Abstract
This special issue focuses on the theme of sensory processing dysfunction in schizophrenia. For more than 50 years, from approximately the time of Bleuler until the early 1960s, sensory function was considered one of the few preserved functions in schizophrenia (Javitt1). Fortunately, the last several decades have brought a renewed and accelerating interest in this topic. The articles included in the issue range from those addressing fundamental bases of sensory dysfunction (Brenner, Yoon, and Turetsky) to those that examine how elementary deficits in sensory processing affect the sensory experience of individuals with schizophrenia (Butler, Kantrowitz, and Coleman) to the question of how sensory-based treatments may lead to improvement in remediation strategies (Adcock). Although addressing only a small portion of the current complex and burgeoning literature on sensory impairments across modalities, the present articles provide a cross-section of the issues currently under investigation. These studies also underscore the severe challenges that individuals with schizophrenia face when trying to decode the complex world around them.
Keywords: sensory, auditory, visual, cognition, NMDA receptor, schizophrenia
Introduction
Bleuler,2 while aware that patients often complained of altered sensory experience, nevertheless, attributed them to more basic deficits in association and emotion. Sensory processing was considered one of the intact simple functions. While correct about a great many issues in schizophrenia, recent findings suggest that in this case Bleuler may have been wrong.
The recent focus on sensory dysfunction in schizophrenia arises from a confluence of findings. First, careful psychophysiological studies beginning in the 1960s began to document objective deficits in sensory processing that could not be attributed easily to either attention or emotion. Key events included the description by McGhie and Chapman3 of sensory distortions spontaneously reported by individuals experiencing early symptoms of schizophrenia as well as subsequent studies begun by Holzman4 documenting objective psychophysiological deficits using eye tracking among other measures.
Subsequent studies documented deficits in processes such as backward masking5 or sensory gating.6 Much of the renewed interest in sensory processing arises from the development of new techniques, such as event-related potentials (ERP) or functional magnetic resonance imaging (fMRI) that permit objective assessment of neural activation at the level of primary sensory cortices. These techniques permit objective evaluation of “early sensory processes,” ie, responses that occur both early in time following delivery of a sensory stimulus (generally within the first 100–200 ms) and also within hierarchically early brain regions such as primary and secondary sensory regions.
Second, within the basic neuroscience community, there has been increasing appreciation for the complexity of processing that takes place even within early sensory regions. These regions were once thought to play a mostly “hard wired” role in cognition—simply passing along veridical electrical representations of the sensory environment to higher cognitive brain regions, where the information was further processed. More recent models, however, focus on the complexity of processing that is found even within primary sensory regions.
For example, Baddeley7 discussed the existence of auditory and visual “slave” memory systems that function outside the control of the central executive. Other theorists, such as Cowan,8 emphasized the preconscious filtering out and filtering in of information that occurs at the level of sensory cortex. Overall, these studies pointed out that much more occurs within early sensory regions than is detected with routine visual or audiometric testing and that breakdown of these early processes can significantly impair the type and complexity of information that is available for subsequent processing.
Etiological Models of Schizophrenia
In addition to newer developments within the basic sensory research literature, newer etiological models of schizophrenia also push increasingly toward renewed focus on sensory dysfunction. For decades, the dopamine model served as the primary organizing theme for schizophrenia. In rodents, dopamine systems target primarily frontal and limbic brain regions, leading to strong focus on those regions in the pathophysiology of schizophrenia.
In primates, dopaminergic systems project more widely than in rodents, but nevertheless sensory cortices remain sparsely innervated,9 leading to a conceptual disinterest in sensory processes. Over recent years, however, limitations of the dopamine model have become increasingly apparent. In particular, although dopamine models account well for positive symptoms of schizophrenia, they account poorly for negative symptoms and cognitive dysfunction. More recent models focus on more generalized neurotransmitter systems such as glutamate,10,11 gamma-amino-butyric acid (GABA),12 and nicotine13 and particularly on dysfunction of transmission at N-methyl-D-aspartate (NMDA)-type glutamate receptors.14
NMDA models of schizophrenia are supported by the ability of phencyclidine (PCP), ketamine, and other NMDA antagonists to induce not only symptoms but also cognitive deficits that closely resemble those of schizophrenia. As opposed to dopaminergic agents, acute administration of NMDA antagonists reproduces the pattern of positive and negative symptoms commonly seen in early schizophrenia and exacerbates symptoms in remitted patients.15 Similarly, NMDA antagonists, even following acute administration, induce a generalized pattern of cognitive deficits similar to that seen in schizophrenia. Following chronic administration, more prolonged schizophrenia-like symptoms may emerge.16 Given the widespread distribution of transmitters such as glutamate, GABA, and nicotine and of receptors such as NMDA, the critical issue in schizophrenia may not be what brain regions are involved but rather what specific processes are impaired within each region. Patients with schizophrenia as a group are neither blind nor deaf. However, the fact that some processes within each modality are intact no longer precludes the possibility that other functions may be impaired.
Auditory Dysfunction in Schizophrenia
Deficits in sensory processing in schizophrenia are perhaps best documented in the auditory system, where integrity of sensory function can be assessed using well-characterized ERP such as P50, N100, and, most recently, mismatch negativity (MMN). Deficits in P50 gating were first described in schizophrenia in the early 1980s and provided some of the first evidence for deficits in inhibitory processes17 and subsequently for impaired nicotinic function.18 Deficits in N100 generation have similarly been documented for over 25 years19 and have been widely replicated over that time20 with strong specificity for schizophrenia over other disorders.21 Both of these potentials are generated within auditory sensory regions and point to breakdown of processing at early stages of stimulus evaluation.
As opposed to P50 and N100, which are elicited in response to simple repetitive stimuli, MMN is elicited only in response to stimuli that deviate from a predictable sequence. As such, it occupies the interface between sensory/perceptual and cognitive processing. Nevertheless, MMN, as commonly recorded, is generated independent of attention. Deficits in MMN generation to attributes such as stimulus pitch22 or duration23 deviance were first reported in the early 1990s and have been confirmed repeatedly since that time.24 Deficits in MMN generation are accompanied as well by impaired function of the auditory “echoic” memory system, which maintains brief representations of the physical attributes of individual auditory stimuli and thus participates in processes such as delayed tone matching.25 Because the echoic memory system, like MMN, functions preattentively, deficits cannot be easily ascribed to impaired attention, emotion, or motivation.
ERP indices, such as P50, N1, or MMN, have also been useful for investigating neurophysiological bases of cortical dysfunction in schizophrenia. An advantage of studying sensory processes in general, and auditory response in particular, is that they are highly amenable to implementation in animal models. MMN is elicited in monkeys using stimuli identical to those used in clinical studies and can thus be studied at the level of the cortical microcolumn.26 Deficits similar to those observed in schizophrenia are induced by administration of PCP, ketamine, or other NMDA antagonists in both monkeys27 and normal human volunteers.28–30 Similar deficits have recently been demonstrating in rodents31,32 as well, supporting the use of rodent models of sensory processing dysfunction in schizophrenia.33
The latest in the series of informative auditory paradigms is the auditory 40 Hz response.34–36 This response is elicited by a series of rapidly presented brief stimuli that entrain the elementary auditory responses and so index functioning of local circuit excitatory/inhibitory networks within auditory cortex. This circuit underlying generation of the auditory steady-state response is amenable to modeling at the basic neurophysiological level, as described by Brenner et al in this volume. As further detailed by Brenner et al, however, deficits in oscillatory responses in schizophrenia are not limited to “gamma range” responding but occur even at slower frequencies in other systems.
Visual Dysfunction in Schizophrenia
Most psychiatric researchers are well aware of the degree to which prefrontal cortices increase in size from rodent to primate to human. However, less well publicized is the extraordinary increase in size and complexity of visual regions in primates compared with other lineages. Even carnivores, such as cats, ferrets and canines have relatively simple visual systems designed primarily to permit capture of attention by motion and convey low-resolution visual representations. Other mammalian orders, such as rodents, lagomorphs, and artiodactyla, rely even less on visual information. By contrast, primates, and especially simians, are visual animals with a far more complex visual system than is present in any other mammalian order or primate suborder.
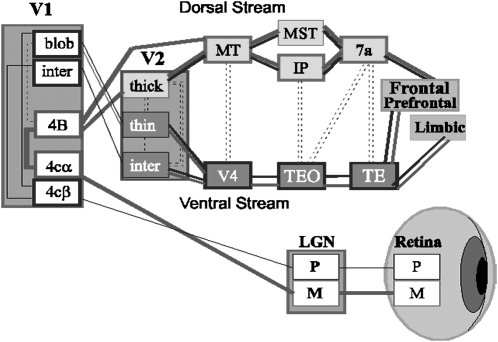
Our visual system is thought to have evolved primarily to allow us to recognize colored fruit standing out against green leaves37 or possibly snakes, which were primary predators or early simians.38 These days, however, it is used far more often to decipher the complex symbolic representations that we use to represent language or to “read” other people’s facial expressions. In order to process fine environmental details, we evolved 2 separate visual pathways, a magnocellular system that is most closely related to the ancestral mammalian visual system and a parvocellular system with unique sensitivity to wavelength (color) of light and the unique ability to produce high-resolution visual images (figure 1).
Fig. 1.
Schematic Representation of the Human Visual System. The magnocellular (M) system and the dorsal stream projection pathway are shown with thick lines. The parvocellular (P) system and ventral stream projection pathway are shown with thin lines.
The parvocellular system extracts far more information but functions far more slowly than the older magnocellular system but is concentrated foveally and thus can engage only limited portions of the complex visual scene at any single time. Complex local circuit and magnocellular/parvocellular interactions within the early visual system are used to extract patterns from the complex visual environment to which we are exposed. The magnocellular system, in particular, plays a key role in guiding parvocellular processing, eg, providing a general low-resolution “frame” for the visual environment, which is then “filled in” by details from the slower parvocellular system. In schizophrenia, physiological deficits affect functioning of the magnocellular visual pathway, which relies heavily on NMDA-based mechanisms and nonlinear gain mechanisms, leading to loss in these framing functions. The parvocellular system, however, can also function nonlinearly and in some circumstances may show impairments as well.39 Impairments in early visual processing have now been well documented in schizophrenia using methods, including steady-state40 and transient41 ERP approaches, along with fMRI42, and lead to impairments in processes such as motion detection43,44, object recognition,45 and reading.46
Several of the articles in this theme focus on the still emerging literature regarding visual dysfunction in schizophrenia. Brenner et al reviews the neurophysiological literature regarding visual function and concludes that deficits most likely represent interplay among magnocellular and parvocellular systems occurs at multiple levels. Yoon et al show deficits in center surround suppression in schizophrenia, also supporting an early visual deficit. Because the magnocellular system conducts more rapidly than the parvocellular system, humans, in general, show a “global advantage” in which they focus on the overall structure of a visual scene (the “forest”) before they focus on the fine details (the “trees”). Coleman et al shows loss of the normal global advantage in patients performing a global/local discrimination task, consistent with dysfunction of magnocellular systems.
Kantrowitz et al show how early visual deficits contribute to impairments in visual depth perception in individuals with schizophrenia and also how it renders patients more susceptible to some illusions even while they remain less susceptible to others. Finally, Butler et al shows how deficits in early visual processing, particularly involving the magnocellular system, interfere with ability of patients to read visual expressions. Overall, these studies not only highlight the importance to early-stage visual dysfunction to higher order cognitive processing but also highlight that even small changes in the visual characteristics of stimuli (eg, size, contrast, illumination) may fundamentally alter the processing pathways engaged and thus the degree of impairment in schizophrenia.
Other Sensory Systems
Although most work has concentrated on the auditory and visual systems in schizophrenia, disturbances within other sensory systems should not be excluded. For example, the somatosensory system, like the auditory system, maintains brief representations of sensory information, permitting individuals to compare weights, textures, or other stimulus qualities across brief periods of time (seconds to tens of seconds). As in the auditory system, patients show reduced sensitivity to stimulus features (eg, weight) but normal ability to retain representations once they are formed.47 Similar deficits are seen following treatment with NMDA antagonists in normal volunteers.15 Other sensory deficits, like impaired 2-point discrimination48 or elevated pain thresholds49 are also well documented. Thus, sensory deficits in schizophrenia are in no way limited to the auditory and visual systems, although psychopathological significance of other sensory deficits has yet to be fully explored.
One system that appears ripe for psychopathological investigation is the olfactory system. Deficits in odor discrimination in schizophrenia have, by now, also become well documented.50,51 Although early literature on olfactory deficits tended to focus on issues such as hedonics and potential limbic involvement, subsequent studies demonstrated severe impairments even at the level of identification and discrimination. NMDA receptors are known to play a critical role in olfactory processing, particularly in processes such as discrimination and habituation.52 Other transmitters and receptors also play well-defined roles, potentially permitting the behavioral dissection of the role of different neurotransmitter systems in olfactory dysfunction in schizophrenia.
The olfactory system also provides a unique model system in which to investigate neurochemical and neurophysiological mechanisms underlying sensory-level impairments in schizophrenia. As with other sensory systems, neural substrates are well known and amenable to investigation using ERP, magnetic resonance imaging, and other brain imaging modalities. Uniquely, however, olfactory cells can be harvested noninvasively through nasal epithelial biopsy and cultured for further investigation. As described by Turetsky et al (this volume), these cells contain many of the receptors (eg, dopamine, NMDA, nicotine, GABA) and second messenger systems (eg, cyclic adenosine monophosphate) that are of most interest in schizophrenia research. This system thus provides the unique opportunity to relate objective behavioral and neurophysiological changes in schizophrenia to alterations in neurochemistry and physiology of specific neuronal elements.
Bottom-up vs Top-down Models
The contributions of sensory dysfunction to overall cognitive impairment in schizophrenia is perhaps easiest to conceptualize as “bottom up” vs “top down.” However, such dichotomization runs the risk of oversimplifying the critical involvement of sensory dysfunction in schizophrenia. Few investigators would claim that the complex constellation of neuropsychological deficits in schizophrenia are due entirely to sensory dysfunction. Indeed, distributed neurochemical models predict that similar neurophysiological deficits should be present throughout cortex. From a theoretical point of view, therefore, the competing models of overall neurocognitive dysfunction in schizophrenia are best conceptualized as “generalized” vs top down, with the critical issue being whether specific regions are preferentially involved in schizophrenia or whether deficits are widespread, with characteristic impairments observed across all brain regions, including those responsible for sensory processing.
Nevertheless, in looking to correct deficits in cognitive functioning, it is clear that bottom-up sensory dysfunction may be a bottleneck. For example, “social cognition”—the ability to infer another person’s internal emotional state—depends upon the ability to interpret their facial expressions and tone of voice. If patients cannot accurately process faces because of early visual deficits, as argued by Butler et al in this issue, or process the pitch changes that allow one to interpret tone of voice as has been proposed previously,53–55 then trying to remediate social cognition as a construct becomes futile.
Bottom-up remediation thus becomes the logical first step in remediation of generalized cognitive dysfunction. Adcock et al in this issue lays out the case for both bottom-up and top-down remediation approaches. In medicine, the “proof of the pudding” is in the treating. If bottom-up remediation approaches are successful, as suggested by Adcock et al, then discussions of whether cognitive dysfunction in schizophrenia is driven bottom up or top down become purely academic.
Overall, this issue highlights the importance of sensory dysfunction to overall impairment in schizophrenia. It is axiomatic that if patients experience the world around them differently, they will react to it differently as well. Until such deficits can be effectively treated or remediated, clinicians and caregivers must be aware of the impact that these early deficits have on the ability of patients to interact effectively with the complex world that surrounds them.
Supplementary Material
Figure 1 is available in color at http://schizophreniabulletin.oxfordjournals.org.
Funding
National Institute of Mental Health (R37 MH49334); National Institute on Drug Abuse (R01 DA03383); New York University Conte Center (P50 MH086385).
References
- 1.Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleuler E. Dementia Praecox of the Group of the Schizophrenias. New York, NY: International Universities Press; 1950. [Google Scholar]
- 3.McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 4.Holzman PS. Assessment of perceptual functioning in schizophrenia. Psychopharmacologia. 1972;24(1):29–41. doi: 10.1007/BF00402904. [DOI] [PubMed] [Google Scholar]
- 5.Saccuzzo DP, Braff DL. Early information processing deficit in schizophrenia. New findings using schizophrenic subgroups and manic control subjects. Arch Gen Psychiatry. 1981;38:175–179. doi: 10.1001/archpsyc.1981.01780270061008. [DOI] [PubMed] [Google Scholar]
- 6.Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- 7.Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 8.Cowan N. On short and long auditory stores. Psychol Bull. 1984;96:341–370. [PubMed] [Google Scholar]
- 9.Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH. The distribution of tyrosine hydroxylase-immunoreaction fibers in primate neocortex is widespread but regionally specific. J Neurosci. 1987;7:279–290. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Javitt DC, Zukin SR. The role of excitatory amino acids in neuropsychiatric illness. J Neuropsychiatry Clin Neurosci. 1990;2:44–52. doi: 10.1176/jnp.2.1.44. [DOI] [PubMed] [Google Scholar]
- 11.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin LF, Freedman R. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- 14.Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 15.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 16.Linn GS, O'Keeffe RT, Lifshitz K, Schroeder C, Javitt DC. Behavioral effects of orally administered glycine in socially housed monkeys chronically treated with phencyclidine. Psychopharmacology. 2007;192(1):27–38. doi: 10.1007/s00213-007-0771-6. [DOI] [PubMed] [Google Scholar]
- 17.Freedman R, Adler LE, Waldo MC, Pachtman E, Franks RD. Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol Psychiatry. 1983;18:537–551. [PubMed] [Google Scholar]
- 18.Adler LE, Olincy A, Waldo M, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- 19.Roth WT, Pfefferbaum A, Kelly AF, Berger PA, Kopell BS. Auditory event-related potentials in schizophrenia and depression. Psychiatry Res. 1981;4:199–212. doi: 10.1016/0165-1781(81)90023-8. [DOI] [PubMed] [Google Scholar]
- 20.Turetsky BI, Bilker WB, Siegel SJ, Kohler CG, Gur RE. Profile of auditory information-processing deficits in schizophrenia. Psychiatry Res. 2009;165:27–37. doi: 10.1016/j.psychres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ford JM, Mathalon DH, Kalba S, Marsh L, Pfefferbaum A. N1 and P300 abnormalities in patients with schizophrenia, epilepsy, and epilepsy with schizophrenialike features. Biol Psychiatry. 2001;49:848–860. doi: 10.1016/s0006-3223(00)01051-9. [DOI] [PubMed] [Google Scholar]
- 22.Javitt DC, Doneshka P, Zylberman I, Ritter W, Vaughan HG., Jr Impairment of early cortical processing in schizophrenia: an event-related potential confirmation study. Biol Psychiatry. 1993;33:513–519. doi: 10.1016/0006-3223(93)90005-x. [DOI] [PubMed] [Google Scholar]
- 23.Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry. 1991;30:1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- 24.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. J Abnorm Psychol. 1997;106:315–324. doi: 10.1037//0021-843x.106.2.315. [DOI] [PubMed] [Google Scholar]
- 26.Javitt DC. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol Neurootol. 2000;5:207–215. doi: 10.1159/000013882. [DOI] [PubMed] [Google Scholar]
- 27.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreitschmann-Andermahr I, Rosburg T, Demme U, Gaser E, Nowak H, Sauer H. Effect of ketamine on the neuromagnetic mismatch field in healthy humans. Brain Res Cogn Brain Res. 2001;12:109–116. doi: 10.1016/s0926-6410(01)00043-x. [DOI] [PubMed] [Google Scholar]
- 29.Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 30.Heekeren K, Daumann J, Neukirch A, et al. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology. 2008;199(1):77–88. doi: 10.1007/s00213-008-1129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tikhonravov D, Neuvonen T, Pertovaara A, et al. Effects of an NMDA-receptor antagonist MK-801 on an MMN-like response recorded in anesthetized rats. Brain Res. 2008;1203:97–102. doi: 10.1016/j.brainres.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Ehrlichman RS, Maxwell CR, Majumdar S, Siegel SJ. Deviance-elicited changes in event-related potentials are attenuated by ketamine in mice. J Cogn Neurosci. 2008;20:1403–1414. doi: 10.1162/jocn.2008.20097. [DOI] [PubMed] [Google Scholar]
- 33.Bickel S, Javitt DC. Neurophysiological and neurochemical animal models of schizophrenia: focus on glutamate. Behav Brain Res. 2009;204:352–362. doi: 10.1016/j.bbr.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong LE, Summerfelt A, McMahon R, et al. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Kwon JS, O'Donnell BF, Wallenstein GV, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Light GA, Hsu JL, Hsieh MH, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 37.Barton RA. Visual specialization and brain evolution in primates. Proc Biol Soc. 1998;265:1933–1937. doi: 10.1098/rspb.1998.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isabell L. Snakes as agents of evolutionary change in primate brains. J Hum Evol. 2006;51:1–35. doi: 10.1016/j.jhevol.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18:151–157. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butler PD, Zemon V, Schechter I, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler PD, Martinez A, Foxe JJ, et al. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130(Pt 2):417–430. doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez A, Hillyard SA, Dias EC, et al. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. J Neurosci. 2008;28:7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Nakayama K, Levy D, Matthysse S, Holzman P. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophr Res. 2003;61:215–227. doi: 10.1016/s0920-9964(02)00222-0. [DOI] [PubMed] [Google Scholar]
- 44.Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophr Res. 2006;82:1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- 46.Revheim N, Butler PD, Schechter I, Jalbrzikowski M, Silipo G, Javitt DC. Reading impairment and visual processing deficits in schizophrenia. Schizophr Res. 2006;87:238–245. doi: 10.1016/j.schres.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Javitt DC, Liederman E, Cienfuegos A, Shelley AM. Panmodal processing imprecision as a basis for dysfunction of transient memory storage systems in schizophrenia. Schizophr Bull. 1999;25:763–775. doi: 10.1093/oxfordjournals.schbul.a033417. [DOI] [PubMed] [Google Scholar]
- 48.Chang BP, Lenzenweger MF. Somatosensory processing and schizophrenia liability: proprioception, exteroceptive sensitivity, and graphesthesia performance in the biological relatives of schizophrenia patients. J Abnorm Psychol. 2005;114(1):85–95. doi: 10.1037/0021-843X.114.1.85. [DOI] [PubMed] [Google Scholar]
- 49.Dworkin RH. Pain insensitivity in schizophrenia: a neglected phenomenon and some implications. Schizophr Bull. 1994;20:235–248. doi: 10.1093/schbul/20.2.235. [DOI] [PubMed] [Google Scholar]
- 50.Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL. Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology. 1999;21:325–340. doi: 10.1016/S0893-133X(99)00019-6. [DOI] [PubMed] [Google Scholar]
- 51.Atanasova B, Graux J, El Hage W, Hommet C, Camus V, Belzung C. Olfaction: a potential cognitive marker of psychiatric disorders. Neurosci Biobehav Rev. 2008;32:1315–1325. doi: 10.1016/j.neubiorev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Wilson DA, Linster C. Neurobiology of a simple memory. J Neurophysiol. 2008;100:2–7. doi: 10.1152/jn.90479.2008. [DOI] [PubMed] [Google Scholar]
- 53.Leitman DI, Laukka P, Juslin PN, Saccente E, Butler P, Javitt DC. Getting the Cue: sensory contributions to auditory emotion recognition impairments in schizophrenia. Schizophr Bull. doi: 10.1093/schbul/sbn115. September 23, 2008; doi:10.1093/schbul/sbn115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leitman DI, Hoptman MJ, Foxe JJ, et al. The neural substrates of impaired prosodic detection in schizophrenia and its sensorial antecedents. Am J Psychiatry. 2007;164:474–482. doi: 10.1176/ajp.2007.164.3.474. [DOI] [PubMed] [Google Scholar]
- 55.Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC. Sensory contributions to impaired prosodic processing in schizophrenia. Biol Psychiatry. 2005;58:56–61. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]