Abstract
The major psychotic disorders schizophrenia and bipolar disorder are etiologically complex involving both heritable and nonheritable factors. The absence of consistently replicated major genetic effects, together with evidence for lasting changes in gene expression after environmental exposures, is consistent with the concept that the biologic underpinnings of these disorders are epigenetic in form rather than DNA sequence based. Psychosis-associated environmental exposures, particularly at key developmental stages, may result in long-lasting epigenetic alterations that impact on the neurobiological processes involved in pathology. Although direct evidence for epigenetic dysfunction in both schizophrenia and bipolar disorder is still limited, methodological technologies in epigenomic profiling have advanced. This means that we are at the exciting stage where it is feasible to start investigating molecular modifications to DNA and histones and examine the mechanisms by which environmental factors can act upon the genome to bring about epigenetic changes in gene expression involved in the etiology of these disorders. Given the dynamic nature of the epigenetic machinery and potential reversibility of epigenetic modifications, the understanding of such mechanisms is of key relevance for clinical psychiatry and for identifying new targets for prevention and/or intervention.
Keywords: DNA methylation, histone modifications, development, schizophrenia, bipolar disorder, gene-environment interaction
Introduction
The major psychotic disorders of schizophrenia and bipolar disorder are etiologically complex, involving multiple factors from multiple domains with minimal to moderate effect sizes. Twin and adoption studies highlight the importance of both heritable and nonheritable factors in these disorders, but specific etiological pathways remain elusive. The advent of whole-genome association studies has heralded new interest in the genetics of major psychotic disorders, but associated polymorphisms are characterized by very small effect sizes and have yet to be conclusively replicated.1,2 The absence of clear genetic effects in schizophrenia and bipolar disorder supports the concept that the biological risk factors for major psychotic disorders are epigenetic in form rather than solely DNA sequence based.3,4 A recent review by Oh and Petronis3 described some of the complexities facing epidemiological studies of schizophrenia and highlighted how the “prism of epigenetics” may enable one to bypass some of the limitations of traditional environmental paradigms. In this article, we summarize current evidence supporting the role of epigenetic processes in schizophrenia and bipolar disorder and describe how they may mediate the action of known environmental risks for major psychotic disorders. These aspects are of key relevance for clinical psychiatry given the potential reversibility of epigenetic marks under the influence of nutrition, social factors, behavioral interventions, and drugs.
Epigenetic Mechanisms
Epigenetics refers to the reversible regulation of various genomic functions, occurring independently of DNA sequence, mediated principally through changes in DNA methylation and chromatin structure.5 Although DNA methylation and histone modifications are the most studied epigenetic mechanisms, other epigenetic processes are known to regulate gene function, eg, the small noncoding RNA-mediated regulation of gene expression and chromatin remodeling.6 Epigenetic processes are essential for normal cellular development and differentiation and allow the long-term regulation of gene function through nonmutagenic mechanisms.7 Like the DNA sequence, the epigenetic profile of somatic cells is inherited from maternal to daughter chromatids during mitosis. In addition to being mitotically heritable, there is evidence that epigenetic mechanisms may be heritable during meiosis in humans and thus potentially transmitted across generations.8–11 This blurs the demarcation between epigenetic- and DNA sequence–based inheritance and challenges the assumption that the “heritable” component to psychosis, and other complex disorders, is entirely genetic.
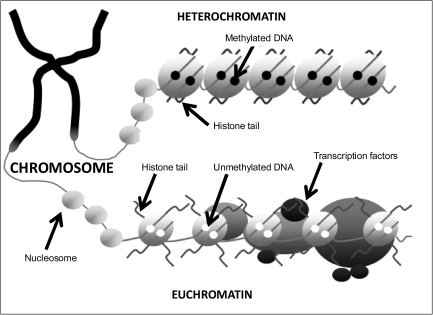
Cytosine methylation, occurring at position 5 of the cytosine pyrimidine ring in CpG dinucleotides is the best understood and most stable epigenetic modification modulating the transcriptional plasticity of mammalian genomes. It is intrinsically linked to the regulation of gene expression, with many genes demonstrating an inverse correlation between the degree of promoter DNA methylation and the level of expression.5 The methylation of CpG sites, overrepresented in CpG islands in the promoter regulatory regions of many genes, disrupts the binding of transcription factors and attracts methyl binding proteins that initiate chromatin compaction and gene silencing. The posttranslational modification of histones, the basic proteins around which DNA is wrapped to form nucleosomes, comprises the other major type of epigenetic mechanism related to gene expression. A number of covalent histone modifications, occurring at specific residues, have been described (eg, acetylation, methylation, phosphorylation, SUMOylation, and ubiquitylation), which together constitute a complex “histone code” modulating gene expression via alterations in chromatin structure.12 Condensed chromatin (heterochromatin), in which the DNA and histone proteins are tightly packed, acts to block the access of transcription factors and other instigators of gene expression to DNA and is thus associated with repressed transcription. Conversely, an open chromatin conformation (euchromatin) allows the cells transcriptional machinery to access DNA and drive transcription (see figure 1). While often investigated independently, epigenetic modifications to DNA and histones are not mutually exclusive and clearly interact in a number of ways, and it is apparent that the classification of epigenetic mechanisms in terms of either gene activation or suppression is too simplistic.12 The methyl binding protein MeCP2, eg, binds specifically to methylated cytosines, attracting histone deacetylases that deacetylate histones,13 and a recent study has shown that histone H3 residues unmethylated at the lysine 4 position recruit DNA methyltransferases resulting in de novo DNA methylation.14
Fig. 1.
The Main Components of the Epigenetic Code. The 2 main components of the epigenetic code comprise DNA methylation and histone modifications. The addition of methyl groups to CpG dinucleotides acts to repress gene activity by blocking the binding of transcription factors and attracting methyl-binding proteins. A combination of different modifications to the “tails” of histones can alter the activity of genes via structural changes to the DNA molecule.
Dynamic Epigenetic Changes and Gene X Environment Interaction
Mounting evidence suggests that epigenetic processes can be influenced by exposure to a range of external environmental factors, either globally or at specific loci.15 DNA methylation, eg, has been shown to vary as a function of nutritional, chemical, physical, and even psychosocial factors. As epigenetic changes are inherited mitotically in somatic cells, they provide a possible mechanism by which the effects of external environmental factors at specific stages in the life course can be propagated through development, producing long-term phenotypic changes. It appears that the epigenome is particularly susceptible to disruption during a number of key developmental periods, especially during prenatal growth when rapid cell replication is occurring and the standard epigenetic signals driving development are being established.16
Environmental mediation of the epigenome can provide a mechanism for the gene-environment interactions currently being uncovered in psychiatric disorders, including psychosis.17,18 The pathogenic effect of a polymorphism associated with disrupted gene function is likely to be dependent upon the degree to which that particular variant is actually expressed. It is thus plausible that risk could be exaggerated or suppressed if expression is directly influenced by environmental factors via processes such as DNA methylation. Of particular interest are so-called “metastable epialleles,” loci that can be epigenetically modified to produce a range of phenotypes from genetically identical cells.19 Many of these loci have been shown to be environmentally sensitive and particularly affected by the prenatal environment of the developing fetus. An example of how such a mechanism could explain gene-environment interactions is provided by the agouti viable yellow allele (Avy) inbred mouse strain, which demonstrates a range of coat color phenotypes, depending upon the epigenetic state of a large transposable element inserted upstream of the agouti gene. The transposon contains a cryptic promoter, which expresses a phenotype characterized by yellow fur and various detrimental metabolic features such as diabetes and obesity. When the transposon is methylated, this phenotype is not expressed; the mice have brown fur and are metabolically healthy. Interestingly, DNA methylation across this region (and thus phenotype) can be manipulated in offspring by altering the diet of pregnant mothers.20,21 Enriching the maternal diet with methyl donor supplements increases offspring DNA methylation, leading to gene expression changes associated with brown fur and metabolic health. Gene-environment interactions may also result when genetic polymorphisms alter the ability of a specific region of the genome to be epigenetically altered in response to an environmental pathogen.
Given the recent evidence suggesting that epigenetic marks may be transmitted meiotically across generations, and the fact that the environment itself can alter the epigenetic regulation of gene expression, the boundary between “environmental” and “heritable” risks for disorders such as schizophrenia and bipolar disorder is likely to be far less clear-cut than is currently recognized. The interplay between the genome, the environment, and epigenetic processes is further complicated by the fact that some DNA alleles and haplotypes are themselves associated with a specific epigenetic profile. For example, allele-specific epigenetic modifications have been associated with “risk” polymorphisms in psychiatric candidate genes including the C/T(102) polymorphism in the serotonin receptor 2A gene22 and the Val66met polymorphism in the brain-derived neurotrophic factor gene (BDNF).23
Role for Epigenetics in Major Psychotic Disorders
There is considerable epidemiological evidence to support a role for epigenetic dysfunction in major psychotic disorders (see Mill and Petronis24 for a detailed review). Of particular note is the high degree of discordance between monozygotic (MZ) twins for both disorders. Such phenotypic discordance between MZ twins is often attributed to nonshared environmental factors, although the empirical evidence for such a large environmental contribution to either disorder is still lacking, with no specific environmental risk factors being conclusively linked to etiology. The partial stability of epigenetic signals provides an alternative explanation for phenotypic discordance between MZ twins. A recent study has demonstrated that fairly profound epigenetic differences across the genome of peripheral lymphocytes arise during the lifetime of MZ twins, highlighting the dynamic nature of epigenetic processes.25 Interestingly, MZ twin methylation differences have been reported for CpG sites in a number of specific genes previously implicated in schizophrenia including the dopamine D2 receptor gene26 and the catechol-O-methyltransferase (COMT) gene.27 This suggests that even between genetically identical individuals the expression and function of the genome is not equivalent. Other characteristics of major psychotic disorders suggesting a role for epigenetics include sex differences in prevalence, parent-of-origin effects, and evidence for abnormal levels of folate and homocysteine in the plasma of affected individuals,28 a marker indicative of dysregulated DNA methylation.
Until recently, few studies had empirically investigated the role of epigenetic factors in major psychotic disorders, with the majority focusing on the role of DNA methylation changes in the vicinity of specific candidate genes. Early studies reported DNA methylation differences associated with schizophrenia in the vicinity of both COMT29 and reelin (RELN),30 although these findings were not confirmed by other groups using fully quantitative methylation profiling methods.31,32 Furthermore, cortical γ-aminobutyric acid–mediated (GABAergic) neurons in schizophrenia have been shown to express increased levels of DNA-methyltransferase-1 that is associated with altered expression of both RELN and GAD67.30,33 Recently, 2 studies have employed genome-wide approaches to identify DNA methylation changes associated with major psychotic disorder. The first investigated DNA methylation differences between MZ twins discordant for bipolar disorder.34 They found evidence for increased methylation in affected twins upstream of the spermine synthase gene (SMS) and lower methylation upstream of the peptidylprolyl isomerase E-like gene (PPIEL). While DNA methylation upstream of SMS was not correlated with expression of the gene, a strong inverse correlation between PPIEL gene expression and DNA methylation was observed. More recently, Mill et al23 utilized frontal cortex brain tissue from patients with schizophrenia and bipolar disorder to assess DNA methylation across approximately 12 000 regulatory regions of the genome using CpG island microarrays. Consistent with increasing evidence for altered glutamatergic and GABAergic neurotransmission in the pathogenesis of major psychotic disorder,35,36 this study identified epigenetic changes in loci associated with both these neurotransmitter pathways. Glutamate is the most abundant fast excitatory neurotransmitter in the mammalian nervous system, with a critical role in synaptic plasticity. Several lines of evidence link the glutamate system to psychosis, in particular the observation that glutamate receptor agonists can cause psychotic symptoms in unaffected individuals.
The potential role of skewed X-inactivation in major psychotic disorders was highlighted by a study examining X chromosome inactivation in a series of 63 female MZ twin pairs concordant or discordant for bipolar disorder or schizophrenia and healthy MZ control subjects.37 They found that discordant female bipolar disorder twins showed greater differences in the methylation of the maternal and paternal chromosome X alleles than concordant twin pairs suggesting a potential contribution from X-linked loci to discordance within twin pairs for bipolar disorder.
Environment and Major Psychotic Disorders
Quantitative genetic studies and a wealth of epidemiological data highlight a crucial role for environmental factors in the etiology of major psychotic disorders.18 Notwithstanding several methodological considerations on the validity and analyses of environmental measures, a number of environmental exposures have consistently been associated with both schizophrenia and bipolar disorder.38 The mechanism through which these environmental factors act upon molecular and cellular biological machineries in the human brain and ultimately give rise to psychosis-related phenotypes and pathology remains poorly understood. Research into behavioral and psychiatric disorders was for decades directed by a perceived dichotomy between “nature” and “nurture” that we now know to be false; it is clear that neither genes nor the environment act in isolation to influence complex behaviors or increase susceptibility for psychopathology.3,39,40 Table 1 summarizes examples of environmental factors that have been proposed to interact with genetic factors in major psychotic disorders.18 One problem with these findings, however, is that they represent purely statistical interactions and provide little information about actual etiological mechanisms. In this respect, it is attractive to argue that the environmental risk factors for major psychotic disorders act, at least in part, via epigenomic alterations. The epigenetic machinery of the cell, which acts to directly control gene expression, can both modify the effects of pathogenic DNA sequence polymorphisms and be influenced by adverse environmental exposures.41 Based upon the currently available evidence from both animal and human research, we focus here on examples of environmental factors putatively associated with schizophrenia and bipolar disorder, for which evidence exists to suggest a pathway via epigenetic mechanisms to increased risk for major psychotic disorder.
Table 1.
Environmental Factors for Major Psychotic Disorders
| Environmental Factor | Developmental Stage |
| Paternal age43–46 | Prenatal |
| Hypoxia47,48 | Prenatal |
| Nutritional deficiency49,50 | Prenatal |
| Folate51–53 | Prenatal |
| Maternal infection47 | Prenatal |
| Maternal stress47,54 | Prenatal |
| Rearing environment55–57 | Childhood |
| Chronic stress58 | Childhood/adolescence |
| Urban environment39,59 | Childhood/adolescence |
| Migration60 | Childhood/adolescence |
| Cannabis61–64 and other drugs of abuse65,66 | Childhood/adolescence |
Note: Environmental factors for major psychotic disorders with evidence for involvement in gene-environmental interactions and the developmental stage of exposure are presented in table 1.
Paternal Age
Convincing evidence from epidemiologic studies implicates advanced paternal age with an increased risk of schizophrenia in the offspring.44,66–68 Offspring of fathers aged 35 years and older have increased risk (up to 3-fold) of developing schizophrenia as compared with offspring of younger fathers.44,69 Several mechanisms have been proposed to underlie this effect.45 Initial explanations focused solely on genetic mutagenesis, with advanced paternal age leading to de novo mutations, copy number changes, or chromosomal breakages, which accumulate in successive generations of sperm-producing cells. Studies in a number of other disorders that show a clear paternal age effect, however, suggest that mutations in sperm cannot fully explain the association.70 An alternative explanation is that epigenetic dysfunction underlies the paternal age effect, eg, via alterations to normal patterns of genomic imprinting.45,71 Genomic imprinting is the molecular mechanism whereby a small subset of all genes is expressed in a tissue of the offspring according to their parent of origin.72 Less than 1% of all genes is estimated to be parentally imprinted,72 and many of these are expressed in the brain, playing a critical role in (neuro)development.73 Interestingly, a study by Flanagan et al74 uncovered significant intra- and interindividual epigenetic variability in the male germ-line and found a number of genes that demonstrated age-related DNA methylation changes. In addition to de novo mutations/epimutations induced by multiple cell divisions, it is also possible that the accumulated exposure to various environmental toxins over the life course could result in germ line alterations in older men. Given that such toxins have been shown in mice to induce germ line mutations, DNA damage, and global hypermethylation,75 it is highly plausible that such changes could increase with age and thus be more prevalent in older fathers.
Nutritional Deficiency—Folate and Related Dietary Factors
Evidence from 2 large independent population samples in The Netherlands and China suggests that in utero nutritional deficiency, as indicated by maternal exposure to severe famine during pregnancy, is associated with an increased risk of schizophrenia in adult life.52,76,77 The exact mechanism linking prenatal nutritional deficiency and schizophrenia is unknown, but considerable evidence exists to support a role for epigenetic changes. It is known that the availability of methyl donors and cofactors is a major external influence on DNA methylation. Methyl donors, usually contained in the diet, are required for the formation of S-adenosylmethionine (SAM). SAM, in turn, acts as a methyl donor for the methylation of cytosine DNA residues. Examples of dietary factors required for the formation of SAM include folate, methionine, choline, vitamin B12, vitamin B6, and vitamin B2. Given the role of DNA methylation in coordinating the correct pattern of gene expression during embryogenesis and development, it is likely that exposure to a diet lacking such components at specific developmental time points could have detrimental phenotypic effects. A recent study found that individuals from the Dutch Hunger winter who were prenatally exposed to famine were relatively hypomethylated at the imprinted IGF2 gene when compared with their unexposed same sex siblings 6 decades after the period of nutritional deficiency.78 The same study elegantly demonstrated that this association is specific for periconceptional exposure, emphasizing the relevance of epigenetic processes at specific stages in very early mammalian development.78 Of course, these effects may not be solely attributed to impaired nutrient uptake during pregnancy; famine may also evoke psychological stress, which is also a risk factor for schizophrenia and interestingly has also been associated with epigenetic changes (see below).
Several nutritional factors such as protein malnutrition, deficiencies of vitamin A or D, essential fatty acids, and folate have been proposed to be responsible for the increased risk of schizophrenia in offspring subjected to famine during fetal development.48 A large birth cohort study with data on schizophrenia in adulthood indicated that elevated homocysteine levels in the third trimester of pregnancy are associated with an increased risk of schizophrenia of the offspring.50 Further indirect evidence for an association between folate and schizophrenia arises from a study in which birth interval was used as an indication of folate levels during pregnancy (as postpartum restoration to normal maternal folate values may take up to 1 y after pregnancy).79 Smits et al79 found that such an index of folate was associated with the risk of schizophrenia in the offspring. Methylenetetrahydrofolate reductase (encoded by the gene MTHFR) is a key enzymatic molecule in the one carbon metabolic pathway, acting to catalyze the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, a cosubstrate for homocysteine remethylation to methionine. Genetic association studies of 2 single-nucleotide poylmorphisms in the MTHFR gene provided evidence of an association with both schizophrenia and bipolar disorder.80–82 In addition, epidemiological studies identified overlapping patterns in the incidence of schizophrenia with neural tube defects, suggesting the existence of one or more shared etiological risk factors between these disorders, with particular emphasis on nutritional deficiencies in utero, in particular folate.83 In further support of a role for folate deficiency in major psychotic disorders is the observation that elevated levels of homocysteine are detected in the blood plasma of patients with schizophrenia.28 Taken together, there is thus accumulating evidence for a critical role for nutritional factors, especially folate levels, in linking epigenetic variation, early development, and the risk of major psychotic disorders.
Rearing Environment and Childhood Abuse
Adoption studies provide evidence for an association between rearing environment, childhood stress, and an increased risk of psychosis later in life. For example, adoptees with a positive family history for psychosis who had been brought up in a dysfunctional adoptive family environment have an increased risk of developing a psychotic spectrum disorder.56,84–86 Although only a few cases of psychosis were identified in families without a history of psychotic disorder, the environmental effects were strong in these cases.87 Conversely, a positive rearing environment may decrease the risk of psychotic disorder later in life. It has been shown that high-risk children with positive parental relationships have a lower risk for developing psychotic disorders.88 Studies on the effects of an adverse early psychosocial environment on major psychotic disorders have been reinvigorated by recent prospective epidemiological findings showing that victims of bullying at ages 8 and/or 10 years have about a 2-fold risk of psychotic symptoms at age 12 years with an even more elevated risk when victimization is chronic or severe.57 No studies have yet examined the link between childhood stress, epigenetic changes, and the onset of psychotic disorders in humans. Recent breakthrough articles from experimental animal research, however, have shown that the psychosocial environment and stress in particular can mediate changes in gene expression during key developmental periods via epigenetic mechanisms. Meaney et al showed that postnatal maternal care in rats led to epigenetic modification of a transcription factor–binding site in the promoter region of the glucocorticoid receptor gene (NR3C1) that in turn altered gene expression and behavioral phenotypes in the offspring that persisted into adult life.89 Subsequent experiments demonstrated that methyl supplementation during the same early postnatal period can reverse the epigenetic modification induced by maternal care, with related gene expression changes and behavioral phenotypes in adult offspring.90 Transcriptomic studies by the same group identified over 900 genes in the hippocampus that are stably regulated by maternal care,91 suggesting an even more widespread effect of the early social environment on gene expression through the life course. A recent study in humans investigated epigenetic differences in a homologous NR3C1 promoter region, comparing DNA methylation in postmortem hippocampus samples obtained from suicide victims with a history of childhood abuse to that seen in samples from either suicide victims with no childhood abuse or control subjects. In line with the animal findings, abused suicide victims had increased CpG methylation of the NR3C1 promoter with concomitant changes in messenger RNA (mRNA).92 Other studies, however, have found little or no evidence for between-individual epigenetic variation in the human NR3C1 promoter,93 suggesting that further replication is needed before any firm conclusions can be drawn. However, these findings may be of relevance for major psychotic disorders given the evidence suggesting links between disturbances in the hypothalamic-pituitary-adrenal axis, altered stress reactivity, and psychosis.94,95
Another line of animal research has identified that chronic psychosocial stress (eg, defeat stress) alters gene expression, particularly of BDNF, via a range of epigenetic mechanisms including histone tail modifications and DNA methylation. For example, chronic exposure to social defeat stress in mice significantly downregulated mRNA levels of histone deacetylase-5 in the nucleus accumbens.96 Chronic defeat stress in mice also induced long-lasting downregulation of BDNF transcripts and increased histone methylation.97 Interestingly, chronic treatment with the tricyclic antidepressant imipramine reversed this downregulation of BDNF transcripts while increasing histone acetylation at the corresponding promoters.97 Altered BDNF transcription has been linked with psychosis,98 while epigenetic moderation of BDNF transcription has also been shown to be involved in neuroplasticity and experience-dependent learning and memory.99,100 Thus, although a direct link between childhood trauma, epigenetic modifications, and psychosis is not established in humans, indirect evidence suggests a potentially major role for epigenetic mechanisms in mediating this environmental influence on psychosis.
Cannabis and Other Drugs of Abuse
Over recent years, evidence from epidemiological studies and meta-analyses has established cannabis as a clear risk factor for later psychotic symptoms or psychotic disorder.60,61,101 Interestingly, the age (or developmental stage) at which individuals start using cannabis influences this association.102 Further evidence suggests that cannabis use is also associated with a decreased age of onset of psychotic disorder103 and that gene-environment interactions are likely implicated in the association between cannabis and psychosis.60,104,105 For example, a longitudinal study by Caspi et al104 showed that cannabis use increased the risk for developing psychotic symptoms and schizophreniform disorder only in carriers with the valine158 allele in the gene encoding COMT. The primary psychoactive component of cannabis is Δ9-tetrahydrocannabinol (THC), which is thought to exert its psychological effects via the disruption of normal cannabis-1 receptor–mediated signaling in the brain.106 Administration of THC or cannabis elicits long-term molecular and cellular changes in the brains of mice and humans.107–110 In animals, cannabis had prolonged impact on electrophysiological and biochemical measures of neuronal signaling in brain structures such as the nucleus accumbens and hippocampus,111 with differential effects depending upon duration of exposure and timing during development.111–114 The underlying mechanisms mediating these prolonged effects have, however, remained elusive. It is tempting to suggest a role for epigenetic factors herein, especially given the recent observation that THC induces expression of histone deacetylase 3.115
The abuse of other psychostimulant drugs, such as cocaine, amphetamine, and phencyclidine has also consistently been associated with major psychotic disorders; patients with major psychotic disorders frequently use, abuse, and become dependent on these drugs.116 Moreover, psychostimulant drugs are well known to elicit psychotic symptoms in individuals without schizophrenia or bipolar disorder. It has been suggested that these drugs of abuse may act via the common mechanism of sensitization and that sensitization may play a key role in psychosis. In humans and animals, repeated exposure to stimulants induces a sensitization state.117 This sensitization state is characterized by an enhanced response to subsequent low-dose challenges.118 Sensitized animals (to different drugs) share a number of neurobiological changes such as long-term alterations of mesolimbic and prefrontal dopaminergic neurotransmission while also expressing behavioral similarities to positive symptoms and long-lasting cognitive deficits such as seen in schizophrenia.117
Acute exposure to drugs of abuse provokes transient increases in cFos and other members of the Fos family of transcription factors in the striatum.119,120 The majority of these Fos factors become desensitized during chronic abuse, with the exception of ΔFOSB, which itself stimulates the expression of other genes, such as Cdk5.121–123 ΔFOSB accumulates in the nucleus accumbens and dorsal striatum—2 brain regions important for reward and locomotor drug responses—and remains for several weeks after cessation of drug administration, suggesting a role in the onset of addiction.123 Addictive behavior, however, continues long after this, and it has been postulated that epigenetic mechanisms may be involved in both rapid and persistent drug reactions. Mouse models of cocaine response have shown that the upregulation of cFos and ΔFOSB upon acute administration is accompanied by rapid and transient H4 hyperacetylation at the cFos and FosB gene promoters.124 Chronic exposure, however, does not result in H4 hyperacetylation but appears to be associated with H3 hyperacetylation of the FosB promoter, with no effect on the cFos promoter. H3 hyperacetylation of 2 genes associated with chronic, but not acute, drug use—Cdk5 and BDNF—was also detected after chronic cocaine administration and persisted even 1 week after treatment ceased.124 Interestingly, the induced overexpression of histone deacetylases in the mouse brain was found to counteract this hyperacetylation and dampen the rewarding effect of cocaine. These findings implicate epigenetic mechanisms in the development of addiction and particularly in the immediate and sustained behavioral effects of cocaine.124 Given the high levels of comorbidity between addiction and psychosis, and the known onset of psychosis-like symptoms in users of drugs such as amphetamines, these animal data provide an insight into the molecular changes induced by drugs of abuse and their potential role in mediating the symptoms of schizophrenia and bipolar disorder.
Evidence from human studies also highlights the occurrence of long-lasting changes in gene expression in subjects exposed to psychostimulants. For example, prolonged exposure to amphetamine is associated with long-term reductions of dopamine transporter density in the brain as measured by in vivo imaging studies.125 Exposure to cannabis, cocaine, and phencyclidine is known to cause long-lasting effects on intracerebral gene expression, and recent evidence suggests that in fact these drugs may all affect common neurobiological pathways. In a recent postmortem study, exposures to cannabis, cocaine, and phencyclidine in humans shared many transcriptional changes in the brain.126 Hierarchical clustering of these transcripts indicated that genes from the calmodulin signaling cluster were predominantly affected in the brains of abusers, a finding that may be of particular importance given that enhanced dopamine release due to sensitization depends on calmodulin signaling.127,128 Thus, chronic exposure to drugs may elicit a sensitization state with long-lasting changes of intracerebral gene transcription, and evidence—at least from animal studies—has started to accumulate for the crucial, mediating role of the epigenetic machinery.
Synergism in Environmental Factors Leading to Psychosis Through Epigenetics?
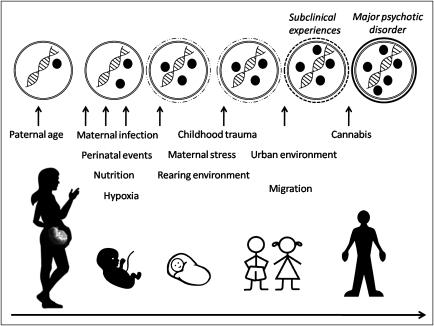
It has been proposed that major psychotic disorders arise slowly from subclinical psychotic symptoms that become abnormally persistent when synergistically combined with various environmental exposures during development (such as pre- and perinatal factors, cannabis abuse, and psychosocial trauma) that may impact on behavioral and neurotransmitter sensitization.129,130 On the basis of the limited evidence from human and animal studies, it is attractive to suggest that the dynamic framework of epigenetic gene regulation may provide a window into the molecular mechanisms accounting for these synergistic effects (figure 2).
Fig. 2.
Epigenetic Mediation of Environmental Factors During Development. Model of epigenetic mediation of environmental influences during development and the relation with the phenotypic pathway leading to major psychotic disorders. From the epigenetic perspective, environmental exposures can have long-lasting effects on gene expression and phenotype (ie, major psychotic disorders) through epigenetic mechanisms such as DNA methylation. Black circles represent epigenetic modifications to DNA (eg, methylated cytosines or histone modifications). The top row represents a nucleus of a postmitotic cells with dynamic changes to DNA modifications that may accumulate within the individual throughout development as a consequence of the synergistical combination of multiple exposures. These dynamic changes in DNA methylation may be associated with the appearance of subclinical psychotic symptoms (reflected by dashed line around the nucleus in the top row) that can become abnormally persistent and ultimately lead to the onset of a major psychotic disorder (reflected by black line around the nucleus in the top row). While this figure illustrates that major psychotic disorders are associated with accumulation of DNA modifications of a gene, scenarios are equally possible where the gene is associated with the removal of epigenetic modifications.
Summary and Challenges
Increasing evidence from case-control analyses of epigenetic alterations and findings from epidemiological studies complemented by experimental animal work suggest a role of the cells’ epigenetic machinery in mediating the effects of environmental exposures in the etiology of major psychotic disorders. However, direct and replicated evidence for clear epigenetic mediation of environmental exposures in psychosis is currently very sparse, and the preliminary findings nominating specific epigenetic alterations need carefully employed replication studies. While it is easy to theorize about the role of epigenetic processes in mediating susceptibility to psychiatric disorders, actually investigating these modifications at a molecular level is not so straightforward. Unlike for other disorders such as cancer where epigenetic investigations are more advanced, epigenomic studies in psychiatry are beset by a number of limitations related to subjective diagnostic paradigms, sample heterogeneity, and the fact that the primary tissue of interest (the brain) is not easily accessible. Difficulties in establishing valid etiological classifications of psychiatric disorders and phenotypes have spurred formulations of intermediate phenotypes or subclasses of conventional disease categories that may be more proximal to the actual neurobiological causal factors.131–133 The first epigenetic studies in psychiatric disorders have increased awareness of the numerous technical challenges that require exploration and watchful attention.134 The limited accessibility to high-quality human brain tissue from well-phenotyped patients, in combination with the cell type–specific and temporal specific nature of epigenetic programming, poses great challenges for epigenetic studies. Despite the problems of tissue specificity, one compromise is to use other, more accessible tissue sources like lymphocytes for epigenetic profiling in the hope that the patterns observed will reflect those of the real tissue of interest. There is increasing evidence from other disorders that many epimutations are not only limited to the affected tissue or cell type but can also be detected in other tissues—this may be especially the case for environmentally induced changes where “exposure” occurs throughout the body. As a “normal” epigenome has not yet been characterized, especially for tissues such as the brain, and correlations across tissue types have not been rigorously performed, there is need for physiological studies that establish these key features.135 Expansion of translational studies that combine findings from (a) carefully employed epidemiological studies, (b) molecular biologic studies on prospectively collected, easily accessible human tissue (such as blood lymphocytes, bucchal mucosa, or germ line cells) and (c) experimental animal studies on the effects of environmental exposures will allow for further exploration on the role of epigenetics in human physiology and patho(psycho)physiology. Such approaches can take full advantage of the recent developments in genome-wide analyses of genetic variations, gene expression, and epigenetic marks that allow for explorative, hypothesis-free investigations. While posing significant challenges on statistical analyses (with risk for spurious and false-positive findings), these explorative analyses may significantly complement conventional hypothesis-testing analyses. Taken together, these issues create a lively research atmosphere in studying the role of epigenetic dysfunction in psychiatric phenotypes, and the next few years are likely to bring important discoveries about the mechanisms linking environmental pathogens to disease.
Future Prospects
Technological advances in epigenomic profiling mean we are now at the exciting stage where it is feasible to start investigating the ways in which environmental factors act upon the genome to bring about epigenetic changes in gene expression and risk for disorders such as schizophrenia and bipolar disorder. A first step in exploring putative epigenetic mediation of environmental exposures in psychosis would be to test for gene-environment interactions by investigating genetic variants in epigenetic-relevant genes for their interaction with environmental exposures in large, well-characterized samples of subjects with psychosis, their siblings, and control subjects.18 Second, longitudinal studies in “at-risk” mental states for psychosis136 and the MZ twin samples discordant for major psychotic disorders and psychosis-related phenotypes137 may yield breakthrough findings for a role of epigenetic alterations in the onset and/or course of major psychotic disorders. Third, animal studies may be of particular virtue for deciphering the exact intracerebral epigenetic alterations as both genetic and environmental factors can be (relatively) well controlled and brain tissue easily obtained. In conclusion, the field of epigenetic research in major psychotic disorders deserves carefully employed translational studies and (as a consequence of the dynamic and the potentially reversible nature of epigenetic marks) appears very promising for indentifying new preventative and interventional strategies.
Acknowledgments
Rutten is the recipient of a Kootstra fellowship award and Mill is the recipient of a National Alliance for Research on Schizophrenia And Depression Young Investigator Award.
References
- 1.Stefansson H, Ophoff RA, Steinberg S, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh G, Petronis A. Environmental studies of schizophrenia through the prism of epigenetics. Schizophr Bull. 2008;34:1122–1129. doi: 10.1093/schbul/sbn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crow TJ. Craddock & Owen vs Kraepelin: 85 years late, mesmerised by “polygenes”. Schizophr Res. 2008;103:156–160. doi: 10.1016/j.schres.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 6.Allis CD, Jenuwein T, Reinberg D. Overview and Concepts. New York, NY: Cold Spring Harbor Laboratory Press; 2007. pp. 23–61. [Google Scholar]
- 7.Henikoff S, Matzke MA. Exploring and explaining epigenetic effects. Trends Genet. 1997;13:293–295. doi: 10.1016/s0168-9525(97)01219-5. [DOI] [PubMed] [Google Scholar]
- 8.Sandovici I, Kassovska-Bratinova S, Loredo-Osti JC, et al. Interindividual variability and parent of origin DNA methylation differences at specific human Alu elements. Hum Mol Genet. 2005;14:2135–2143. doi: 10.1093/hmg/ddi218. [DOI] [PubMed] [Google Scholar]
- 9.Rakyan V, Whitelaw E. Transgenerational epigenetic inheritance. Curr Biol. 2003;13:R6. doi: 10.1016/s0960-9822(02)01377-5. [DOI] [PubMed] [Google Scholar]
- 10.Klar AJ. Propagating epigenetic states through meiosis: where Mendel's gene is more than a DNA moiety. Trends Genet. 1998;14:299–301. doi: 10.1016/s0168-9525(98)01535-2. [DOI] [PubMed] [Google Scholar]
- 11.Richards EJ. Inherited epigenetic variation–revisiting soft inheritance. Nat Rev Genet. 2006;7:395–401. doi: 10.1038/nrg1834. [DOI] [PubMed] [Google Scholar]
- 12.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 13.Robertson KD, Wolffe AP. DNA methylation in health and disease. Nat Rev Genet. 2000;1:11–19. doi: 10.1038/35049533. [DOI] [PubMed] [Google Scholar]
- 14.Ooi SK, Qiu C, Bernstein E, et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature. 2007;448:714–717. doi: 10.1038/nature05987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutherland JE, Costa M. Epigenetics and the environment. Ann N Y Acad Sci. 2003;983:151–160. doi: 10.1111/j.1749-6632.2003.tb05970.x. [DOI] [PubMed] [Google Scholar]
- 16.Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation:linking early developmental environment to adult disease. Reprod Toxicol. 2007;23:297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 18.van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34:1066–1082. doi: 10.1093/schbul/sbn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakyan VK, Blewitt ME, Druker R, Preis JI, Whitelaw E. Metastable epialleles in mammals. Trends Genet. 2002;18:348–351. doi: 10.1016/s0168-9525(02)02709-9. [DOI] [PubMed] [Google Scholar]
- 20.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132(suppl):2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- 22.Polesskaya OO, Aston C, Sokolov BP. Allele C-specific methylation of the 5-HT2A receptor gene: evidence for correlation with its expression and expression of DNA methylase DNMT1. J Neurosci Res. 2006;83:362–373. doi: 10.1002/jnr.20732. [DOI] [PubMed] [Google Scholar]
- 23.Mill J, Tang T, Kaminsky Z, et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mill J, Petronis A. The relevance of epigenetics to major psychosis. In: Ferguson-Smith A, Greally J, Martienssen R, editors. Epigenomics. Springer; 2009. pp. 411–434. [Google Scholar]
- 25.Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petronis A, Gottesman, Kan P, et al. Monozygotic twins exhibit numerous epigenetic differences: clues to twin discordance? Schizophr Bull. 2003;29:169–178. doi: 10.1093/oxfordjournals.schbul.a006988. [DOI] [PubMed] [Google Scholar]
- 27.Mill J, Dempster E, Caspi A, Williams B, Moffitt T, Craig I. Evidence for monozygotic twin (MZ) discordance in methylation level at two CpG sites in the promoter region of the catechol-O-methyltransferase (COMT) gene. Am J Med Genet B Neuropsychiatr Genet. 2006;141:421–425. doi: 10.1002/ajmg.b.30316. [DOI] [PubMed] [Google Scholar]
- 28.Haidemenos A, Kontis D, Gazi A, Kallai E, Allin M, Lucia B. Plasma homocysteine, folate and B12 in chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1289–1296. doi: 10.1016/j.pnpbp.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Abdolmaleky HM, Cheng KH, Faraone SV, et al. Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum Mol Genet. 2006;15:3132–3145. doi: 10.1093/hmg/ddl253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grayson DR, Jia X, Chen Y, et al. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci U S A. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dempster EL, Mill J, Craig IW, Collier DA. The quantification of COMT mRNA in post mortem cerebellum tissue: diagnosis, genotype, methylation and expression. BMC Med Genet. 2006;7:10. doi: 10.1186/1471-2350-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tochigi M, Iwamoto K, Bundo M, et al. Methylation status of the reelin promoter region in the brain of schizophrenic patients. Biol Psychiatry. 2008;63:530–533. doi: 10.1016/j.biopsych.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci U S A. 2005;102:2152–2157. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuratomi G, Iwamoto K, Bundo M, et al. Aberrant DNA methylation associated with bipolar disorder identified from discordant monozygotic twins. Mol Psychiatry. 2008;13:429–441. doi: 10.1038/sj.mp.4002001. [DOI] [PubMed] [Google Scholar]
- 35.Coyle JT. The GABA-glutamate connection in schizophrenia: which is the proximate cause? Biochem Pharmacol. 2004;68:1507–1514. doi: 10.1016/j.bcp.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 36.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 37.Rosa A, Picchioni MM, Kalidindi S, et al. Differential methylation of the X-chromosome is a possible source of discordance for bipolar disorder female monozygotic twins. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:459–462. doi: 10.1002/ajmg.b.30616. [DOI] [PubMed] [Google Scholar]
- 38.van Os J, Krabbendam L, Myin-Germeys I, Delespaul P. The schizophrenia envirome. Curr Opin Psychiatry. 2005;18:141–145. doi: 10.1097/00001504-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Crow TJ. How and why genetic linkage has not solved the problem of psychosis: review and hypothesis. Am J Psychiatry. 2007;164:13–21. doi: 10.1176/ajp.2007.164.1.13. [DOI] [PubMed] [Google Scholar]
- 40.Wong AH, Gottesman, Petronis A. Phenotypic differences in genetically identical organisms: the epigenetic perspective. Hum Mol Genet. 2005;14 Spec No 1:R11–R18. doi: 10.1093/hmg/ddi116. [DOI] [PubMed] [Google Scholar]
- 41.Feinberg AP. Epigenetics at the epicenter of modern medicine. JAMA. 2008;299:1345–1350. doi: 10.1001/jama.299.11.1345. [DOI] [PubMed] [Google Scholar]
- 42.Brown AS, Schaefer CA, Wyatt RJ, et al. Paternal age and risk of schizophrenia in adult offspring. Am J Psychiatry. 2002;159:1528–1533. doi: 10.1176/appi.ajp.159.9.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malaspina D. Paternal factors and schizophrenia risk: de novo mutations and imprinting. Schizophr Bull. 2001;27:379–393. doi: 10.1093/oxfordjournals.schbul.a006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malaspina D, Harlap S, Fennig S, et al. Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry. 2001;58:361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- 45.Perrin MC, Brown AS, Malaspina D. Aberrant epigenetic regulation could explain the relationship of paternal age to schizophrenia. Schizophr Bull. 2007;33:1270–1273. doi: 10.1093/schbul/sbm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull. 2008;34:1083–1094. doi: 10.1093/schbul/sbn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicodemus KK, Marenco S, Batten AJ, et al. Serious obstetric complications interact with hypoxia-regulated/vascular-expression genes to influence schizophrenia risk. Mol Psychiatry. 2008;13:873–877. doi: 10.1038/sj.mp.4002153. [DOI] [PubMed] [Google Scholar]
- 48.Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull. 2008;34:1054–1063. doi: 10.1093/schbul/sbn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu MQ, Sun WS, Liu BX, et al. Prenatal malnutrition and adult schizophrenia: further evidence from the 1959-1961 Chinese famine. Schizophr Bull. 2009;35:568–576. doi: 10.1093/schbul/sbn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown AS, Bottiglieri T, Schaefer CA, et al. Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Arch Gen Psychiatry. 2007;64:31–39. doi: 10.1001/archpsyc.64.1.31. [DOI] [PubMed] [Google Scholar]
- 51.Susser E, Brown AS, Klonowski E, Allen RH, Lindenbaum J. Schizophrenia and impaired homocysteine metabolism: a possible association. Biol Psychiatry. 1998;44:141–143. doi: 10.1016/s0006-3223(97)00427-7. [DOI] [PubMed] [Google Scholar]
- 52.Susser E, Neugebauer R, Hoek HW, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 53.Khashan AS, Abel KM, McNamee R, et al. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65:146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 54.Cannon M, Clarke MC. Risk for schizophrenia–broadening the concepts, pushing back the boundaries. Schizophr Res. 2005;79:5–13. doi: 10.1016/j.schres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 55.Cannon TD, Mednick SA, Parnas J. Antecedents of predominantly negative- and predominantly positive-symptom schizophrenia in a high-risk population. Arch Gen Psychiatry. 1990;47:622–632. doi: 10.1001/archpsyc.1990.01810190022003. [DOI] [PubMed] [Google Scholar]
- 56.Tienari P, Wynne LC, Sorri A, et al. Genotype-environment interaction in schizophrenia-spectrum disorder. Long-term follow-up study of Finnish adoptees. Br J Psychiatry. 2004;184:216–222. doi: 10.1192/bjp.184.3.216. [DOI] [PubMed] [Google Scholar]
- 57.Schreier A, Wolke D, Thomas K, et al. Prospective study of peer victimization in childhood and psychotic symptoms in a nonclinical population at age 12 years. Arch Gen Psychiatry. 2009;66:527–536. doi: 10.1001/archgenpsychiatry.2009.23. [DOI] [PubMed] [Google Scholar]
- 58.Krabbendam L, van Os J. Schizophrenia and urbanicity: a major environmental influence–conditional on genetic risk. Schizophr Bull. 2005;31:795–799. doi: 10.1093/schbul/sbi060. [DOI] [PubMed] [Google Scholar]
- 59.Cantor-Graae E, Selten JP. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162:12–24. doi: 10.1176/appi.ajp.162.1.12. [DOI] [PubMed] [Google Scholar]
- 60.Henquet C, Di Forti M, Morrison P, Kuepper R, Murray RM. Gene-environment interplay between cannabis and psychosis. Schizophr Bull. 2008;34:1111–1121. doi: 10.1093/schbul/sbn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murray RM, Morrison PD, Henquet C, Di Forti M. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci. 2007;8:885–895. doi: 10.1038/nrn2253. [DOI] [PubMed] [Google Scholar]
- 62.Luzi S, Morrison PD, Powell J, di Forti M, Murray RM. What is the mechanism whereby cannabis use increases risk of psychosis? Neurotox Res. 2008;14:105–112. doi: 10.1007/BF03033802. [DOI] [PubMed] [Google Scholar]
- 63.Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 64.Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, Murray RM. Pathways to schizophrenia: the impact of environmental factors. Int J Neuropsychopharmacol. 2004;7(suppl 1):S7–S13. doi: 10.1017/S1461145704004122. [DOI] [PubMed] [Google Scholar]
- 65.Chen CK, Lin SK, Sham PC, Ball D, Loh el W, Murray RM. Morbid risk for psychiatric disorder among the relatives of methamphetamine users with and without psychosis. Am J Med Genet B Neuropsychiatr Genet. 2005;136B:87–91. doi: 10.1002/ajmg.b.30187. [DOI] [PubMed] [Google Scholar]
- 66.Zammit S, Allebeck P, Dalman C, et al. Paternal age and risk for schizophrenia. Br J Psychiatry. 2003;183:405–408. doi: 10.1192/bjp.183.5.405. [DOI] [PubMed] [Google Scholar]
- 67.Byrne M, Agerbo E, Ewald H, Eaton WW, Mortensen PB. Parental age and risk of schizophrenia: a case-control study. Arch Gen Psychiatry. 2003;60:673–678. doi: 10.1001/archpsyc.60.7.673. [DOI] [PubMed] [Google Scholar]
- 68.Sipos A, Rasmussen F, Harrison G, et al. Paternal age and schizophrenia: a population based cohort study. BMJ. 2004;329:1070. doi: 10.1136/bmj.38243.672396.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wohl M, Gorwood P. Paternal ages below or above 35 years old are associated with a different risk of schizophrenia in the offspring. Eur Psychiatry. 2007;22:22–26. doi: 10.1016/j.eurpsy.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 70.Tiemann-Boege I, Navidi W, Grewal R, et al. The observed human sperm mutation frequency cannot explain the achondroplasia paternal age effect. Proc Natl Acad Sci U S A. 2002;99:14952–14957. doi: 10.1073/pnas.232568699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flint J. Implications of genomic imprinting for psychiatric genetics. Psychol Med. 1992;22:5–10. doi: 10.1017/s0033291700032669. [DOI] [PubMed] [Google Scholar]
- 72.Davies W, Isles AR, Humby T, Wilkinson LS. What are imprinted genes doing in the brain? Epigenetics. 2007;2:201–206. doi: 10.4161/epi.2.4.5379. [DOI] [PubMed] [Google Scholar]
- 73.Wilkinson LS, Davies W, Isles AR. Genomic imprinting effects on brain development and function. Nat Rev Neurosci. 2007;8:832–843. doi: 10.1038/nrn2235. [DOI] [PubMed] [Google Scholar]
- 74.Flanagan JM, Popendikyte V, Pozdniakovaite N, et al. Intra- and interindividual epigenetic variation in human germ cells. Am J Hum Genet. 2006;79:67–84. doi: 10.1086/504729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yauk C, Polyzos A, Rowan-Carroll A, et al. Germ-line mutations, DNA damage, and global hypermethylation in mice exposed to particulate air pollution in an urban/industrial location. Proc Natl Acad Sci U S A. 2008;105:605–610. doi: 10.1073/pnas.0705896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.St Clair D, Xu M, Wang P, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA. 2005;294:557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- 77.Susser ES, Lin SP. Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944-1945. Arch Gen Psychiatry. 1992;49:983–988. doi: 10.1001/archpsyc.1992.01820120071010. [DOI] [PubMed] [Google Scholar]
- 78.Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smits L, Pedersen C, Mortensen P, van Os J. Association between short birth intervals and schizophrenia in the offspring. Schizophr Res. 2004;70:49–56. doi: 10.1016/j.schres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 80.Allen NC, Bagade S, McQueen MB, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 81.Jonsson EG, Larsson K, Vares M, et al. Two methylenetetrahydrofolate reductase gene (MTHFR) polymorphisms, schizophrenia and bipolar disorder: an association study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:976–982. doi: 10.1002/ajmg.b.30671. [DOI] [PubMed] [Google Scholar]
- 82.Roffman JL, Weiss AP, Purcell S, et al. Contribution of methylenetetrahydrofolate reductase (MTHFR) polymorphisms to negative symptoms in schizophrenia. Biol Psychiatry. 2008;63:42–48. doi: 10.1016/j.biopsych.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 83.Zammit S, Lewis S, Gunnell D, Smith GD. Schizophrenia and neural tube defects: comparisons from an epidemiological perspective. Schizophr Bull. 2007;33:853–858. doi: 10.1093/schbul/sbl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tienari P, Wynne LC, Moring J, et al. The Finnish adoptive family study of schizophrenia. Implications for family research [see comments] Br J Psychiatry Suppl. 1994;23:20–26. [PubMed] [Google Scholar]
- 85.Wahlberg KE, Wynne LC, Oja H, et al. Gene-environment interaction in vulnerability to schizophrenia: findings from the Finnish Adoptive Family Study of Schizophrenia. Am J Psychiatry. 1997;154:355–362. doi: 10.1176/ajp.154.3.355. [DOI] [PubMed] [Google Scholar]
- 86.Wahlberg KE, Wynne LC, Hakko H, et al. Interaction of genetic risk and adoptive parent communication deviance: longitudinal prediction of adoptee psychiatric disorders. Psychol Med. 2004;34:1531–1541. doi: 10.1017/s0033291704002661. [DOI] [PubMed] [Google Scholar]
- 87.Carter JW, Schulsinger F, Parnas J, Cannon T, Mednick SA. A multivariate prediction model of schizophrenia. Schizophr Bull. 2002;28:649–682. doi: 10.1093/oxfordjournals.schbul.a006971. [DOI] [PubMed] [Google Scholar]
- 88.Carter JW, Parnas J, Cannon TD, Schulsinger F, Mednick SA. MMPI variables predictive of schizophrenia in the Copenhagen High-Risk Project: a 25-year follow-up. Acta Psychiatr Scand. 1999;99:432–440. doi: 10.1111/j.1600-0447.1999.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 89.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 90.Weaver IC, Champagne FA, Brown SE, et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McGowan PO, Sasaki A, D'Alessio AC, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moser D, Molitor A, Kumsta R, Tatschner T, Riederer P, Meyer J. The glucocorticoid receptor gene exon 1-F promoter is not methylated at the NGFI-A binding site in human hippocampus. World J Biol Psychiatry. 2007;8:262–268. doi: 10.1080/15622970701429862. [DOI] [PubMed] [Google Scholar]
- 94.Pariante CM. Pituitary volume in psychosis: the first review of the evidence. J Psychopharmacol. 2008;22(suppl):76–81. doi: 10.1177/0269881107084020. [DOI] [PubMed] [Google Scholar]
- 95.Myin-Germeys I, van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27:409–424. doi: 10.1016/j.cpr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 96.Renthal W, Maze I, Krishnan V, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 97.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 98.Buckley PF, Pillai A, Evans D, Stirewalt E, Mahadik S. Brain derived neurotropic factor in first-episode psychosis. Schizophr Res. 2007;91:1–5. doi: 10.1016/j.schres.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–117. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- 102.Houston JE, Murphy J, Adamson G, Stringer M, Shevlin M. Childhood sexual abuse, early cannabis use, and psychosis: testing an interaction model based on the National Comorbidity Survey. Schizophr Bull. 2008;34:580–585. doi: 10.1093/schbul/sbm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sugranyes G, Flamarique I, Parellada E, et al. Cannabis use and age of diagnosis of schizophrenia. Eur Psychiatry. 2009;24:282–286. doi: 10.1016/j.eurpsy.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 104.Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 105.Henquet C, Rosa A, Delespaul P, et al. COMT ValMet moderation of cannabis-induced psychosis: a momentary assessment study of ‘switching on’ hallucinations in the flow of daily life. Acta Psychiatr Scand. 2009;119:156–160. doi: 10.1111/j.1600-0447.2008.01265.x. [DOI] [PubMed] [Google Scholar]
- 106.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;28:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 107.Villares J. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience. 2007;145:323–334. doi: 10.1016/j.neuroscience.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 108.Ellgren M, Spano SM, Hurd YL. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2007;32:607–615. doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- 109.Casu MA, Pisu C, Sanna A, et al. Effect of delta9-tetrahydrocannabinol on phosphorylated CREB in rat cerebellum: an immunohistochemical study. Brain Res. 2005;1048:41–47. doi: 10.1016/j.brainres.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 110.Fernandez-Ruiz J, Gomez M, Hernandez M, de Miguel R, Ramos JA. Cannabinoids and gene expression during brain development. Neurotox Res. 2004;6:389–401. doi: 10.1007/BF03033314. [DOI] [PubMed] [Google Scholar]
- 111.Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, Manzoni OJ. A single in-vivo exposure to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci. 2004;7:585–586. doi: 10.1038/nn1251. [DOI] [PubMed] [Google Scholar]
- 112.Scallet AC. Neurotoxicology of cannabis and THC: a review of chronic exposure studies in animals. Pharmacol Biochem Behav. 1991;40:671–676. doi: 10.1016/0091-3057(91)90380-k. [DOI] [PubMed] [Google Scholar]
- 113.Heath RG, Fitzjarrell AT, Fontana CJ, Garey RE. Cannabis sativa: effects on brain function and ultrastructure in rhesus monkeys. Biol Psychiatry. 1980;15:657–690. [PubMed] [Google Scholar]
- 114.Hoffman AF, Oz M, Caulder T, Lupica CR. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci. 2003;23:4815–4820. doi: 10.1523/JNEUROSCI.23-12-04815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Khare M, Taylor AH, Konje JC, Bell SC. Delta9-tetrahydrocannabinol inhibits cytotrophoblast cell proliferation and modulates gene transcription. Mol Hum Reprod. 2006;12:321–333. doi: 10.1093/molehr/gal036. [DOI] [PubMed] [Google Scholar]
- 116.Barnett JH, Werners U, Secher SM, et al. Substance use in a population-based clinic sample of people with first-episode psychosis. Br J Psychiatry. 2007;190:515–520. doi: 10.1192/bjp.bp.106.024448. [DOI] [PubMed] [Google Scholar]
- 117.Featherstone RE, Kapur S, Fletcher PJ. The amphetamine-induced sensitized state as a model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1556–1571. doi: 10.1016/j.pnpbp.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 118.Castner SA, Williams GV. From vice to virtue: insights from sensitization in the nonhuman primate. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1572–1592. doi: 10.1016/j.pnpbp.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 119.Uslaner J, Badiani A, Norton CS, et al. Amphetamine and cocaine induce different patterns of c-fos mRNA expression in the striatum and subthalamic nucleus depending on environmental context. Eur J Neurosci. 2001;13:1977–1983. doi: 10.1046/j.0953-816x.2001.01574.x. [DOI] [PubMed] [Google Scholar]
- 120.Uslaner J, Badiani A, Norton CS, et al. Amphetamine and cocaine induce different patterns of c-fos mRNA expression in the striatum and subthalamic nucleus depending on environmental context. Eur J Neurosci. 2001;13:1977–1983. doi: 10.1046/j.0953-816x.2001.01574.x. [DOI] [PubMed] [Google Scholar]
- 121.Bibb JA, Chen J, Taylor JR, et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- 122.Nestler EJ, Barrot M, Self DW. Delta FosB: A sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98:11042. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kumar A, Choi KH, Renthal W, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 125.Sekine Y, Iyo M, Ouchi Y, et al. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- 126.Lehrmann E, Colantuoni C, Deep-Soboslay A. Transcriptional changes common to human cocaine, cannabis and phencyclidine abuse. PLoS One. 2006;1 doi: 10.1371/journal.pone.0000114. e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Greenstein R, Novak G, Seeman P. Amphetamine sensitization elevates CaMKIIbeta mRNA. Synapse. 2007;61:827–834. doi: 10.1002/syn.20429. [DOI] [PubMed] [Google Scholar]
- 128.Iwata SI, Hewlett GH, Ferrell ST, Kantor L, Gnegy ME. Enhanced dopamine release and phosphorylation of synapsin I and neuromodulin in striatal synaptosomes after repeated amphetamine. J Pharmacol Exp Ther. 1997;283:1445–1452. [PubMed] [Google Scholar]
- 129.Cougnard A, Marcelis M, Myin-Germeys I, et al. Does normal developmental expression of psychosis combine with environmental risk to cause persistence of psychosis? A psychosis proneness-persistence model. Psychol Med. 2007;37:513–527. doi: 10.1017/S0033291706009731. [DOI] [PubMed] [Google Scholar]
- 130.Dominguez MD, Wichers M, Lieb R, Wittchen HU, van Os J. Evidence that onset of clinical psychosis is an outcome of progressively more persistent subclinical psychotic experiences: an 8-year cohort study. Schizophr Bull. May 23, 2009 doi: 10.1093/schbul/sbp022. doi:10.1093/schbul/sbp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.MacQueen GM, Hajek T, Alda M. The phenotypes of bipolar disorder: relevance for genetic investigations. Mol Psychiatry. 2005;10:811–826. doi: 10.1038/sj.mp.4001701. [DOI] [PubMed] [Google Scholar]
- 132.Cannon TD. The inheritance of intermediate phenotypes for schizophrenia. Curr Opin Psychiatry. 2005;18:135–140. doi: 10.1097/00001504-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 133.Fiedorowicz JG, Epping EA, Flaum M. Toward defining schizophrenia as a more useful clinical concept. Curr Psychiatry Rep. 2008;10:344–351. doi: 10.1007/s11920-008-0055-9. [DOI] [PubMed] [Google Scholar]
- 134.Schumacher A, Petronis A. Epigenetics of complex diseases: from general theory to laboratory experiments. Curr Top Microbiol Immunol. 2006;310:81–115. doi: 10.1007/3-540-31181-5_6. [DOI] [PubMed] [Google Scholar]
- 135.Callinan PA, Feinberg AP. The emerging science of epigenomics. Hum Mol Genet. 2006;15 Spec No 1:R95–R101. doi: 10.1093/hmg/ddl095. [DOI] [PubMed] [Google Scholar]
- 136.Borgwardt SJ, McGuire PK, Aston J, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 137.Picchioni MM, Toulopoulou T, Landau S, Davies N, Ribchester T, Murray RM. Neurological abnormalities in schizophrenic twins. Biol Psychiatry. 2006;59:341–348. doi: 10.1016/j.biopsych.2005.07.007. [DOI] [PubMed] [Google Scholar]