Abstract
Objective
To examine the opinions of obstetrician-gynecologists regarding hormone therapy (HT) and the results from the Women’s Health Initiative (WHI).
Design
Separate surveys were sent to two groups of practicing obstetrician-gynecologists: 1) respondents to a 2004–2005 survey (Follow-up #1), 2) members of the American College of Obstetricians and Gynecologists’ Collaborative Ambulatory Research Network (Follow-up #2 CARN). These studies complete a longitudinal study investigating obstetrician-gynecologists’ opinions of the evidence from WHI.
Results
Response rates were 64.5% and 58.8%, respectively. Responses from both surveys were generally consistent with the results from the 2004–2005 survey. A majority of physicians from both survey populations were skeptical of the combined HT results. Respondents were more likely to find the results of the unopposed estrogen trial convincing. Similar to the results from the 2004–2005 study CARN physicians generally disagreed with the decision to end the WHI trials. Unlike the 2004–2005 study there was no consistent effect of either age or year residency was completed on physician opinions. Similar to the 2004–2005 study physicians that considered alternative therapies viable treatment options were more likely to report they found the trial results convincing. The results from Follow-up #2 CARN indicate that physicians in the south were most likely to prescribe and physicians in the east were least likely, suggesting that unmeasured socio-cultural parameters might influence HT prescribing practice.
Conclusions
Obstetrician-gynecologists remain generally skeptical of the WHI results, though less so of the estrogen only trial. The early end to the trials may have contributed to their skepticism.
Introduction
The Women’s Health initiative (WHI) trial of combined estrogen and progestin hormone therapy (HT) was ended May 31, 2002 and the preliminary results were first published shortly afterward.1 The estrogen only arm of the WHI HT study was stopped in early 2004, and the first publication of its results was in April, 2004.2 The results of these large, randomized, placebo controlled trials showed no decreased risk from HT for cardiovascular disease. There was a significant increase in risk of stroke and a significant decrease in risk of fracture for both combined HT and estrogen alone. Combined estrogen and progestin had additional significant increases in the risks of breast cancer and blood clots, and a significant decrease in the risk of colon cancer. The overall mortality indices showed no significant difference between HT and placebo. These results were somewhat unexpected, based on many previous cohort and case-controlled studies that had indicated an overall benefit to HT users and especially a decreased risk of cardiovascular disease.3
This lack of concordance between epidemiological studies and the WHI randomized, placebo controlled trials has led to significant controversy regarding the use of HT for peri and postmenopausal women. Obstetrician-gynecologists in particular appear to be very skeptical of the WHI research findings.4,5
The Research Department of the American College of Obstetricians and Gynecologists (ACOG) undertook a series of survey studies of practicing Fellows of ACOG to examine their attitudes towards and clinical practice patterns regarding HT, and to ascertain their opinions regarding the results of the WHI studies. A preliminary study was conducted in November 2003,4 after publication of the combined HT results but before the results of the estrogen only trial were published. This study helped form the basis for a series of three survey studies undertaken between November 2004 and January 2007 designed to investigate changes in attitudes and clinical practice regarding HT among practicing obstetrician-gynecologists.
The preliminary study4 found that virtually all (97.4%) respondents were aware of the recent research findings from the WHI clinical trial on combined HT and that the trial was terminated early due to the preliminary results (98.4%). However, almost half of the respondents to the preliminary study (49.1%) did not find the reported research convincing, and almost half of the respondents (48.1%) disagreed with the decision to stop the trial. In both cases residency year was a significant factor, with those that had completed residency more recently being more likely to find the results convincing and to agree with the trial stoppage. Physicians self-rated confidence in their ability to interpret the scientific literature also affected their opinions regarding the WHI combined HT trial. Physicians that were confident or very confident of their ability to interpret the scientific literature were significantly more likely to be skeptical (P<.001). For example, among physicians that were very confident about interpreting the scientific literature, 55.7% responded that they did not find the results convincing, and 55.9% disagreed with the decision to end the trial.
Despite this apparent skepticism regarding the evidence from the WHI combined HT trial the publication of the data from this trial appears to have affected patient preferences and physician prescribing practices. Although just over half of respondents expected their prescribing practices for HT to remain the same in the near future, four of ten expected to prescribe HT to fewer patients.4 This is consistent with findings that, after publication of the WHI combined HT results there was an increased rate of patients discontinuing HT6,7 and that prescriptions for HT had been reduced by 32% over nine months.8
The first of the three survey studies derived from the preliminary survey results was undertaken between November 2004 and March 2005 and the results have been published.5 The 2004–2005 study found that many obstetrician-gynecologists remained skeptical of the combined HT results. Compared to the 2003 survey, men were more likely to be skeptical of the combined HT results (58.8% reported they found the evidence not convincing in 2004 versus 53.4% in 2003, P=.045) and women tended to be less skeptical (39.5% in 2004 versus 45.3% in 2003, p = .056). There was less skepticism about the estrogen-only trial, although four of ten respondents did not find the results convincing. Men were more skeptical than women; a majority of men disagreed with the decisions to stop either trial. In agreement with the preliminary study, physicians that completed their residency more recently were more likely to accept the results of the trials. Respondents reported a reduction in HT prescription practice relative to the year 2000, but 62.7% reported they did not expect their prescribing practices to change further in the near future. The proportion of respondents that considered alternate therapies to HT viable treatment options increased between 2003 (28.1%) and 2004 (37.1%, p < .001). There was strong support for the use of HT for vasomotor symptoms, vaginal dryness, and osteoporosis, but most physicians did not consider HT useful for cardiovascular disease or dementia.
In this paper we present the results from two follow-up studies that completed the research study. These follow-up surveys were conducted to track changes in the attitudes and clinical practice of obstetrician-gynecologists over time. The first follow-up study (Follow-up #1), conducted in late 2005, used a shortened version of the 2004–2005 survey, and was sent to the 1,029 physicians out of the 2,500 recipients of the original 2004–2005 survey who had returned a completed survey and were included in the published analysis.5 The final study in the series was conducted from November 2006 to January 2007 (Follow-up #2 CARN), used the shortened version of the 2004–2005 survey with some additional questions, and was sent to members of the Collaborative Ambulatory Research Network (CARN), practicing obstetrician-gynecologists who have agreed to participate in ACOG Research Department surveys.
Methods
All participants in these two follow-up studies are Fellows and Junior Fellows in Practice of the American College of Obstetricians and Gynecologists. A Fellow is a member who has a current medical license, is in medical practice focused on women’s health, and is board certified in obstetrics and gynecology. A Junior Fellow in Practice is a practicing member who meets all the requirements to be a Fellow of the College except that they are not board certified. ACOG Fellows and Junior Fellows in Practice comprise at least 90% of the practicing obstetricians and gynecologists in the United States. The characteristics of these studies and the basic demographics of the participants are compared in Table 1.
Table 1.
Study Characteristics and Demographics for 2004–2005 Study, Follow-up #1, and Follow-Up #2 CARN
| 2004–2005 Study | Follow-up #1 | Follow-Up #2 CARN | ||
|---|---|---|---|---|
| Survey | Survey | Letter | Survey | |
| Year Conducted | 2004 | 2005–2006 | 2006 | |
| Sample Size | 2,500 | 1029 | 365 | 486 |
| # of respondents | 1029 | 664 | 185 | 286 |
| 95% confidence interval for proportion data | 3.1% | 3.8% | 7.2% | 5.8% |
| Survey | 51 item survey | Survey from 2004–2005 Study shortened to 31 items | 2 questions from the 31-item survey | Survey from Follow-up #1 with additional questions regarding ACOG publications |
| Mailings | 3 survey mailings | 3 survey mailings | 1 letter mailing | 3 survey mailings |
| Sample Population | Random sample of ACOG Fellows | Eligible Respondents from 2004–2005 Study included in Power et al., 2007 (5) | Those who did not respond to the survey after 3 mailings. | Random sample of ACOG members who are part of the Collaborative Ambulatory Research Network (CARN) |
| % of Male | 54.3% | 54.6% | 54.7% | 51.0% |
| Mean age (years) | 47.4 ± .3 | 49.4 ± .4 | 48.6 ± .8 | 47.7 ± .6 |
| Median age (years) | 47 | 49 | 49 | 47.5 |
Follow-up #1
The subjects for this study were the 1,029 physicians that returned a completed 2004–2005 survey and were included in the published analysis.5 The first survey mailing was sent on September 16, 2005, with follow-up mailings October 12, 2005 and November 18, 2005. On January 20, 2006, a letter was sent to all subjects that had yet to return a completed survey. That letter asked them to return answers to two questions from the survey: “Do you find the reported research findings from the WHI trial of combined HT convincing?” and “Do you find the reported research findings from the WHI trial of unopposed estrogen convincing?” The purpose of the letter was two-fold: 1) to maximize the number of physicians whose answers to these two questions could be compared between the 2004–2005 study and Follow-up #1; and 2) to assess whether there was any participation bias that would be reflected in differing opinions on these two questions between those physicians that completed the survey and those that chose not to.
Follow-up #2 CARN
In November 2006, 486 obstetrician-gynecologists that were members of the Collaborative Ambulatory Research Network (CARN) were sent a survey that contained the same questions as in Follow-up #1 plus a few questins from the 2004–2005 survey that were excluded in Follow-up #1, and an additional short questionnaire concerning ACOG publications regarding HT. CARN is comprised of Fellows and Junior Fellows in Practice of ACOG who have agreed to complete four to five surveys throughout the year. The network was designed to increase response rates to survey studies and in turn facilitate the assessment of clinical practice patterns. CARN members are representative of the total ACOG population in male/female ratio, age and geographic location.9,10,11 Because none of the recipients of the 2004–2005 survey were CARN members there was no overlap between the samples from Follow-up #1 and Follow-up #2 CARN. Two follow-up mailings were sent, one in December and one in January, 2007.
Surveys
The surveys contained questions on physician demographics, self-evaluation of knowledge of issues relating to HT, clinical practice, and knowledge of and opinions regarding the WHI trials of both combined HT and estrogen-only HT. Questions for these surveys were selected as a subset of the survey conducted in 2004–2005. The wording and answer choices for the questions from the 2004–2005 survey were exactly the same on both the follow-up surveys and appeared in the same order. More detailed information about the 2004–2005 survey questions can be found elsewhere.5 The Follow-up #2 CARN contained additional questions concerning ACOG publications regarding HT.
Data Analysis
For both surveys, responses were entered into a personal computer-based software package (SPSS 15.0, SPSS, Inc, Chicago, IL) data file for analysis. The data were screened for highly implausible answers and for obvious coding errors. Any identified errors were corrected by reviewing the original survey. Differences between respondents and non respondents were examined based on sex, birth year, and geographic location.
Respondents to Follow-up #1 were divided into three approximately equal subgroups based on the year they completed residency: before 1985, 1985 – 1994, and after 1994. These categories were used in the published analysis of the 2004–2005 survey.5 The data on year of residency completion came from the 2004–2005 survey; the question was omitted from the shortened survey. Age and year residency was highly correlated. Accordingly, for Follow-up #2 CARN using CARN members, age was used as the grouping variable (born before 1952, born 1953–1964, or born 1965 or later.) The respondents to the Follow-up #2 CARN study were also grouped by geographic region, as defined in Table 2. Finally, respondents to the Follow-up #2 CARN study were also characterized as confident if they responded that they were confident or very confident to all three of the following questions: their ability to counsel patients concerning hormone therapy, their ability to understand and interpret scientific knowledge concerning hormone therapy, and their ability to counseling patients concerning alternatives to hormone therapy.
Table 2.
Geographic Regions were as follows (number of respondents in parentheses):
| South (n = 95): Tennessee, Alabama, Louisiana, Arkansas, Oklahoma, Texas, Mississippi, West Virginia, Virginia, North Carolina, South Carolina, Georgia, Florida, Puerto Rico |
| Mid-West (n = 29): Kansas, Missouri, North Dakota, South Dakota, Nebraska, New Mexico, Arizona, Utah, Colorado, Wyoming, Nevada, Idaho, Montana, Alberta, and Saskatchewan |
| West (n = 43): Washington, Oregon, California, Hawaii, Alaska, British Columbia |
| Mid-East (n = 54): Minnesota, Wisconsin, Iowa, Illinois, Kentucky, Ohio, Indiana, Michigan, Ontario, Manitoba |
| East (n = 65): District of Columbia, Maryland, Delaware, Pennsylvania, New Jersey, New York, Connecticut, Rhode Island, Massachusetts, New Hampshire, Vermont, Maine, Nova Scotia, New Brunswick, Quebec |
Values for continuous variable are reported as means ± SEM. The 95% confidence intervals for proportion data for each study were calculated based on the number of returned surveys (Table 1). Categorical variables were analyzed using chi-square tests; continues variables were analyzed by analysis of variance (ANOVA). Exact P values are reported.
Results
Follow-up #1
There were 664 returned surveys out of 1,029 mailed (64.5%). There were 185 letters returned from the 365 Fellows that did not return a survey (50.7%). Thus there are approximately 800 obstetrician-gynecologists (not every survey responder answered both questions) whose opinions regarding the evidence from the WHI combined HT and estrogen only trials can be directly compared between the 2004–2005 study and Follow-up #1. There was no difference in the rates at which men and women responded by returning either the survey or the letter. There was no difference in age and years since completion of residency between physicians that returned the letter versus the survey, however, physicians that did not respond (i.e. returned neither the letter nor the survey) were younger (median of 43 years versus 49 years, P=.001) and had fewer years of practice (median of 11 years versus 16 years, P<.001). Among survey responders the pattern of responses to the questions did not vary between mailings.
Men and women obstetrician-gynecologists appear to disagree about how convincing they found the results from the WHI trials of HT based on data from both the survey and the letter (Table 3). Men were significantly more likely to find the evidence unconvincing. Women respondents to the letter did not differ from women respondents to the survey on these two questions (P=.152 and P=.320, respectively); however, men respondents to the letter were significantly more likely than men respondents to the survey to find the evidence unconvincing (P=.036 and P=.006, for combined HT and estrogen only trials, respectively).
Table 3.
Comparison of men and women among respondents to the survey and the letter.
| Respondents to the survey (95% CI ± 3.8%) | |||
| Did you find the results of the WHI combined HT trial convincing? | |||
| Men N = 359 | Women N = 298 | P value | |
| Yes | 27.2% | 38.8% | .001 |
| No | 54.2% | 39.8% | |
| Not sure | 18.5% | 21.4% | |
| Did you find the results of the WHI estrogen only trial convincing? | |||
| Yes | 38.4% | 50.2% | .002 |
| No | 38.4% | 24.4% | |
| Not sure | 20.5% | 22.3% | |
| Not aware | 2.8% | 3.1% | |
| Respondents to the letter (95% CI ± 7.2%) | |||
| Did you find the results of the WHI combined HT trial convincing? | |||
| Men N = 108 | Women N = 77 | P value | |
| Yes | 19.2% | 46.3% | <.001 |
| No | 68.7% | 41.5% | |
| Not sure | 12.1% | 12.2% | |
| Did you find the results of the WHI estrogen only trial convincing? | |||
| Yes | 24.2% | 58.3% | <.001 |
| No | 56.5% | 22.6% | |
| Not sure | 17.2% | 14.3% | |
| Not aware | 1.0% | 4.8% | |
A majority of responding obstetrician-gynecologists that finished their residency before 1985 were men (81.6%) and a majority that finished their residency after 1995 were women (69.6%); between 1985 and 1994 the proportions were equal (49.8% men and 50.2% women). There was a significant negative correlation between the year residency was completed and physician skepticism for both trials (r = -.172 and r = -.142, respectively, P<.001). Physicians that had completed their residency more recently were less skeptical. After accounting for the year residency was completed, men and women did not differ; however, only women showed a significant pattern of decreasing skepticism with more recent completion of residency, and only of their opinion of the evidence from the combined HT trial (P=.019).
The responding physicians reported that they found the estrogen only trial results significantly more convincing that the combined HT results (P<.001). In regards to the combined HT trial, only one of five men and women who completed residency before 1985 found the results convincing, increasing to about one of three for residency years 1985–1994, and about four of ten after 1995 (Table 4). For the unopposed estrogen trial, about half of the women found the results convincing. One of three men who completed residency before 1995 found the unopposed estrogen results convincing, increasing to half of the men who completed residency in 1995 or later.
Table 4.
The proportion of respondents who found the evidence from the trials convincing by sex and year of residency completion. There was no statistical difference between men and women after accounting for year of residency completion. The respondents were significantly more likely to find the results of the estrogen only trial convincing (P<.001). 95% confidence interval is ± 3.8%.
| Completed residency before 1985 | Completed residency 1985 – 1994 | Completed residency 1995 or later | |
|---|---|---|---|
| Combined HT trial | |||
| Men | 21.9% | 31.7% | 36.4% |
| Women* | 22.0% | 34.7% | 46.3% |
| Estrogen only trial | |||
| Men | 34.3% | 38.4% | 50.0% |
| Women | 50.0% | 46.0% | 53.4% |
significant effect of year of residency completion (P=.019).
Both men and women were generally confident of their ability to counsel their patients (97.4% very confident or confident) and interpret the scientific literature (95.6% very confident or confident) in regards to HT. The more confident a physician was the more likely they were to have formed an opinion about the results of the trials, either positive or negative (P<.01). However, the patterns were the opposite for the combined HT and estrogen only trials; the most confident physicians were the least likely to believe the results of the combined HT trial (52.9% not convinced; P<.001), but the most likely to believe the results of the estrogen only trial (50.3% convinced; P<.001).
About three of four (72.9%) physicians responding to Follow-up #1 reported that they believed their prescribing practices would not change in the future. Almost none of the respondents reported that they would not prescribe HT. Compared with the results from the 2004–2005 study, fewer physicians would only prescribe HT if their patient requests it (11.4% in Follow-up #1 versus 23.9% for the 2004–2005 Study, P<.05).
The respondents reported that a majority of their patients appear to be more apprehensive about HT compared with six months previously and are asking for alternatives to HT (Table 5). The physicians reported they are spending more time counseling patients about HT than previously. There were no differences between men and women and no differences across year of residency for these questions about the physicians’ patients.
Table 5.
Follow-up #1 physicians’ opinions regarding patient behavior compared with six months prior to the survey. 95% confidence interval is ± 3.8%. Rows do not add to 100% because some physicians did not answer every question.
| Compared to six months ago: | TRUE | FALSE |
|---|---|---|
| More of my patients are asking for alternatives to HT | 63.4% | 31.0% |
| My patients generally appear more apprehensive about HT | 62.5% | 31.5% |
| I am spending more time counseling patients on HT | 59.8% | 34.8% |
| Fewer of my patients are requesting HT | 51.5% | 42.5% |
| More of my patients on HT are choosing to discontinue its use | 45.2% | 49.4% |
| More of my patients on HT are choosing to resume its use | 40.7% | 53.9% |
| More of my patients are requesting HT | 23.2% | 71.5% |
Patients are asking about alternatives to HT (Table 5) and the physicians appear to have a generally positive opinion of alternative therapies; very few consider them harmful and should not be prescribed (0.4%). Almost half of the responding physicians consider alternatives to HT to be either viable treatment options (26.5%) or that they probably do more good than harm (23.2%); 41% consider them at best a placebo. Women were more likely than men to answer that alternative therapies were viable treatment options (37.9% versus 18.9%; P<.001).
The responding physicians reported that HT was a viable treatment for menopausal symptoms (hot flashes 97.4%; vaginal atrophy 92.2%) and osteoporosis (73.6%). Few consider HT a viable treatment for dementia (5.4%) or cardiovascular disease (3.5%). About one of four (26.8%) did consider it a viable treatment for depression; physicians who had completed residency more recently were less likely to respond that HT was a viable treatment for depression.
2004–2005 Study and Follow-Up #1 Analyses
By combining the answers to the survey and the two-question letter in Follow-up #1 there are approximately 800 obstetrician-gynecologists (not every survey responder answered the questions) whose opinions regarding the evidence from the WHI combined HT and estrogen only trials can be directly compared between the 2004–2005 Study and Follow-up #1. Not surprisingly, the general pattern for opinion on both the combined HT and estrogen only trials was for there to be a decrease in the proportion of respondents who were unsure about the evidence. Opinion regarding the estrogen only trial was more variable between the two studies; 71% of responders gave the same answer in each survey regarding the combined HT trial compared to 56.9% who answered the same in both surveys regarding the estrogen only trial. Regarding the combined HT trial, there was a net 10% decease in the number of responders that were unsure whether they found the evidence convincing, a 10% increase in the number who answered yes, and a 2% decrease in the number who said no. The corresponding values for the estrogen only trial were a net 23% decrease in the number of physicians who were unsure about the evidence, a 6% increase in the number of physicians who answered yes, and a 13% increase in the number who answered no.
Follow-up #2 CARN
A total of 286 (150 male and 136 female) CARN members returned the survey for a response rate of 58.8% after the three mailings. There was no difference in the rate in which men (59.5%) and women (58.1%) returned the survey and the response rates for each region varied between 50% in the mid-west to 64.7% in the mid-east. The median age of responders was 47.5 years with men being significantly older than the women (53 years versus 41.5 years, p < .001). This age distribution was comparable to the values from 2004–2005 Study (overall median of 47 years; 52 years for men and 42 years for women). There was no significant difference between respondents and non-respondents by age, year of birth, or geographic region. Answers to the questions did not differ by mailing.
Most CARN obstetrician-gynecologists reported that the training they received during residency concerning hormone therapy was comprehensive or adequate (22.5% and 50.5% respectively). Most respondents reported HT has a positive effect on hot flashes (100%), vaginal dryness (99%), bone fractures (98.6%), sleep disturbance (92%), colon cancer (87%), quality of life (85%), overall wellbeing (78%), and sexual desire (72.3%). About half of respondents reported HT has a neither a positive nor negative effect on mental activity (52%), memory (50%), and depression (49%), and the majority of other respondents thought HT had positive effects on mental activity (33%), memory (33%), and depression (41%).
Virtually all (99%) the physicians were aware of the findings from the Women’s Health Initiative (WHI) concerning the risks and benefits of combined estrogen and progestin therapy. Similarly, nearly all (99%) of ob-gyns were aware that the WHI estrogen and progestin trial was stopped in 2002 due to the preliminary results. The majority of responding physicians (59.9%) did not find the reported research about the combined trials convincing; only 27.7% did find the reported research convincing, 12.1% were not sure and .03% were not aware of the findings. Physicians’ opinions about the results of the estrogen only trial were more split; 45.5% were convinced, 33.7% were not convinced, 17.0% were not sure, and 3.8% were not aware of the finding. In contrast to the results from the previous studies, no age group was any more or less convinced (when running separate analyses with males and females to control for gender). There were no significant gender differences (when controlling for age) in their awareness or opinions about the HT trail results.
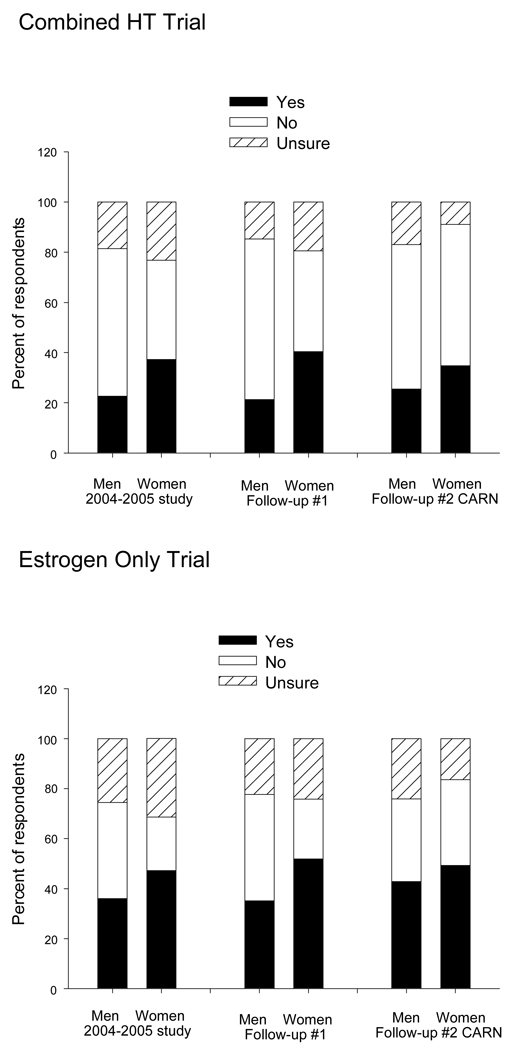
Compared to the 2004–2005 Study, fewer CARN physicians responded that they were unsure whether they found the results of either trial convincing. The percentage of CARN physicians that responded that they did not find the results of the combined HT trial convincing was higher than in previous surveys for both men and women (Figure 1); for the estrogen only trial the percentage that were not convinced was higher for women but lower for men, resulting in no significant difference between men and women (Figure 1).
Figure 1.
Responses to: “Do you find the evidence from the WHI trial convincing?” for 2004–2005 Study, Follow-up #1, and Follow-up #2 CARN.
Almost half of the CARN members (48.8%) did not agree with the decision to stop the combined trial (48.8%) or the estrogen only trial (45.0%). In all age categories a substantial proportion of physicians were critical of the decision to stop either trial; however, younger physicians were more likely to agree with the decision to stop the trials than were older physicians (combined HT, p = .030; estrogen only, P=.046). There was no difference in opinion between men and women.
The surveyed obstetrician-gynecologists were asked to rate the current state of knowledge of both HT and alternatives to HT. Those who reported believing that the current state of knowledge concerning hormone therapy was comprehensive tended to rate the current state of knowledge concerning alternatives to hormone therapy comprehensive as well ( r = .331, p < .001); however, more physicians rate knowledge of HT as either comprehensive (13.3%) or adequate (48.6%) compared to their collective opinion about knowledge about alternatives to HT (0.7% comprehensive and 22.9% adequate). When asked about the viability of alternatives to HT, 39.9% reported that alternatives to HT are viable treatment options, 20.6% reported they probably do more good than harm, 38.5% reported they are at best placebo, and 1% reported they are probably do more harm than good.
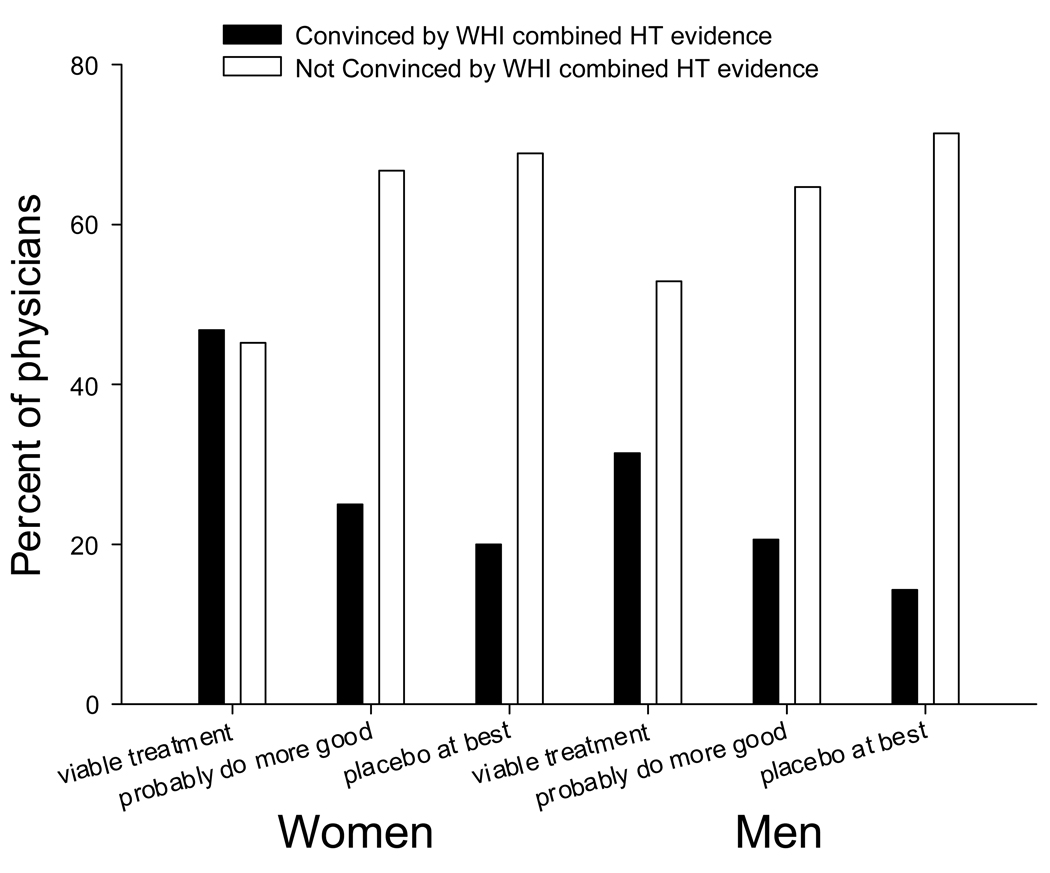
Opinions about the alternatives to HT treatments were not associated with whether obstetrician-gynecologists find the results of the combined trial convincing in males or females; however, if the analysis is restricted to only those physicians who had an opinion about the results of the trial (excluding those who answered not sure), physicians who viewed alternatives as viable treatment options were more likely to find the results of the trial convincing, though in men this was only a trend (women p=.019; men p=.068; Figure 2).
Figure 2.
Physicians who responded that alternative therapies to HT are viable treatment options were more likely to respond that they found the WHI combined HT evidence convincing.
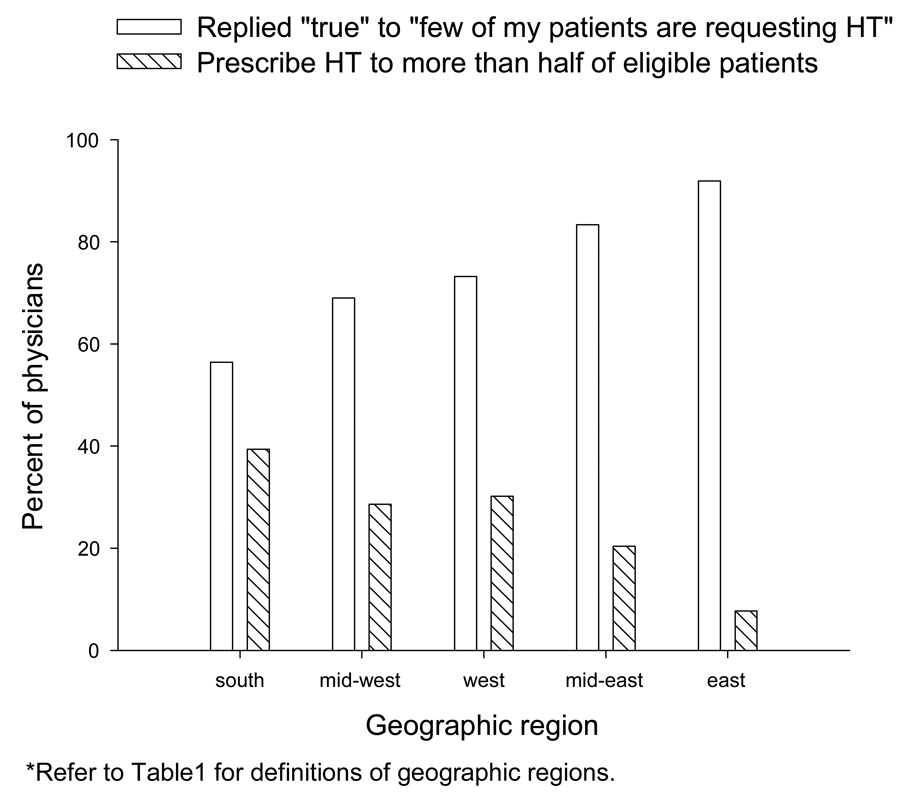
Most physicians reported that their patients were apprehensive about HT (84.7%) and ask about alternatives (86.9%). A majority (63.6%) reported that many of their patients were choosing to stop using HT. Obstetrician-gynecologists in the east were the least likely to have patients request HT (8.9% indicating that few of my patients are requesting HT; Figure 3) and the least likely to prescribe HT to over half of their eligible patients (10.9%; Figure 3). The percent of respondents that would only prescribe HT upon patient request (23.9%) was similar to the result from the 2004–2005 Study (21.2%). Obstetrician-gynecologists in the mid-west were the least likely to only prescribe HT if it was requested by the patient (10.7% indicating doing so, compared with 21.3% in the south, 22.2% in the mid-east, 30.2% in the west, and 32.3% in the east).
Figure 3.
There was consistent geographic variation in the following responses from survey used in Follow-up #2 CARN: “Few of my patients are requesting HT”, and “I prescribe HT to more than half of my eligible patients.”
Most (85.4%) respondents were aware that ACOG produces a Patient Education pamphlet on HT. Of those who were aware, 16.3% indicated it was very useful in their practice, 54.9% indicated somewhat useful, 8.1% indicated not useful and 20.7% indicated that they do not use the pamphlet. Similarly, most respondents (70.7%) were aware of the published report from the ACOG Task Force on Hormone Therapy (ACOG, 2004); of those who were aware, 43.3% found the report very useful, 53.2% found it somewhat useful and 3.4% found it not useful. Awareness of the ACOG Task Force report was not associated with either the respondents’ opinions regarding the evidence from the trials or whether the trials should have been stopped.
Most obstetrician-gynecologists reported they were very confident (64.7%) or confident (32.9%) in counseling their patients about hormone therapy. They were similarly very confident (45.3%) or confident (52.6%) in their ability to understand and interpret the scientific literature concerning hormone therapy. Fewer were very confident (11.1%) or confident (54.7%) in their ability to counsel patients about alternatives to hormone therapy. There was no association between the level of confidence and how convinced they were about the combined or the estrogen only trial results.
Of all the CARN obstetrician-gynecologists, 65.1% fit the conditions for the confident group and 33.6% not confident group. Those who reported being confident or not confident did not significantly differ by gender, birth year, region, reported adequacy of training in residency, family or personal experience with HT, or opinion about the current state of knowledge concerning HT. Those who were aware of ACOG’s patient education pamphlet and supplement published by the ACOG Task Force on Hormone Therapy in October of 2004 were more likely to be confident (p = .013). Those who were more likely to be confident reported that their prescribing patterns were unlikely to change in the near future (p = .008). Those who were confident were more likely to disagree with the decision to stop the combined estrogen and progestin trials (p = .041) and the estrogen only trial (p = .010).
Discussion
Overall, the results of Follow-up #1 were similar to the 2004 – 2005 study results. In aggregate the opinions of these physicians regarding the evidence from both WHI trials did not change for either men or women, except for a decrease in the proportion of physicians who were unsure or unaware of the evidence (Figure 1). The CARN physicians, however, differed in several aspects. Regarding the evidence from the combined HT trials the opinions of CARN men did not differ from the opinions expressed by the male physicians in the previous surveys; majorities found the evidence unconvincing. The opinions of CARN women regarding the combined HT evidence differed from the opinions of the women in the previous surveys, with a greater percentage of CARN women finding the evidence unconvincing (Figure 1). Regarding opinion of the estrogen only trial evidence, both CARN men and women differed from the previous results; a smaller percentage of CARN men found the evidence unconvincing while a larger percentage of CARN women found it unconvincing (Figure 1). These differences cannot be explained by age, as the CARN men and women were of identical age as the men and women in the 2004–2005 survey (Table 1). From these data we cannot distinguish between whether these differences are due to differences between the samples of physicians or if they reflect changes in opinion over time.
Obstetrician-gynecologists appear to regard the evidence from the two WHI trials differently. They are quite critical of the results from the combined HT trial, with a majority of respondents from both follow-up studies being skeptical of the results. The responding physicians were generally more accepting of the evidence from the estrogen only arm of the WHI trials. Physicians from 2004–2005 Study and Follow-up #1 that are confident in their ability to counsel patients and interpret the scientific literature are the most skeptical of the combined HT trial results, but the least skeptical of the estrogen only trial results. This result did not hold for the CARN physicians.
A finding from the preliminary study was that physicians that found the evidence from the combined HT trial convincing and agreed with the decision to end the trial had 1) completed their residency more recently, 2) rated evidence from randomized controlled trials as more important, 3) were more concerned with causing harm by action versus inaction, and 4) were more likely to have a favorable opinion of alternatives to HT.12 Year of residency (or age) continued to be a significant factor in physician acceptance of the evidence from the WHI trials in 2004–2005 Study; however the effect appears to have weakened in the follow-up studies, with only women in Follow-up #1 showing a significant result. A favorable opinion of alternatives continues to be associated with finding the evidence convincing. The proportion of obstetrician-gynecologists that responded alternatives to HT either are viable treatment options or at least do more good than harm, has remained relatively constant over the course of these studies (52.2% for the preliminary study, 56.3% for the 2004–2005 Study, 49.7% in Follow-up #1 and 60.5% in Follow-up #2 CARN).
An interesting finding from Follow-up #2 CARN is that physician prescribing patterns vary with geographic region in association with physician opinion of their patients’ preferences. This suggests that there may be social and cultural interactions that influence the interpretation of the evidence from the WHI trials among practicing obstetrician-gynecologists.
A consistent result from these studies is that a majority of obstetrician-gynecologists disagreed with the decision to end the trial of combined HT prematurely (preliminary study, 2004–2005 survey, and Follow-up #2 CARN; unfortunately the question was deleted from the survey used in Follow-up #1). A large number of respondents from the 2004–2005 survey and Follow-up #2 CARN disagreed with the decision to end the estrogen only arm of the WHI study as well. Physicians confident in their ability to interpret scientific evidence were more likely to disagree with the early termination of the trials. It is possible that the early termination of the WHI trials is viewed by some obstetrician-gynecologists as weakening the evidence from these clinical trials. However, the trials were stopped according to accepted ethical standards for the conduct of such clinical trials. Some have suggested that this tension between ethical conduct of trials and effective translation of evidence into practice indicates a need to educate clinicians more fully regarding the function and importance of data and safety monitoring boards for clinical trials.13
The response rates for both Follow-up #1 and Follow-up #2 CARN were higher than those of the previously published studies. This was to be expected as these sample populations consisted of physicians that either had returned a previous survey or have agreed to participate in survey studies. Although the response rates were near or above 60% for these studies, we cannot exclude the possibility of participation bias. There was limited evidence against participation bias. The pattern of responses to the questions did not vary over the three mailings for either study, indicating that the length of time it took a physician to respond did not significantly affect their responses. There were no differences between responders and nonresponders in follow-up #2 CARN in any demographic parameters. However, responders to Follow-up #1 were slightly but significantly older than nonresponders, and the comparison of men (but not women) who returned the letter as opposed to the survey indicates that men who did not respond to the survey were likely to be more skeptical of the WHI results.
Another potential limitation concerns the samples of physicians. In Follow-up #1 the surveyed physicians were those who had returned the 2004–2005 survey. This was deliberate to track changes in opinions; however, these physicians may not be representative of ACOG Fellows as a whole. There was no evidence of participation bias in the 2004–2005 survey,5 however the possibility cannot be excluded. In Follow-up #2 CARN the sampled physicians volunteer to answer surveys. The Research Department of ACOG actively manages the membership list of CARN in order to ensure that the members are as representative of all ACOG Fellows as is possible. Several times per year surveys are mailed to both the CARN Fellows and randomly selected Fellows. To date, the responses of CARN and nonCARN Fellows to clinical practice and knowledge questions from these matched surveys have rarely differed. 9,10,11 However, we acknowledge that CARN members might differ from other ACOG Fellows in some respects that we have not been able to measure.
We cannot ascertain from these data exactly what aspects of the WHI results those respondents that were skeptical found unconvincing. One criticism of the WHI trials was that it included a majority of older women many years from menopause. It is possible that some of the skepticism expressed by the physicians responding to these surveys relates to applying the global WHI results to their younger patients entering menopause. The data from the WHI trials continues to be analyzed. Recently published results have indicated that the risks from HT are lower for younger women within a few years of menopause; indeed the risk of mortality from all causes was lower in younger women (50–59 years old) taking HT. However, the evidence still supports an increased risk of stroke associated with HT use for all women, regardless of age.14 Ongoing clinical trials of HT in younger women undergoing menopause should clarify the risks and benefits for these women.
These two follow-up studies found that individual opinion regarding the evidence from the WHI trials of combined HT and unopposed estrogen has become more certain. Fewer practicing obstetrician-gynecologists are reporting being unsure of their opinion regarding the evidence. There has not been a move toward consensus, however. A significant proportion of obstetrician-gynecologists continue to express skepticism regarding the WHI results. With further analyses of the WHI data being reported in the literature and data from other trials forthcoming we expect the opinions of obstetrician-gynecologists regarding HT to continue to evolve.
Acknowledgement
Supported under contract NO1-HO-34205 from the National Heart, Lung, and Blood Institute, with support from the Office of Women’s Health Research, National Institutes of Health, Human Resources and Services Administration, Department of Health and Human Services; and by Grant #R60 MC 05674 from the Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services‥
Footnotes
Summary
A significant proportion of obstetrician-gynecologists remain skeptical of the evidence from the WHI trials and critical of the decision to end the trials.
References
- 1.Rossouw JE, Anderson GL, Prentice RL, La Croix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 3.Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, Ernster VL, Cummings SR. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992 Dec 15;117(12):1016–1037. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- 4.Power ML, Zinberg S, Schulkin J. A survey of obstetrician-gynecologists concerning practice patterns and attitudes towards hormone therapy. Menopause. 2006;13:434–441. doi: 10.1097/01.gme.0000185753.77704.65. [DOI] [PubMed] [Google Scholar]
- 5.Power ML, Schulkin J, Rossouw JE. The evolving attitudes of obstetrician-gynecologists towards hormone therapy. Menopause. 2007;14:20–28. doi: 10.1097/01.gme.0000229571.44505.cb. [DOI] [PubMed] [Google Scholar]
- 6.Ettinger B, Grady D, Tosteson AN, Pressman A, Macer JL. Effect of the Women’s Health Initiative on women’s decisions to discontinue postmenopausal hormone therapy. Obstet Gynecol. 2003;102:1225–1232. doi: 10.1016/j.obstetgynecol.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Bestul MB, McCollum M, Hansen LB, Saseen JJ. Impact of the women’s health initiative trial results on hormone replacement therapy. Pharmacotherapy. 2004;24:495–499. doi: 10.1592/phco.24.5.495.33349. [DOI] [PubMed] [Google Scholar]
- 8.Majumdar SR, Almasi EA, Stafford RS. Promotion and prescribing of hormone therapy after report of harm by the Women’s Health Initiative. JAMA. 2004;292:1983–1988. doi: 10.1001/jama.292.16.1983. [DOI] [PubMed] [Google Scholar]
- 9.Hill LD, Erickson K, Holzman GB, Power ML, Schulkin J. Practice tends in outpatient obstetrics and gynecology: findings of the Collaborative Ambulatory Research Network, 195–2001. Obstetrical & Gynecological Survey. 2001;56:505–516. doi: 10.1097/00006254-200108000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Coleman VH, Power ML, Zinberg S, Schulkin J. Contemporary clinical issues in outpatient obstetrics and gynecology: Findings of the collaborative ambulatory research network, 2001–2004: Part I. Obstetrical & Gynecological Survey. 2004a;59(11):780–786. doi: 10.1097/01.ogx.0000143775.07827.bb. [DOI] [PubMed] [Google Scholar]
- 11.Coleman VH, Power ML, Zinberg S, Schulkin J. Contemporary clinical issues in outpatient obstetrics and gynecology: Findings of the collaborative ambulatory research network, 2001–2004: Part II. Obstetrical & Gynecological Survey. 2004b;59(11):787–794. doi: 10.1097/01.ogx.0000143776.92687.ea. [DOI] [PubMed] [Google Scholar]
- 12.Power ML, Baron J, Schulkin J. Factors associated with obstetrician-gynecologists’ response to the Women’s Health Initiative trial of combined hormone therapy. Med Dec Making. 2008 doi: 10.1177/0272989X07312722. [DOI] [PubMed] [Google Scholar]
- 13.Ecker JL. Evidence and opinion: closing the gap. Menopause. 2007;14:3–4. doi: 10.1097/01.gme.0000247018.48630.e4. [DOI] [PubMed] [Google Scholar]
- 14.Rossouw Jacques E, MD, Prentice Ross L, PhD, Manson JoAnn E, MD DrPH, Wu LieLing, MSc, Barad David, MD, Barnabei Vanessa M, MD PhD, Ko Marcia, MD, LaCroix Andrea Z, PhD, Margolis Karen L, MD, Stefanick Marcia L. Postmenopausal Hormone Therapy and Risk of Cardiovascular Disease by Age and Years Since Menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]