spatial reasoning; Gc = verbal knowledge; WJ = Woodcock–Johnson Psycho-Educational Battery-Revised; Gs = processing speed; Gm = episodic memory; rOE = odd-even split half reliability corrected with the Spearman-Brown prophecy formula.
One way to evaluate the selectivity of a sample involves a comparison of the scores on a number of standardized measures to the scores for the normative sample of the Wechsler Adult Intelligence Scale (3rd ed.) and the Wechsler Memory Scale (3rd ed.; Wechsler, 1997b), which have been matched to the U.S. population on a number of demographic variables including gender, ethnicity, years of education, and region of residence in the country. Age-adjusted scaled scores had means of 10 and standard deviations of 3 in the normative sample, but the scaled score means in the current sample were all above 11. Although this indicates that the individuals in this sample were functioning above the average of the normative sample, this was true to nearly the same extent at all ages, as the correlations between age and the scaled scores were all quite small. Results from this dataset may therefore be most applicable to people with higher-than-average levels of functioning, but the age comparisons should be meaningful because there is little evidence that participants of different ages were differentially representative of their age groups.
Measures
All of the studies included a battery of between 14 and 16 cognitive tests (3 or 4 for each ability) selected to measure fluid reasoning (Gf), spatial reasoning (Gv), verbal knowledge (Gc), processing speed (Gs), and episodic memory (Gm). Because the pattern of missingness in the data was largely a function of the study to which participants were assigned, full information maximum likelihood estimation was used to handle missing data under the missing at random assumption (cf. Salthouse, 2004, 2005). The specific cognitive tests are described in earlier publications (e.g., Salthouse, 2004). For the current study, all variables were standardized to the IQ metric (M = 100, SD = 15) for the youngest age group.
Reliabilities and standard deviations of the cognitive variables by age group are presented in Table 1. It can be seen that the reliabilities are all very high, and that there is little evidence of systematic trends in the magnitudes of reliabilities or standard deviations with age.
Analytical Approach
We applied a recent method for scaling latent ability constructs (Little, Slegers, & Card, 2006) that is in line with the earlier stated goals in that it (a) allows for the separation of information concerning the absolute magnitudes of manifest variable relations—specifically relevant to the dedifferentiation hypothesis—from the relative patterns by which manifest variables are indicative of the latent ability constructs and that it (b) does not require that an arbitrary parameter (i.e., factor loading, factor variance) be fixed (and consequently set invariant across groups), a convention that has the potential to have nonarbitrary consequences (e.g., Millsap, 2001; Steiger, 2002). The method simply requires that the average of the factor loadings for each latent factor (in a given age group) be fixed to 1, which scales the factor variance in a metric that can be directly interpreted as the average amount of variance in each indicator accounted for by the factor, or the amount of systematic variance in the system of factor indicators. Increases in factor variances across age groups would therefore indicate dedifferentiation at the level of specific ability indicators. Dedifferentiation at the level of ability interrelations can then be examined through the use of covariances or correlations among the factors. Because the factor loadings, which only indicate relative factor representation, are free to differ across groups, we can be sure that we are examining a broad range of the ability space, as it might be idiosyncratically manifest for each given age group (cf. Nesselroade, 1970; Nesselroade et al., 2007). We further constrained the sum of the indicators' intercepts to 0, such that the latent mean retains the observed metric of the indicators and that it is optimally weighted by the factor loadings. Little et al. (2006) have, in fact, suggested that this nonarbitrary method is ideal for use with Nesselroade's (2007) idiographic method.
Finally, rather than merely inspecting freely estimated parameter values for the different groups (for which an objective criterion would be lacking) or testing models with freely estimated parameters against those with cross-group equality constraints (which does not directly address the presence or absence of systematic trends), we statistically evaluated the extent to which parameter values systematically differed as functions of age. We imposed cross-group linear and quadratic age-based constraints in the form of
where P[A] is the parameter (i.e., factor variance or interfactor correlation) value for a given age group (1–7); P[4] is the parameter for the 4th (middle) age group (50–59 years); [A] is a 7-unit vector corresponding to age group (centered; i.e., −3, −2, −1, 0, 1, 2, 3); [A2] is a 7-unit vector that corresponds to age group squared (i.e., 9, 4, 1, 0, 1, 4, 9); and L and Q are freely estimated coefficients, which are similar to multilevel model parameters (McArdle & Hamagami, 1996), that correspond to linear and quadratic effects, both with standard errors. Significant positive linear effects would support dedifferentiation in the form of increased parameter magnitudes with age, and significant positive quadratic effects would support dedifferentiation in the form of accelerated increases in parameter magnitudes at later ages (cf. de Frias et al., 2007).
Results
Primary Analyses
All models were fit as multiple group models, with group membership determined by age at testing, as shown in Table 1. To avoid Type I error as a result of the large number of statistical comparisons, we set alpha values to .01.
The first model fit was one in which factor loadings were constrained to be invariant across groups, and one in which factor variances and covariances were allowed to vary freely across groups, χ2(790) = 2,088.7, with a root-mean-square error of approximation (RMSEA) = .070. In a second model, these factor loading equality constraints were removed. This resulted in a substantial improvement in model fit, χ2(724) = 1,582.0, RMSEA = .061, which suggests that the behavioral manifestations of the factors differed by age group. Follow-up analyses suggested that these failures of measurement invariance could not be isolated to a few specific constructs or a few specific age groups. For the remaining models, loadings were allowed to vary freely across groups.
In order to determine whether the amounts of systematic variance in the systems of factor indicators differed as functions of age, we fit a model with linear and quadratic cross-group parameter constraints on the factor variances. Two linear parameter constraints (one on the variance of Gc and one on the variance of Gv) were significantly different from zero and retained. The remaining linear and quadratic parameter constraints were removed, which resulted in cross-group equality constraints for these variances. The resulting model, χ2(752) = 1,629.0, RMSEA = .061, fit better than a model in which all factor variances were constrained to equality across groups, χ2(754) = 1,734.0, RMSEA = .064, and no worse than the earlier, less parsimonious, model in which factor variances were freely estimated for each age group. This structure with free loadings and linear age trends in two variances was retained for the remaining analyses.
In a final set of models, age relations in factor interrelations (correlations) were examined. Because we already had information about the magnitudes of individual differences from the factor variances, correlations were chosen so as to focus exclusively on the degrees of correspondence between the relative ordering of individuals. A model with linear and quadratic parameter constraints on the correlations was fit that resulted in two significant linear parameters (on rGv–Gm and on rGv–Gc) and one significant quadratic parameter (on rGf–Gc). With these parameters retained, a final model, χ2(809) = 1,695.1, RMSEA = .059 was constructed that fit better than a model in which correlations were constrained to equality across groups, χ2(812) = 1,719.5, RMSEA = .059, and no worse than the earlier, less parsimonious, model in which correlations were free to vary across groups.
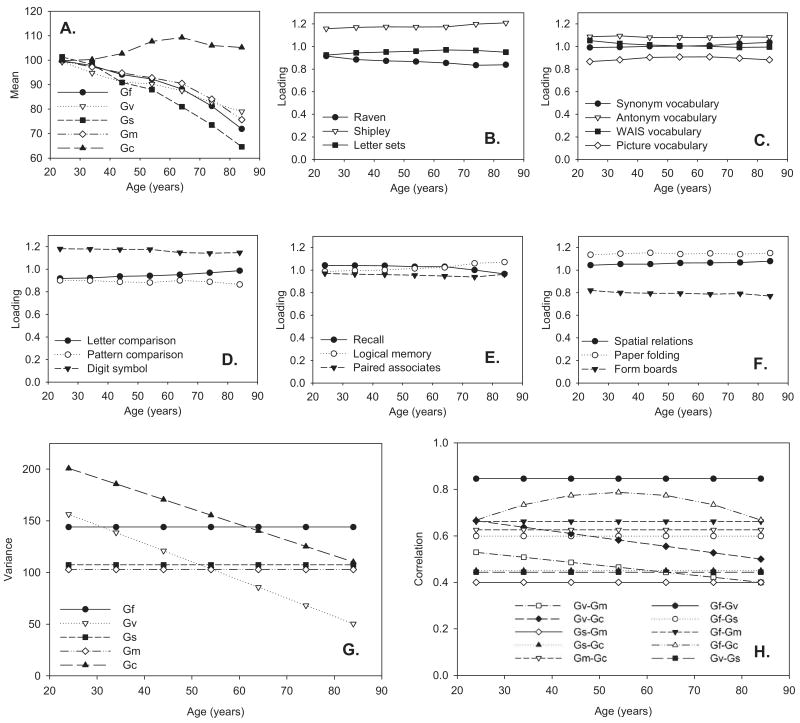
Age trends in key parameters of the final model are presented in Figure 1. In Panel A, it can be seen that the means of all factors except for the Gc factor exhibit monotonic negative age trends that—on the basis of Spearman's hypothesis and recent versions of the dedifferentiation hypothesis (e.g., Li et al., 2004)—we would expect to be accompanied by increasing interrelations. In Panel B through Panel F, the relative loadings of the indicators on the factors drift with age to some extent but on the whole exhibit a fair amount of stability. Finally, Panel G and Panel H show that, in contrast to the dedifferentiation hypothesis, the common variances in the systems of Gc and Gv indicators actually decrease with age, the Gv–Gc relation decreases with age, and the Gf–Gc relation is at its peak in middle adulthood. In the final model, the linear age trend in the Gv–Gm relation is no longer significant. The remaining factor variances and factor interrelations are invariant across age groups.
Figure 1.
Parameters are plotted according to age group, as indicated by the final model, χ2 (809) = 1695.1, comparative fit index = 0.95, Tucker-Lewis fit index = 0.95. RMSEA = 0.059. Age is represented in years. Panel A shows age trends in factor means. Panels B–F show age trends in factor loadings for indicators of Gf (fluid reasoning; Panel B), Gc (verbal knowledge; Panel C), Gs (processing speed; Panel D), Gm (episodic memory; Panel E), and Gv (spatial reasoning; Panel F). Panel G shows age trends in factor variances. Panel H shows age trends in factor intercorrelations. WAIS = Wechsler Adult Intelligence Scale; Raven = Raven's Progressive Matrices; Shipley = Shipley Institute of Living Scale, Abstraction subtest.
Alternative Analyses
Many previous examinations of dedifferentiation (e.g., Juan-Espinosa et al., 2002; Li et al., 2004) have focused on age-group comparisons of the percentage of variance accounted for by a single common factor. When this approach was applied in the current project, the results were also inconsistent with dedifferentiation. The percentages of variance accounted for by a single common factor were 43, 47, 46, 42, 40, 40, and 43 in the youngest through the oldest age groups, respectively. Moreover, a model in which cross-group linear and quadratic age-based constraints were fit to the variance of a single common factor, scaled in the nonarbitrary fashion described earlier (i.e., the common factor's variance is proportional to the amount of variance that it accounts for in the indicators), revealed that there was a significant linear age-related decrease in the amount of unstandardized variance (9.9 units per variable per approximate 10-year age group) accounted for by the common factor. These alternative analyses indicate that the above findings are robust and not obscured by the unconventional approach advocated earlier. Dedifferentiation was not supported through the use of any of the three approaches.
Discussion
The major finding from the analyses reported in this project was that there was little evidence to support the dedifferentiation hypothesis which states that the relations among cognitive abilities systematically increase with adult age. In fact, of the 15 parameters representative of the magnitudes of relations among indicators of abilities (factor variances) and among latent abilities themselves (factor intercorrelations), only 4 were found to vary according to age, and these were all in directions opposite to those predicted by the dedifferentiation hypothesis. The reasons for the weaker relations with increased age are not yet clear, but because these trends were opposite to those expected, we can be confident that dedifferentiation was not supported by these data.
The analytical method that we employed was novel in that instead of requiring factor loadings to remain invariant, as is conventionally the case, we allowed them to vary freely according to age group. This allowed for the behavioral manifestations of the abilities to differ by age, while invariance of the relations among the unobserved factors was tested. This was advantageous in that it ensured that the full amount of common variance was sampled in each age group (regardless of its source). It is important, however, to acknowledge that the patterns of factor loadings were very similar across the age groups and that while its fit was significantly worse than the noninvariant model, the invariant model fit the data adequately in absolute terms. It is also possible that although it is standard practice, the specification of a linear measurement model and the assumption of interval measurement properties of the cognitive tests may not be justified. Therefore, the proposition that the behavioral manifestations of unobserved abilities may qualitatively differ with age warrants a great deal of further investigation.
The failure to find age-related increases in the strengths of the interrelations among variables and among constructs is inconsistent with the dedifferentiation hypothesis and instead suggests that variables and constructs retain their distinctiveness—and perhaps become even more distinct—across nearly all of adulthood. There is considerable evidence that age-related influences on different cognitive variables are not independent (e.g., Salthouse, 2004, 2005; Salthouse & Ferrer-Caja, 2003). However, the current results suggest that even if broad or systemic influences are operating to affect average levels of functioning, they do not necessarily result in age-related increases in the relations among cognitive abilities.
Acknowledgments
This research was supported by National Institute on Aging Grants R37 AG02427042 and R01 AG19627 to Timothy A. Salthouse. Elliot M. Tucker-Drob was supported as a trainee by National Institute on Aging Grant T32AG020500.
Footnotes
An earlier version of this article was presented at the 2006 Cognitive Aging Conference in Atlanta, Georgia. We appreciate valuable suggestions made by John R. Nesselroade and Ulman Lindenberger.
Although this was a longitudinal study, the comparisons that addressed dedifferentiation were across cohorts only at the follow-up measurement occasion.
This study was longitudinal in the sense that correlations were computed between initial levels and between rates of change. These correlations, however, were compared across cross-sectional age groups.
Because we were interested in the relations among more stable traits (i.e., abilities) we did not take a time series approach, but rather we took a cross-sectional approach. Although it was likely that cognitive performance fluctuates systematically in the short term, the mechanisms responsible for short-term stationary change are likely to be different from the determinants of absolute levels of ability that change slowly in the long term. We therefore focused this article on interindividual relations, which might not be ergodic relative to short-term intraindividual relations. However, by disaggregating our data into narrow age cohorts, we were still able to allow for potentially differing manifestations of factors with age.
References
- Anstey KJ, Hofer SM, Luszcz MA. Cross-sectional and longitudinal patterns of dedifferentiation in late-life cognitive and sensory function: The effects of age, ability, attrition, and occasion of measurement. Journal of Experimental Psychology: General. 2003;132:470–487. doi: 10.1037/0096-3445.132.3.470. [DOI] [PubMed] [Google Scholar]
- Balinsky B. An analysis of the mental factors of various age groups from nine to sixty. Genetic Psychology Monographs. 1941;23:191–234. [Google Scholar]
- Baltes PB, Cornelius SW, Spiro A, Nesselroade JR, Willis SL. Integration vs. differentiation of fluid-crystallized intelligence in old age. Developmental Psychology. 1980;16:625–635. [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychology and Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Bickley PG, Keith TZ, Wolf LM. The three-stratum theory of cognitive abilities: Test of the structure of intelligence across the life span. Intelligence. 1995;20:309–328. [Google Scholar]
- Carroll JB. Human cognitive abilities: A survey of factor-analytic studies. New York: Cambridge University Press; 1993. [Google Scholar]
- Cattell RB. Abilities: Their structure, growth, and action. Boston: Houghton Mifflin; 1971. [Google Scholar]
- Cattell RB. Intelligence: Its structure, growth, and action. Amsterdam: North-Holland; 1987. [Google Scholar]
- Coan RW. Child personality and developmental psychology. In: Cattell RB, editor. Handbook of multivariate experimental psychology. Chicago: Rand McNally; 1966. pp. 732–752. [Google Scholar]
- Deary IJ, Whiteman MC, Starr JM, Whalley LJ, Fox HC. The impact of childhood intelligence on later life: Following up the Scottish mental surveys of 1932 and 1947. Journal of Personality and Social Psychology. 2004;86:130–147. doi: 10.1037/0022-3514.86.1.130. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Lovden M, Lindenberger U, Nilsson LG. Revisiting the dedifferentiation hypothesis with longitudinal multicohort data. Intelligence. 2007;35:381–392. [Google Scholar]
- Garrett HE. Differentiable mental traits. Psychological Record. 1938;2:259–298. [Google Scholar]
- Garrett HE. A developmental theory of intelligence. American Psychologist. 1946;1:372–378. doi: 10.1037/h0056380. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, de Ribaupierre A. A dynamic investigation of cognitive dedifferentiation with control for retest: Evidence from the Swiss Interdisciplinary Longitudinal Study on the Oldest Old. Psychology and Aging. 2005;20:671–682. doi: 10.1037/0882-7974.20.4.671. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Lindenberger U. Age-based structural dynamics between perceptual speed and knowledge in the Berlin Aging Study: Direct evidence for ability dedifferentiation in old age. Psychology and Aging. 2003;18:696–713. doi: 10.1037/0882-7974.18.4.696. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Lindenberger U. Static and dynamic longitudinal structural analyses of cognitive changes in old age. Gerontology. 2004;50:12–16. doi: 10.1159/000074383. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Sliwinski MJ. Understanding ageing: An evaluation of research designs for assessing the interdependence of ageing-related changes. Gerontology. 2001;47:341–352. doi: 10.1159/000052825. [DOI] [PubMed] [Google Scholar]
- Jensen AR. Vehicles of g. Psychological Science. 1992;3:275–278. [Google Scholar]
- Juan-Espinosa M, García LF, Escorial S, Rebollo I, Colom R, Abad FJ. Age dedifferentiation hypothesis: Evidence from the WAIS III. Intelligence. 2002;30:1–14. [Google Scholar]
- Kaufman AS, Kaufman NL. Manual for the Kaufman Adolescent and Adult Intelligence Test (KAIT) Circle Pines, MN: American Guidance Service; 1993. [Google Scholar]
- Li SC, Lindenberger U. Cross-level unification: A computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age. In: Nilsson LG, Markowitsch HJ, editors. Cognitive neuroscience of memory. Kirkland, WA: Hogrefe & Huber; 1999. pp. 103–146. [Google Scholar]
- Li SC, Lindenberger U, Hommel B, Aschersleben G, Prinz W, Baltes PB. Lifespan transformations in the couplings of mental abilities and underlying cognitive processes. Psychological Science. 2004;15:155–163. doi: 10.1111/j.0956-7976.2004.01503003.x. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Sikström S. Aging cognition: From neuromodulation to representation. Trends in Cognitive Sciences. 2001;5:479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Lienert GA, Crott HW. Studies on the factor structure of intelligence in children, adolescents, and adults. Vita Humana. 1964;7:147–163. doi: 10.1159/000270108. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: Cross-sectional results from the Berlin Aging Study. Psychology and Aging. 1997;12:410–432. doi: 10.1037//0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- Little TD, Slegers DW, Card NA. A non-arbitrary method of identifying and scaling latent variables in SEM and MACS models. Structural Equation Modeling. 2006;13:59–72. [Google Scholar]
- Lovden M, Ghisletta P, Lindenberger U. Cognition in the Berlin Aging Study (BASE): The first 10 years Aging. Neuropsychology, and Cognition. 2004;11:104–133. [Google Scholar]
- McArdle JJ, Hamagami F. Multilevel models from a multiple group structural equation perspective. In: Marcoulides G, Schumacker R, editors. Advanced structural equation modeling techniques. Hillsdale, NJ: Erlbaum; 1996. pp. 89–124. [Google Scholar]
- McHugh RB, Owens WA. Age changes in mental organization—A longitudinal study. Journal of Gerontology. 1954;9:296–302. doi: 10.1093/geronj/9.3.296. [DOI] [PubMed] [Google Scholar]
- Millsap RE. When trivial constraints are not trivial: The choice of uniqueness constraints in confirmatory factor analysis. Structural Equation Modeling. 2001;8:1–17. [Google Scholar]
- Nesselroade JR. Application of multivariate strategies to problems of measuring and structuring long-term change. In: Goulet LR, Baltes PB, editors. Life-span developmental psychology: Research and theory. New York: Academic Press; 1970. pp. 193–211. [Google Scholar]
- Nesselroade JR. Factoring at the individual level: Some matters for the second century of factor analysis. In: MacCallum RC, Cudeck R, editors. 100 years of factor analysis. Mahwah, NJ: Erlbaum; 2007. pp. 249–264. [Google Scholar]
- Nesselroade JR, Gerstorf D, Hardy SA, Ram N. Idiographic filters for psychological constructs. Measurement: Interdisciplinary Research and Perspectives. 2007;5:217–235. [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson N, Smith AD, Smith P. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- The Psychological Corporation. Manual for the Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Author; 1999. [Google Scholar]
- Reinert G. Comparative factor analytic studies of intelligence through the human life-span. In: Goulet LR, Baltes PB, editors. Life-span developmental psychology: Research and theory. New York: Academic Press; 1970. pp. 468–485. [Google Scholar]
- Salthouse TA. Localizing age-related individual differences in a hierarchical structure. Intelligence. 2004;32:541–561. doi: 10.1016/j.intell.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Relations between cognitive abilities and measures of executive functioning. Neuropsychology. 2005;19:532–545. doi: 10.1037/0894-4105.19.4.532. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Implications of within-person variability in cognitive and neuropsychological functioning on the interpretation of change. Neuropsychology. 2007;21:401–411. doi: 10.1037/0894-4105.21.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology: General. 2003;132:566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Berish DE, Siedlecki KL. Construct validity and age sensitivity of prospective memory. Memory & Cognition. 2004;32:1133–1148. doi: 10.3758/bf03196887. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Ferrer-Caja E. What needs to be explained to account for age-related effects on multiple cognitive variables? Psychology and Aging. 2003;18:91–110. doi: 10.1037/0882-7974.18.1.91. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Nesselroade JR, Berish DE. Short-term variability and the calibration of change. Journal of Gerontology: Psychological Sciences. 2006;61:144–151. doi: 10.1093/geronb/61.3.p144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Pink JE, Tucker-Drob EM. Contextual analysis of fluid intelligence. Intelligence. doi: 10.1016/j.intell.2007.10.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Siedlecki KL. Efficiency of route selection as a function of adult age. Brain and Cognition. 2007;63:279–287. doi: 10.1016/j.bandc.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Siedlecki KL, Krueger LE. An individual differences analysis of memory control. Journal of Memory and Language. 2006;55:102–125. doi: 10.1016/j.jml.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW, Maitland SB, Willis SL, Intrieri RL. Longitudinal invariance of adult psychometric ability factor structures across seven years. Psychology and Aging. 1998;13:8–20. doi: 10.1037/0882-7974.13.1.8. [DOI] [PubMed] [Google Scholar]
- Spearman C. “General intelligence” objectively determined and measured. American Journal of Psychology. 1904;15:201–293. [Google Scholar]
- Spearman C. The abilities of man. London: Macmillan; 1927. [Google Scholar]
- Steiger JH. When constraints interact: A caution about reference variables, identification constraints, and scale dependencies in structural equation modeling. Psychological Methods. 2002;7:210–227. doi: 10.1037/1082-989x.7.2.210. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS–R: Manual: Wechsler Adult Intelligence Scale—Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd. San Antonio, TX: The Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3rd. San Antonio, TX: The Psychological Corporation; 1997b. [Google Scholar]
- Zelinski EM, Lewis KL. Adult age differences in multiple cognitive functions: Differentiation, dedifferentiation, or process-specific change? Psychology and Aging. 2003;18:727–745. doi: 10.1037/0882-7974.18.4.727. [DOI] [PubMed] [Google Scholar]