Abstract
A substantial number of patients who meet criteria for a prodromal syndrome for first psychosis are treated with antipsychotic and/or antidepressant medications. There is suggestive evidence that both classes of medication may reduce prodromal symptoms. This longitudinal study examined the relation of antipsychotic and antidepressant medication with prodromal symptom severity at baseline and 6-month follow-up. Participants met Structured Interview for Prodromal Syndromes (SIPS) criteria for the prodrome, and were evaluated at eight centers as part of the North American Prodrome Longitudinal Study (NAPLS).Symptom ratings (positive, negative, disorganized and general) and data on antipsychotics, SSRIs, and other antidepressant medications were obtained at baseline and 6-month follow-up. Analyses revealed that all symptom dimensions declined in severity over time, but there were differences in the magnitude of the decline as a function of antipsychotic medication. Those never on antipsychotics showed less reduction in positive and disorganized symptoms over time. SSRIs and other antidepressants were not linked with declines in symptom severity. Consistent with findings from small-sample, clinical trials, the present results suggest that atypical antipsychotics may be effective in reducing the severity of attenuated positive symptoms associated with the prodrome to psychotic disorders. Limitations of the present study are noted, including the fact that it is not a randomized trial, and data on duration and dosage of medication and 2-year follow-up were not available for most participants. The results are discussed in light of the relative risks and benefits of preventive interventions, both medication and cognitive therapies, and the importance of future clinical trials.
Keywords: psychosis, prodrome, medication
1. Introduction
Research has demonstrated that the onset of psychosis is typically preceded by a period of subclinical symptoms. This is referred to as the prodrome, with duration varying from one to six years (Cannon et al., 2008; Thomas and Woods, 2006). The onset of the prodrome is usually in the young adult period, and is characterized by behavioral dysfunction and subpsychotic symptoms that gradually increase in severity (Cannon, 2008; Sun et al., 2009; Walker, 2002). Interest in the prodrome has escalated recently in response to evidence that longer durations of untreated psychosis are associated with poorer prognosis. Thus, identifying individuals at risk for psychosis has the potential to hasten the provision of treatment if a psychotic episode occurs.
Several groups have developed structured interviews for assessing prodromal symptoms, and have significantly advanced the prediction of psychosis. Using the Comprehensive Assessment of At-Risk Mental States (CAARMS), researchers in Australia have conducted studies on prodromal individuals and initially reported that, within two years, 30 to 40% meet diagnostic criteria for a psychotic disorder (Yung and McGorry, 1996). In a more recent cohort from this project, a 2-year conversion rate of 16% was reported (Yung et al., 2008). It is suggested that this may reflect earlier detection of high risk individuals and/or greater provision or effectiveness of interventions in recent cohorts. Kloesterkoetter et al., (2001), in Germany, used another measure of prodromal symptoms, the Bonn Scale for the Assessment of Basic Symptoms (BSABS), to identify 110 prodromal patients who were followed for an average of 9.6 years. Of those with at least one prodromal symptom, 70% subsequently developed schizophrenia (within 4.3 years among women and after 6.7 years in men). In the US., McGlashan et al. (2006) developed the Structured Interview for Prodromal Syndromes (SIPS), as well as a severity scale, the Scale of Prodromal Symptoms (SOPS), and found that about 30 % of individuals who meet criteria for attenuated positive symptoms manifest a psychotic disorder within 2 years (Miller et al., 2002). Most recently, a consortium has pooled data from prodromal studies conducted at eight sites, all using the SIPS. The sites comprising the North American Prodrome Longitudinal Study (NAPLS) ascertained clinical high-risk (CRH) individuals and followed them at regular intervals for up to 2 and 1/2 years (Addington et al., 2007). Approximately 35% of these subjects converted to psychosis over the 2 and ½ year period (Cannon et al., 2008).
A pivotal issue currently under discussion in the field is whether preventive intervention, especially antipsychotics, might be effective in delaying or reducing the onset of psychosis in prodromal individuals. Investigators have debated the potential benefits and risks in administering psychotropics to youth at risk for psychosis based on prodromal signs. Since 2000, several reports have been published on the effects of antipsychotics on prodromal symptoms and conversion to psychosis. The first, conducted by McGorry et al. (2002), in Australia, randomized prodromal patients to either usual care (n=28) or open-label risperidone plus cognitive therapy and usual care (n=31) for six months. Six month conversion to psychosis rates were 10% for the risperidone and therapy treatment versus 36% for usual care (p<.05), suggesting that the medication and cognitive therapy delayed onset of disorder, and possibly reduced incidence. In a follow-up of this sample 3–4 years later, however, there were no group differences in conversion or on any symptom measures (Phillips et al., 2007). A second study by the Yale group randomly assigned 60 prodromal youth to olanzapine or to placebo, with all receiving supportive psychotherapy (McGlashan et al., 2006; Woods et al., 2003). After one year, 16% of olanzapine-treated patients vs. 38% of placebo-treated patients had become psychotic. All of the conversions in the olanzapine group took place within the first month, while conversion continued beyond this period in the placebo group, suggesting a latency to the antipsychotic effect or doses that were too low. Treatment effects on symptoms were evident by eight weeks, with olanzapine producing greater symptom reductions, particularly in positive symptoms, than placebo. There was, however, significantly greater weight gain and fatigue among the olanzapine patients. In a treatment study from the German group, described above, prodromal individuals were randomly assigned to a needs-focused intervention without (n=59) or with amisulpride (n=65) (Ruhrmann, et al., 2007). After 12 weeks, amisulpride plus the needs-focused intervention produced a greater reduction in symptoms, especially positive symptoms, as well as depression and functioning deficits. Although prolactin levels were higher with amisulpride, only a small number developed clinical elevations. Finally, in a nonrandomized clinical trial of risperidone with a small sample of youth yielded similar results, with greater reductions in positive prodromal symptoms (Cannon et al., 2002).
Subsequently, the Yale team completed an open-label, trial of aripiprazole in fifteen young prodromal patients (mean age 17.1years) (Woods et al., 2007). The principal outcome was the reduction in the SOPS total score after 8 weeks. Improvement from baseline on the SOPS scores was statistically significant by the first week. Thirteen of 15 subjects (87%) completed the planned eight weeks of treatment. Weight gain averaged 1.2 kg over 8 weeks, and akathisia emerged in 8 of 15 patients, but mean akathisia ratings fell to baseline levels by the final visit. Adverse events were otherwise minimal. In summary, the findings from trials of antipsychotics with prodromal patients suggest conservative optimism about the potential benefits with respect to symptom reduction in the short term, but the findings are inconsistent with regard to prevention or delay of psychosis onset.
A recently reported naturalistic study indicates that antidepressants may also benefit clinical high risk youth (Cornblatt et al., 2007). The researchers examined non-randomly prescribed antidepressants (N = 20) and second-generation antipsychotics (N = 28). The two medication groups did not differ in baseline symptom profiles, with the exception of disorganized thinking, which was more severe in the second-generation antipsychotic group. Improvement in 3 of 5 positive symptoms over time was significant and similar for both medications. Disorganized thought, however, did not improve with either medication. Twelve of the 48 developed a psychotic disorder, with all converters having been on antipsychotics, and none on antidepressants. However, treatment outcome was confounded with medication adherence, in that 11 of the 12 converters were nonadherent, and participants were more likely to be nonadherent to second-generation antipsychotics than to antidepressants.
It should be noted that a randomized controlled trial of cognitive therapy (CT) was recently reported on a sample of prodromal subjects identified with the CAARMS (French et al. 2007; Morrison et al., 2007). Participants received either CT (n=35) or monitoring (n=23) over a 6 month period and 47% were followed up over a 3-year period. Within the first year, CT was associated with a greater reduction in positive symptoms (French et al., 2007). At the 3-year follow-up, CT significantly reduced the likelihood of being prescribed antipsychotic medication, but it did not affect transition to psychosis. Thus, to date, the limited findings on CT are similar to those on antipsychotic medication in showing reductions in positive symptom severity, but limited effect on conversion to psychosis.
In light of these findings, it is clear that additional research is needed on the relation between psychotropic medications and prodromal symptom progression. The NAPLS data set offers a unique opportunity for naturalistic investigations of this issue with a large sample. In this study, we examine the progression of prodromal symptoms in relation with the two most commonly prescribed classes of psychotropic medications: antipsychotics and antidepressants (SSRIs). Data on symptoms and medication status were collected at both baseline and follow-up. Based on past reports, it is predicted that prodromal patients on antipsychotics will show a more pronounced reduction in prodromal positive symptoms over time.
2. Methods
2.1 Sample and Assessment Procedures
A total of 191 patients, recruited between 1999 and 2004, who met at-risk or “prodromal” criteria, as defined by the SOPS, were the subjects of this study. None had any history of Axis I psychotic disorder. Demographics at baseline are listed in Table 1. Distribution by race is 80% white, 8% African American, 4.5% Asian, 5% multiracial and 3% unidentified.
Table 1.
Demographic Characteristics and Baseline Medication for Prodromal Patients with and without 6-month Follow-up.
| 6-month Follow-up Prodromal sample (N=191) | Baseline Mean Age (SD) | 18.65 (4.7) |
| % Male | 56a | |
| % on Antipsychotic | 9 | |
| % on SSRI | 27b | |
| % on Antidepressant | 15 | |
| % High school graduate | 41 | |
| Converted Subgroup (n=43) | 18.51 | |
| Baseline Mean Age (SD) | (3.79) | |
| % Male | 56 | |
| % on Antipsychotic | 10 | |
| % on SSRI | 21 | |
| % on Antidepressant | 9 | |
| % High school graduate | 40 | |
| Prodromal Subjects with no 6-month Follow-up (n=144) | ||
| Baseline Mean Age (SD) | 17.87 (4.5) | |
| % Male | 68a | |
| % on Antipsychotic | 15 | |
| % on SSRI | 12b | |
| % on Antidepressant | 10 | |
| % High school graduate | 32 |
Chi2 = 5.00, p=.03 significantly more males in group without 6-month follow-up
Chi2 = 5.43, p=.02 significantly fewer on SSRIs in group without 6-month follow-up
This sample is a subgroup from the NAPLS project described above. Although originally independent studies, the NAPLS sites employed similar ascertainment and assessment methods, making it possible to form a standardized protocol for converting acquired data into a new scheme representing the common measures across sites, yielding a large longitudinal database (Addington et al., 2007; Cannon et al., 2008).
The study protocols and informed consent documents, including procedures for data pooling, were reviewed and approved by the IRBs of the eight study sites (Emory University, Harvard University, University of California Los Angeles [UCLA], University of California San Diego [UCSD], University of North Carolina Chapel Hill, University of Toronto, Yale University, and Zucker Hillside Hospital in New York). Study methods and details of NAPLs are described elsewhere in detail (Addington et al., 2007; Cannon et al., 2008).
The SIPS criteria were used at study entry (Miller et al., 2002), in addition to a general assessment instrument, usually with the Structured Clinical Interview for DSM-IV (SCID) (American Psychiatric Association, 2000; First et al., 2002). Procedures for establishing SIPS reliability are described previously (Cannon et al., 2008; Addington et al., 2008).
The dependent measures in the present analyses were scores from four SOPS symptom dimensions: positive (unusual thought content, delusional ideas, suspiciousness, persecutory ideas, grandiosity, perceptual abnormalities, hallucinations, conceptual disorganization), negative (social isolation and withdrawal, avolition, decreased expression of emotion, decreased experience of emotion, decreased ideational richness, deterioration in role functioning), disorganized (odd behavior or appearance, bizarre thinking, trouble with focus and attention, impairment in personal hygiene or social attentiveness), and general (sleep disturbance, motor disturbance, dysphoric mood, impaired tolerance of normal stress). Individual symptoms are rated from 0 to 6, with levels 0 to 2 (none, questionable, mild) reflective of normal to sub-prodromal functioning, levels 3 to 5 (moderate, moderately severe, severe) indicative of a prodromal state, and level 6 indicative of a fully psychotic state. The ratings are summed to derive a score for each of the four dimensions.
The SOPS criteria for a prodromal syndrome emphasize onset or worsening in the past 12 months of attenuated positive symptoms (APS) in one or more of the positive symptoms rated from 3 to 5. A subject may also qualify for a prodromal syndrome on the SIPS due to onset in the past 3 months of brief intermittent psychotic syndrome (BIPS), which entails positive symptoms of psychotic intensity, but below the threshold required for a DSM-IV psychotic diagnosis, or by manifesting a deterioration of 30% or greater on the General Assessment of Functioning scale in the past 12 months, plus having either a first-degree relative with a psychotic disorder or a diagnosis of schizotypal personality disorder. This syndrome is called the Genetic Risk and Deterioration Syndrome (GRD). The present sample includes those predominantly classified by the SIPs as having APS, with only 4 listed as BIPS and 3 as GRD.
The sample used in this paper are all 191 subjects from the NAPLS database who; 1) met the SIPS criteria for prodromal status, 2) had baseline and 6-month follow-up symptom ratings and data on medication, and 3) had not participated in any randomized clinical trials. When compared to the 144 prodromal subjects for whom 6-month follow-up symptom data were unavailable, the present sample did not differ in baseline age, racial composition, proportion on antipsychotics and other antidepressants, or ratings of negative, disorganized, and general symptoms. However, as shown in Table 1, compared to those who did not complete 6-month follow-up, the present sample contained fewer males (56% versus 68%) (Chi sq=5.00, p=.03), and a higher proportion on SSRIs (27% versus 12%) (Chi sq=11.26, p=.001).
2.2 Medication
Each NAPLS site provided data on all psychotropic medications coded by specific medication type at each assessment. Medications were prescribed by independent practitioners, were not a component of the studies, and prescribers were not made aware that the participants had been identified as being at risk for psychosis by the research teams. Also, some participants reported that they had received various forms of psychological treatment in the community.
Because treatment was not standardized across patients or sites, information on the dosing and duration of medication between follow-ups was not available for the majority of cases. For the sample in the present study, the most commonly prescribed psychotropic medications at baseline and follow-up assessment were atypical antipsychotics (n=48), SSRIs (n=71), and other antidepressants (n=40). Medications in each category are listed in Table 2. Of the present sample of 191, 7 subjects were from the previous report from the Hillside research group (Cornblatt et. al., 2007).
Table 2.
Medications by Class at Baseline and Follow-up*
| Category | Generic Name | Frequency |
|---|---|---|
| Atypical | ||
| Antipsychotics | Olanzapine | 10 |
| Quetiapine | 7 | |
| Risperidone | 24 | |
| Ziprasidone | 3 | |
| Aripiprazole | 3 | |
| Total | 47 | |
| SSRIs | Fluoxetine | 17 |
| Paroxetine | 16 | |
| Paroxetine CR | 1 | |
| Citalopram | 10 | |
| Fluvoxamine | 1 | |
| Escitalopram Oxalate | 7 | |
| Sertraline | 25 | |
| Total | 77 | |
| Other Antidepressants | Bupropion | 16 |
| Trazadone | 9 | |
| Nefazodone | 1 | |
| Mirtazapine | 5 | |
| Venlafaxine | 15 | |
| Venlafaxine XR | 1 | |
| Hypericum Perforatum | 1 | |
| Total | 48 |
Some patients were switched from one medication to another, within class, from baseline to follow-up.
For each medication type, subjects were classified into one of four categories, based on their status at baselines and follow-up; Group 1, off medication at both times, Group 2, on medication at baseline and off at follow-up, Group 3, off medication at baseline but on at follow-up, and Group 4, on medication at both times. Those in group 3, off at baseline and on at follow-up, were prescribed medication at some point during the 6-month interval between baseline and follow-up. (It is reasonable to assume that a substantial number received medication as a result of the symptoms that were present at baseline or emerged shortly thereafter.)
Using this categorization scheme, and the criterion of at least 10 subjects each group, there were insufficient numbers in group 2 (n=4 on medication at baseline but not follow-up) for two of the medications; atypical antipsychotics and other antidepressants. Thus, only three medication status groups were compared in the analyses of these two medication classes, whereas all subjects were included in the analysis of the SSRIs. Further, it should be noted that a minority of patients was on more than one medication simultaneously, with 16 on both an antipsychotic and SSRI, and 7 on both an antipsychotic and other antidepressant.
2.3 Statistical Analyses
The dependent measures in the analyses were the scores from four SOPS symptom dimensions.
Analyses were conducted using the General Linear Model (GLM) with time (baseline and follow-up) as the within subjects factor and SSRIs, other Antidepressants, and Antipsychotics as the between subjects factors. The main effects and all two-way interactions were tested. Because this is an unbalanced between-subjects model with missing cells, the Type IV sum-of-squares was used to calculate the sums of squares. The numbers of participants (and dfs) vary due to missing ratings on some symptom dimensions at one or both time points, however, all 191 subjects had complete data on positive symptoms..
3. Results
3.1 Demographic and Clinical Characteristics
Analyses conducted to test the relation of demographic factors with symptoms showed only one significant sex difference, with males scoring higher on negative symptoms at baseline, t(1,188)=3.59, p<.001, and follow-up, t(1,188)=2.55, p<.05.
Mean symptom ratings by medication status are listed in Table 3. GLM analysis of positive symptoms yielded a significant main effect for Time (F(1,164)=70.36, p<.001), reflecting the reduction across groups in rated severity of positive symptoms over time, independent of medication (see Figure 1). In addition, there is a significant Time X Antipsychotic interaction (F(2,182)=3.03, p<.04). No other main or interaction effects were significant.
Table 3.
Mean Symptom Dimension Severity Ratings
| Baseline Symptom Ratings | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Antipsychotics |
SSRIs* |
Other Antidepressants (OD) |
|||||||
| Symptom Dimension | No Antipsychotic(n = 143) Mean (SD) | Follow-up only (n = 31 Mean (SD)) | Baseline & Follow-up (n = 13) Mean (SD) | No SSRI (n = 116) Mean (SD) | Follow-up only (n = 20) Mean (SD) | Baseline & Follow-up (n = 40) Mean (SD) | No OD (n = 151) Mean (SD) | Follow-up only (n = 12) Mean (SD) | Baseline & Follow up (n = 24) Mean (SD) |
| Positive | a 11.91 (4.04) | 12.81 (3.51) | a 14.42 (5.00) | 12.74 (4.34) | 12.30 (4.65) | 11.15 (2.90) | 12.30 (4.28) | 10.94 (3.65) | 11.81 (2.94) |
| Negative | 10.75 (7.11) | 14.83 (6.14) | 14.45 (8.26) | 11.65 (7.57) | 12.15 (6.45) | 11.47 (6.84) | 11.73 (7.26) | 16.07 (6.68) | 11.08 (7.51) |
| Disorganized | ab 5.63 (3.54) | a 7.90 (3.81) | b 9.28 (3.96) | 6.49 (3.74) | 6.42 (4.13) | 5.84 (3.84) | 6.23 (3.72) | 7.21 (4.64) | 5.44 (3.78) |
| General | 7.47 (4.00) | 10.74 (4.13) | 8.72 (4.75) | 7.32 (4.16) | 10.15 (4.25) | 9.05 (4.23) | 7.65 (4.26) | 9.59 (3.48) | 9.42 (4.06) |
| Follow-up Symptom Ratings (Corrected for covariates) | |||||||||
| Antipsychotics |
SSRIs |
Other Antidepressants |
|||||||
| Symptom Dimension | No Antipsychotic(n = 143) Mean (SD) | Follow-up only(n = 31) Mean (SD) | Baseline & Follow-up (n = 13) Mean (SD) | No SSRI (n = 116) Mean (SD) | Follow-up only (n = 20) Mean (SD) | Baseline & Follow-up (n = 40) Mean (SD) | No OD (n = 151) Mean (SD) | Follow-up only (n = 12) Mean (SD) | Baseline & Follow-up (n = 24) Mean (SD) |
| Positive | 7.20 (5.17) | 6.33 4.69) | 6.75 (5.51) | 7.31 (5.36) | 6.65 (5.05) | 6.58 (4.75) | 7.20 (5.20) | a 9.12 (5.93) | a 5.60 (3.32) |
| Negative | 7.82 (7.67) | 9.55 (7.38) | 11.39 (7.38) | 8.94 (7.75) | 6.53 (6.64) | 7.66 (7.08) | 8.34 (7.46) | 13.77 (9.94) | 6.38 (6.05) |
| Disorganized | 3.99 (3.71) | 4.64 (2.95) | 5.03 (4.20) | 4.19 (3.79) | 4.39 (3.31) | 4.17 (3.27) | 4.19 (3.71) | 5.95 (4.09) | 3.09 (2.57) |
| General | 4.80 (4.50) | 6.85 (4.32) | 4.01 (4.96) | 4.85 (4.42) | 6.60 (3.71) | 5.48 (5.31) | 4.89 (4.44) | 8.13 (6.18) | 5.24 (3.99) |
denotes pairs of means significantly different at p<.05
11 subjects on SSRIs at baseline but not follow-up are not included in this table
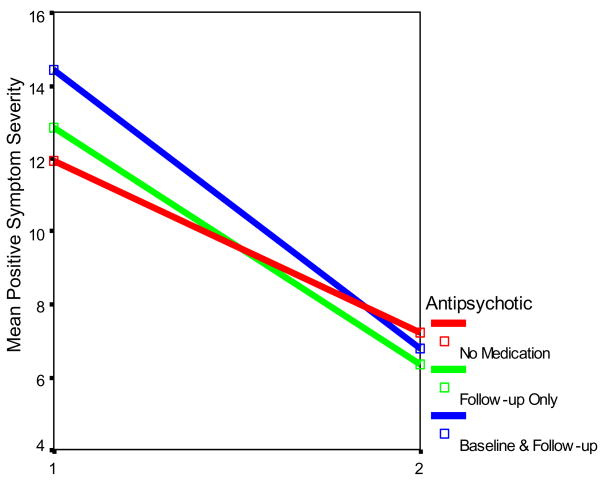
Figure 1.
Total Positive Symptom scores by Antipsychotic Medication Status and Time
This significant interaction is illustrated in Figure 1, which shows that the decline in positive symptom severity (i e., sum of positive symptom ratings) was greatest for the group on antipsychotic medication at both baseline and follow-up. Those not on an antipsychotic at either assessment showed the least decline, and those on an antipsychotic only at follow-up manifested a decline that was intermediate between the other two groups.
Comparisons of positive symptoms scores as a function of antipsychotic medication showed that the prodromal patients positive symptoms at baseline differed as a function of antipsychotic medication status (F(2,186)=3.71, p<.02), with pairwise comparisons showing that those who were on antipsychotic medication at baseline manifested higher positive symptom severity ratings than those never on medication (F(1,152)=3.71, p<.04). Although, there was no significant medication group difference in the severity of positive symptoms at follow-up, as illustrated in Figure 1, the medicated groups shifted their positions relative to the nonmedicated group, scoring below them at 6 months.
The GLM analysis of disorganized symptoms yielded a significant main effect for Time (F(1,150)=36.41, p<.001), again reflecting the trend for a reduction in the rated severity of symptoms over time across groups. In addition, there was a significant Time X Antipsychotic interaction (F(2,150)=3.87, p=.01). This is illustrated in Figure 2, which shows that the decline in total disorganized symptom severity was greatest for the groups that were on antipsychotic medication at baseline and/or follow-up, when compared to those not receiving antipsychotics at either time point. No other main or interaction effects were significant.
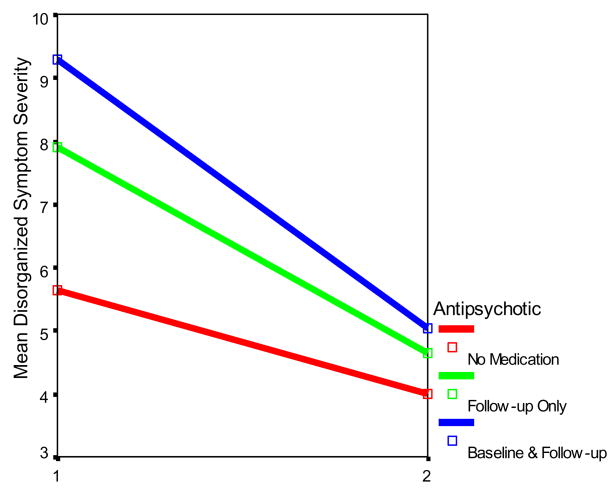
Figure 2.
Total Disorganized Symptom scores by Antipsychotic Medication Status and Time
Further analyses to explore the Time X Antipsychotic Medication interaction showed that the prodromal patients disorganized symptom ratings at baseline differed as a function of antipsychotic medication (F(2,181)=7.74, p=.001). Those on antipsychotic medication at baseline and follow-up (F(1,151)=11.38, p<.01), or follow-up only (F(1,169)=6.92, p<.01), manifested more severe disorganized symptom severity ratings than those never on medication. However, there was no trend toward a medication group difference in disorganized symptoms at follow-up.
The GLM analysis of negative symptom scores (F(1,154)=30.66, p<.001) and general symptoms (F(1,170)=26.43, p<.001) revealed a significant Time main effect, indicating a decline in symptoms for all groups from baseline to follow-up. There was no interaction of time with medication group, however, indicating that the declines in negative and disorganized symptoms did not vary as a function of psychotropics.
3.2 Regression Analyses Controlling for Baseline Symptoms
Because there were differences in baseline positive and disorganized symptom severity as a function of antipsychotic medication, with those on medication scoring higher, the greater symptom decline observed in patients on antipsychotics might be attributed to regression toward the mean. We therefore conducted a more stringent test of the longitudinal change, using hierarchical regression analysis in which baseline symptom severity was controlled. In these analyses, baseline symptom severity was entered first in the equation, then medication status for antipsychotics in the second block. The corresponding measure of symptom severity at follow-up was the dependent variable.
For positive symptoms, the regression analyses revealed a significant increment in R2 change when antipsychotic medication status was entered on the second block (R2 (1,183) = .18, F= 13.30, p<.01; R2 change = .04, F change = 3.51, p<.05), and the t-test for the significance of the individual predictors was significant for antipsychotic medication (Beta = .18, t=1.87, p<.05). Comparisons of the residual follow-up positive symptom scores (standardized) showed that patients on an antipsychotic at one or both time periods had lower residual follow-up scores than those not on an antipsychotic, t(1,189)=1.70, p<.05. Thus, antipsychotic medication was associated with lower positive symptom severity at follow-up, when baseline positive symptom severity was controlled. In other words, the regression findings indicate that the patients on antipsychotic medication had lower positive symptom scores (residuals) at follow-up than would have been predicted based on their higher baseline symptom levels.
The same regression analysis conducted on the disorganized symptom ratings revealed no significant effect of antipsychotic medication. Thus, when controlling for baseline disorganized symptoms, antipsychotic medication was not significantly related with follow-up symptom severity.
3.3 Conversion in Relation to Antipsychotic Medication
One of the first published papers from the NAPLs study utilized the entire sample and found no significant association between baseline medication status and conversion to psychosis based on meeting DSM IV criteria for an Axis I psychotic disorder (Cannon et al., 2008), however, many had not yet completed the 2-year follow-up. Of the sample included in this study, only 69 had 2-year follow-up data on psychiatric status. (The other subjects in the present sample were either not yet followed to two years, or were lost to 2-year follow-up.) Subjects were classified as converted (n=43) if they met DSM IV criteria for psychotic disorder between the 6-month follow-up and 2-year follow-up period. A logistic regression analyses was conducted to determine whether the three classes of medication were linked with conversion to psychosis. There was no significant association between any of the medications and conversion. These results must be interpreted with caution, however, given the relatively small number of patients for whom data on psychiatric outcome were available. Moreover, as suggested by Kloesterkoetter et al., (2001), conversions to psychosis would be expected to continue well beyond the modal 2 years follow-up period for NAPLs subjects.
4. Discussion
The present study utilized a database of prodromal patients to examine the relation of symptom progression with atypical antipsychotics, SSRIs and other antidepressant medications. The findings are generally consistent with reports from previous controlled studies of the effects of antipsychotics on prodromal symptoms.
It is important to note that, independent of medication, we find a generalized trend toward a reduction in symptom severity ratings within the 6-month period between baseline and follow-up. This converges with previous reports on the progression of prodromal symptoms, and suggests that some youth may show an acute high level of prodromal symptoms that is transient and declines over a 6-month period, in the absence of medication (McGlashan et al., 2006; McGorry et al., 2002).
It does appear, however, that the administration of atypical antipsychotics is associated with a greater decline in positive symptoms. As shown in Figures 1, those receiving an antipsychotic at baseline and/or subsequently, score higher on positive symptoms at baseline. This undoubtedly reflects the influence of baseline symptom severity on the likelihood that the patient will receive an antipsychotic prescription. At follow-up, however, positive symptom severity of the medicated groups is lower than for the nonmedicated group. Thus, the rate of decline in positive and disorganized symptoms severity was greater for those who received antipsychotic medication. Further, when baseline positive symptoms were controlled in regression analyses, the results suggest that the medicated patients had lower positive symptom ratings than would have been predicted by their elevated baseline scores. In contrast, we found no significant relation between antipsychotic status and progression of negative or general symptom ratings. This mirrors previous reports of a more pronounced effect of antipsychotics on positive prodromal symptoms (McGlashan et al., 2006; Cannon et al., 2002).
In contrast to antipsychotics, neither SSRIs or other antidepressants were significantly associated with a differential decline in symptoms. The absence of an antidepressant effect for prodromal individuals is contrasts with the earlier report of Cornblatt et al. (2007), following a younger sample for a considerably longer time period. It is possible that, in the current sample, subjects had already been treated with anti-depressants for some time (information on duration is not available), and that symptoms are already at least partially under control. This is suggested by the observation that in several instances subjects treated with antidepressants, especially those in the baseline/follow-up group, tended to display lower symptom severity at baseline and thus had less room for improvement with follow-up. Also, Cornblatt et al. (2007) note that anti-depressants have the advantage of being well tolerated by teenagers, as opposed to anti-psychotics which were associated with high rates of non-adherence.
Several limitations of the present study should be taken into consideration when interpreting the findings. First, this is a naturalistic study, and this is reflected in the fact that those on antipsychotics at baseline and/or subsequently have higher positive and disorganized symptoms at baseline. Second, data on dosage, compliance and adverse events were not available for the majority of the patients, so it cannot be established that those recorded as being on medication received a therapeutic dose. Third, as noted, it is possible that those on both SSRIs and other antidepressants had been on these medications for a longer period of time than those on antipsychotics. Thus, a dampening effect of antidepressants on symptoms may have occurred prior to the baseline and was, therefore, not measured in the present study. Finally, because it is difficult to obtain reliable retrospective data on the duration of symptoms and the nature of past pharmacologic and psychological treatments (e g., dose, duration and compliance), past history of treatment and symptom duration were not examined in this study.
Nonetheless, the present findings are consistent with reports that atypical antipsychotics may be effective in reducing prodromal symptoms, especially positive symptoms. However, although sufficient follow-up data were only available for a subgroup, like previous studies of antipsychotic medication (e g., Cannon et al., 2008), there is no evidence that the likelihood of conversion is changed. The findings highlight the importance of future clinical trials aimed at testing the efficacy of antipsychotics and other treatments for reducing the severity of the prodromal syndrome, and potentially delaying, preventing, or reducing the severity of psychosis. At the same time, previous work in this area also indicates the importance of monitoring side effects in at-risk populations. As mentioned, previous reports have noted adverse side effects of antipsychotics for prodromal subjects (e g., McGlashan, et al., 2006). Given the lack of an evidence base on preventive effects of psychiatric medications, a cautious approach is needed with a cost/benefit analysis to determine whether treating prodromal symptoms with any psychotropic is in the best interests of patients.
Acknowledgments
Role of Funding Source
Funding/Support: This study was supported by investigator-initiated grants from the NIMH and a gift from the Staglin Music Festival for Mental Health.
Footnotes
Contributors
All authors contributed to and have approved the final manuscript.
Conflict of Interest
Dr. Addington reports that she currently or in the past 12 months has received investigator-initiated research funding support from multiple not-for-profit entities including the National Institute for Mental Health. Dr. Cadenhead reports that she currently or in the past 12 months has received investigator-initiated research funding support from the National Institute for Mental Health. Dr. Cannon reports that he currently or in the past 12 months has received investigator-initiated research funding support from multiple not-for-profit entities including the National Institute for Mental Health, the National Alliance for Research on Schizophrenia and Depression, and the Staglin Music Festival for Mental Health. Dr Cannon reports that he has served as a consultant for Janssen Pharmaceuticals and Eli Lilly. Dr. Cornblatt reports that she currently or in the past 12 months has received investigator-initiated research funding support from not-for-profit entities including the National Institute for Mental Health and the Stanley Medical Research Institute. She has also served as a consultant for Lilly, Bristol-Meyers Squibb and Janssen Pharmaceuticals and has received unrestricted educational grants from Janssen. Dr. McGlashan reports that he currently or in the past 12 months he has received investigator-initiated research funding support from the National Institute of Mental Health, the Personality Disorder Research Foundation, and Eli Lilly Company. Dr. McGlashan reports that he has served as a consultant for Lilly, Pfizer, Solvay/Wyeth, and Roche pharmaceuticals. In the past 12 months Dr. Perkins reports having received research funding from AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Otsuka Pharmaceutical Co. Ltd, Eli Lilly and Co., Janssen Pharmaceutica Products, and Pfizer Inc.; and consulting and educational fees from AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Eli Lilly and Co., Janssen Pharmaceuticals, GlaxoSmithKline, Forest Labs, Pfizer Inc and Shire. Dr. Seidman reports that he currently or in the past 12 months has received investigator-initiated research funding support from multiple not-for-profit entities including the National Institute for Mental Health, the National Institute of Aging, the Commonwealth of Massachusetts Department of Mental Health, and the National Association for Research in Schizophrenia and Depression and the Sidney R. Baer Foundation. Dr. Tsuang reports that he has no financial relationships with for-profit or non-profit entities. Dr. Walker reports that she currently or in the past 12 months has received investigator-initiated research funding support from not-for-profit entities including the National Institute for Mental Health, and the National Alliance for Research on Schizophrenia and Depression. She has not received funding from for-profit entities. Dr. Woods reports that he currently or in the past 12 months has received investigator-initiated research funding support from multiple not-for-profit entities including the National Institute for Mental Health and the Donaghue, Stanley, and NARSAD foundations. In addition, he has received investigator-initiated research funding support from multiple for-profit entities including UCB Pharma, and Bristol-Myers Squibb and has consulted to Otsuka and Schering-Plough. Dr. Woods reports that he has not served on speaker’s bureaus. Dr. Heinssen is an employee of the nonprofit National Institutes of Health. He reports having no financial relationships with for-profit entities.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington J, Cadenhead KS, Cannon TD, Cornblatt B, McGlashan TH, Perkins DO, et al. North American Prodrome Longitudinal Study: A collaborative multisite approach to prodromal schizophrenia research. Schizophr Bull. 2007;33(3):665–672. doi: 10.1093/schbul/sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: A multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Huttunen MO, Dahlstrom M, Larmo I, Rasanen P, Juriloo A. Antipsychotic drug treatment in the prodromal phase of schizophrenia. Am J Psychiatry. 2002;159(7):1230–1232. doi: 10.1176/appi.ajp.159.7.1230. [DOI] [PubMed] [Google Scholar]
- Cannon TD. Neurodevelopment and the transition from prodrome to schizophrenia: Research imperatives. Biological Psychiatry. 2008;64:737–738. doi: 10.1016/j.biopsych.2008.07.027. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Lencz T, Smith CW, Olsen R, Auther AM, Nakayama E, et al. Can antidepressants be used to treat the schizophrenia prodrome? Results of a prospective, naturalistic treatment study of adolescents. J Clin Psychiatry. 2007;68(4):546–547. doi: 10.4088/jcp.v68n0410. [DOI] [PubMed] [Google Scholar]
- French P, Shryane N, Bentall RP, Lewis SW, Morrison AP. Effects of cognitive therapy on the longitudinal development of psychotic experiences in people at high risk of developing psychosis. British Journal of Psychiatry - Supplementum. 2007;51:s82–7. doi: 10.1192/bjp.191.51.s82. [DOI] [PubMed] [Google Scholar]
- Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Archives of General Psychiatry. 2001;58(2):158–64. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller T, Woods SW, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. American Journal of Psychiatry. 2006;163(5):790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Yung AR, Phillips LJ, Yuen HP, Francey S, Cosgrave EM, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59(10):921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: Preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159(5):863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Morrison AP, French P, Parker S, Roberts M, Stevens H, Bentall RP, Lewis SW. Three-year follow-up of a randomized controlled trial of cognitive therapy for the prevention of psychosis in people at ultrahigh risk. Schizophrenia Bulletin. 2007;33(3):682–7. doi: 10.1093/schbul/sbl042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LJ, McGorry PD, Yuen HP, Ward J, Donovan K, Kelly D, Francey SM, Yung AR. Medium term follow-up of a randomized controlled trial of interventions for young people at ultra high risk of psychosis. Schizophrenia Research. 2007;96(1–3):25–33. doi: 10.1016/j.schres.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Ruhrmann S, Bechdolf A, Kuhn KU, Wagner M, Schultze-Lutter F, Janssen B, Maurer K, Hafner H, Gaebel W, Moller HJ, Maier W, Klosterkotter J. Acute effects of treatment for prodromal symptoms for people putatively in a late initial prodromal state of psychosis. LIPS study group. British Journal of Psychiatry - Supplementum. 2007;51:s88–95. doi: 10.1192/bjp.191.51.s88. [DOI] [PubMed] [Google Scholar]
- Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, van Erp TGM, Thompson PM, Toga AW, Cannon TD, Pantelis C. Brain structural change during the development of psychosis: A longitudinal MRI study. Schizophrenia Research. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LE, Woods SW. The schizophrenia prodrome: A developmentally informed review and update for psychopharmacologic treatment. Child Adolesc Psychiatr Clin N Am. 2006;15(1):109–133. doi: 10.1016/j.chc.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Walker E. Adolescent neurodevelopment and psychopathology. Current Directions in Psychological Science. 2002;11:24–28. [Google Scholar]
- Woods SW, Brier A, Zipursky RB, Perkins DO, Addington J, Miller TJ, et al. Randomized Trial of Olanzapine versus Placebo in the Symptomatic Acute Treatment of the Schizophrenic Prodrome. Biol Psychiatry. 2003;54(4):453–464. doi: 10.1016/s0006-3223(03)00321-4. [DOI] [PubMed] [Google Scholar]
- Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: Past and current conceptualizations. Schizophr Bull. 1996;22(2):353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- Yung AR, Nelson B, Stanford C, Simmons MB, Cosgrave EM, Killackey E, Phillips LJ, Bechdolf A, Buckby J, McGorry PD. Validation of "prodromal" criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophrenia Research. 2008;105(1–3):10–7. doi: 10.1016/j.schres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Yuen HP, Francey SM, McFarlane CA, Hallgren M, et al. Psychosis prediction: 12-month follow up of a high-risk ("prodromal") group. Schizophr Res. 2003;60(1):21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]