Abstract
Many genes implicated in schizophrenia can be related to glutamatergic transmission and neuroplasticity, oligodendrocyte function, and other families clearly related to neurobiology and schizophrenia phenotypes. Others appear rather to be involved in the life cycles of the pathogens implicated in the disease. For example, aspartylglucosaminidase (AGA), PLA2, SIAT8B, GALNT7, or B3GAT1 metabolize chemical ligands to which the influenza virus, herpes simplex, cytomegalovirus (CMV), rubella, or Toxoplasma gondii bind. The epidermal growth factor receptor (EGR/EGFR) is used by the CMV to gain entry to cells, and a CMV gene codes for an interleukin (IL-10) mimic that binds the host cognate receptor, IL10R. The fibroblast growth factor receptor (FGFR1) is used by herpes simplex. KPNA3 and RANBP5 control the nuclear import of the influenza virus. Disrupted in schizophrenia 1 (DISC1) controls the microtubule network that is used by viruses as a route to the nucleus, while DTNBP1, MUTED, and BLOC1S3 regulate endosomal to lysosomal routing that is also important in viral traffic. Neuregulin 1 activates ERBB receptors releasing a factor, EBP1, known to inhibit the influenza virus transcriptase. Other viral or bacterial components bind to genes or proteins encoded by CALR, FEZ1, FYN, HSPA1B, IL2, HTR2A, KPNA3, MED12, MED15, MICB, NQO2, PAX6, PIK3C3, RANBP5, or TP53, while the cerebral infectivity of the herpes simplex virus is modified by Apolipoprotein E (APOE). Genes encoding for proteins related to the innate immune response, including cytokine related (CCR5, CSF2RA, CSF2RB, IL1B, IL1RN, IL2, IL3, IL3RA, IL4, IL10, IL10RA, IL18RAP, lymphotoxin-alpha, tumor necrosis factor alpha [TNF]), human leukocyte antigen (HLA) antigens (HLA-A10, HLA-B, HLA-DRB1), and genes involved in antigen processing (angiotensin-converting enzyme and tripeptidyl peptidase 2) are all concerned with defense against invading pathogens. Human microRNAs (Hsa-mir-198 and Hsa-mir-206) are predicted to bind to influenza, rubella, or poliovirus genes. Certain genes associated with schizophrenia, including those also concerned with neurophysiology, are intimately related to the life cycles of the pathogens implicated in the disease. Several genes may affect pathogen virulence, while the pathogens in turn may affect genes and processes relevant to the neurophysiology of schizophrenia. For such genes, the strength of association in genetic studies is likely to be conditioned by the presence of the pathogen, which varies in different populations at different times, a factor that may explain the heterogeneity that plagues such studies. This scenario also suggests that drugs or vaccines designed to eliminate the pathogens that so clearly interact with schizophrenia susceptibility genes could have a dramatic effect on the incidence of the disease.
Keywords: influenza, herpes simplex, cytomegalovirus, rubella, toxoplasmosis, T. Gondii, pathogen, genes, gene environment interaction, host pathogen interaction, schizophrenia
Introduction
Over 240 gene variants have been implicated in schizophrenia in genetic association studies. Such studies are notoriously inconsistent, and problems of replication are the norm rather than the exception. It has, however, been noted that many of these genes can be assembled into discrete but interconnected signaling networks related to glutamatergic neurotransmission and neuronal plasticity, oligodendrocyte function, and oxidative stress, all relevant subpathologies or endophenotypes of schizophrenia. The more important genes, eg, neuregulin or DISC1,1 appear to be those residing at key hubs of these signaling networks or those with multiple effects on several areas of the network.2
Several viral or bacterial pathogens as well as famine in prenatal and postnatal periods (see below) have also been implicated in schizophrenia. Such stressors activate an eif2alpha kinase signaling cascade, again composed of proteins encoded for by schizophrenia susceptibility candidates, that shuts off protein synthesis in response to stress. This is achieved by inhibition of the translation initiation factor eif2b, whose malfunction has been associated with oligodendrocyte cell loss in vanishing white matter disease.3 Glutamatergic (eg, N-methyl D-aspartic acid [NMDA] receptors) and growth factor signaling networks (eg, neuregulin 1 [NRG1], brain-derived neurotrophic factor [BDNF]) also control protein translation and synthesis via this network, and such effects are important in the control of synaptic plasticity and dendritic growth. These pathways, created by a lattice of susceptibility genes may represent key intrinsic signaling networks whose malfunction may contribute to many pathological features of schizophrenia including oligodendrocyte cell loss, reduced glutamatergic function, and impaired synaptic connectivity.2
Several studies have shown that maternal viral or parasitic infection during pregnancy is an important risk factor for the subsequent development of schizophrenia in the adult offspring. The pathogens implicated in these maternal effects include influenza,4 herpes simplex virus (HSV)-2,5 rubella,6 and Toxoplasma gondii.7 A large Danish study has also reported that viral infection during early childhood (0–12 years), particularly with cytomegalovirus (CMV) or mumps are associated with an increased risk of psychosis.8 Seropositivity to HSV-1, related to cognitive deficits and cerebral gray matter changes has also been reported in adult schizophrenics9and antibodies to HHV-6 or T gondii prior to diagnosis in adults have also been associated with schizophrenia.10,11 Increased seropositivity for CMV has also been observed at presentation in first-episode schizophrenic patients.12 It would, thus, seem that prenatal, childhood, and adult infections could all act as risk factors, perhaps, in a synergistic or additive fashion. Further examples (borna virus, Borrelia burgdorferi, chlamydial infection, coxsackie B5, poliovirus, retroviruses) are listed at http://www.polygenicpathways.co.uk/. Herpes viruses (HSV-1 and HSV-2 and CMV) are neurotropic and known to be able to infect the central nervous system, although only HSV-1, HSV-2, CMV, and HHV-6 have been detected in schizophrenic brain tissue.13–15 The influenza virus is also capable of cerebral entry through the nasal route via olfactory nerves, at least in mice.16 Toxoplasma gondii is also capable of infecting the brain17 but has not been detected in schizophrenia brain tissue, and indeed, many studies searching for cerebral viral or bacterial DNA or protein postmortem have been negative.18 However, as already suggested,19 given the precocious timing of certain infections related to schizophrenia risk, an inability to detect viral traces many decades later at autopsy is perhaps not unexpected. A viral presence in cerebral tissue might also not be strictly necessary, and several groups have suggested that circulating infection-related cytokines may participate in the risk-promoting process. For example, maternally administered interleukin (IL)-6 or the cytokine releaser polyriboinosinic-polyribocytidylic acid are also known to be able to influence the behavior of the adult offspring in rats and also induce morphological changes in the hippocampus resembling those observed in schizophrenia.20–22 Immune perturbation can also affect behavior in adults. For example, in adult mice, T-cell deficiency can induce cognitive deficits, while T-cell vaccination can reduce the behaviorally excitant and cognitive-disturbing effects of the NMDA receptor antagonist MK-801 or amphetamine.23 Both heat shock (hyperthermia/fever) and cytokines (interferons, IL1B, and TNF) are able to activate the viral-activated eif2alpha kinase pkr,24 (EIF2AK2: eukaryotic translation initiation factor 2-alpha kinase 2) involved in the control of protein translation (see above). Infection or its inflammatory or immune consequences may, thus, affect both neurodevelopment and neurotransmitter-related behavior, although the mechanisms behind these effects remain poorly understood. Again, as with individual susceptibility genes, the implication of individual pathogens as risk factors has been subject to controversy.25
Pathogens purloin different aspects of the hosts' physiology during their life cycle. They have evolved mechanisms to adhere and gain entry to target cells via specific receptors and use endocytic pathways and the intracellular transport and sorting machinery to access specific cellular compartments. They may use cellular junctions for intercellular spread, or lie dormant in vacuoles or vesicles, formed by host and pathogen proteins. Viruses usurp the host's transcriptional machinery to replicate. Each pathogen uses its own and the hosts' cellular genes and proteins during these processes. The host retaliates using the immune system, as well other infection-related defense mechanisms, including cytokine production and free radical–related bactericidal and viricidal effects. Pathogens have also evolved mechanisms to avoid immune detection or to otherwise abrogate immune defense.26
As described below, many genes associated with schizophrenia in genetic studies code for proteins specifically implicated in this host-pathogen battlefield creating a further network of genes, in this case etching out stages of the pathways used by the pathogens during their life cycles and interaction with the host. These extrinsic pathways may play a role in infection, although the host genes involved evidently also play a role in human physiology that is often pertinent to schizophrenia. Again, multifunctional effects seem to dictate the relevance of the gene as certain genes of high interest, notably, DISC1, NRG1, RGS4, and dysbindin, inter alia, can be related to both intrinsic and extrinsic signaling networks. This might suggest that association studies may in some cases relate to genes specifically involved in the host/pathogen physiology of causative pathogens as well as to the disease itself. If these genes are implicated in the life cycles of the pathogens, polymorphisms therein may well affect the virulence of the pathogen, providing a further example of how genes and environmental factors might interact to produce disease. These interactions have important medical implications and suggest that targeting the appropriate pathogens may have dramatic effects on the incidence and progression of schizophrenia as already suggested by several authors (for review, see Yolken and Torrey7).
Methods and Background
The genes associated with schizophrenia have been the subject of previous reviews2,27and are listed together with the environmental risk factors at http://www.polygenicpathways.co.uk. The pathogens included in this review focus on influenza, rubella, herpes viruses (HSV-1 and CMV), or the protozoan parasite T gondii. A literature survey was undertaken to analyze whether any of the susceptibility gene candidates are associated with the cellular processes used during the life cycles of these and other potential pathogens. The Vita database (http://vita.mbc.nctu.edu.tw/)28 was used to predict potential viral targets of human microRNAs. Because of the large volume of data, the bulk of this analysis, and references, are posted in a supplementary table at http://www.polygenicpathways.co.uk/schizmicrobes.htm, and only certain main conclusions are summarized below. The families of genes mentioned in this review are annotated in a supplementary table posted at http://www.polygenicpathways.co.uk/szfam.htm. Association data relating to genes implicated in infection in man are summarized at http://www.polygenicpathways.co.uk/infectassoc.htm. Genes associated with schizophrenia are in bold throughout the text.
Viral Receptors
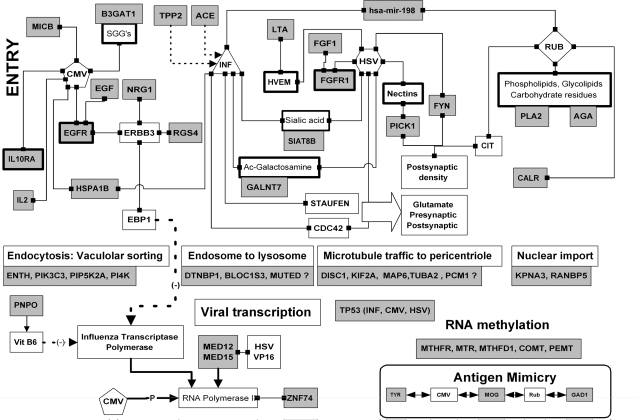
Binding to Chemical Residues on the Cell Surface (figure 1)
Influenza, herpes simplex, and CMV bind to sialic acid residues on the host cell surface, including alpha-2-8-linked sialic acids synthesized by the sialyltransferase SIAT8B.29 Acetylated galactosamine (metabolized by GALNT7) is also a receptor for the influenza C virus glycoprotein,30 while glycoprotein C of the herpes virus acquires N-acetylgalactosamine prior to routing to the host Golgi apparatus.31 The CMV binds to sulfated glucuronyl glycosphingolipids (synthesized by B3GAT1).32 The rubella virus binds to membrane phospholipids and glycolipids, and phospholipase A2 (PLA2G4) digestion of Vero cells markedly reduces viral infectivity. Viral infection is also reduced after beta-N-acetyl-D-glucosaminidase, alpha-glucosidase, and beta-galactosidase treatment, suggesting that diverse carbohydrate residues may contribute to a complex receptor structure for this virus.33 Aspartylglucosaminidase (AGA) metabolizes beta-N-acetyl-D-glucosamine residues.
Ligand-Activated Receptors (figure 1)
The herpes virus uses a number of receptors to gain entry to host cells,34 including nectin receptors (PVRL1-3), for which PICK1 acts as a scaffold35; a TNF-related receptor (TNFRSF14), for which lymphotoxin-alpha (LTA) is the natural ligand; and the fibroblast growth factor receptor 1 (FGFR1).36
The epidermal growth factor (EGF) receptor is used by CMV for entry, and epidermal growth factor receptor (EGFR)/ERBB3 heterodimers bind glycoprotein B of the virus.37 NRG1 is a ligand for such heterodimers,38 while RGS4 is involved in ERBB3 signaling.39 A CMV viral homolog of the cytokine IL10 also binds to the host cognate receptor IL10RA.40
Adhesion Molecules and Cell Junctions
Although none of the adhesion molecules (CHL1, NCAM1) or junctions (CLDN5, GJA8) implicated in schizophrenia are specifically involved in the life cycles of the pathogens in this study, these families play an important role in microbe recognition (adhesion molecules)41 and intercellular spread (gap and tight junctions).42 For example, NCAM1 serves as a viral receptor for the herpes family rabies virus,43 while claudins function as entry receptors for hepatitis C.44 ARVCF is a component of adherens junctions, which play an important role in the invasion of several viruses including herpes simplex.45 The chitinase-like protein, CHI3L1, is also involved in bacterial adhesion and invasion in colonic epithelial cells.46
Intracellular Traffic
Many pathogens exploit the trafficking networks used by the cell to obtain nutrients, or to endocytose membrane receptors, entering cells via these routes (figure 1). These endosomal and other pathways converge on lysozymes, which are able to kill pathogens.47 Dysbindin (DTNBP1), BLOC1S3, or MUTED are part of the biogenesis of lysosomal organelles complex (BLOC-1) which is specifically involved in the sorting of cargoes from vacuolar early endosomes to the lysosomal complex.48 Viruses also use the microtubule network to gain access to the nucleus and are driven along by the host's dynein and kinesin motors toward the microtubule organizing center close to the nucleus.49 Viral inclusions, factories for viral replication, form at pericentriolar sites close to this microtubule organizing center or in specialized nuclear domains known as ND10/PML bodies.50 DISC1 is a component of this microtubule-related dynein motor complex51 and implicated in centriolar function via its association with kendrin, a binding partner of the pericentriolar protein PCM1.52 While neither DISC1 or dysbindin nor the kinesin or microtubule genes (KIF2A, MAP6, TUBA2) have been specifically implicated in viral traffic, they are clearly involved in processes commandeered for viral transport.
Fig. 1.
A Summary of the Interactions Between Viruses and Proteins Coded for by Schizophrenia Susceptibility Genes. Linked boxes represent binding between 2 components. Arrows represent effects on transcription or metabolism. Single lines represent stimulatory and dotted lines inhibitory effects. Genes associated with schizophrenia are represented by gray boxes. Molecular mimicry between pathogen epitopes and schizophrenia proteins is also represented. See text for details. CMV, cytomegalovirus; HSV, herpes simplex virus; INF, influenza; RUB, rubella; T Gon, Toxoplasma gondii; SGGs, sulfated glucuronyl glycosphingolipids; Ac-galactoseamine, acetylgalactoseamine. These figures should be viewed as an “interactome” and although individual interactions between binding partners are all valid, multicomplexes or ternary interactions should not be inferred. The interactions described in each of these figures can be found in the text or at http://www.polygenicpathways.co.uk
The phosphoinositide kinase subtype vps34 (PIK3C3) is involved in the vacuolar protein-sorting pathway used by the influenza virus to gain entry to cells. Phophinositide-5 and -4 kinases (PIP5K2A, PI4K) are also involved in the intracellular traffic of the influenza virus.53
Protein Folding and Heat Shock Proteins
Proteins derived from the influenza, CMV, herpes simplex, and rubella all bind to calreticulin (CALR), which plays an important role in viral protein folding.54 The CMV glycoprotein B binds to a number of heat shock proteins including HSPA1L and HSPA1B, also a binding partner of the influenza ribonucleoprotein and polymerase complex. Subjugation of the host's protein folding machinery by viral polypeptides also leads to endoplasmic reticulum stress.55 ATF6 and XBP1 play a key role in this pathway (see below and figure 4).
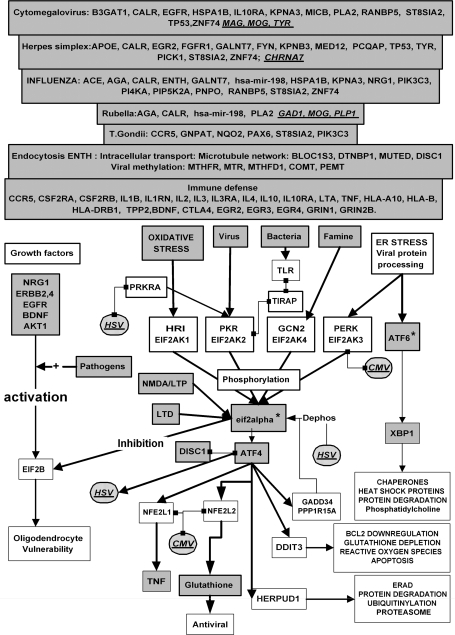
Fig. 4.
Oxidative Stress, Endoplasmic Reticulum Stress, Starvation, Viruses, Bacteria, and Other Pathogens Activate the eif2alpha kinase Signaling Network. Phosphorylated eif2alpha shuts down protein synthesis via inhibition of the translation initiation factor eif2B, a gene that is crucial to oligodendrocyte function. Protein synthesis is also controlled by growth factors via the AKT1 signaling network and by NMDA and metabotropic glutamate receptor activation. Protein synthesis is enhanced by long-term potentiation and repressed by long-term depression via effects on eif2alpha phosphorylation. ATF4 activation by this pathway regulates processes designed to counteract the effects of these stressors, or in unsuccessful, to trigger apoptotic programmes. Pathogens activate the eif2alpha kinase pkr or Toll receptor signaling and can activate or inhibit the AKT1 survival pathway. Gray boxes depict schizophrenia susceptibility genes identified in association studies or as genes within runs of homozygosity regions marked with an asterisk (ATF6, eif2alpha/EIS2S1). Those implicated specifically in the life cycles of each pathogen are also illustrated. Hatched boxes represent viral proteins or genes, which interact directly with certain of the proteins in this network (see text for details). LTP, long-term potentiation; LTD, long-term depression; HSV, herpes simplex virus; CMV, cytomegalovirus.
Viral Replication
Nuclear Import
Many viruses need to enter the cell nucleus, where they subvert the cellular transcriptional machinery (figures 1 and 2). Both RANBP5 and KPNA3 (nuclear importins or karyopherins that control the entry of proteins from the cytoplasm to the nucleus via the nuclear pore) are involved in the nuclear import of the influenza virus.56
Fig. 2.
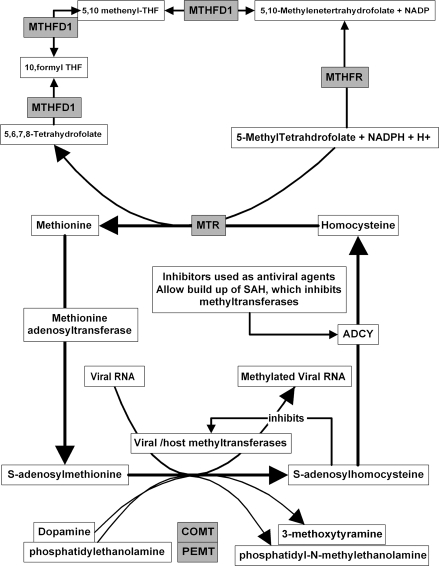
Viral RNA Methylation, Using S-adenosylmethionine, Plays an Important Role in Viral RNA Maturation. The genes involved in S-adenosylmethione and homocysteine metabolism are depicted here. Gray-shaded boxes represent those associated with schizophrenia (modified from De clercq63).
RNA Polymerase and Viral Transcription
Herpes simplex uses the host's RNA polymerase II, which it diverts it to synthesizing viral RNA at the expense of the host's (figure 1).57 The mediator complex (MED12 [aka, HOPA], MED15 [aka, PCQAP]) is a host RNA polymerase coactivator used by herpes simplex, which diverts the polymerase activity toward viral RNA synthesis. A viral protein VP16 binds to components of this mediator complex (MED15, MED17, and MED25).58 Other schizophrenia susceptibility candidates also affect the transcription of the influenza virus. Vitamin B6, the product of pyridoxamine 5′-phosphate oxidase (PNPO), inhibits influenza viral replication by a direct action on the viral transcriptase to which it binds.59 NRG1 signaling via ERBB receptors might also be expected to influence influenza viral transcription. A host protein, EBP1, binds to and acts as an inhibitor of the influenza virus RNA polymerase.60 EBP1 was initially characterized as an ERBB3 binding partner. It dissociates from this receptor following stimulation by its ligand, NRG1, and translocates from the cytoplasm to the nucleus.61 RGS4 is a component of the ERBB3 signaling pathway. The effects of NRG1 on influenza infection have not been directly examined, although interestingly, NRG1 expression levels are markedly increased in the blood of influenza-infected children with febrile convulsions,62 while RGS4 expression levels are increased in the brains of influenza-infected mice.16
Viral RNA Methylation: S-adenosylmethione and Homocysteine Metabolism
Viral RNA methylation, using the methyl donor S-adenosylmethionine, is crucial for the maturation of viral RNA as shown in figure 2 (modified from a review by De clercq63) (figure 2). The product of these methylation reactions, S-adenosylhomocysteine inhibits the diverse methyltransferases involved in this process. S-adenosylhomocysteine (SAH) is normally rapidly metabolized to adenosine and homocysteine by SAH hydrolase (ADCY), and inhibitors of this enzyme inhibit viral RNA methylation by favoring SAH accumulation. SAH hydrolase inhibitors (carbocyclic adenosine, carbocyclic 3-deazaadenosine, neplanocin A, 3-deazaneplanocin A) display broad-spectrum antiviral activity in the laboratory that has not yet translated to the clinic, presumably, because of the difficulty of selectively affecting viral rather than host methylation.63 Methyltransferase inhibitors also inhibit influenza viral replication,64 and methylation inhibition also results in the increased nuclear retention of influenza viral RNA.65 Methylation of viral DNA or proteins also plays a role in the viral life cycles. For example, HSV-1 latency or lytic infection can be influenced by such reactions.66
The availability of S-adenosylmethionine is, thus, a general factor contributing to viral viability. One might also expect that methylation of viral RNA would be a competing factor for the methylation of host DNA or protein, depending on the compartment in which this occurs.
Viruses also affect the methylation of host DNA. For example, the herpes simplex strain HSV-2 produces marked hypomethylation of host DNA in infected rat embryo cells, although this effect may be due to a redistribution of the host DNA methyltransferase rather than to any diversion of methionine/homocysteine metabolism toward viral methylation.67 A generalized decrease in DNA methylation has been observed in leukocytes isolated from male schizophrenic patients.68
Several genes implicated in schizophrenia (MTHFR, MTR, and MTHFD1) are relevant to this area as shown in figure 2. Other methylating enzymes may also influence this cycle indirectly by modifying substrate levels. For example, phosphatidylethanolamine methyltransferase (PEMT) knockout mice have dramatically reduced plasma homocysteine levels69 and catechol-O-methyltransferase (COMT) inhibition increases S-adenosylmethionine levels in the rat brain.70 DNA methyltransferase expression is markedly increased in certain areas of the schizophrenic brain, and S-adenosylmethionine levels are also increased. Such effects have been proposed to be relevant to the hypermethylation of the reelin promoter and a reduction in its messenger RNA (mRNA) and protein levels observed in schizophrenia.71–73 While not wishing to imply that viruses are fully responsible for these effects, or that the effects of genes such as COMT are solely related to viral replication, it is clear that viruses are able to affect host methylation reactions and that these in turn are able to influence viral replication. Interestingly, the prenatal infection of mice with influenza does result in a reduction in cortical and hippocampal reelin immunoreactivity.74
Human microRNA
microRNAs are short RNA transcripts, derived from chromosomal DNA, that are not translated into protein (figure 1). Their sequence is complementary to one or more mRNAs, to which they bind, thus, inhibiting translation of the mRNA to protein. They can bind either to host mRNA or to viral genomes, wherever sequence complementarity exists.
The human microRNAs, hsa-mir-206 and hsa-mir-198, are complementary to a number of host mRNAs and also to several viral RNAs, as suggested by interrogation of the Vita database.4 This bioinformatic analysis suggested that hsa-mir-198 targets viral genes encoding for the influenza virus PB2 protein and for structural proteins E1, E2, and C of the rubella virus, inter alia. hsa-mir-206 is predicted to bind to genes of the human coxsackievirus B2 and human poliovirus 1, inter alia.
Bacteria and Parasites
This review is primarily concerned with viral risk factors, although it is likely that such interactions are also pertinent to the life cycles of bacteria (eg, Chlamydia) or parasites (T gondii) that have been implicated in schizophrenia (figure 3). Only a few examples are given here. Tryptophan, metabolized by tryptophan hydroxylase (TPH)-1, is an essential nutrient for several microorganisms, including T gondii and chlamydial species.75
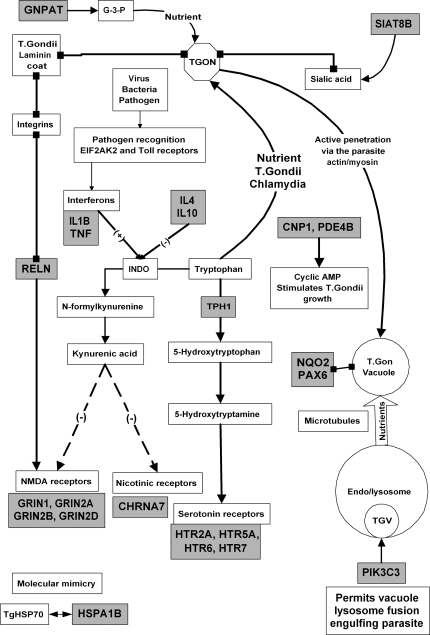
Fig. 3.
Toxoplasma gondii (TGON) Binds to Membrane Constituents (sialic acid residues, N-acetylated galactoseamine or glucoseamine (N-Ac-Gal and N-Ac-Glu) or Galactosylceramide (Gal-Cer)) That Are Metabolized or Influenced by the Products of Diverse Schizophrenia Susceptibility Genes (gray boxes). Other interactions T gondii and host processes are also shown. Cytokines, generated by bacterial attachment to Toll receptors or other inflammatory processes are able to reroute tryptophan to serotonin metabolism via effects on indoleamine 2,3-dioxygenase (INDO) resulting in the formation of kynurenic acid. Linked boxes represent binding between 2 components. Arrows represent effects on transcription or metabolism. Single lines represent stimulatory and dotted lines inhibitory effects. Genes associated with schizophrenia are depicted by gray boxes. G-3-P, glycerone-3-phosphate.
Spirochetes (T gondii) forage phospholipids in the blood. They possess an enzyme that metabolizes dihydroxyacetone phosphate (glycerone-3-phosphate) to glycerol-3-phosphate that may be used as an energy source by the parasite. glyceronephosphate O-acyltransferase (GNPAT) metabolizes glycerone-3-phosphate to acylglyceronephosphate.76
Exogenous cyclic adenosine monophosphate (AMP) also stimulates T gondii growth in cell culture and cyclic AMP phosphodiesterase inhibition or activation and stimulates or inhibits T gondii growth, respectively77 (cf, the cyclic nucleotide phosphodiesterases PDE4B and CNP1). Toxoplasma gondii, like viruses, also binds to sialic acid residues on cell surfaces78 (cf, SIAT8B). Toxoplasma gondii contact with the cell surface in fibroblasts is mediated by laminins on the parasite surface, which bind to host beta 1 integrins.79 Interestingly, the reelin/beta 1 integrin system controls the surface expression of NMDA receptors (GRIN1, GRIN2B),80 although the effects of T gondii on reelin-NMDA receptor interactions have not been studied. A T gondii cyclophilin-like secreted protein also binds to the chemokine receptor CCR5.81 The parasite survives in macrophages by hiding in parasitophorous vacuoles that avoid fusion with lysosomes. This parasitophorous vacuole is derived from invagination of the host plasma membrane following entry of the parasite by active penetration. The parasite uses the host's microtubule network to transport host endosomes or lysosomes to invaginations within this organelle and feeds on their constituents. This vacuole is also closely associated with the host mitochondria and endoplasmic reticulum,82 and its parasite-derived constituents dense granule proteins have been shown to bind to a number of host proteins including the transcriptional regulator PAX6 and the quinone reductase NQO2.83 Activation of the immune response can reroute these vacuoles to the lysosomal system allowing macrophage bactericidal activity. The phosphoinositide kinase PIK3C3 plays a key role in this vacuolar/lysosomal fusion and in enabling macrophages to kill T gondii.84 The cellular microtubule network and cell-sorting machinery, thus, play an important role in both viral and parasitic infection.
General Pathways
Growth Factor and Phosphoinositide Signaling
Many pathogens subvert the phosphoinositide kinase/AKT survival pathway, primarily to activate antiapoptotic pathways that allow the pathogen to stay in residence (figure 4). The NS1 influenza viral protein binds to the p85 beta subunit of phosphoinositide kinase (PIK3R2) and activates AKT1 signaling. These effects limit the viral-induced cell death program allowing further replication.85,86 CMV, herpes simplex, rubella, and T gondii also use this type of subversion of growth factor signaling (see supplementary data on Web site).
Glutathione and Oxidative Stress
Glutathione levels are decreased in the cortex and cerebrospinal fluid (CSF) in schizophrenia (figure 4).87 Glutathione levels are also decreased in Vero cells by HSV-1 infection, and glutathione supplementation markedly inhibits viral replication.88 HSV-1 infection of microglia results in the upregulation of the glutamate/cysteine transporter SLC1A2, which plays an important role in providing the glutamate and cysteine necessary for glutathione biosynthesis by the glutamate cysteine ligases (GCLC, GCLM). High intracellular glutathione levels also inhibit the replication of the influenza virus,89 and cells with high levels of glutathione are also resistant to CMV infection.90 Glutathione is also effective in reducing influenza infection in mice in vivo.91
NMDA receptors possess a redox modulatory site sensitive to glutathione, and glutathione depletion in animal models leads to NMDA receptor hypofunction, mimicking several features of schizophrenia (reviewed in Lavoie et al92). A recent clinical trial using the glutathione precursor N-acetylcysteine has reported significant modifications in auditory evoked potential deficits in schizophrenia and also suggested effects on the improvement of negative symptoms.92 As glutathione-based therapy might be expected to reduce viral load and favorably modify NMDA receptor function, this area clearly deserves further attention. Glutathione also induces the egress of T gondii from infected cells by activating a T gondii–secreted apyrase (nucleoside triphosphate hydrolase) in the parasite vacuole resulting in a rapid depletion of host cell adenosine triphosphate (ATP).90
The Innate Immune System
A large number of cytokine- and chemokine-related genes (CCR5, CSF2RA, CSF2RB, IL1B, IL1RN, IL2, IL3, IL3RA, IL4, IL10, IL10RA, IL12B, IL18, IL18R1, IL18RAP, LTA, TNF) and major histocompatibility complex antigens (HLA-A10, HLA-B, HLA-DRB1) have been implicated in schizophrenia (figure 4). The angiotensin-converting enzyme is also involved in immune processes and cleaves an influenza antigen nucleoprotein.93 Tripeptidyl peptidase 2 is also involved in the antigen processing of the influenza virus.94 A CMV protein (UL16) also binds to the natural killer kell receptor ligand MICB. This receptor triggers natural killer cells and costimulates antigen-specific T cells that would normally destroy invading pathogens. MICB binding by UL16 may circumvent this immune response.95 The CMV IE2 protein also binds to the promoter of the IL2 gene and upregulates its expression.96 Free radical–generating systems also play an important role in pathogen defense. For example, nitric oxide synthase 1 (NOS1) is toxoplasmacidal.97
The immune system is designed to protect against pathogens by recruiting immunocompetent cells, macrophages, and microglia to sites of infection, and in a general sense, polymorphisms in many of these genes would by expected to impinge upon the life cycles of any of the pathogens implicated in schizophrenia. Most of these genes are affected in some way by one or other of the pathogens and several are able to influence pathogen viability. The specific relationships of these genes to each of the pathogens are reviewed and referenced more extensively in the supplementary table at http://www.polygenicpathways.co.uk/schizmicrobes.htm, and their overall effects summarized in table 1. (A number of other genes whose function one would tend to associate with central nervous system (CNS) effects are also expressed on lymphocytes and play a role in the immune system. These include the growth factors BDNF and NTF3, NMDA and metabotropic glutamate receptors, and dopamine and serotonin receptors, inter alia. The effects of polymorphisms on immune function may be as important as their effects in the CNS. Further details are provided at http://www.polygenicpathways.co.uk/schizmicrobes.htm.)
Table 1.
A survey of the Interactions Between the Pathogens and Genes Implicated in Schizophrenia
| Influenza | Cytomegalovirus | Herpes | Rubella | Toxoplasmagondii | Others | |
| Direct interactions (binding, degradation, or synthesis of a binding partner) | ACE | CALR | APOE | CALR | CCR5 | ARVCF/herpes |
| CALR | EGFR | ATF4 | ||||
| HLA-B | HSPA1B | CALR | HLA-B | HLA-B | FEZ1/JC virus | |
| HLA-DRB1 | HSPA1L | FGFR1 | ||||
| HSPA1B | IL1B | FYN | HLA-DRB1 | NQO2 | HLA-A10/Chlamydia | |
| KPNA3 | IL10RA | HLA-B | ||||
| PIK3C3 | MICB | KPNB3 | hsa-mir-198 | PAX6 | HTR2A/JC virus | |
| RANBP5 | PLA2 | PCQAP | ||||
| TPP2 | TP53 | TP53 | TF | Adhesion molecules and junctions? | ||
| hsa-mir-198 | TYR | |||||
| Receptor or association with ligand synthesis | AGA | B3GAT1 | FGFR1 | AGA | ST8SIA2 | |
| EGFR | GALNT7 | PLA2G4A | ||||
| GALNT7 | IL10RA | PICK1 | PLA2G4B | |||
| ST8SIA2 | ST8SIA2 | ST8SIA2 | PLA2G4C | |||
| Transport and nutrition | CALR | CALR | CALR | ENTH | GNPAT | Microtubule network (viral traffic) |
| ENTH | KPNA3 | PIK3C3 | DISC1, KIF2A, TUB2A? | |||
| PIK3C3 | RANBP5 | TPH1 | Endosome/lysosomal pathway | |||
| PI4KA | DTNB1, BLOC1S3, MUTED? | |||||
| PIP5K2A | ||||||
| KPNA3 | ||||||
| RANBP5 | ||||||
| Transcription | PNPO | ZNF74 | EGR2 | |||
| MED12 | ||||||
| TP53 | PCQAP | |||||
| ZNF74 | ZNF74 | |||||
| Viral RNA methylation | MTHFR, MTHFD1, MTR, COMT? PEMT? | |||||
| Pathogen increases expression or activates | AKT1 | AKT1 | ADCYAP1 | AKT1 | ACE | |
| CHN2 | AKT1 | CHI3L1 | AKT1 | |||
| CTLA4 | ATF4 | CCR5 | EGFR | HP | ||
| ERBB4 | CNP | EGR3 | ||||
| FOXP2 | IL1B | ERBB2 | HLA-A | IL1B | ||
| GAD1 | GNAS | HLA-B | ||||
| IL1B | IL2 | IL1B | HOMER1 | IL10 | ||
| IL10 | IL1RN | IL10 | ||||
| LTA | LTA | NOS1 | NRG1 | MDR1 | ||
| MICB | NPY | PDE4B | ||||
| RGS4 | PTGS2 | NTF3 | PLA2G4A | NRG2 | ||
| NOS1 | PLP1 | PTGS2 | PTGS2 | |||
| PLA2 | XBP1 | PTGS2 | RGS4 | SOD2 | ||
| SOD2 | SLC1A2 | SOD2 | TNF | |||
| TNF | TP53 | |||||
| TP53 | ||||||
| Pathogen decreases expression | DLG2 | GRIN1 | APC | ATXN1 | NCAM1 | |
| EGR2 | NCAM1 | GRIN1 | ERBB2 | ND4 | ||
| HOMER1 | TH | HOMER1 | GRM3 | PNPO | ||
| IL2 | HTR2A | PLXNA2 | SLC6A3 | |||
| PLP1 | IL1RN | PRODH | YWHAH | |||
| RELN | PIK3C3 | RBP1 | ||||
| TH | ||||||
| Kills pathogen or reduces replication/infection | Glutathione (GCLM GCLC, GSTM1, GSTT1) | Glutathione (GCLM GCLC, GSTM1, GSTT1) | Glutathione (GCLM GCLC, GSTM1, GSTT1) | CCR5del | NRG1 releases EBP1, an influenza viral transcriptase inhibitor. | |
| CCR5 | IL1B | |||||
| IL1B | IL4 | IL1RN | IL2 | |||
| IL1RN | IL12 | IL12 | IL3 | |||
| IL18 | LTA | IL18 | IL4 | |||
| LTA | LTA | IL12 | ||||
| MDR1 | (−)PTGS2 | MICB | LTA | |||
| PNPO | (−)PTGS2 | Nitric oxide | ||||
| (−)PTGS2 | TNF | TNF | PDE4B | |||
| SOD2 | TNF | |||||
| Favors pathogen | CTLA4 | EGF | CTLA4 | Glutathione (GCLM GCLC, GSTM1, GSTT1) | ||
| IL2 | ||||||
| IL4 | IL1B | IL4 | CTLA4 | |||
| TP53 | IL4 | MICB | DRD2 | |||
| TNF | NMDA receptor activation (GRIN1, GRIN2A, GRIN2B, GRIN2D) | IL10 | ||||
| Cannabis (immunosuppressant) | TP53 | TNF | UCP2 | |||
| TPH | TP53 | TPH | ||||
| Molecular mimicry | GRIN2B | MAG | CHRNA7 | GAD1 | HSPA1B | |
| MOG | ||||||
| MOG, TYR | PLP1 | |||||
| % of schizophrenia candidates to date (N = 245) | 21% | 18% | 22% | 13% | 16% | |
| Genes implicated in infection (association studies related specifically to these pathogens) | HLA-DRB1, IL10 | HLA-B, HLA-DRB1,IL1RN, IL10 ,IL12B , IL18 ,IL18R1, IL18RAP,TNF | APOE, HLA-B, IL10, MICB, IL18RAP | — | HLA-DRB1 | |
| No reports of association with schizophrenia | No reports of association with schizophrenia | No reports of association with schizophrenia | ||||
| IL6 | GJB2, MBL, MICA, TLR2, TLR4 | KIR2DL2, LTF, MBL | ||||
| TLR2 | ||||||
| % of genes implicated in infection also implicated in schizophrenia | 2 of 3 = 66% | 9 of 14 = 64% | 5 of 9 = 55% | — | Not applied | |
| Immune defense | (CCR5, CSF2RA, CSF2RB, IL1B, IL1RN, IL2, IL3, IL3RA, IL4, IL10, IL10RA, IL12B, IL18, IL18R1, IL18RAP, LTA, TNF), HLA antigens (HLA-A10, HLA-B, HLA-DRB1), and genes involved in antigen processing (TPP2) or T- or B-cell signaling and development (BDNF, CTLA4, EGR2/3/4, FOXP2, GRIN1, GRIN2B) | |||||
Note: Most of the interactions summarized here are referenced in a supplementary table posted at http://www.polygenicpathways.co.uk/schizmicrobes.htm. Association data with respect to infection per se are posted at http://www.polygenicpathways.co.uk/infectassoc.htm. See text for details.
Glutamate Receptors and Plasticity
A large proportion of the genes implicated in schizophrenia code for presynaptic and postsynaptic proteins related to glutamatergic neurotransmission and to components of the postsynaptic density (figures 1 and 3). Few of these appear to be directly implicated in the life cycles of the pathogens implicated in schizophrenia, although both FYN and citron kinase, binding partners of the herpes simplex and rubella virus, respectively, are components of the postsynaptic density, while PICK1 is a scaffolding partner for glutamate receptors and the herpes entry portals, nectin receptors35 (figure 1). The binding of T gondii species to integrins, components of the reelin signaling pathway, might also be expected to influence NMDA receptor function (figure 2). The NMDA receptor does bind to components of the HIV virus,98and the use of this receptor by other viruses or pathogens would not be unprecedented.
Viral infection does affect glutamatergic signaling. For example, the influenza nucleoprotein distributes to dendritic spines in cultures of rat hippocampal neurones. Spontaneous excitatory synaptic activity and the amplitude of miniature excitatory postsynaptic currents are reduced at later stages, 17–22 days after transfection.99 Infection of PC12 cells with the herpes virus HSV-1 also results in a reduced expression of the NMDA receptor subunit GRIN1.100 The hippocampal expression of GRIN1 is also reduced following CMV infection in mice, an effect also observed in primary neuronal culture.101
Interestingly, the NS1 influenza protein binds to the RNA transport protein staufen,102 which plays a key role delivering mRNA to neuronal dendrites. Dendrites contain mRNA, thought to code for many of the elements necessary for glutamatergic transmission, protein synthesis, and plasticity.103 Staufen plays a key role in dendritic mRNA transport, allowing delivery of these genes.104
The herpes virus also triggers the formation of varicosities along the axons of sensory neurones that stain for synaptophysin and closely resemble presynaptic terminals.105 These effects are mediated by viral activation of the rho guanosine triphosphate–binding protein CDC42, which also plays a key role in the maturation of presynaptic glutamatergic terminals.106 These varicosities serve as exit sites for the herpes virus, and if they correspond to presynaptic terminals, presumably, contain many of the components of these structures (eg, synapsins, complexins, and syntaxins). CDC42 also controls dendritic spine development,107 a process disrupted in the schizophrenic brain. The M1 protein of the influenza virus also binds to CDC42,108 whose suppression inhibits viral production.
Dopaminergic and Serotonergic Systems
Dopamine or serotonin receptors have not been implicated in the life cycles of any of the pathogens detailed in this review, although the 5HT-2 receptor (HTR2A) is an entry portal for the JC polyoma virus that causes demyelination (figure 3).109 Pathogenic infection does, however, have marked effects on serotonin metabolism. The activation of pathogen recognition receptors (the viral-activated kinase pkr and Toll receptors) stimulates the production of interferons and other type 1 cytokines (IL1B, TNF) involved in immune defense. These stimulate the expression and activity of the tryptophan-degrading enzyme, indoleamine 2,3-dioxygenase (INDO). Type 2 cytokines (eg, IL4, IL10) inhibit INDO activity. Such effects modify the concentrations of the serotonin precursor, tryptophan, and the enzyme product, N-formylkynurenine in different cellular compartments. Tryptophan is an essential nutrient for several microorganisms, including T gondii and chlamydial species,75 while N-formylkynurenine is a precursor of kynurenic acid, an endogenous antagonist of both nicotinic, and NMDA receptors. Kynurenic acid levels are elevated in the CSF of schizophrenic patients, and as already reviewed, such effects may well be due to the activation of inflammatory cascades by invading pathogens and could well contribute to the neurotransmitter imbalances at the root of schizophrenic symptoms.110 Tryptophan depletion also exerts antiviral effects against herpes strains, suggesting a role of the INDO pathway in general immune defense.75 It, thus, appears that infection, or its consequences, can have profound effects on the glutamate/dopamine/serotonin triad that plays such a significant role in the symptomatology of schizophrenia.
Antigenic Molecular Mimicry
Molecular mimicry has been reported between the myelin-oligodendrocyte protein(MOG) and structural glycoprotein E(2)of the rubella, or CMV-UL86 peptide, between a CMV envelope glycoprotein H peptide and tyrosinase (TYR), and between glutamate decarboxylase 1 (GAD1) and a rubella antigen in diabetic patients (figures 1 and 3). CMV infection correlates with immunoglobulin M anti–myelin-associated glycoprotein (anti-MAG)/sulfated glucuronyl paragloboside antibody levels in neuropathic conditions. Mimicry has also been reported between the NMDA receptor subunit GRIN2B and the influenza virus hemagglutinin (see Web site for references).
The eif2alpha Kinase Signaling Network
Viruses activate the eif2alpha kinase pkr (EIF2AK2), which phosphorylates eif2alpha (figure 4). This leads to an inhibition of protein translation because phosphorylated eif2alpha inhibits the actions of the translation initiation factor eif2b. Other stressors including starvation and oxidative and endoplasmic reticulum stress also activate this pathway via the stimulation of other eif2alpha kinases specific for each stressor. The protein translation pathway is also regulated by growth factors (eg, BDNF, EGF, FGF1, NRG1) and by glutamate receptors (eg, GRIN1, GRIN2A). Oligodendrocytes are particularly sensitive to eif2b malfunction because mutations in the genes coding for subunits of this complex cause vanishing white matter disease.3 The malfunction of this signaling network has been suggested as a potential cause of the oligodendrocyte cell death observed in schizophrenia.111 eif2Alpha phosphorylation also activates the transcription factor ATF4 (a binding partner of DISC1), whose outputs control a series of processes designed either to rectify the initial effects of the stressors (eg, the activation of oxidative stress–related or protein folding–related pathways in response to oxidative or endoplasmic reticulum stress).3
The viral pathogens implicated in schizophrenia activate the viral-sensitive kinase pkr and T gondii as well as bacterial pathogens can be linked to this network via their effects on Toll receptors. Toll-like receptors play an important role in activating the immune response defense mechanisms in response to viruses, bacteria, and other pathogens. Their signaling networks also activate the double-stranded DNA-responsive eif2alpha kinase pkr (EIF2AK2).112
A number of herpes viral products affect components of this pathway. For example, the HSV-1 gene ICP34.5 encodes for a phosphatase that mimics the actions of gadd34 (PPP1R15A) whose normal role is to dephosphorylate eif2alpha. The herpes protein US11 prevents pkr (EIF2AK2) activation by inhibiting its activator protein kinase, interferon-inducible double stranded RNA dependent activator (PRKRA).113 The herpes viral glycoprotein B (UL27) also binds to perk (EIF2AK3), preventing its activation by endoplasmic reticulum stress.114 The CMV also activates perk (EIF2AK3), independently of its effects on the viral kinase pkr, and induces splicing of XBP1 to its active form.115 The CMV protein IE2 also binds to nrf1 and nrf2 (NFE2L1 and 2),116 the transcription factors that control mitochondrial biogenesis and glutathione-related genes.
The latency transcript of the HSV is a promoter region of the viral genome responsible for controlling the reactivation of the virus. It contains 2 cyclic AMP response elements, which bind to cAMP response element-binding (CREB) and ATF4 (also known as CREB-2),117 suggesting that disruption of this signaling network may also affect viral latency. The human CMV major immediate-early enhancer also contains 5 functional cyclic AMP response elements and activation of the cyclic AMP signaling cascade by forskolin is able to modify the function of this enhancer and to regulate the progression of viral infection from a quiescent to a lytic form.118 The nonspecific phosphodiesterase inhibitor pentoxifylline also promotes the replication of the CMV119 (cf, CNP1, PDE4B). The presence of cAMP response elements in certain viral promoters of course suggests a host of potential influences of neurotransmitter-related cyclic AMP signaling pathways upon viral activation. Each of these pathogens therefore modulates a common stress-related signaling network, despite their involvement with different host genes and proteins prior to arrival at this convergence point.
Intrinsic and Extrinsic Pathways Etched Out by Susceptibility Genes?
It is of course possible to mimic certain of the symptoms of schizophrenia without advocating a role for exogenous pathogens. For example, amphetamine or phencyclidine can reproduce certain of the features of psychosis.120 DISC1 mutant mice also display a range of schizophrenia-like phenotypes, including ventricular enlargement, reduced parvalbumin immunoreactivity in cortical interneurones, hyperactivity, and modified sensorimotor gating.121 Disruption of ERBB4 signaling can also lead to hypomyelination and to subsequent dopaminergic hyperactivity in mice.122 These features (endophenotypes) could, thus, be induced in man by polymorphisms in any or several of the multiple susceptibility genes implicated in NMDA receptor function and plasticity, or in those involved in NRG1 or DISC1 signaling. The pathways carved out by such genes may well form an intrinsic network whose dysfunction may contribute to much of the schizophrenia pathology.
Prenatal infection or immune activation can also model certain of the features of schizophrenia in mice.123 The pathogens implicated in schizophrenia affect the same signaling network, the eif2alpha kinase pathway, as that influenced by many of the susceptibility genes, and as described above, interact in multiple ways with a number of gene candidates. Because these pathogenic and genetic pathways coalesce, it is possible to advocate some form of synergy between pathogens and susceptibility genes and pathways.
Others genes, eg, the immune-related genes, those coding for receptors (EGFR, FGFR1, IL10RA), or metabolizing compounds (AGA, B3GAT1, SIAT8B, GALNT7, PLA2, PSPA) that these pathogens bind or forage (GNPAT, TPH1), those involved in the nuclear/cytoplasmic transport of the influenza virus (KPNA3, RANBP5), or implicated in RNA polymerase function (MED12, MED15, ZNF74) may trace out pathways more related to the life cycles of the pathogens implicated in the disease than to the disease itself.
In many cases, dual functions may explain the greater importance of a particular gene. For example, NRG1 signaling affects glutamatergic signaling and oligodendrocyte function and might also be expected to affect influenza virulence via its effects on the viral transcriptase inhibitor EBP1.60,61 COMT might be expected to modify dopaminergic function but could also affect viral methylation via its effects on S-adenosylmethionine levels. The involvement of DISC1 in the control of the microtubule network suggests that it might be important both in viral traffic and in the rerouting of microtubules to the vacuoles formed by T gondii, as well as in important aspects of neurobiology. DISC1 also binds to ATF4 that plays such a key role in the stress- and pathogen-activated eif2alpha defence pathway. Currently, these ideas are speculative as the effects of NRG1; COMT or DISC1 variants on viral or parasitic virulence have not been studied. It is known, however, that several immune defense genes implicated in schizophrenia are also associated with infectious agents (see below).
Integrated Pathways: Genetic and Pathogenic Units Contributing to Disruption of the Same Networks
Schizophrenia is a complex disorder with multiple subpathologies or endophenotypes, which broadly match the gene families implicated in the disorder. For example, subsets of genes can be related to glutamatergic function, oligodendrocyte viability, neuronal growth and development, oxidative stress or dopaminergic, serotonergic, or GABAergic function, inter alia. These genes form a number of integrated networks (see “Introduction”). While certain important “hub genes,” eg, DISC1, appear to bridge these distinct families and networks, it seems currently unlikely that any single gene defect is able to provoke all the underlying pathologies that constitute the entire complexity of schizophrenia. The full-blown pathology is, thus, likely to relate to an integration of multiple phenotypes each related to their corresponding underlying genotypic subgroups.
When pathogen genes or proteins interact with components of these signaling networks, regardless of whether they interact with the susceptibility genes themselves, they essentially become a part thereof and could be regarded simply as yet another susceptibility unit (albeit foreign) capable of disrupting the same pathways etched out by the human susceptibility genes (see figures 1–4). The ability of the influenza virus to interfere with staufen or cdc42 could clearly affect both presynaptic and postsynaptic glutamatergic networks, while the effects of many pathogens on the PI3K/AKT survival pathway could evidently interfere with multiple growth factor signaling networks involved in plasticity or survival. Certain viruses are known to modify synaptic activity. Both herpes simplex and murine CMV infection results in the downregulation of NMDA receptor expression in PC12100 cells or in the hippocampus.101 In neuronal/glial coculture, the infection of murine astrocytes by CMV increases their sensitivity to ATP and glutamate, while the noninfected overlying neurons exhibit decreased synaptic activity as a result.124
Because each pathogen is able to activate the pkr signaling network that controls translation initiation (eif2B), that in turn appears to be crucial to oligodendrocyte viability and to synaptic plasticity, it seems plausible that compromised oligodendrocyte and synaptic function, as observed in schizophrenia, might be related to such pathogens. Indeed, prenatal influenza infection in mice results in white matter thinning in the corpus callosum125as well as atrophy of cortical pyramidal cells.126 Oligodendrocyte cell loss or demyelination has also been observed in response to infection with rubella127or herpes simplex in vitro.128 The CMV appears to preferentially infect human astrocytes and neurons, although oligodendrocyte infection has been reported. Interestingly, in a human oligodendrocyte cell line, resistance to CMV infection can be overcome by treatment of the cells with the protein kinase C activator, phorbol 12-myristate 13-acetate, or with a combination of dibutyryl cyclic AMP and a phosphodiesterase inhibitor (cf, PDE4B, CNP), suggesting that cellular activity can modulate the infection of such cells.129
Other schizophrenia-related endophenotypes induced by these pathogens include defects in prepulse inhibition in the offspring of mice infected with the influenza virus,130 while in adult mice, influenza infection results in decreased anxiety and impaired spatial learning.16 Thus, viruses, per se, can impact on several endophenotypes involved in schizophrenia. Prenatal immune challenge in mice also leads to dopaminergic-related hyperactivity and cognitive impairments in the adult progeny,123 although the mechanisms leading to such effects are poorly understood.
As with each susceptibility gene, it seems unlikely that any single pathogen could produce all the pathological features of schizophrenia per se, but each may affect certain signaling networks, related to different susceptibility gene subsets, that in turn impact on particular endophenotypes.
Comparative Figures in Relation to Other Gene Families and Pathological Processes
Viruses and pathogens interact with many human genes and proteins during their life cycle, and by chance, one would expect any large gene data set to contain a number of pathogen-interacting elements. To be able to define any overall statistical relevance, one would need a comprehensive data set of all the pathogen-host interactions for each pathogen. While several protein interaction databases exist for human proteins, such data sets are not currently available for human-pathogen interactions for the pathogens reviewed in this study. Perhaps, the most intensively studied virus is the Acquired immune deficiency syndrome (AIDS) human immunodeficiency virus (HIV) virus, for which a comprehensive data set is available at the HIV-1, Human Protein Interaction Database http://www.ncbi.nlm.nih.gov/RefSeq/HIVInteractions. This data set collates interactions between the virus and human genes and proteins (protein-protein binding, effects on transcription, phosphorylation, expression, etc). Currently, 1510 viral-host interactions are reported from a total of 29 277 known human proteins. The HIV-1 virus, thus, interacts in some way with about 5.2% of all known human proteins. Comparative figures as a percentage of 245 schizophrenia-related gene candidates are influenza = 21%, HSV-1 = 22%, CMV = 18%, rubella = 12.6%, and T gondii = 16%, suggesting a generalized enrichment of pathogen-related genes in the schizophrenia gene data set. A more rigorous analysis is not currently possible given the incomplete data sets, both for host-pathogen interactions and for the growing list of genes implicated in schizophrenia.
In total, 114 of 245 susceptibility genes (46%) can be related to the life cycles of one or other of these pathogens, a figure that is likely to increase when other species such as the Chlamydia family or the borna virus, also implicated as risk factors, are taken into account. These figures also compare favorably with the percentage of genes involved in endogenous brain processes related to the pathology of schizophrenia. For example, 24% of the suspect genes encode for proteins related to the postsynaptic density, 16% are involved in growth-related processes (eg, neuritic or axonal growth), 14% can be related to oligodendrocyte function, 7% to oxidative stress, and 7% to dopaminergic function (see supplementary table 1 at http://www.polygenicpathways.co.uk/szfam.htm).
Susceptibility Genes Involved in Infection
Human gene variants also modify infection, although there are relatively few genetic association studies in relation to the pathogens or genes reviewed in this study. Data from the Hugenet phenopedia131 (http://www.hugenavigator.net), which lists gene association studies for a number of infectious and other diseases, and a Medline search, are shown in supplementary table 2 (http://www.polygenicpathways.co.uk/infectassoc.htm), details of which are summarized in table 1. In all, 5 of 10 genes (50%) (APOE, HLA-B, IL10, MICB, IL18RAP) implicated in HSV-1 infection and 9 of 14 genes (61%) (HLA-B, HLA-DRB1, IL1RN, IL10, IL12B, IL18, IL18R1, IL18RAP, TNF) implicated in CMV infection have also been implicated in schizophrenia. Although the numbers are small, and the genes involved almost exclusively related to the innate immune system, the enrichment does illustrate that certain genes implicated in schizophrenia are also related to infection. Clearly, it would be interesting to investigate the effects of polymorphisms in more relevant genes related to cerebral function such as NRG1 or DISC1.
Of course, the physiological significance of any particular interaction relates to the role of the human gene/protein in the context of schizophrenia pathology, whether the pathogen-host interaction is able to disrupt the role of the human gene in a relevant manner, or whether the host polymorphism is able to affect the pathogen life cycle.
A number of whole-genome association analyses are in progress in relation to schizophrenia and related disorders. Antibody serotyping, pathogen DNA/RNA detection in the same blood samples, or a detailed knowledge of infection history would permit multifactorial analysis of any permutations of gene-pathogen-disease interactions. Such analyses are clearly warranted and would help to answer many of these questions. More direct analyses, eg, viral/human 2-hybrid experiments using “normal” and variant susceptibility genes would also be informative.
Support From Microarray and Expression Studies
Indirect support for pathogen involvement, or immune activation in schizophrenia, derives from a series of microarray studies on schizophrenic brain tissue, which have shown marked changes in the expression of genes related to the immune and inflammatory system.132,133 In a collective analysis of multiple brain gene expression studies from the Stanley Research Consortium, genes related to multiple histocompatibility complex receptor activity and antigen processing and presentation figure among the top 10 of the Gene Ontology classified processes.134 In addition, a recent study has found that alpha-defensins are selectively elevated in T-cell lysates from schizophrenic patients.135 Defensins, which are produced by immunocompetent cells, and triggered by infection, are small endogenous peptide antibiotics with antiviral, bactericidal, and immunomodulatory properties.136 These studies clearly highlight the importance of pathogen and immune defense in relation to schizophrenia in adulthood.
Natural Selection and the Evolutionary Perspective
It has been suggested that schizophrenia susceptibility genes have been maintained in the human population at a frequency of about 1%, despite negative fitness, because of some form of positive selection pressure.137 In other words, certain gene variants must be of some benefit to the population. Many of these genes are implicated in pathogen life cycles and may well influence their virulence. For example, NRG1 signaling releases a factor that inhibits the replication of the influenza virus.60,61 Certain gene variants may favor the pathogens, eg, polymorphisms in genes related to the IL18 signaling pathway138 or in the natural killer cell ligand MICB139 are associated with both herpes viral infection and with schizophrenia. Other variants associated with schizophrenia could well protect in some way against these agents. This is the case for a CCR5 delta32 deletion which protects against T gondii and other infections.140 This deletion has also been implicated in schizophrenia but only in late-onset patients, suggesting either that it was associated with this particular type of disease or that it delays the onset of schizophrenia,141 a supposition that would be compatible with an anti-infective potential.
The mothers of schizophrenic patients born during influenza pandemics evidently survived the virus, but their progeny may bear the scars of the infection, eg, activation of the eif2alpha kinase signaling cascade by viral, cytokine, or stress-activated kinases. The selection pressure maintaining certain genes in the population could, thus, well be related to anti- as well as proviral effects, which would diminish mortality, at the expense of collateral damage, that nevertheless has devastating psychiatric consequences.
Genetic Implications
Association studies compare the frequencies of a particular gene variant between a normal and disease-affected population. They are notoriously inconsistent, and problems of replication between populations are the norm rather than the exception. Because these pathogens so clearly interact with many of the schizophrenia susceptibility genes during the course of their life cycles, many of the genetic associations could plausibly be related to extrinsic factors that contribute to the disease, as well as to the intrinsic disease process itself. It seems reasonable to assume that polymorphisms in such pathogen-related genes may well affect the infectivity or virulence of the pathogen, or the ability of the host to retaliate, and that the pathogens will in turn affect the function of the susceptibility genes, with which they interact.
Specific infections have distinct geographical and temporal distributions. If any particular virus interacts with a particular susceptibility gene or its product, the risk-promoting effects of the gene may be conditional on the presence of the virus at a particular time, when its effects are relevant to the genesis of particular subpathologies of schizophrenia (see also Pearce19). In addition, genes and proteins are expressed at different times and in different tissues and cellular compartments during our lifespan, at times, which could coincide (or not) with infection or with the localization of the pathogen. Such conditional effects could well explain some of the heterogeneity in genetic studies. As shown in table 1, the relationship of different pathogens to different genes is similarly heterogeneous. It seems likely that a stratification of genetic association data in relation to pathogenic history could markedly influence the strength of association for many candidate genes.
Medical Implications and the Timing of Infection
A less than 100% concordance rate for schizophrenia in identical twins142 suggests that genes per se do not cause the disease. The pathogens implicated in schizophrenia are also common and are unlikely to be implicated in schizophrenia in all individuals. However, the convergence of genes and pathogens on a common signaling cascade and a plethora of direct interactions between host and pathogen genes and proteins suggests how genetic variants could contribute to disease in infected individuals and how infections might contribute to disease in populations with particular genetic variants.
One could reasonably argue that, in some cases, these pathogens may well contribute to certain features of the disease but only in genetically susceptible individuals. If so, then elimination of the pathogens or prevention by vaccination could have a major effect on the incidence and perhaps the progression of the disease, as already suggested by several authors.7,143–145 Several infections implicated in schizophrenia occur many years before the onset of the disease, eg, maternal influenza infections during pregnancy or CMV or mumps infections during early childhood. There is no obvious indication that a small percentage of such patients might later develop a psychiatric disease and no reason why antiviral agents should be effective in later life when psychosis develops. However, vaccinations preventing such infections could have a significant effect on the incidence of psychiatric disease at later periods. A decline in the incidence of schizophrenia has, indeed, been reported in relation to the introduction of the polio vaccine.146 In addition, common childhood diseases, usually considered as benign, should perhaps be viewed from another perspective given the accumulating evidence that such infections can markedly increase the risk of developing psychiatric disorders later in life and a genetic rationale as to how this might affect particular individuals.
Other pathogens that have been detected in adulthood (eg, herpes viruses, Chlamydia, and T gondii) suggest that infections in adult life may also contribute to schizophrenia pathology. These may themselves be amenable to therapy, and their detection and elimination may have beneficial effects in schizophrenia. Interestingly, the use of valacyclovir has been reported to reduce symptomatology in schizophrenia patients seropositive for CMV.144 The use of activated immune cells coupled with antibiotic treatment has also been reported to improve psychiatric symptomatology in schizophrenic patients with Chlamydia infection.145 The situation may be complicated by the possibility that different and, perhaps, multiple pathogens may be implicated in different populations, although all converge on the same defence network (figure 4). Pathogen screening, perhaps, using antibody or gene arrays may well be useful in identifying these and other potential pathogens, some of which are amenable to therapy. Such therapy might, however, need to be tailored to individual pathogens.
Conclusion
Genes and pathogens both have a role to play in the genesis of schizophrenia. These factors appear to be interdependent. The pathways traced out by schizophrenia susceptibility genes relate both to intrinsic signaling networks and to the pathways specifically used by pathogens to commandeer host cell physiology. The host and pathogen genes and proteins together form an integrated transgenomic network (figure 4) that may govern the risk of developing schizophrenia. The genetic and medical implications are far reaching and likely to apply to the many other polygenic diseases in which pathogens also act as risk factors. Perhaps, an important take-home message is that many infections, generally considered as relatively benign, could in fact be a contributory factor to schizophrenia and that their identification, prevention, and treatment could have marked effects on the disease.
Supplementary Material
Supplementary tables 1 and 2 and other data are available at http://schizophreniabulletin.oxfordjournals.org.
References
- 1.Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, et al. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- 2.Carter CJ. Schizophrenia susceptibility genes converge on interlinked pathways related to glutamatergic transmission and long-term potentiation, oxidative stress and oligodendrocyte viability. Schizophr Res. 2006;86:1–14. doi: 10.1016/j.schres.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 3.van der Knaap MS, Pronk JC, Scheper GC. Vanishing white matter disease. Lancet Neurol. 2006;5:413–423. doi: 10.1016/S1474-4422(06)70440-9. [DOI] [PubMed] [Google Scholar]
- 4.Adams W, Kendell RE, Hare EH, Munk-Jorgensen PE. pidemiological evidence that maternal influenza contributes to the aetiology of schizophrenia. An analysis of Scottish, English, and Danish data. Br J Psychiatry. 1993;163:522–534. doi: 10.1192/bjp.163.4.522. [DOI] [PubMed] [Google Scholar]
- 5.Buka SL, Cannon TD, Torrey EF, Yolken RH. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol Psychiatry. 2007;63:809–815. doi: 10.1016/j.biopsych.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol Psychiatry. 2008;13:470–479. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]
- 8.Dalman C, Allebeck P, Gunnell D, Harrison G, Kristensson K, Lewis G, et al. Infections in the CNS during childhood and the risk of subsequent psychotic illness: a cohort study of more than one million Swedish subjects. Am J Psychiatry. 2008;165:59–65. doi: 10.1176/appi.ajp.2007.07050740. [DOI] [PubMed] [Google Scholar]
- 9.Prasad KM, Shirts BH, Yolken RH, Keshavan MS, Nimgaonkar VL. Brain morphological changes associated with exposure to HSV1 in first-episode schizophrenia. Mol Psychiatry. 2007;12:105–113. doi: 10.1038/sj.mp.4001915. 1. [DOI] [PubMed] [Google Scholar]
- 10.Niebuhr DW, Millikan AM, Yolken R, Li Y, Weber NS. Results from a hypothesis generating case-control study: herpes family viruses and schizophrenia among military personnel. Schizophr Bull. doi: 10.1093/schbul/sbm139. December 21, 2007; doi:10.1093/schbul/sbm139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niebuhr DW, Millikan AM, Cowan DN, Yolken R, Li Y, Weber NS. Selected infectious agents and risk of schizophrenia among U.S. military personnel. Am J Psychiatry. 2008;165:99–106. doi: 10.1176/appi.ajp.2007.06081254. [DOI] [PubMed] [Google Scholar]
- 12.Torrey EF, Leweke MF, Schwarz MJ, Mueller N, Bachmann S, Schroeder J, et al. Cytomegalovirus and schizophrenia. CNS Drugs. 2006;20:879–885. doi: 10.2165/00023210-200620110-00001. [DOI] [PubMed] [Google Scholar]
- 13.Conejero-Goldberg C, Torrey EF, Yolken RH. Herpesviruses and Toxoplasma gondii in orbital frontal cortex of psychiatric patients. Schizophr Res. 2003;60:65–69. doi: 10.1016/s0920-9964(02)00160-3. [DOI] [PubMed] [Google Scholar]
- 14.Gordon L, McQuaid S, Cosby SL. Detection of herpes simplex virus (types 1 and 2) and human herpesvirus 6 DNA in human brain tissue by polymerase chain reaction. Clin Diagn Virol. 1996;6:33–40. doi: 10.1016/0928-0197(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 15.Moises HW, Ruger R, Reynolds GP, Fleckenstein B. Human cytomegalovirus DNA in the temporal cortex of a schizophrenic patient. Eur Arch Psychiatry Neurol Sci. 1988;238:110–113. doi: 10.1007/BF00452786. [DOI] [PubMed] [Google Scholar]
- 16.Beraki S, Aronsson F, Karlsson H, Ogren SO, Kristensson K. Influenza A virus infection causes alterations in expression of synaptic regulatory genes combined with changes in cognitive and emotional behaviors in mice. Mol Psychiatry. 2005;10:299–308. doi: 10.1038/sj.mp.4001545. [DOI] [PubMed] [Google Scholar]
- 17.Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophr Bull. 2007;33:745–751. doi: 10.1093/schbul/sbm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yolken RH, TorreyViruses, schizophrenia EF, bipolar disorder Clin Microbiol Rev. 1995;8:131–145. doi: 10.1128/cmr.8.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearce BD. Schizophrenia and viral infection during neurodevelopment: a focus on mechanisms. Mol Psychiatry. 2001;6:634–646. doi: 10.1038/sj.mp.4000956. [DOI] [PubMed] [Google Scholar]
- 20.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Zuckerman L, Weiner I. Post-pubertal emergence of disrupted latent inhibition following prenatal immune activation. Psychopharmacology (Berl) 2003;169:308–313. doi: 10.1007/s00213-003-1461-7. [DOI] [PubMed] [Google Scholar]
- 23.Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci USA. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams BR, PKR a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 25.Selten JP, Brown AS, Moons KG, Slaets JP, Susser ES, Kahn RS. Prenatal exposure to the 1957 influenza pandemic and non-affective psychosis in The Netherlands. Schizophr Res. 1999;38:85–91. doi: 10.1016/s0920-9964(99)00005-5. [DOI] [PubMed] [Google Scholar]
- 26.Baron S. Medical Microbiology. (4th ed) 1996 Galreston, TX: The University of Texas Medical Branch at Galveston. [PubMed] [Google Scholar]
- 27.Carter CJ. EIF2B and oligodendrocyte survival: where nature and nurture meet in bipolar disorder and schizophrenia? Schizophr Bull. 2007;33:1343–1353. doi: 10.1093/schbul/sbm007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu PW, Lin LZ, Hsu SD, Hsu JB, Huang HD. ViTa: prediction of host microRNAs targets on viruses. Nucleic Acids Res. 2007;35:D381–D385. doi: 10.1093/nar/gkl1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varki A, Cummings R, Esko J, Freeze H, Hunt G, Marth J. Essentials of Glycobiology. Woodbury, NY: Cold Spring Harbor Laboratory press; 2008. [PubMed] [Google Scholar]
- 30.Luther P, Cushley W, Holzer C, Desselberger U, Oxford JS. Acetylated galactosamine is a receptor for the influenza C virus glycoprotein. Arch Virol. 1988;101:247–254. doi: 10.1007/BF01311005. [DOI] [PubMed] [Google Scholar]
- 31.Serafini-Cessi F, Dall'Olio F, Malagolini N, Campadelli-Fiume G. Temporal aspects of O-glycosylation of glycoprotein C from herpes simplex virus type-1. Biochem J. 1989;262:479–484. doi: 10.1042/bj2620479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogawa-Goto K, Arao Y, Ito Y, Ogawa T, Abe T, Kurata T, et al. Binding of human cytomegalovirus to sulfated glucuronyl glycosphingolipids and their inhibitory effects on the infection. J Gen Virol. 1998;79(pt 10):2533–2541. doi: 10.1099/0022-1317-79-10-2533. [DOI] [PubMed] [Google Scholar]
- 33.Mastromarino P, Cioe L, Rieti S, Orsi N. Role of membrane phospholipids and glycolipids in the Vero cell surface receptor for rubella virus. Med Microbiol Immunol. 1990;179:105–114. doi: 10.1007/BF00198531. [DOI] [PubMed] [Google Scholar]
- 34.Campadelli-Fiume G, Cocchi F, Menotti L, Lopez M. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev Med Virol. 2000;10:305–319. doi: 10.1002/1099-1654(200009/10)10:5<305::aid-rmv286>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 35.Reymond N, Garrido-Urbani S, Borg JP, Dubreuil P, Lopez M. PICK-1: a scaffold protein that interacts with Nectins and JAMs at cell junctions. FEBS Lett. 2005;579:2243–2249. doi: 10.1016/j.febslet.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Kaner RJ, Baird A, Mansukhani A, Basilico C, Summers BD, Florkiewicz RZ, et al. Fibroblast growth factor receptor is a portal of cellular entry for herpes simplex virus type 1. Science. 1990;248:1410–1413. doi: 10.1126/science.2162560. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- 38.Frolov A, Schuller K, Tzeng CW, Cannon EE, Ku BC, Howard JH, et al. ErbB3 expression and dimerization with EGFR influence pancreatic cancer cell sensitivity to erlotinib. Cancer Biol Ther. 2007:6. doi: 10.4161/cbt.6.4.3849. [DOI] [PubMed] [Google Scholar]
- 39.Thaminy S, Auerbach D, Arnoldo A, Stagljar I. Identification of novel ErbB3-interacting factors using the split-ubiquitin membrane yeast two-hybrid system. Genome Res. 2003;13:1744–1753. doi: 10.1101/gr.1276503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spencer JV. The cytomegalovirus homolog of interleukin-10 requires phosphatidylinositol 3-kinase activity for inhibition of cytokine synthesis in monocytes. J Virol. 2007;81:2083–2086. doi: 10.1128/JVI.01655-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerr JR. Cell adhesion molecules in the pathogenesis of and host defence against microbial infection. Mol Pathol. 1999;52:220–230. doi: 10.1136/mp.52.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sousa S, Lecuit M, Cossart P. Microbial strategies to target, cross or disrupt epithelia. Curr Opin Cell Biol. 2005;17:489–498. doi: 10.1016/j.ceb.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Hotta K, Motoi Y, Okutani A, Kaku Y, Noguchi A, Inoue S, et al. Role of GPI-anchored NCAM-120 in rabies virus infection. Microbes Infect. 2007;9:167–174. doi: 10.1016/j.micinf.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Zheng A, Yuan F, Li Y, Zhu F, Hou P, Li J, et al. Claudin-6 and claudin-9 function as additional co-receptors for hepatitis C virus. J Virol. 2007;81:12465–12471. doi: 10.1128/JVI.01457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoon M, Spear PG. Disruption of adherens junctions liberates nectin-1 to serve as receptor for herpes simplex virus and pseudorabies virus entry. J Virol. 2002;76:7203–7208. doi: 10.1128/JVI.76.14.7203-7208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawada M, Hachiya Y, Arihiro A, Mizoguchi E. Role of mammalian chitinases in inflammatory conditions. Keio J Med. 2007;56:21–27. doi: 10.2302/kjm.56.21. [DOI] [PubMed] [Google Scholar]
- 47.Sieczkarski SB, Whittaker GR. Dissecting virus entry via endocytosis. J Gen Virol. 2002;83:1535–1545. doi: 10.1099/0022-1317-83-7-1535. [DOI] [PubMed] [Google Scholar]
- 48.Setty SR, Tenza D, Truschel ST, Chou E, Sviderskaya EV, Theos AC, et al. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol Biol Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leopold PL, Pfister KK. Viral strategies for intracellular trafficking: motors and microtubules. Traffic. 2006;7:516–523. doi: 10.1111/j.1600-0854.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 50.Wileman T. Aggresomes and pericentriolar sites of virus assembly: cellular defense or viral design? Annu Rev Microbiol. 2007;61:149–167. doi: 10.1146/annurev.micro.57.030502.090836. [DOI] [PubMed] [Google Scholar]
- 51.Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1067–1078. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- 52.Miyoshi K, Asanuma M, Miyazaki I, Diaz-Corrales FJ, Katayama T, Tohyama M, et al. DISC1 localizes to the centrosome by binding to kendrin. Biochem Biophys Res Commun. 2004;317:1195–1199. doi: 10.1016/j.bbrc.2004.03.163. [DOI] [PubMed] [Google Scholar]
- 53.Khor R, McElroy LJ, Whittaker GR. The ubiquitin-vacuolar protein sorting system is selectively required during entry of influenza virus into host cells. Traffic. 2003;4:857–868. doi: 10.1046/j.1398-9219.2003.0140.x. [DOI] [PubMed] [Google Scholar]
- 54.Braakman I, van Anken E. Folding of viral envelope glycoproteins in the endoplasmic reticulum. Traffic. 2000;1:533–539. doi: 10.1034/j.1600-0854.2000.010702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006;13:393–403. doi: 10.1038/sj.cdd.4401833. [DOI] [PubMed] [Google Scholar]
- 56.Mayer D, Molawi K, Martinez-Sobrido L, Ghanem A, Thomas S, Baginsky S, et al. Identification of cellular interaction partners of the influenza virus ribonucleoprotein complex and polymerase complex using proteomic-based approaches. J Proteome Res. 2007;6:672–682. doi: 10.1021/pr060432u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rice SA, Long MC, Lam V, Spencer CA. RNA polymerase II is aberrantly phosphorylated and localized to viral replication compartments following herpes simplex virus infection. J Virol. 1994;68:988–1001. doi: 10.1128/jvi.68.2.988-1001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ikeda K, Stuehler T, Meisterernst M. The H1 and H2 regions of the activation domain of herpes simplex virion protein 16 stimulate transcription through distinct molecular mechanisms. Genes Cells. 2002;7:49–58. doi: 10.1046/j.1356-9597.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- 59.Romanos MA, Hay AJ. Identification of the influenza virus transcriptase by affinity-labeling with pyridoxal 5′-phosphate. Virology. 1984;132:110–117. doi: 10.1016/0042-6822(84)90095-3. [DOI] [PubMed] [Google Scholar]
- 60.Honda A, Okamoto T, Ishihama A. Host factor Ebp1: selective inhibitor of influenza virus transcriptase. Genes Cells. 2007;12:133–142. doi: 10.1111/j.1365-2443.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- 61.Yoo JY, Wang XW, Rishi AK, Lessor T, Xia XM, Gustafson TA, et al. Interaction of the PA2G4 (EBP1) protein with ErbB-3 and regulation of this binding by heregulin. Br J Cancer. 2000;82:683–690. doi: 10.1054/bjoc.1999.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawada J, Kimura H, Kamachi Y, Nishikawa K, Taniguchi M, Nagaoka K, et al. Analysis of gene-expression profiles by oligonucleotide microarray in children with influenza. J Gen Virol. 2006;87:1677–1683. doi: 10.1099/vir.0.81670-0. [DOI] [PubMed] [Google Scholar]
- 63.De Clercq E. 2001 ASPET Otto Krayer Award Lecture: Molecular targets for antiviral agents. J Pharmacol Exp Ther. 2001;297:1–10. [PubMed] [Google Scholar]
- 64.Woyciniuk P, Linder M, Scholtissek C. The methyltransferase inhibitor Neplanocin A interferes with influenza virus replication by a mechanism different from that of 3-deazaadenosine. Virus Res. 1995;35:91–99. doi: 10.1016/0168-1702(94)00085-q. [DOI] [PubMed] [Google Scholar]
- 65.Vogel U, Kunerl M, Scholtissek C. Influenza A virus late mRNAs are specifically retained in the nucleus in the presence of a methyltransferase or a protein kinase inhibitor. Virology. 1994;198:227–233. doi: 10.1006/viro.1994.1025. [DOI] [PubMed] [Google Scholar]
- 66.Minarovits J. Epigenotypes of latent herpesvirus genomes. Curr Top Microbiol Immunol. 2006;310:61–80. doi: 10.1007/3-540-31181-5_5. [DOI] [PubMed] [Google Scholar]
- 67.Macnab JC, Adams RL, Rinaldi A, Orr A, Clark L. Hypomethylation of host cell DNA synthesized after infection or transformation of cells by herpes simplex virus. Mol Cell Biol. 1988;8:1443–1448. doi: 10.1128/mcb.8.4.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shimabukuro M, Sasaki T, Imamura A, Tsujita T, Fuke C, Umekage T, et al. Global hypomethylation of peripheral leukocyte DNA in male patients with schizophrenia: a potential link between epigenetics and schizophrenia. J Psychiatr Res. 2007;41:1042–1046. doi: 10.1016/j.jpsychires.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 69.Shields DJ, Lingrell S, Agellon LB, Brosnan JT, Vance DE. Localization-independent regulation of homocysteine secretion by phosphatidylethanolamine N-methyltransferase. J Biol Chem. 2005;280:27339–27344. doi: 10.1074/jbc.M504658200. [DOI] [PubMed] [Google Scholar]
- 70.Yassin MS, Cheng H, Ekblom J, Oreland L. Inhibitors of catecholamine metabolizing enzymes cause changes in S-adenosylmethionine and S-adenosylhomocysteine in the rat brain. Neurochem Int. 1998;32:53–59. doi: 10.1016/s0197-0186(97)00047-8. [DOI] [PubMed] [Google Scholar]
- 71.Guidotti A, Ruzicka W, Grayson DR, Veldic M, Pinna G, Davis JM, et al. S-adenosyl methionine and DNA methyltransferase-1 mRNA overexpression in psychosis. Neuroreport. 2007;18:57–60. doi: 10.1097/WNR.0b013e32800fefd7. [DOI] [PubMed] [Google Scholar]
- 72.Levenson JM. DNA (cytosine-5) methyltransferase inhibitors: a potential therapeutic agent for schizophrenia. Mol Pharmacol. 2007;71:635–637. doi: 10.1124/mol.106.033266. [DOI] [PubMed] [Google Scholar]
- 73.Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, et al. reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci USA. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, et al. Defective corticogenesis and reduction in reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry. 1999;4:145–154. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- 75.MacKenzie CR, Heseler K, Muller A, Daubener W. Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion versus production of toxic kynurenines. Curr Drug Metab. 2007;8:237–244. doi: 10.2174/138920007780362518. [DOI] [PubMed] [Google Scholar]
- 76.Schwan TG, Battisti JM, Porcella SF, Raffel SJ, Schrumpf ME, Fischer ER, et al. Glycerol-3-phosphate acquisition in spirochetes: distribution and biological activity of glycerophosphodiester phosphodiesterase (GlpQ) among Borrelia species. J Bacteriol. 2003;185:1346–1356. doi: 10.1128/JB.185.4.1346-1356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi WY, Nam HW, Youn JH, Kim DJ, Kim WK, Kim WS. The effect of cyclic AMP on the growth of Toxoplasma gondii in vitro. Kisaengchunghak Chapchi. 1990;28:71–78. doi: 10.3347/kjp.1990.28.2.71. [DOI] [PubMed] [Google Scholar]
- 78.Blumenschein TM, Friedrich N, Childs RA, Saouros S, Carpenter EP, Campanero-Rhodes MA, et al. Atomic resolution insight into host cell recognition by Toxoplasma gondii. EMBO J. 2007;26:2808–2820. doi: 10.1038/sj.emboj.7601704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Furtado GC, Cao Y, Joiner KA. Laminin on Toxoplasma gondii mediates parasite binding to the beta 1 integrin receptor alpha 6 beta 1 on human foreskin fibroblasts and Chinese hamster ovary cells. Infect Immun. 1992;60:4925–4931. doi: 10.1128/iai.60.11.4925-4931.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Groc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, Chavis P. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein reelin. J Neurosci. 2007;27:10165–10175. doi: 10.1523/JNEUROSCI.1772-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aliberti J, Valenzuela JG, Carruthers VB, Hieny S, Andersen J, Charest H, et al. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat Immunol. 2003;4:485–490. doi: 10.1038/ni915. [DOI] [PubMed] [Google Scholar]
- 82.Coppens I, Dunn JD, Romano JD, Pypaert M, Zhang H, Boothroyd JC, et al. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell. 2006;125:261–274. doi: 10.1016/j.cell.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 83.Ahn HJ, Kim S, Kim HE, Nam HW. Interactions between secreted GRA proteins and host cell proteins across the paratitophorous vacuolar membrane in the parasitism of Toxoplasma gondii. Korean J Parasitol. 2006;44:303–312. doi: 10.3347/kjp.2006.44.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest. 2006;116:2366–2377. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hale BG, Jackson D, Chen YH, Lamb RA, Randall RE. Influenza A virus NS1 protein binds p85beta and activates phosphatidylinositol-3-kinase signaling. Proc Natl Acad Sci USA. 2006;103:14194–14199. doi: 10.1073/pnas.0606109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ehrhardt C, Wolff T, Pleschka S, Planz O, Beermann W, Bode JG, et al. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J Virol. 2007;81:3058–3067. doi: 10.1128/JVI.02082-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 88.Palamara AT, Perno CF, Ciriolo MR, Dini L, Balestra E, D'Agostini C, et al. Evidence for antiviral activity of glutathione: in vitro inhibition of herpes simplex virus type 1 replication. Antiviral Res. 1995;27:237–253. doi: 10.1016/0166-3542(95)00008-a. [DOI] [PubMed] [Google Scholar]
- 89.Nencioni L, Iuvara A, Aquilano K, Ciriolo MR, Cozzolino F, Rotilio G, et al. Influenza A virus replication is dependent on an antioxidant pathway that involves GSH and Bcl-2. FASEB J. 2003;17:758–760. doi: 10.1096/fj.02-0508fje. [DOI] [PubMed] [Google Scholar]
- 90.Stommel EW, Cho E, Steide JA, Seguin R, Barchowsky A, Schwartzman JD, et al. Identification and role of thiols in Toxoplasma gondii egress. Exp Biol Med (Maywood) 2001;226:229–236. doi: 10.1177/153537020122600311. [DOI] [PubMed] [Google Scholar]
- 91.Cai J, Chen Y, Seth S, Furukawa S, Compans RW, Jones DP. Inhibition of influenza infection by glutathione. Free Radic Biol Med. 2003;34:928–936. doi: 10.1016/s0891-5849(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 92.Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. doi: 10.1038/sj.npp.1301624. November 14, 2007;doi:10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- 93.Sherman LA, Burke TA, Biggs JA. Extracellular processing of peptide antigens that bind class I major histocompatibility molecules. J Exp Med. 1992;175:1221–1226. doi: 10.1084/jem.175.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guil S, Rodriguez-Castro M, Aguilar F, Villasevil EM, Anton LC, Del Val M. Need for tripeptidyl-peptidase II in major histocompatibility complex class I viral antigen processing when proteasomes are detrimental. J Biol Chem. 2006;281:39925–39934. doi: 10.1074/jbc.M608522200. [DOI] [PubMed] [Google Scholar]
- 95.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 96.Geist LJ, Monick MM, Stinski MF, Hunninghake GW. Cytomegalovirus immediate early genes prevent the inhibitory effect of cyclosporin A on interleukin 2 gene transcription. J Clin Invest. 1992;90:2136–2140. doi: 10.1172/JCI116099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zheng C, Lin J. [The role of L-arginine and L-citrulline in activated macrophage against Toxoplasma gondii infection in vitro] Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 1998;16:326–330. [PubMed] [Google Scholar]
- 98.Lipton SA. Models of neuronal injury in AIDS: another role for the NMDA receptor? Trends Neurosci. 1992;15:75–79. doi: 10.1016/0166-2236(92)90013-x. [DOI] [PubMed] [Google Scholar]
- 99.Brask J, Chauhan A, Hill RH, Ljunggren HG, Kristensson K. Effects on synaptic activity in cultured hippocampal neurons by influenza A viral proteins. J Neurovirol. 2005;11:395–402. doi: 10.1080/13550280500186916. [DOI] [PubMed] [Google Scholar]
- 100.Holmes KD, Cassam AK, Chan B, Peters AA, Weaver LC, Dekaban GA. A multi-mutant herpes simplex virus vector has minimal cytotoxic effects on the distribution of filamentous actin, alpha-actinin 2 and a glutamate receptor in differentiated PC12 cells. J Neurovirol. 2000;6:33–45. doi: 10.3109/13550280009006380. [DOI] [PubMed] [Google Scholar]
- 101.Kosugi I, Kawasaki H, Tsuchida T, Tsutsui Y. Cytomegalovirus infection inhibits the expression of N-methyl-D-aspartate receptors in the developing mouse hippocampus and primary neuronal cultures. Acta Neuropathol (Berl) 2005;109:475–482. doi: 10.1007/s00401-005-0987-7. [DOI] [PubMed] [Google Scholar]
- 102.Falcon AM, Fortes P, Marion RM, Beloso A, Ortin J. Interaction of influenza virus NS1 protein and the human homologue of Staufen in vivo and in vitro. Nucleic Acids Res. 1999;27:2241–2247. doi: 10.1093/nar/27.11.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bramham CR, Wells DG. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci. 2007;8:776–789. doi: 10.1038/nrn2150. [DOI] [PubMed] [Google Scholar]
- 104.Hillebrand J, Barbee SA, Ramaswami M. P-body components, microRNA regulation, and synaptic plasticity. Scientific World J. 2007;7:178–190. doi: 10.1100/tsw.2007.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Regge N, Nauwynck HJ, Geenen K, Krummenacher C, Cohen GH, Eisenberg RJ, et al. Alpha-herpesvirus glycoprotein D interaction with sensory neurons triggers formation of varicosities that serve as virus exit sites. J Cell Biol. 2006;174:267–275. doi: 10.1083/jcb.200510156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen W, Wu B, Zhang Z, Dou Y, Rao ZR, Chen YR, et al. Activity-induced rapid synaptic maturation mediated by presynaptic cdc42 signaling. Neuron. 2006;50:401–414. doi: 10.1016/j.neuron.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 107.Scott EK, Reuter JE, Luo L. Small GTPase Cdc42 is required for multiple aspects of dendritic morphogenesis. J Neurosci. 2003;23:3118–3123. doi: 10.1523/JNEUROSCI.23-08-03118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hui EK, Barman S, Tang DH, France B, Nayak DP. YRKL sequence of influenza virus M1 functions as the L domain motif and interacts with VPS28 and Cdc42. J Virol. 2006;80:2291–2308. doi: 10.1128/JVI.80.5.2291-2308.2006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Elphick GF, Querbes W, Jordan JA, Gee GV, Eash S, Manley K, et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306:1380–1383. doi: 10.1126/science.1103492. [DOI] [PubMed] [Google Scholar]
- 110.Muller N, Schwarz MJ. The immunological basis of glutamatergic disturbance in schizophrenia: towards an integrated view. J Neural Transm Suppl. 2007;72:269–280. doi: 10.1007/978-3-211-73574-9_33. [DOI] [PubMed] [Google Scholar]
- 111.Carter CJ. EIF2B and oligodendrocyte survival: where nature and nurture meet in bipolar disorder and schizophrenia? Schizophr Bull. 2007;33:1343–1353. doi: 10.1093/schbul/sbm007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Horng T, Barton GM, Medzhitov RTIRAP. an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2:835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 113.Mohr I. Neutralizing innate host defenses to control viral translation in HSV-1 infected cells. Int Rev Immunol. 2004;23:199–220. doi: 10.1080/08830180490265600. [DOI] [PubMed] [Google Scholar]
- 114.Mulvey M, Arias C, Mohr I. Maintenance of endoplasmic reticulum (ER) homeostasis in herpes simplex virus type 1-infected cells through the association of a viral glycoprotein with PERK, a cellular ER stress sensor. J Virol. 2007;81:3377–3390. doi: 10.1128/JVI.02191-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Isler JA, Skalet AH, Alwine JC. Human cytomegalovirus infection activates and regulates the unfolded protein response. J Virol. 2005;79:6890–6899. doi: 10.1128/JVI.79.11.6890-6899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang CF, Wang YC, Tsao DA, Tung SF, Lin YS, Wu CW. Antagonism between members of the CNC-bZIP family and the immediate-early protein IE2 of human cytomegalovirus. J Biol Chem. 2000;275:12313–12320. doi: 10.1074/jbc.275.16.12313. [DOI] [PubMed] [Google Scholar]
- 117.Millhouse S, Kenny JJ, Quinn PG, Lee V, Wigdahl B. ATF/CREB elements in the herpes simplex virus type 1 latency-associated transcript promoter interact with members of the ATF/CREB and AP-1 transcription factor families. J Biomed Sci. 1998;5:451–464. doi: 10.1007/BF02255935. [DOI] [PubMed] [Google Scholar]
- 118.Keller MJ, Wu AW, Andrews JI, McGonagill PW, Tibesar EE, Meier JL. Reversal of human cytomegalovirus major immediate-early enhancer/promoter silencing in quiescently infected cells via the cyclic AMP signaling pathway. J Virol. 2007;81:6669–6681. doi: 10.1128/JVI.01524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Staak K, Prosch S, Stein J, Priemer C, Ewert R, Docke WD, et al. Pentoxifylline promotes replication of human cytomegalovirus in vivo and in vitro. Blood. 1997;89:3682–3690. [PubMed] [Google Scholar]
- 120.Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, et al. Psychosis pathways converge via D2high dopamine receptors. Synapse. 2006;60:319–346. doi: 10.1002/syn.20303. [DOI] [PubMed] [Google Scholar]
- 121.Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, et al. From the cover: dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci USA. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roy K, Murtie JC, El Khodor BF, Edgar N, Sardi SP, Hooks BM, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci USA. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 124.Ho WS, van den Pol AN. Bystander attenuation of neuronal and astrocyte intercellular communication by murine cytomegalovirus infection of glia. J Virol. 2007;81:7286–7292. doi: 10.1128/JVI.02501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, et al. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr Res. 2008;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, et al. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Domegan LM, Atkins GJ. Apoptosis induction by the Therien and vaccine RA27/3 strains of rubella virus causes depletion of oligodendrocytes from rat neural cell cultures. J Gen Virol. 2002;83:2135–2143. doi: 10.1099/0022-1317-83-9-2135. [DOI] [PubMed] [Google Scholar]
- 128.Bello-Morales R, Fedetz M, Alcina A, Tabares E, Lopez-Guerrero JA. High susceptibility of a human oligodendroglial cell line to herpes simplex type 1 infection. J Neurovirol. 2005;11:190–198. doi: 10.1080/13550280590924179. [DOI] [PubMed] [Google Scholar]
- 129.Spiller OB, Borysiewicz LK, Morgan BP. Development of a model for cytomegalovirus infection of oligodendrocytes. J Gen Virol. 1997;78(pt 12):3349–3356. doi: 10.1099/0022-1317-78-12-3349. [DOI] [PubMed] [Google Scholar]
- 130.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu W, Gwinn M, Clyne M, Yesupriya A, Khoury MJ. A navigator for human genome epidemiology. Nat Genet. 2008;40:124–125. doi: 10.1038/ng0208-124. [DOI] [PubMed] [Google Scholar]
- 132.Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007;7:46. doi: 10.1186/1471-244X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2007;62:711–721. doi: 10.1016/j.biopsych.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Higgs BW, Elashoff M, Richman S, Barci B. An online database for brain disease research. BMC Genomics. 2006;7:70. doi: 10.1186/1471-2164-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Craddock RM, Huang JT, Jackson E, Harris N, Torrey EF, Herberth M, et al. Increased alpha defensins as a blood marker for schizophrenia susceptibility. Mol Cell Proteomics. doi: 10.1074/mcp.M700459-MCP200. March 18, 2008. [DOI] [PubMed] [Google Scholar]
- 136.Lehrer RI. Multispecific myeloid defensins. Curr Opin Hematol. 2007;14:16–21. doi: 10.1097/00062752-200701000-00005. [DOI] [PubMed] [Google Scholar]
- 137.Crespi B, Summers K, Dorus S. Adaptive evolution of genes underlying schizophrenia. Proc Biol Sci. 2007;274:2801–2810. doi: 10.1098/rspb.2007.0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shirts BH, Wood J, Yolken RH, Nimgaonkar VL. Comprehensive evaluation of positional candidates in the IL-18 pathway reveals suggestive associations with schizophrenia and herpes virus seropositivity. Am J Med Genet B Neuropsychiatr Genet. 2007;147:343–350. doi: 10.1002/ajmg.b.30603. [DOI] [PubMed] [Google Scholar]
- 139.Shirts BH, Kim JJ, Reich S, Dickerson FB, Yolken RH, Devlin B, et al. Polymorphisms in MICB are associated with human herpes virus seropositivity and schizophrenia risk. Schizophr Res. 2007;94:342–353. doi: 10.1016/j.schres.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 140.Ashton LJ, Stewart GJ, Biti R, Law M, Cooper DA, Kaldor JM. Heterozygosity for CCR5-Delta32 but not CCR2b-64I protects against certain intracellular pathogens. HIV Med. 2002;3:91–96. doi: 10.1046/j.1468-1293.2002.00106.x. [DOI] [PubMed] [Google Scholar]
- 141.Rasmussen HB, Timm S, Wang AG, Soeby K, Lublin H, Fenger M, et al. Association between the CCR5 32-bp deletion allele and late onset of schizophrenia. Am J Psychiatry. 2006;163:507–511. doi: 10.1176/appi.ajp.163.3.507. [DOI] [PubMed] [Google Scholar]
- 142.Gottesman I. Schizophrenia Genesis: The Origins of Madness. New York, NY: Freeman; 1991. [Google Scholar]
- 143.Brown AS. The risk for schizophrenia from childhood and adult infections. Am J Psychiatry. 2008;165:7–10. doi: 10.1176/appi.ajp.2007.07101637. [DOI] [PubMed] [Google Scholar]
- 144.Dickerson FB, Boronow JJ, Stallings CR, Origoni AE, Yolken RH. Reduction of symptoms by valacyclovir in cytomegalovirus-seropositive individuals with schizophrenia. Am J Psychiatry. 2003;160:2234–2236. doi: 10.1176/appi.ajp.160.12.2234. [DOI] [PubMed] [Google Scholar]
- 145.Fellerhoff B, Laumbacher B, Wank R. High risk of schizophrenia and other mental disorders associated with chlamydial infections: hypothesis to combine drug treatment and adoptive immunotherapy. Med Hypotheses. 2005;65:243–252. doi: 10.1016/j.mehy.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 146.Suvisaari J. Enteroviruses and Schizophrenia. 2005:31–37. [Google Scholar]