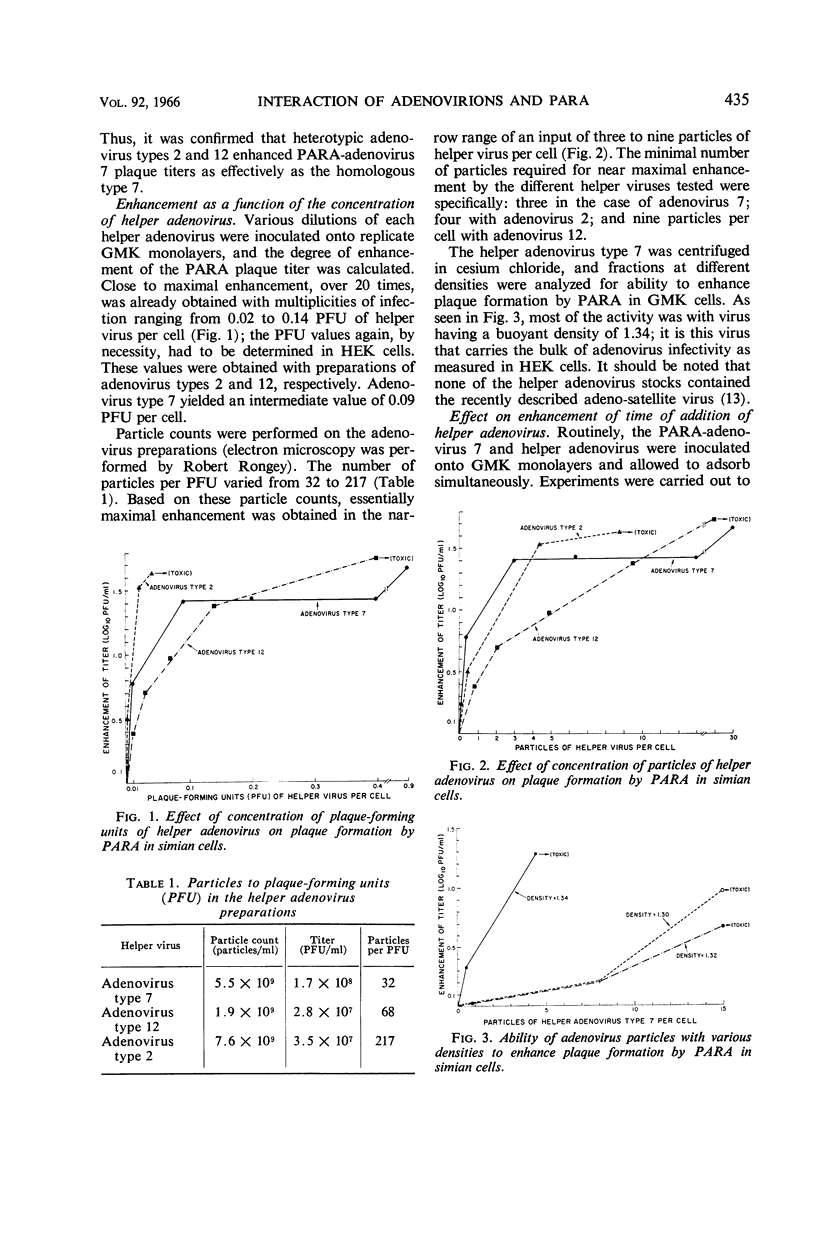
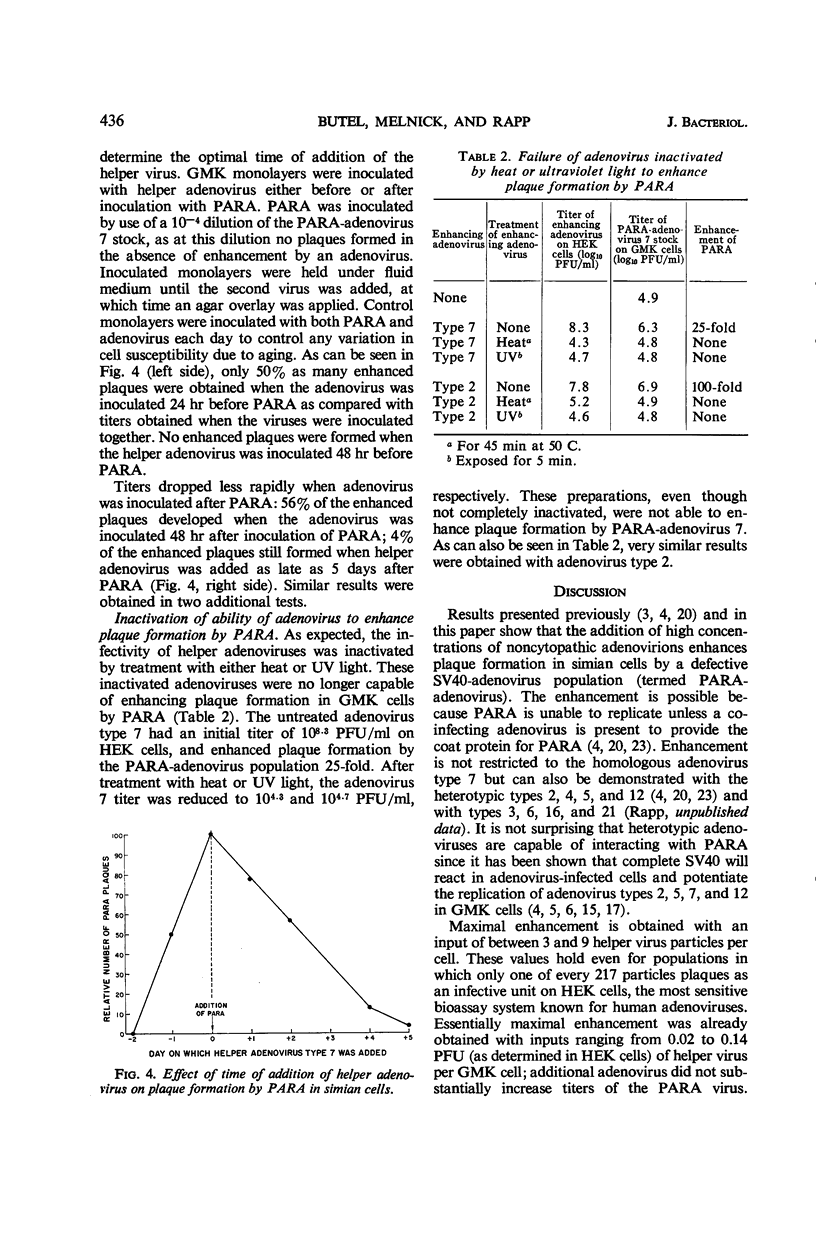
Abstract
Butel, Janet S. (Baylor University College of Medicine, Houston, Tex.), Joseph L. Melnick, and Fred Rapp. Detection of biologically active adenovirions unable to plaque in human cells. J. Bacteriol. 92:433–438. 1966.—Plaque formation in green monkey kidney (GMK) cells by a defective simian virus 40-adenovirus 7 “hybrid” population (PARA-adenovirus 7) was enhanced by the addition of excess adenovirions. Adenovirus types 2, 7, and 12 were capable of providing enhancement, although none of these viruses gives rise to plaques in simian cells in the absence of PARA (particle aiding replication of adenovirus). Near maximal enhancement of the PARA plaque titer on simian cells was obtained with input multiplicities ranging from 0.02 to 0.14 plaque-forming units (PFU) of helper adenovirus per GMK cell. The PFU of helper adenoviruses tested (types 2, 7, and 12) were measured in the most sensitive assay system, human kidney cells. This input corresponded to three to nine helper virus particles per GMK cell. The majority of particles capable of enhancing plaque formation by PARA banded at a density of 1.34 in CsCl. Adenoviruses inactivated by heat or ultraviolet light were not capable of enhancing plaque formation by PARA. Highest titers were obtained when PARA and helper adenovirus were inoculated simultaneously. Inoculation of the helper adenovirus 24 hr prior to the inoculation of PARA resulted in the formation of only 50% as many plaques, and no enhanced plaques developed when the adenovirus preceded PARA by 48 hr. Conversely, the addition of adenovirus 48 hr after the inoculation of PARA initiated 56% as many plaques as simultaneous inoculation; 4% of the enhanced plaques still formed when helper virus was added as late as 5 days after inoculation of PARA. These results suggest that adenovirus particles unable to plaque on human or monkey kidney cells are nevertheless capable of interacting with PARA in simian cells, thereby facilitating replication of both particles.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK P. H., ROWE W. P., TURNER H. C., HUEBNER R. J. A SPECIFIC COMPLEMENT-FIXING ANTIGEN PRESENT IN SV40 TUMOR AND TRANSFORMED CELLS. Proc Natl Acad Sci U S A. 1963 Dec;50:1148–1156. doi: 10.1073/pnas.50.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOEYE A., MELNICK J. L., RAPP F. ADENOVIRUS-SV40 "HYBRIDS": PLAQUE PURIFICATION INTO LINES IN WHICH THE DETERMINANT FOR THE SV40 TUMOR ANTIGEN IS LOST OR RETAINED. Virology. 1965 Jul;26:511–512. doi: 10.1016/0042-6822(65)90016-4. [DOI] [PubMed] [Google Scholar]
- Boeyé A., Melnick J. L., Rapp F. SV40-adenovirus "hybrids": presence of two genotypes and the requirement of their complementation for viral replication. Virology. 1966 Jan;28(1):56–70. doi: 10.1016/0042-6822(66)90306-0. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Rapp F. Replication in simian cells of defective viruses in an SV40-adenovirus "hybrid" population. J Bacteriol. 1966 Jan;91(1):278–284. doi: 10.1128/jb.91.1.278-284.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENNER F., SAMBROOK J. F. THE GENETICS OF ANIMAL VIRUSES. Annu Rev Microbiol. 1964;18:47–94. doi: 10.1146/annurev.mi.18.100164.000403. [DOI] [PubMed] [Google Scholar]
- Feldman L. A., Butel J. S., Rapp F. Interaction of a simian papovavirus and adenoviruses. I. Induction of adenovirus tumor antigen during abortive infection of simian cells. J Bacteriol. 1966 Feb;91(2):813–818. doi: 10.1128/jb.91.2.813-818.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L. A., Melnick J. L., Rapp F. Influence of SV40 Genome on the Replication of an Adenovirus-SV40 "Hybrid" Population. J Bacteriol. 1965 Sep;90(3):778–782. doi: 10.1128/jb.90.3.778-782.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILDEN R. V., CARP R. I., TAGUCHI F., DEFEND V. THE NATURE AND LOCALIZATION OF THE SV 40-INDUCED COMPLEMENT-FIXING ANTIGEN. Proc Natl Acad Sci U S A. 1965 Mar;53:684–692. doi: 10.1073/pnas.53.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANOFF A. Studies on mixed infection with Newcastle disease virus. III. Activation of nonplaque-forming virus by plaque-forming virus. Virology. 1961 May;14:143–144. doi: 10.1016/0042-6822(61)90142-8. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROWE W. P., BLACK P. H., HUEBNER R. J. PRODUCTION OF "TUMOR-SPECIFIC" ANTIGENS BY ONCOGENIC VIRUSES DURING ACUTE CYTOLYTIC INFECTIONS. Proc Natl Acad Sci U S A. 1965 Jan;53:12–19. doi: 10.1073/pnas.53.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUEBNER R. J., CHANOCK R. M., RUBIN B. A., CASEY M. J. INDUCTION BY ADENOVIRUS TYPE 7 OF TUMORS IN HAMSTERS HAVING THE ANTIGENIC CHARACTERISTICS OF SV40 VIRUS. Proc Natl Acad Sci U S A. 1964 Dec;52:1333–1340. doi: 10.1073/pnas.52.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDINKO N., HIRST G. K. Mixed infection of HeLa cells with polioviruses types 1 and 2. Virology. 1961 Jun;14:207–219. doi: 10.1016/0042-6822(61)90196-9. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Mayor H. D., Smith K. O., Rapp F. Association of 20-Millimicron Particles with Adenoviruses. J Bacteriol. 1965 Jul;90(1):271–274. doi: 10.1128/jb.90.1.271-274.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'CONOR G. T., RABSON A. S., BEREZESKY I. K., PAUL F. J. MIXED INFECTION WITH SIMIAN VIRUS 40 AND ADENOVIRUS 12. J Natl Cancer Inst. 1963 Oct;31:903–917. [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABSON A. S., O'CONOR G. T., BEREZESKY I. K., PAUL F. J. ENHANCEMENT OF ADENOVIRUS GROWTH IN AFRICAN GREEN MONKEY KIDNEY CELL CULTURES BY SV40. Proc Soc Exp Biol Med. 1964 May;116:187–190. doi: 10.3181/00379727-116-29197. [DOI] [PubMed] [Google Scholar]
- RAPP F., BUTEL J. S., FELDMAN L. A., KITAHARA T., MELNICK J. L. DIFFERENTIAL EFFECTS OF INHIBITORS ON THE STEPS LEADING TO THE FORMATION OF SV40 TUMOR AND VIRUS ANTIGENS. J Exp Med. 1965 Jun 1;121:935–944. doi: 10.1084/jem.121.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., BUTEL J. S., MELNICK J. L. VIRUS-INDUCED INTRANUCLEAR ANTIGEN IN CELLS TRANSFORMED BY PAPOVAVIRUS SV40. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:1131–1135. doi: 10.3181/00379727-116-29472. [DOI] [PubMed] [Google Scholar]
- RAPP F., KITAHARA T., BUTEL J. S., MELNICK J. L. SYNTHESIS OF SV40 TUMOR ANTIGEN DURING REPLICATION OF SIMIAN PAPOVAVIRUS (SV40). Proc Natl Acad Sci U S A. 1964 Nov;52:1138–1142. doi: 10.1073/pnas.52.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., MELNICK J. L., BUTEL J. S., KITAHARA T. THE INCORPORATION OF SV40 MATERIAL INTO ADENOVIRUS 7 AS MEASURED BY INTRANUCLEAR SYNTHESIS OF SV40 TUMOR ANTIGEN. Proc Natl Acad Sci U S A. 1964 Dec;52:1348–1352. doi: 10.1073/pnas.52.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., BAUM S. G. EVIDENCE FOR A POSSIBLE GENETIC HYBRID BETWEEN ADENOVIRUS TYPE 7 AND SV40 VIRUSES. Proc Natl Acad Sci U S A. 1964 Dec;52:1340–1347. doi: 10.1073/pnas.52.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Butel J. S., Melnick J. L. SV40-adenovirus "hybrid" populations: transfer of SV40 determinants from one type of adenovirus to another. Proc Natl Acad Sci U S A. 1965 Sep;54(3):717–724. doi: 10.1073/pnas.54.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Bau S. G. Studies of adenovirus SV40 hybrid viruses. II. Defectiveness of the hybrid particles. J Exp Med. 1965 Nov 1;122(5):955–966. doi: 10.1084/jem.122.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P. Studies of adenovirus-SV40 hybrid viruses. 3. Transfer of SV40 gene between adenovirus types. Proc Natl Acad Sci U S A. 1965 Sep;54(3):711–717. doi: 10.1073/pnas.54.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABIN A. B., KOCH M. A. SOURCE OF GENETIC INFORMATION FOR SPECIFIC COMPLEMENT-FIXING ANTIGENS IN SV40 VIRUS-INDUCED TUMORS. Proc Natl Acad Sci U S A. 1964 Nov;52:1131–1138. doi: 10.1073/pnas.52.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH K. O., MELNICK J. L. A method for staining virus particles and identifying their nucleic acid type in the electron microscope. Virology. 1962 Jul;17:480–490. doi: 10.1016/0042-6822(62)90143-5. [DOI] [PubMed] [Google Scholar]
- Wassermann F. E. The effect of UV-radiation on HeLa cells infected with adenovirus. Virology. 1965 Oct;27(2):193–198. doi: 10.1016/0042-6822(65)90159-5. [DOI] [PubMed] [Google Scholar]
- Wood H. A., Bancroft J. B. Activation of a plant virus by related incomplete nucleoprotein particles. Virology. 1965 Sep;27(1):94–102. doi: 10.1016/0042-6822(65)90146-7. [DOI] [PubMed] [Google Scholar]


