Abstract
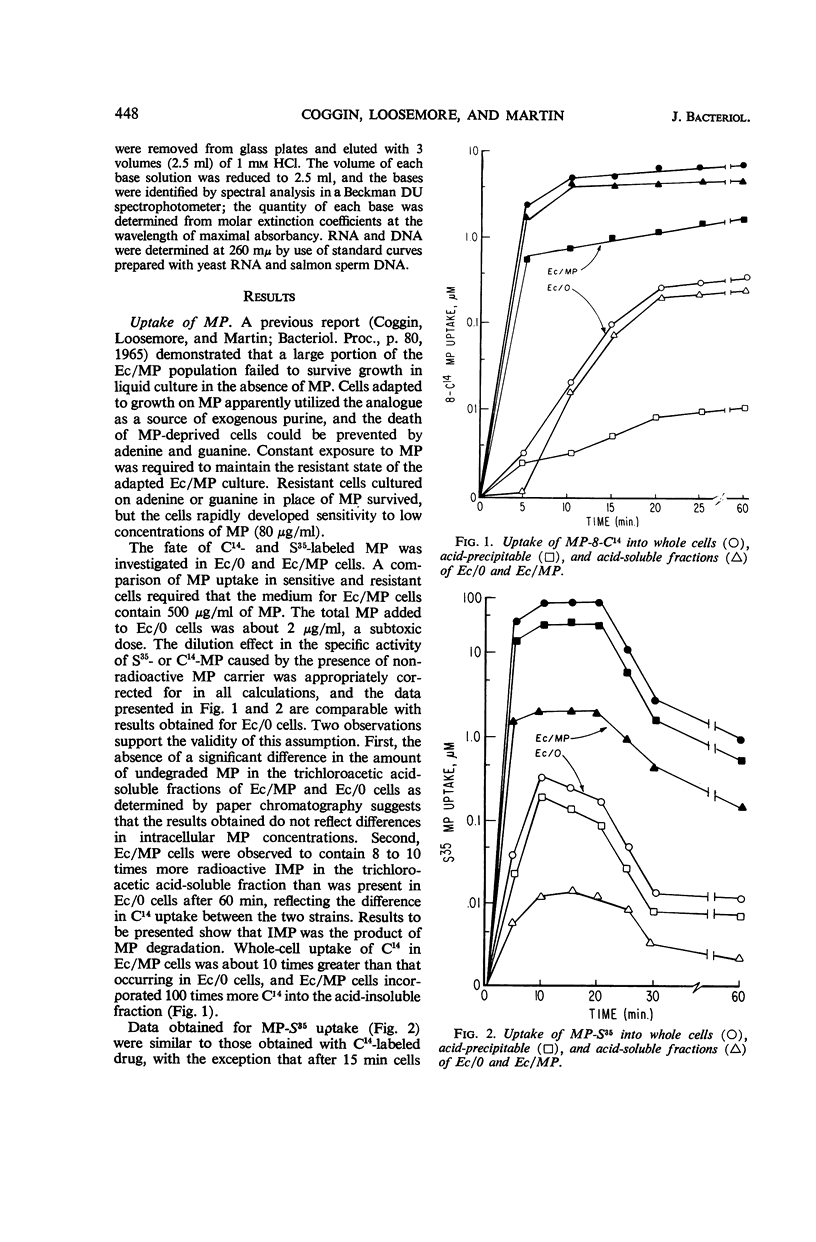
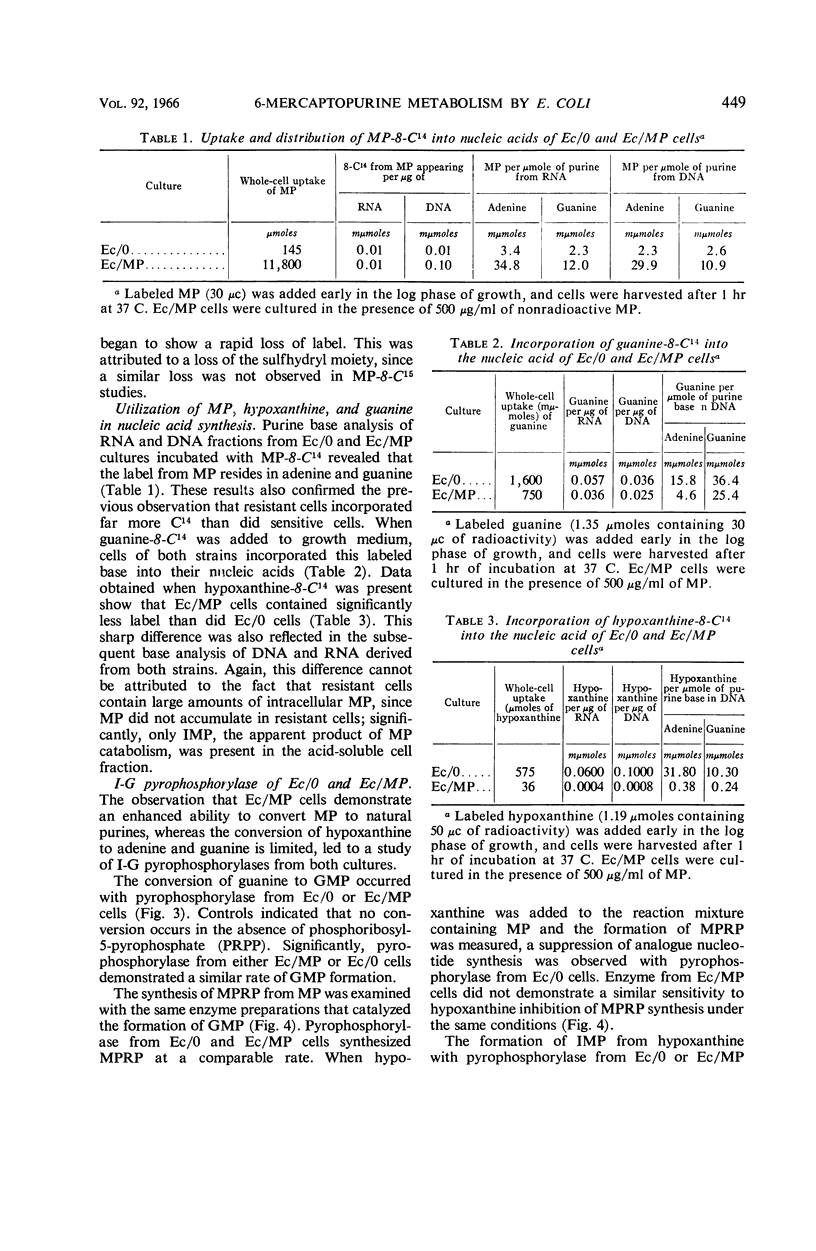
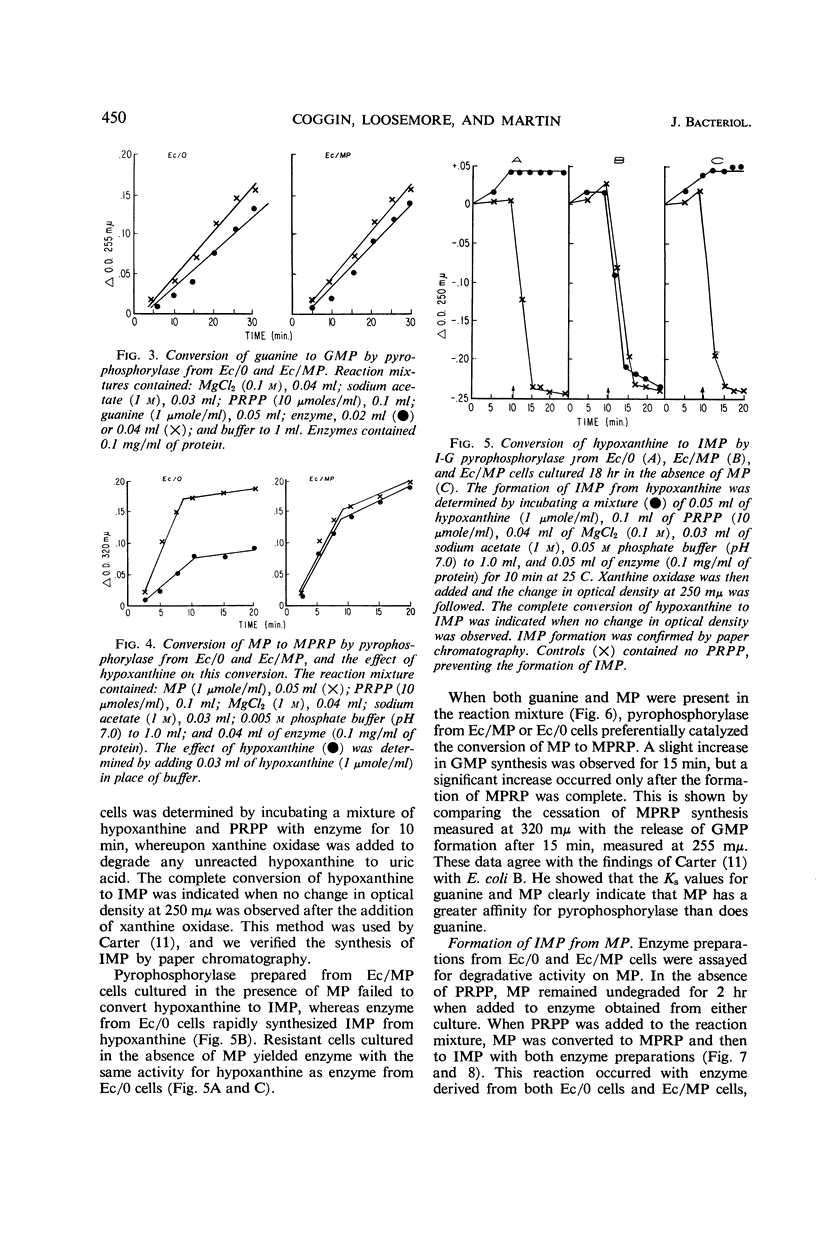
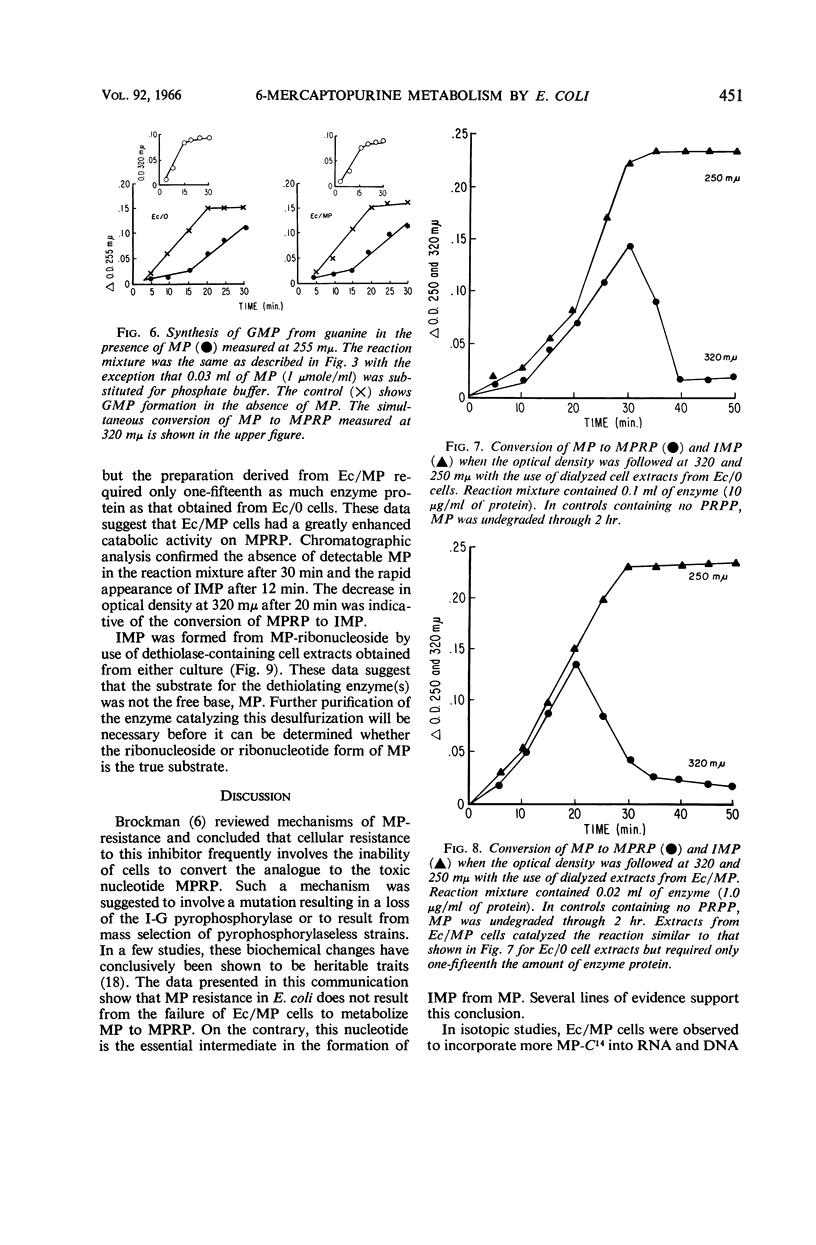
Coggin, Joseph H. (University of Chicago, Chicago, Ill.), Muriel Loosemore, and William R. Martin. Metabolism of 6-mercaptopurine by resistant Escherichia coli cells. J. Bacteriol. 92:446–454. 1966.—6-Mercaptopurine (MP) utilization as a source of purine in MP-sensitive and -resistant cultures of Escherichia coli was investigated. The label of MP-8-C14 appeared in adenine and guanine of ribonucleic acid and deoxyribonucleic acid in sensitive and resistant cultures. Studies using MP-S35 further demonstrated that the MP moiety was degraded, as shown by a rapid decrease in radioactivity from cells upon exposure to MP for 20 min. Enzymatic analysis showed that MP was converted to 6-mercaptopurine ribonucleotide (MPRP) by extracts derived from both sensitive and resistant cells. Resistant cell preparations, however, degraded MPRP to inosine monophosphate (IMP) rapidly when compared with analogue degradation by sensitive cells. Inosineguanosine-5′-phosphate pyrophosphorylase from resistant cells did not catalyze the synthesis of IMP from hypoxanthine when the cells were cultured in the presence of MP, but these enzyme preparations actively converted guanine to guanosine monophosphate (GMP). Pyrophosphorylase derived from resistant cells cultured in medium without MP catalyzed the conversion of hypoxanthine to IMP and also guanine to GMP. These observations suggest that inosine-guanosine-5′-phosphate pyrophosphorylase is composed of two distinct enzymes. The mode of resistance to MP in E. coli is related to an enhancement of the enzymatic degradation of MPRP to the pivotal purine intermediate, IMP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALIS M. E., HYLIN V., COULTAS M. K., HUTCHINSON D. J. Metabolism of resistant mutants of Streptococcus faecalis. III. The action of 6-mercaptopurine. Cancer Res. 1958 May;18(4):440–444. [PubMed] [Google Scholar]
- BALIS M. E., HYLIN V., COULTAS M. K., HUTCHISON D. J. Metabolism of resistant mutants of Streptococcus faecalis. II. Incorporation of exogenous purines. Cancer Res. 1958 Feb;18(2):220–225. [PubMed] [Google Scholar]
- BROCKMAN R. W., DAVIS J. M., STUTTS P. Metabolism of uracil and 5-fluorouracil by drug-sensitive and by drug-resistant bacteria. Biochim Biophys Acta. 1960 May 6;40:22–32. doi: 10.1016/0006-3002(60)91311-1. [DOI] [PubMed] [Google Scholar]
- BROCKMAN R. W. MECHANISMS OF RESISTANCE TO ANTICANCER AGENTS. Adv Cancer Res. 1963;7:129–234. doi: 10.1016/s0065-230x(08)60983-5. [DOI] [PubMed] [Google Scholar]
- BROCKMAN R. W., SPARKS C., HUTCHISON D. J., SKIPPER H. E. A mechanism of resistance to 8-azaguanine. I. Microbiological studies on the metabolism of purines and 8 azapurines. Cancer Res. 1959 Feb;19(2):177–188. [PubMed] [Google Scholar]
- Britten R. J., Roberts R. B., French E. F. AMINO ACID ADSORPTION AND PROTEIN SYNTHESIS IN Escherichia Coli. Proc Natl Acad Sci U S A. 1955 Nov 15;41(11):863–870. doi: 10.1073/pnas.41.11.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAREY N. H., MANDEL H. G. The metabolism of 6-mercaptopurine by Bacillus cereus. Biochem Pharmacol. 1960 Oct;5:64–78. doi: 10.1016/0006-2952(60)90009-5. [DOI] [PubMed] [Google Scholar]
- CARTER C. E. Reaction of 6-mercaptopurine with inosine- and guanosine-5'-phosphate pyrophosphorylase purified from E. coli. Biochem Pharmacol. 1959 Aug;2:105–111. doi: 10.1016/0006-2952(59)90077-2. [DOI] [PubMed] [Google Scholar]
- KALLE G. P., GOTS J. S. Alterations in purine nucleotide pyrophosphorylases and resistance to purine analogues. Biochim Biophys Acta. 1961 Oct 14;53:166–173. doi: 10.1016/0006-3002(61)90803-4. [DOI] [PubMed] [Google Scholar]
- LAW L. W. Differences between cancers in terms of evolution of drug resistance. Cancer Res. 1956 Aug;16(7):698–716. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUKENS L. N., HERRINGTON K. A. Enzymic formation of 6-mercaptopurine tibo tide. Biochim Biophys Acta. 1957 May;24(2):432–433. doi: 10.1016/0006-3002(57)90220-2. [DOI] [PubMed] [Google Scholar]
- SALSER J. S., HUTCHISON D. J., BALIS M. E. Studies on the mechanism of action of 6-mercaptopurine in cell-free preparations. J Biol Chem. 1960 Feb;235:429–432. [PubMed] [Google Scholar]
- TOMIZAWA S., ARONOW L. Studies on drug resistance in mammalian cells. II. 6-Mercaptopurine resistance in mouse fibroblasts. J Pharmacol Exp Ther. 1960 Feb;128:107–114. [PubMed] [Google Scholar]
- Tomisek A. J., Hoskins A. P., Reid M. R. Chromatographic studies of purine metabolism. VI. Inhibition of inosine phosphorylase by 6-mercaptopurine. Cancer Res. 1965 Dec;25(11):1925–1932. [PubMed] [Google Scholar]
- WAY J. L., PARKS R. E., Jr Enzymatic synthesis of 5'-phosphate nucleotides of purine analogues. J Biol Chem. 1958 Mar;231(1):467–480. [PubMed] [Google Scholar]
- ZIMMERMAN E. F., MAGASANIK B. UTILIZATION AND INTERCONVERSION OF PURINE BASES AND RIBONUCLEOSIDES BY SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jan;239:293–300. [PubMed] [Google Scholar]



