Abstract
Visual perception of a stimulus is a function of the visual context in which it is displayed. Surround suppression is a specific form of contextual modulation whereby the perceived contrast of a center stimulus is decreased by a high-contrast surround. Recent studies have demonstrated that individuals with schizophrenia are less prone to visual contextual effects, suggesting impairments in cortical lateral connectivity. We tested whether altered contextual modulation in schizophrenia is stimulus orientation selective. Participants viewed an annulus consisting of contrast-reversing sinusoidal gratings and determined if any one segment of the annulus had lower contrast relative to the other segments. Three stimulus configurations were tested: no surround (NS), parallel surround (PS), and orthogonal surround (OS). In the PS condition, the annulus was embedded in a 100% contrast grating parallel to the annulus gratings. In the OS condition, the surround grating was rotated 90° relative to the orientation of the annulus gratings. The main dependent measure was the suppression index—the change in contrast threshold in the OS and PS conditions relative to the NS condition. There was a group × condition interaction such that patients had significantly lower PS suppression index than controls, but there were no group differences in the OS suppression index. We conclude that individuals with schizophrenia possess an abnormality in surround suppression that is specific for stimulus orientation. In conjunction with physiological and anatomical evidence from basic and postmortem studies, our results suggest a deficit of inhibition in primary visual cortex in schizophrenia.
Keywords: schizophrenia, psychophysics, visual processing, contextual modulation
Introduction
Cognitive and information processing deficits are core symptoms of schizophrenia1 that confer significant disability2 and are mostly refractory to currently available treatments.3–5 The study of cognition in schizophrenia, therefore, may lead to a deeper understanding of the neural mechanisms of illness and the development of new therapies. Investigation of deficits in visual processing in schizophrenia represents a particularly promising line of research. Visual psychophysics provides a set of sophisticated tools for measuring components of visual perception, and much is known regarding the processes underlying visual perception and their neural substrates. These tools and knowledge can be used to facilitate the identification of fundamental neural mechanisms of illness in schizophrenia.
Impairments in contextual modulation of perception, a process by which perception of a stimulus is influenced by the visual context in which it is displayed, have recently been documented in schizophrenia.6–12 In healthy subjects, the presence of a high-contrast surround decreases perceived contrast in the central surrounded region (surround suppression).13,14 In subjects with schizophrenia, this surround suppression is diminished, resulting in better (more veridical) performance compared with controls in judging the contrast of the center region.7 While this enhanced performance addresses the generalized deficit confound in schizophrenia15 and demonstrates a differential and specific abnormality,16 the neural bases of this alteration in contextual modulation remain unclear. In this study, we examined the orientation selectivity of contextual modulation abnormalities in schizophrenia. Such a finding would have implications for identifying the neural correlates of these phenomena.
For sinusoidal grating stimuli, surround suppression is orientation specific, with the greatest suppression occurring when the orientation of the surround grating is parallel to that of the center.14,17 This suggests that the neural circuits mediating surround suppression should also be selective for stimulus orientation. The earliest stage in which orientation-selective neurons are found in the visual processing pathways is the primary visual cortex (area V1), and these neurons also exhibit orientation-specific surround suppression.17,18 In addition, psychophysical measures of the magnitude of suppression by a parallel surround (PS) are in agreement with the magnitude of inhibition of visual responses in cortical area V1 (but not in V2 or V3), as measured with functional magnetic resonance imaging.19 Finally, electroencephalogaphic and magnetoencephalographic correlates of orientation-specific surround suppression have been described, and source localization of these signals suggested a locus in primary visual cortex.20 Thus, the available evidence indicates that the demonstration of an orientation-specific impairment in contextual modulation in schizophrenia would imply a deficit in primary visual cortex.
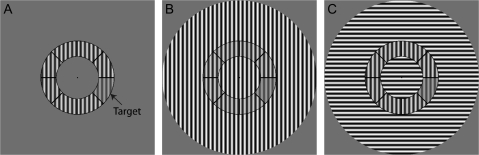
In this study, we examined the magnitude and orientation specificity of surround suppression in schizophrenia. In a group of subjects with schizophrenia and a demographically matched healthy control group, we measured contrast discrimination thresholds under 3 conditions: no surround (NS), vertically oriented surround grating (parallel to the annulus grating, PS), and horizontally oriented surround grating (orthogonal to the annulus grating, orthogonal surround [OS]) (figure1). We predicted that patients would show diminished orientation-specific surround suppression: reduced contextual effects in the PS but not the OS condition. Such a reduction in PS suppression in schizophrenia would result in preserved or even improved contrast discrimination in the PS condition in patients relative to controls. These findings would demonstrate a specific abnormality in schizophrenia as opposed to generalized deficits,16 thereby facilitating investigations of the neural correlates of the disease. Furthermore, given the orientation specificity of surround suppression of responses in primary visual cortex, these results would implicate dysfunction of cortical area V1 in schizophrenia.
Fig. 1.
Paradigm. Subjects performed a contrast decrement detection task in which they indicated whether they perceived a difference in contrast between any one segment (target) and the remaining 7 segments (pedestal) of the annulus. For half of the trials, the target had a lower contrast than the pedestal, which always had a constant contrast of 75%. In the remaining trials, all segments of the annulus displayed 75% contrast. The task was performed under 3 conditions: A) no surround, B) parallel surround, and C) orthogonal surround. The identical annulus is displayed in all 3 conditions in this figure.
Methods
Subjects
Seventeen individuals with schizophrenia and 20 healthy controls participated in this study (table 1). All patients were clinically stable and were recruited as outpatients at the time of study. This sample consisted of a mixture of individuals with chronic and recent onset schizophrenia. Diagnosis was made by master- or doctoral-level clinicians using Structured Clinical Interview for DSM-IV and confirmed by consensus conference. Exclusion criteria were the following: IQ < 70, drug or alcohol dependence or abuse within 3 months of testing, a positive urine drug screen on day of testing, major medical or neurological illness, significant head trauma, and history of more than 1 h/wk exposure to “first person shooter” or similar video games. The latter exclusion was employed because this activity is thought to significantly enhance spatial attention.21 Exclusion criteria for controls were lifetime diagnosis of Axis I disorder or first-degree relative with a psychotic disorder. Groups were matched demographically except for years of education, 14.3 and 15.8 for schizophrenia individuals and controls, respectively (P = .05). At the time of testing, all patients but one were taking atypical antipsychotics, and none were taking typical agents. Written informed consent was obtained from all subjects. This study was approved by the Institutional Review Board at the University of California Davis.
Table 1.
Subject Demographics and Patient Clinical Characteristics
| Patient (N = 17) |
Control (N = 20) |
||||
| Mean | SD | Mean | SD | P Value | |
| Age (y) | 32.0 | 9.4 | 30.7 | 8.5 | .73 |
| Gender (% male) | 70 | 60 | .4 | ||
| WRAT | 109.4 | 8.5 | 106.3 | 7.3 | .29 |
| Education (y) | 14.1 | 2.1 | 15.6 | 2.1 | .051 |
| Parental education (y) | 13.8 | 2.7 | 15.2 | 2.9 | .23 |
| On medications (%) | 94 | ||||
| BPRS | 37.7 | 6.0 | |||
| SAPS | 4.2 | 2.5 | |||
| SANS | 10.2 | 3.9 | |||
| CPZ equivalents (mg) | 443 | 369 | |||
Note: BPRS, Brief Psychiatric Rating Scale; CPZ, chlorpromazine; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; WRAT, Wide Range Achievement Test.
Visual Psychophysics Experiment
All testing was conducted in an enclosed, soundproofed room with constant ambient light levels for all sessions. Stimuli were presented and responses recorded with the Psychophysics Toolbox (http://psychtoolbox.org)22,23 for Matlab running on an Apple G4 laptop. An LCD monitor with 1280 × 1024 resolution and 60-Hz refresh rate was used. Subjects stabilized their heads on a chin rest equipped with a forehead strap. The monitor was placed 50 cm from subjects’ eyes.
The stimulus configuration and contrast discrimination task were adapted from Zenger-Landolt and Heeger.19 The stimulus was a circular patch consisting of a contrast-reversing (4 Hz), grayscale sinusoidal grating with a spatial frequency of 1.1 cycles per degree (figure 1). The stimulus was divided into annulus and surround regions by concentric black lines. The inner and outer radii of the annulus were 4.5° and 7.8° of visual angle, respectively. The surround region contained the remainder of the stimulus: the central portion, extending from the central fixation point to the inner border of the annulus, and the outer portion, extending from the outer border of the annulus to an eccentricity of 16.4° radius. The surround contrast was 100%. Contrast was defined as C = 100 × (Lmax − Lmin)/(Lmax + Lmin), where Lmin and Lmax were the minimal and maximal luminances in a given stimulus, respectively.
The annulus was divided into 8 equal segments, and the subject's task was to determine if any one segment (target) had lower contrast relative to the other 7 annulus segments (pedestal), which contained uniform 75% contrast. Participants pressed one button to indicate “target present” and another button to indicate “target absent.” In half the trials, the contrast decrement target was presented in a random location within the 8 segments, and the correct response for these trials was “target present.” The remaining trials had no target (all 8 segments had pedestal contrast levels of 75%), and the correct response for these trials was “target absent.” On a given trial, the annulus gratings and the surround (when present) were all presented synchronously for a duration of 750 milliseconds. The contrast difference between the pedestal and target segment was varied trial to trial according to a 3-up, 1-down adaptive staircase procedure24 converging to 79% accuracy. The staircase always started with 37% contrast difference between the target and the pedestal (pedestal fixed at 75%, initial target contrast was 38%), and the step increments in the staircase were 5% contrast. Each run contained 150 trials. The use of an adaptive staircase ensured that all participants were performing the task near their psychophysical contrast discrimination thresholds. As a result, difficulty of task performance was equated across all participants and for all 3 surround conditions.
In all conditions, the grating within the annulus portion of the stimulus was vertically oriented. The presence and orientation of grating in the surround portion of the stimulus differed for the 3 experimental conditions.
NS: The stimulus contained only the vertical annulus gratings (figure 1A).
PS: The surround sinusoidal grating shared the same vertical orientation as the annulus gratings (figure 1B).
OS: The surround grating had horizontal orientation, orthogonal to that of the vertical annulus gratings (figure 1C).
The relative temporal phase differences between annulus and surround were 0 (contrast reversals were synchronous in the annulus and surround), and the relative spatial phase differences were 0 in the PS condition, when both were vertical (ie, the annulus and surround gratings were aligned).
Behavioral performance (percent correct) at different levels of contrast was computed from all trials in the staircase. The threshold in each condition was derived by fitting a Weibull cumulative distribution function25 to the percent correct vs contrast plot, where contrast was expressed as the difference between pedestal and target contrasts. Thresholds were defined as the point of the Weibull function corresponding to 79% correct performance. Suppression indices were then quantified as ratios of these thresholds: PS/NS suppression index = PS threshold/NS threshold and OS/NS suppression index = OS threshold/NS threshold.
Individual variability in performance was assessed using a bootstrapping procedure.26 Individual trials from a given condition and subject were resampled with replacement, and a psychometric curve was fit to this bootstrap sample. This procedure was repeated 10 000 times to produce a distribution of thresholds. The variability in performance corresponded to the 95th central percentile range of this distribution. The advantage of employing a bootstrapping procedure is that it allows quantification of the reliability of each subject's responses for each surround condition without making any assumptions about the shape of the distribution of thresholds.
Results
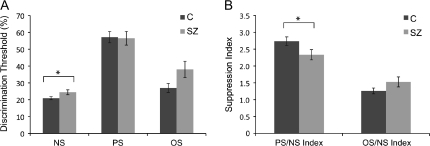
Mean contrast discrimination thresholds results are displayed in figure 2A. Group comparisons revealed a significant difference only in the NS condition, with controls exhibiting lower thresholds (better discrimination) (2-tailed t test, P < .05). No significant differences between groups were observed in the PS (P = .84) or OS conditions (P = .06).
Fig. 2.
Results. A) Contrast discrimination thresholds (the minimum contrast difference between target and pedestal segments necessary for accurate contrast discrimination) for no surround (NS), parallel surround (PS), and orthogonal surround (OS) conditions for patients and controls. *Significant group difference, P < .05. B) PS/NS and OS/NS suppression indices for patients and controls. *Significant group difference, P < .05.
PS/NS and OS/NS suppression indices, a measure of the impairment in task performance (elevation of contrast discrimination threshold) induced by the surround, are displayed in figure 2B. Repeated-measures analysis of variance (ANOVA), with factors of group and condition, demonstrated a significant main effect of condition (F1,35 = 110.5, P < .001) but not of group (F1,35 = 0.37, P = .55) and a significant group × condition interaction (F1,35 = 8.93, P < .01). Post hoc t tests showed that patients had a significantly smaller PS/NS suppression index (P < .05), meaning that the PS caused less suppression of contrast discrimination in patients compared with controls. There was no difference between groups in the OS/NS suppression index (P = .21), demonstrating orientation specificity of the group differences in surround suppression. Given that the only difference between the PS and OS conditions was the orientation of the surround, the finding of reduced surround suppression in patients in only the PS condition provides a control for a number of possible confounds, including group differences in motivation and attention.
The significant group difference in the NS condition raises the possibility that lower NS discrimination thresholds for controls are driving the group differences in PS/NS suppression index. To evaluate this possibility, we compared control subjects in the top half of the distribution of NS thresholds (the controls with the worst NS performance, N = 10) with the full sample of patients (N = 17). These 2 groups demonstrated virtually identical NS thresholds: controlmean = 24.0% (SD = 2.1) and schizophreniamean = 24.4% (SD = 5.5), P = .78. Despite this matched performance in the NS condition, there was a nearly significant difference in PS threshold: controlmean = 68.3% (SD = 11.2) and schizophreniamean = 56.1% (SD = 17.0), P = .055, and a significant group difference in PS/NS suppression index: controlmean = 2.9 (SD = 0.6) and schizophreniamean = 2.3 (SD = 0.7), P = .03. This finding demonstrates a genuine difference between patients and controls in the magnitude of PS suppression, with patients showing less suppression and enhanced contrast discrimination in the presence of a PS relative to control subjects.
Differential reliability of performance across groups presents another potential confound. This was assessed using a bootstrap analysis,26 in which the central 95th percentile range of discrimination thresholds was estimated from 10 000 random samples of trials of each subject in each surround condition. ANOVA on this measure with factors of condition and group revealed a main effect of condition (F2,54 = 18.53, P < .001) but not of group (F2,27 = 0.54, P = .82). In addition, there was not a group × condition interaction (F2,54 = 0.20, P = .82), demonstrating that the schizophrenia group did not exhibit differential variability in task performance in any of the surround conditions compared with controls. Thus, the differences between patients and controls in surround suppression do not stem from a difference in variability in task performance between the groups.
Three patients and 4 controls exhibited very robust suppression in the PS condition. As a result, a reliable threshold could not be calculated for these participants. In these cases, the maximal possible contrast difference (0% in the target and 75% in the pedestal) was below the psychophysical discrimination threshold. For these subjects, the threshold is at least 75%, so we set their thresholds to be equal to this lower bound. We reanalyzed the data, excluding these subjects, and found nearly identical results to those obtained from the full sample: There was a highly significant group × condition interaction (P < .01), with patients showing a trend toward greater PS/NS suppression index relative to controls (P = .07) but no difference in the OS/NS suppression index (P = .21).
Discussion
We tested the hypothesis that subjects with schizophrenia possess a specific deficit in contextual modulation of visual processing by examining the orientation selectivity of surround suppression. We found that presentation of a high-contrast PS resulted in robust reduction in contrast discrimination performance and that this PS suppression was markedly reduced in patients compared with controls. In the presence of an OS, there was substantially less suppression than in the PS case for both groups, and there was no difference between patients and controls in the OS suppression index. We conclude that patients with schizophrenia have altered orientation-specific surround suppression and that these results are not secondary to a generalized performance deficit. Furthermore, we interpret these results as suggesting a deficit in cortical inhibition in schizophrenia.
The finding of altered surround suppression in schizophrenia is consistent with several recent reports of altered contextual modulation of visual processing in this disease.6–8,10,12 The current results extend these findings by demonstrating an orientation-selective abnormality in contextual modulation in subjects with schizophrenia. These results, combined with those from the literature, suggest an alteration of neural inhibition in the primary visual cortex in schizophrenia. Physiological studies in animals17,18 and humans19,20 are consistent with a primary visual cortical locus for the surround suppression effects we describe. The most commonly cited neural circuit models of surround suppression share a common final stage in which inhibition mediates the suppressive effects of the surround.27 The spatial extent28,29 and temporal properties30 of surround suppression in V1 have been taken as evidence for a neural substrate involving feedback connections from extrastriate cortex to V1 (reviewed in Angelucci and Bressloff31). These feedback connections may modulate V1 neuronal responses through γ-aminobutyric acid–mediated interneurons.29,32 The involvement of γ-aminobutyric acid (GABA) in altered contextual processing in schizophrenia is consistent with postmortem findings pointing to decreased GABA neurotransmission in cerebral cortex,33,34 including area V1.35 Taken together, the available evidence suggests a circuit-based model of inhibitory dysfunction in V1 in schizophrenia. Direct testing of this model awaits future studies incorporating physiological measures of cortical inhibition and top-down modulation of visual cortex.
This study adds to a growing body of literature characterizing visual processing abnormalities in schizophrenia. In recognition of the potential importance of these impairments for our understanding of schizophrenia, visual processing has been selected as a domain of study in the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) project.36 Our findings relate to 2 of the sensory functions identified by the CNTRICS project: gain control and integration.
Gain control is the process by which sensory systems adapt the dynamic range of their responses to the prevailing conditions of the environment. For example, in the contrast discrimination task used in the present study, it would be advantageous to adjust the dynamic range of operation of the visual system so that it becomes most sensitive to changes in intensities similar to the intensity of the pedestal contrast. Therefore, diminished performance in contrast discrimination in the NS condition may imply a gain control impairment in visual perception in schizophrenia. However, impaired performance on a single task or condition (in this case, the NS condition) is difficult to interpret due to the generalized deficit confound in schizophrenia.16 In this particular case, reduced contrast discrimination not only could reflect a gain control deficit but could also be the result of a number of other factors, including attention and motivation.
The phenomenon of surround suppression can be considered to be an example of gain control. In natural vision, contextual information is used by the visual system to allow invariant perception of objects, features, and colors across a wide range of illumination conditions. One implementation of this process in the visual system is antagonism between center and surround. This antagonism serves to highlight boundaries and to suppress responses to uniform portions of the visual field. Our finding of reduced PS suppression in schizophrenia implies a deficit in gain control. Note that this is unlikely to be due to the generalized deficit confound, as the PS suppression index is the ratio of PS and NS thresholds for a given subject, effectively controlling for individual and group differences in overall task performance. Additionally, when patients were compared with a subset of control subjects who had equivalent performance in the NS condition, the patients performed as well as or even better than control subjects in the PS condition. In other words, the patients exhibited less contextual modulation from the PS.
The reduction in surround suppression in schizophrenia was observed for the PS but not for the OS condition. This orientation selectivity may be an indication of reduced integration,9 another sensory process identified by the CNTRICS project.11 Integration is the process whereby the visual system links basic features such as color, luminance, contrast, and orientation into coherent interpretations of the visual scene. In the PS condition, the surround gratings were vertical and spatially aligned with those of the annulus. This arrangement results in integration of the contours of the surround and the stimulus, facilitating a perceptual interpretation of the display as one large circular grating. For OS stimuli, the collinearity between the annulus gratings and surround was broken, effectively segmenting the stimulus into 2 portions. Contour integration in visual perception is facilitated by collinear interactions, presumably reflecting connections between visual cortical neurons with similar preferred stimulus orientations. The dependence of the magnitude of surround suppression on the relative orientations of the annulus and surround gratings suggests that it reflects an interaction of contour integration and gain control. In the surround suppression task, differences between patients and controls were only observed for PS (collinear) suppression but not for OS suppression. The fact that patients only differed from controls for the collinear stimulus configuration raises the possibility that abnormal spatial integration in schizophrenia underlies the group differences in surround suppression.
This reduced contextual modulation was advantageous for performing this particular task, but in general, a deficit in processing of contextual information in the visual scene in schizophrenia would be expected to have significant negative consequences for visual perception. This is supported by previous studies that have described impaired contextual processing in visual perception in schizophrenia, including deficits in facilitation of contrast detection by flankers,12 contour integration,37 and perceptual closure of fragmented images of objects.38
A number of studies have provided evidence for a dysfunction in the magnocellular pathway in schizophrenia (reviewed in Butler and Javitt39). The present study was not designed to address the question of a specific magnocellular deficit in schizophrenia, and the stimuli we used to measure surround suppression are not obviously biased toward either the magnocellular or parvocellular pathways. Specifically, both the spatial and temporal frequency of the gratings were relatively low (1.1 cycle per degree and 4 Hz). The magnocellular system is specialized for high temporal frequencies and low spatial frequencies and is complementary to the parvocellular system, which is specialized for low temporal frequencies and high spatial frequencies. Finally, the trials in which a surround was presented involve visual stimulation of a large part of the visual field (a circle 32.8° in diameter). The relative contributions of the magnocellular and parvocellular systems vary as a function of visual field eccentricity, and our stimuli included a wide range of eccentricities from central to peripheral vision. In summary, our stimuli and task do not clearly favor either the magnocellular or parvocellular systems and are not suitable for evaluating hypotheses regarding specific magnocellular impairments in schizophrenia.
Generalized deficits likely do not account for the present results. First, patients performed equivalently to control subjects in the PS condition. Second, the results of the bootstrap analysis suggest that patients did not exhibit differential reliability in performance. Third, the nearly equal magnitude of suppression across groups in the OS condition argues against effects of distraction by the high-contrast surround stimulus. Finally, the finding that patients demonstrated significantly less surround suppression in the PS condition compared with a subgroup of control subjects, matched for NS thresholds, suggests that the group difference in the PS/NS suppression index was not a consequence of higher NS thresholds for patients.
All patients but one were taking neuroleptics, raising the possibility of a medication confound. While this study cannot directly address this possibility, the following lines of evidence argue against it. First, all medicated subjects were taking atypical neuroleptics, which have been shown to not significantly alter contrast sensitivity.40 Second, the arguments presented above regarding generalized deficits are also applicable to a medication confound, ie, if present, medication effects would have to be selective for stimulus orientation. Third, the available evidence in the literature suggests that antipsychotic drugs do not account for orientation-specific visual contextual abnormalities in schizophrenia. Specifically, Keri et al12 found no significant difference between medicated and unmedicated patients with schizophrenia in orientation-specific modulation of contrast detection. Finally, the one unmedicated subject in our study demonstrated a PS/NS suppression index in the bottom half of the patient distribution.
In conclusion, we have demonstrated reduced surround suppression in schizophrenia that is selective for stimulus orientation. This finding, combined with substantial evidence for orientation-specific contextual modulation in primary visual cortical neurons, provides a basis for further investigation of the physiological substrates of abnormalities in this cortical region in schizophrenia.
References
- 1.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 2.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”. Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter WT, Gold JM. Another view of therapy for cognition in schizophrenia. Biol Psychiatry. 2002;51:969–971. doi: 10.1016/s0006-3223(02)01399-9. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg TE, Goldman RS, Burdick KE, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- 5.Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Norton D, Ongur D. Altered center-surround motion inhibition in schizophrenia. Biol Psychiatry. 2008;64:74–77. doi: 10.1016/j.biopsych.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dakin S, Carlin P, Hemsley D. Weak suppression of visual context in chronic schizophrenia. Curr Biol. 2005;15:R822–R824. doi: 10.1016/j.cub.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Must A, Janka Z, Benedek G, Keri S. Reduced facilitation effect of collinear flankers on contrast detection reveals impaired lateral connectivity in the visual cortex of schizophrenia patients. Neurosci Lett. 2004;357:131–134. doi: 10.1016/j.neulet.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 9.Silverstein SM, Knight RA, Schwarzkopf SB, West LL, Osborn LM, Kamin D. Stimulus configuration and context effects in perceptual organization in schizophrenia. J Abnorm Psychol. 1996;105:410–420. doi: 10.1037//0021-843x.105.3.410. [DOI] [PubMed] [Google Scholar]
- 10.Tadin D, Kim J, Doop ML, et al. Weakened center-surround interactions in visual motion processing in schizophrenia. J Neurosci. 2006;26:11403–11412. doi: 10.1523/JNEUROSCI.2592-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol Psychiatry. 2008;64:40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keri S, Kelemen O, Benedek G, Janka Z. Lateral interactions in the visual cortex of patients with schizophrenia and bipolar disorder. Psychol Med. 2005;35:1043–1051. doi: 10.1017/s0033291705004381. [DOI] [PubMed] [Google Scholar]
- 13.Chubb C, Sperling G, Solomon JA. Texture interactions determine perceived contrast. Proc Natl Acad Sci U S A. 1989;86:9631–9635. doi: 10.1073/pnas.86.23.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing J, Heeger DJ. Measurement and modeling of center-surround suppression and enhancement. Vision Res. 2001;41:571–583. doi: 10.1016/s0042-6989(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 15.Bilder RM, Goldman RS, Robinson D, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- 16.Knight RA, Silverstein SM. A process-oriented approach for averting confounds resulting from general performance deficiencies in schizophrenia. J Abnorm Psychol. 2001;110:15–30. doi: 10.1037//0021-843x.110.1.15. [DOI] [PubMed] [Google Scholar]
- 17.Blakemore C, Tobin EA. Lateral inhibition between orientation detectors in the cat's visual cortex. Exp Brain Res. 1972;15:439–440. doi: 10.1007/BF00234129. [DOI] [PubMed] [Google Scholar]
- 18.Cavanaugh JR, Bair W, Movshon JA. Selectivity and spatial distribution of signals from the receptive field surround in macaque V1 neurons. J Neurophysiol. 2002;88:2547–2556. doi: 10.1152/jn.00693.2001. [DOI] [PubMed] [Google Scholar]
- 19.Zenger-Landolt B, Heeger DJ. Response suppression in v1 agrees with psychophysics of surround masking. J Neurosci. 2003;23:6884–6893. doi: 10.1523/JNEUROSCI.23-17-06884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haynes JD, Roth G, Stadler M, Heinze HJ. Neuromagnetic correlates of perceived contrast in primary visual cortex. J Neurophysiol. 2003;89:2655–2666. doi: 10.1152/jn.00820.2002. [DOI] [PubMed] [Google Scholar]
- 21.Green CS, Bavelier D. Action video game modifies visual selective attention. Nature. 2003;423:534–537. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- 22.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 23.Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- 24.Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49(suppl 2):467+. [PubMed] [Google Scholar]
- 25.Weibull W. A statistical distribution function of wide applicability. J Appl Mech. 1951;18:293–297. [Google Scholar]
- 26.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993. [Google Scholar]
- 27.Smith MA. Surround suppression in the early visual system. J Neurosci. 2006;26:3624–3625. doi: 10.1523/JNEUROSCI.0236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angelucci A, Levitt JB, Walton EJ, Hupe JM, Bullier J, Lund JS. Circuits for local and global signal integration in primary visual cortex. J Neurosci. 2002;22:8633–8646. doi: 10.1523/JNEUROSCI.22-19-08633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavanaugh JR, Bair W, Movshon JA. Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. J Neurophysiol. 2002;88:2530–2546. doi: 10.1152/jn.00692.2001. [DOI] [PubMed] [Google Scholar]
- 30.Bair W, Cavanaugh JR, Movshon JA. Time course and time-distance relationships for surround suppression in macaque V1 neurons. J Neurosci. 2003;23:7690–7701. doi: 10.1523/JNEUROSCI.23-20-07690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angelucci A, Bressloff PC. Contribution of feedforward, lateral and feedback connections to the classical receptive field center and extra-classical receptive field surround of primate V1 neurons. Prog Brain Res. 2006;154:93–120. doi: 10.1016/S0079-6123(06)54005-1. [DOI] [PubMed] [Google Scholar]
- 32.Schwabe L, Obermayer K, Angelucci A, Bressloff PC. The role of feedback in shaping the extra-classical receptive field of cortical neurons: a recurrent network model. J Neurosci. 2006;26:9117–9129. doi: 10.1523/JNEUROSCI.1253-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 34.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33:1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverstein SM, Kovács I, Corry R, Valone C. Perceptual organization, the disorganization syndrome, and context processing in chronic schizophrenia. Schizophr Res. 2000;43:11–20. doi: 10.1016/s0920-9964(99)00180-2. [DOI] [PubMed] [Google Scholar]
- 38.Doniger GM, Silipo G, Rabinowicz EF, Snodgrass JG, Javitt DC. Impaired sensory processing as a basis for object-recognition deficits in schizophrenia. Am J Psychiatry. 2001;158:1818–1826. doi: 10.1176/appi.ajp.158.11.1818. [DOI] [PubMed] [Google Scholar]
- 39.Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18:151–157. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Levy DL, Sheremata S, Nakayama K, Matthysse S, Holzman PS. Effects of typical, atypical, and no antipsychotic drugs on visual contrast detection in schizophrenia. Am J Psychiatry. 2003;160:1795–1801. doi: 10.1176/appi.ajp.160.10.1795. [DOI] [PubMed] [Google Scholar]