Abstract
Among the sensory modalities, olfaction is most closely associated with the frontal and temporal brain regions that are implicated in schizophrenia and most intimately related to the affective and mnemonic functions that these regions subserve. Olfactory probes may therefore be ideal tools through which to assess the structural and functional integrity of the neural substrates that underlie disease-related cognitive and emotional disturbances. Perhaps more importantly, to the extent that early sensory afferents are also disrupted in schizophrenia, the olfactory system—owing to its strategic anatomic location—may be especially vulnerable to such disruption. Olfactory dysfunction may therefore be a sensitive indicator of schizophrenia pathology and may even serve as an “early warning” sign of disease vulnerability or onset. In this article, we review the evidence supporting a primary olfactory sensory disturbance in schizophrenia. Convergent data indicate that structural and functional abnormalities extend from the cortex to the most peripheral elements of the olfactory system. These reflect, in part, a genetically mediated neurodevelopmental etiology. Gross structural and functional anomalies are mirrored by cellular and molecular abnormalities that suggest decreased or faulty innervation and/or dysregulation of intracellular signaling. A unifying mechanistic hypothesis may be the epigenetic regulation of gene expression. With the opportunity to obtain olfactory neural tissue from live patients through nasal epithelial biopsy, the peripheral olfactory system offers a uniquely accessible window through which the pathophysiological antecedents and sequelae of schizophrenia may be observed. This could help to clarify underlying brain mechanisms and facilitate identification of clinically relevant biomarkers.
Keywords: neurodevelopment, genetics, biomarkers, evoked potentials, olfactory receptor neurons, olfactory bulb
Introduction
Neuropsychological and neuroimaging studies offer convergent evidence that schizophrenia patients have selective impairments in the functional domains of memory, attention, executive planning, and affect regulation1,2 and have neuroanatomical and physiological abnormalities in the temporolimbic and frontal lobe regions that underlie these behavioral domains.3 Among the sensory modalities, olfaction is most closely associated with these neuroanatomical regions and most intimately related to the affective and mnemonic functions that they subserve. Olfactory probes may therefore be ideal tools through which to assess the structural and functional integrity of the neural substrates underlying disease-related cognitive and emotional disturbances.4–6 Perhaps more importantly, to the extent that early sensory afferents are disrupted in schizophrenia, the olfactory system—owing to its strategic anatomic location—may be especially vulnerable to such disruption. As such, olfactory dysfunction may be a sensitive indicator of schizophrenia pathology and, perhaps, may even serve as an “early warning” sign of disease vulnerability or onset. In this article, we review the evidence supporting a primary olfactory sensory disturbance in schizophrenia, with particular emphases on (1) the specific neuroanatomical substrates that may be involved, (2) the likely neurodevelopmental etiology of this disturbance, and (3) the cellular and molecular mechanisms that may be implicated. This growing body of evidence, along with the unique opportunity to obtain olfactory neural tissue from live patients through nasal epithelial biopsy, suggests that the peripheral olfactory system may be a promising entry point for understanding the brain mechanisms underlying schizophrenia pathology.7
Neuroanatomy of the Olfactory System
We begin with a description of the olfactory system, highlighting several unusual features that make it especially informative. The olfactory system is the only sensory modality that does not utilize the thalamus as a central relay station.8 Primary olfactory neurons arising in the nasal epithelium project unmyelinated afferent fibers through the cribriform plate into the brain cavity, where they terminate on mitral and tufted cells whose dendrites are clustered in glomeruli in the ipsilateral olfactory bulb (OB) (figure 1, left). Axons from these second-order OB neurons form the olfactory tracts, which project to the ipsilateral pyriform and entorhinal cortices (figure 1, right).9 Olfactory information passes, in turn, from these primary recipient zones to the amygdala, hippocampus, thalamus, and orbitofrontal cortex.10 It also passes through the anterior commissure to olfactory cortical areas in the contralateral hemisphere, facilitating the integration of olfactory inputs to left and right nostrils. With only 2 synapses between olfactory receptors and secondary cortical and subcortical targets,9 the olfactory system provides the most direct environmental access to the neuroanatomical substrates that have been implicated in schizophrenia.
Fig. 1.
Left: Primary olfactory receptor neurons originate in the superior nasal cavity and project axons through the cribriform plate into the cranium, where they terminate in the overlying olfactory bulb (Patrick J. Lynch, medical illustrator; C. Carl Jaffe, MD, cardiologist. http://creativecommons.org/licenses/by/2.5/). Right: Ventral surface of the brain, showing the olfactory bulbs (arrows) and olfactory tracts projecting to the ipsilateral medial temporal lobe.
The olfactory system is also unique in that it retains plasticity and exhibits robust neurodevelopment throughout life. The olfactory epithelium regenerates approximately every 2–3 months.11 Basal stem cells divide to give rise to new immature neurons that migrate toward the epithelial surface. These neurons differentiate into mature olfactory receptor neurons (ORNs) that project new axons back to re-innervate the glomeruli of the OB and form new synapses with target neurons. The olfactory system thus offers an unparalleled opportunity to observe the developmental processes of neurogenesis, axon guidance, and synapse formation that are no longer evident to any significant extent in other adult brain areas. Given the growing clinical and postmortem evidence implicating abnormal neurodevelopment in the pathogenesis of schizophrenia, the olfactory system may therefore hold special promise for understanding neurodevelopmental contributions to schizophrenia pathophysiology.
Developmental Neurobiology of Olfaction
Morphological differentiation of the human olfactory system occurs early in embryonic development.12 The nasal placodes begin to invaginate to form nasal pits at about the sixth week of gestation, and the nasal cavities are fully sculpted by week 11.13 ORN axons reach the bulb by the end of the first trimester and there is evidence of mitral cell synapse formation by the 17th week of gestation. The olfactory system is believed to be functional in the late fetus, and newborns can discriminate among a wide variety of odorants. Consistent with its early development, the olfactory system is vulnerable to disruption, in utero, by physical and chemical teratogens.12 The evidence that such disruptions may also play a role in the etiology of schizophrenia is now clear. A history of gestational or perinatal complications, including second trimester influenza infection,14 rhesus and ABO blood-type incompatibility,15 and perinatal anoxic birth injury,16 all increase the risk of illness. A neurodevelopmental etiology is also supported by findings of reduced cranial size17 and, in particular, minor midline physical anomalies (eg, reduced palate height and cleft palate) that arise from the same embryological processes that produce the olfactory structures.18 It is plausible, therefore, that early developmental abnormalities in the structure, growth, or functioning of olfactory neurons would not only result in olfactory deficits but would also be representative of neurodevelopmental abnormalities that were more diffusely extant in the brain during gestation.
Behavioral Evidence of Olfactory Impairment
Abnormalities in Schizophrenia Patients
Since the pioneering psychophysical studies of olfactory recognition memory in patients with schizophrenia by Campbell and Gregson,19 a number of investigators have reported that schizophrenia patients exhibit olfactory dysfunction [See Moberg et al20 for a review]. In an early study, Bradley21 reported that psychotic patients, most notably schizophrenic men, were hypersensitive to the odorant 5α-16-androsten-3-one. However, more recent studies have not confirmed this hypersensitivity, with some studies finding intact olfactory sensitivity 22–24 and others demonstrating decreased sensitivity.25,26 With only one exception 27 deficits in odor identification,23,24,26,28–38 odor detection threshold sensitivity25,26 and odor memory 19,38,39 have all been reported.
Despite the increasing interest in olfactory abnormalities in patients with schizophrenia, very few studies have explored the relative severity of different olfactory deficits (ie, identification, threshold, memory, and intensity/hedonics). Given the presumed differences in neuroanatomical loci for these different olfactory domains, it may be expected that they would be differentially impaired in the disorder. In addition, the effects of potential moderator variables, such as gender, medication, and smoking history, have not been consistently assessed across studies. However, a comprehensive meta-analytic review of the literature pertaining to olfactory function in schizophrenia20 revealed that (1) comparable deficits are seen for all psychophysical measures (odor detection, identification, and memory); (2) no differences exist between male and female patients, except as they reflect more general gender differences in olfaction; (3) there are no significant effects of antipsychotic medications on olfactory performance; and (4) patient deficits persist after accounting for the effects of smoking.
Family and “At-Risk” Studies
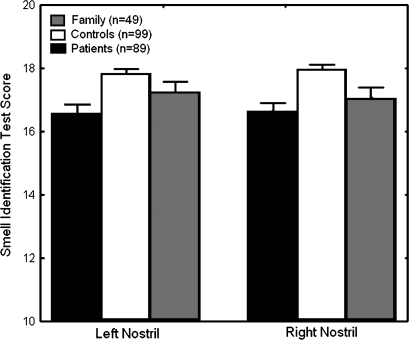
Responses to odorants often appear to be instinctual, and highly consistent responses are observed across individuals within a species. This reflects the fact that there are important genetic contributions to the development of the olfactory system.40 So, it is reasonable to consider whether the olfactory abnormalities observed in schizophrenia might also be genetically mediated. There have now been several studies of olfactory performance in unaffected family members of patients.41–43 Although results have been inconsistent, some deficits have been found. In one of the most compelling studies, Kopala and colleagues42 examined 19 probands, 27 nonpsychotic family members, and 43 healthy controls using the University of Pennsylvania Smell Identification Test (UPSIT) and an ascending staircase odor detection threshold test. They found that the UPSIT performance of the nonpsychotic family members was intermediate to that of the patient probands and the healthy controls. Fifty-eight percent of the probands and 34% of the nonpsychotic family members performed in the microsmic range, compared with only 9% of healthy comparison subjects.
Roalf et al43 expanded on these earlier efforts by testing schizophrenia patients, unaffected family members, and healthy comparison subjects unirhinally (ie, each nostril separately) rather than birhinally. In addition to exposing potentially important laterality differences, unirhinal testing eliminates the effects of birhinal facilitation, whereby olfactory performance is improved through secondary integration of the separate left and right nostril afferent inputs. Unirhinal testing is therefore a “purer” test of the integrity of primary olfactory afferents. Odor identification and detection threshold sensitivity measures were acquired from 22 Diagnostic and Statistical Manual of Mental Disorder (Fourth Edition) diagnosed schizophrenia patients, 30 nonill first-degree family members, and 45 healthy comparison subjects, who did not differ with regard to age, sex, or level of education. This study provided the first description of unirhinal olfactory function in a relatively large sample of schizophrenia patients and their unaffected family members. Both patients and first-degree relatives showed significant deficits in their ability to correctly identify odorants. Mean scores of family members were nominally intermediate to those of patients and controls. However, family members’ odor identification scores were not statistically different from those of patients. Patients were also impaired in their ability to simply detect the presence of a low concentration odorant, relative to healthy controls. In contrast, there were no significant differences in detection threshold between healthy participants and family members or between patients and family members. These results have now been replicated in a larger sample (figure 2). The finding of comparable odor identification deficits in patients and nonill first-degree relatives suggests that this is likely to be a genetic marker of vulnerability to the illness, rather than a manifestation of the disease itself. The presence of both identification and threshold sensitivity deficits in patients, but only identification deficits in the nonill family members, may indicate dissociation between olfactory deficits that represent genetically mediated vulnerability factors and deficits that are manifestations of the overt disease process.
Fig. 2.
Performance on the University of Pennsylvania Smell Identification Test. Both patients and unaffected first-degree relatives have significant bilateral impairments.
Consistent with the hypothesis that at least some olfactory abnormalities denote a genetic vulnerability to schizophrenia, olfactory performance deficits have also been reported in individuals with schizotypal personality disorder,44 who are thought to share the same genetic vulnerability as patients with schizophrenia. Studies of “psychosis-prone” individuals, who do not meet criteria for any disorder but score high on measures of perceptual aberration, physical anhedonia, and magical ideation, have similarly shown that these subclinical symptoms are correlated both with increases in deviant olfactory experiences (eg, misperceptions and hallucinations).45–47 The relationship between olfaction and aberrant cognitive and perceptual experiences extends beyond mere correlation. In a 10-year longitudinal study,48 the presence of such deviant olfactory experiences was found to significantly predict the development of future psychosis. More importantly, a similar investigation49 examining actual psychophysical olfactory deficits, as opposed to aberrant olfactory experiences, found that odor identification performance was significantly impaired in those “high-risk” individuals who subsequently developed schizophrenia but not in those who went on to develop affective psychoses or remained symptom free. This finding has been recently replicated in a sample of 26 well-characterized adolescents with early onset psychosis.50 Results revealed that deficits in odor identification existed across youths with psychotic disorder and were specifically related to typical characteristics of schizophrenia, such as negative symptoms and lower intelligence, but not to features of bipolar disorder.
Diagnostic Specificity
The causes of olfactory impairments are numerous, including chemical, infectious, traumatic, metabolic, and hormonal disturbances. Within the realm of neuropsychiatry, several neurodegenerative disorders have been shown to compromise olfaction, including Alzheimer's disease, Down's syndrome, Huntington's disease, Parkinson's disease, and multiple sclerosis.51 Among these, the relationship of olfaction to Alzheimer's disease is perhaps of greatest interest because the anterior medial temporal lobe areas that receive afferents from the OB are among the earliest to exhibit the characteristic neuropathology of that disorder. It has therefore been suggested that olfactory deficits may be an early indicator of disease onset, prior to the development of clinically observable memory loss. The question of diagnostic specificity or lack thereof is much less certain with respect to the major psychiatric disorders. For reasons that are not clear, there have been only a few studies of olfaction in affective illnesses, and the data that do exist are inconsistent. Patients with major depression or seasonal affective disorder have variously been reported to exhibit increased,52,53 decreased,54 and normal55 olfactory acuity, as well as normal27,33 and reduced26 olfactory identification ability.
There is even less information concerning bipolar affective disorder (BPD) patients. One early study30 found no impairments in either acuity or identification among BPD patients being treated with antipsychotic medications. A subsequent investigation56 found that affective disorder patients were indistinguishable from schizophrenia patients on olfactory identification performance. However, those affective disorder patients with psychotic symptoms also performed significantly worse than those without evidence of psychosis. Most recently, a study of heterogeneous first-episode psychosis patients57 reported no difference, across the diagnostic subgroups, in the level of odor identification impairment. The authors concluded that olfactory deficits exist at the onset of psychotic illness, but they are not specific to schizophrenia or schizophreniform disorder. The accumulating evidence from these studies, and from the high-risk studies cited above, indicate that olfactory deficits are relatively specific to psychotic disorders, as distinct from nonpsychotic psychiatric conditions. However, the specificity to schizophrenia, as opposed to BPD with psychosis, is less clear. The finding cited above,49,50 that olfactory impairments during the prodromal phase significantly predict a subsequent diagnosis of schizophrenia, supports the hypothesis that this is a true disease-specific vulnerability marker.
Summary and Limitations
These investigations indicate that (1) olfactory abilities are impaired in schizophrenia; (2) similar impairments are evident in individuals who are symptom free but genetically at risk; (3) these deficits may be diagnostically specific for schizophrenia as opposed to other major psychiatric conditions; and (4) they may be predictive of those high-risk individuals who will develop overt illness. However, it is important to emphasize that these findings were all based on behavioral performance measures, which rely upon a subject's cooperation, motivation, attention, and cognitive capacity to ensure accurate assessment. It remains unclear, therefore, whether olfactory deficits denote a primary disturbance within the olfactory neural substrate or whether these are merely reflections of more diffuse cognitive and/or affective impairment. With few exceptions, prior studies have not included structural or physiological assessments of the integrity of the olfactory system. Structural and functional measures that do not rely upon nonspecific factors such as motivation or attention are likely to be more sensitive to differential deficits across diagnostic groups and more specific in localizing abnormalities to discrete neural substrates. We consider, below, the neurobiological evidence supporting specific disruptions in each of the 3 components of the afferent olfactory system—the primary olfactory cortex, the bulbs and tracts, and the nasal cavity.
Structural Abnormalities
Olfactory Cortex
There have been a vast number of quantitative magnetic resonance imaging (MRI) studies in schizophrenia.58 Areas of the temporal lobe, including the superior temporal gyrus, hippocampus, and amygdala, have been among the most extensively studied. Interest in these particular regions stems from their association with the verbal, cognitive memory, and affective symptoms that are prominent in the disorder. Primary olfactory brain regions, in contrast, have been virtually ignored. However, a growing number of studies have now examined the entorhinal cortex (Brodmann area 28),59–65 which functions as a critical relay between the hippocampus and cortical association areas and, importantly, also receives direct afferent inputs from the OB. Patient decrements were identified in 6 of 7 studies, including 2 that focused exclusively on neuroleptic-naive first-episode patients.63,64 In one of these,63 however, a small sample of nonschizophrenic psychotic patients also exhibited reduced entorhinal cortical volumes, raising a question regarding the diagnostic specificity of this volume reduction.
Only 2 studies have specifically assessed the perirhinal cortex (Brodmann areas 35 and 36), which receives most of the afferent inputs from the OB.61,65 Both found volume decrements in this region. However, a similar abnormality was not observed in the first-degree relatives of schizophrenia patients (B.I. Turetsky, MD, unpublished data, 2003). Importantly, Turetsky et al61 also found that this volume decrement was specific to the primary olfactory cortex and did not extend into the adjacent temporopolar cortex (Brodmann area 38), which is functionally distinct and does not receive any olfactory afferents. This finding of intact temporal pole morphology in schizophrenia has since been independently replicated.66 Embryologically, the temporal pole develops separately from the olfactory system and it is one of the last cortical regions to emerge.67 Selective changes in olfactory cortical regions may therefore point to a developmental disturbance occurring during the late first or early second trimester of gestation, preceding the development of the temporopolar cortex but coincident with the emergence of the olfactory sensory apparatus. If this developmental hypothesis is correct, then we might expect to see similar abnormalities in more peripheral elements of the olfactory system that develop in association with the cortex in an integrated manner over the same time course.
Olfactory Bulb and Tract
Although MRI studies of the OBs have been conducted in the context of normal fetal development, congenital and acquired anosmias, Parkinson's disease, and Kallmann's syndrome,68–74 only 2 such studies have been conducted in schizophrenia patients.75,76 In an initial study of 26 schizophrenia patients and 22 healthy comparison subjects, Turetsky et al75 reported a 23% volume reduction, bilaterally, in patients. In healthy individuals, there was a strong association between OB volume and odor detection threshold sensitivity, but this normal structural-functional relationship was disrupted in schizophrenia. A second study by the same investigators76 examined bulb volumes in an independent patient sample, as well as a sample of unaffected first-degree relatives of patients. This investigation replicated the finding of reduced bilateral bulb volumes in schizophrenia patients. It also revealed volume reductions of the right, but not the left, OB among genetically at-risk family members (figure 3). The presence of a bulb abnormality in the family members supports the hypothesis of a genetically mediated vulnerability factor affecting primary sensory components of the olfactory system. These 2 studies are the first demonstration of such a deficit in any sensory modality, in either schizophrenia patients or their family members. The restriction of the deficit to the right bulb, in relatives, may be related to the well-established finding that the right hemisphere is better adapted to processing olfactory inputs than the left.77 This functional asymmetry presumably extends to the level of the bulb and recent studies have demonstrated larger right OBs as a consequence of normal development in diverse species.78,79 Right bulb development may therefore be more susceptible to developmental disruption or genetic mediation.
Fig. 3.
Left: High-resolution sagittal MRI scan of the olfactory bulb (arrows). Right: Mean bulb volumes for patients, unaffected family members, and control subjects. Asterisks indicate significant volume reductions relative to controls (adapted from Turetsky et al76).
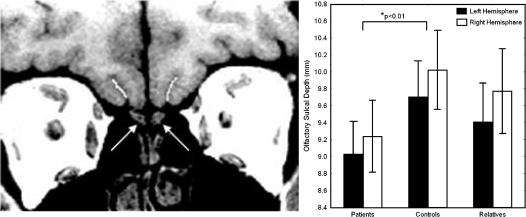
There are no volumetric studies of the olfactory tracts, as opposed to the bulbs, and such studies would likely shed little light on the status of these white matter fiber bundles. Diffusion tensor imaging, however, has been shown to be extremely sensitive to disruptions of olfactory tract integrity in early Parkinson's disease.80 It is likely that this approach would prove to be similarly sensitive to such disruptions in schizophrenia. Unfortunately, these studies have yet to be conducted. Indirect inferences regarding the developmental integrity of the olfactory tracts can, however, be obtained by examining the olfactory sulcus. This sulcus, which resides in the basal forebrain directly overlying the olfactory tracts, is among the earliest to develop in utero and is identifiable in the fetal forebrain around week 16 of gestation.81 The depth of the olfactory sulcus is a measure that appears to be directly linked to embryonic development of both the olfactory system and the cerebral cortex. It develops in association with the projection of the olfactory tract from the OB to the olfactory cortex and it appears to be dependent upon the integrity of this afferent fiber tract for its own development. In developmental disorders such as Kallmann’s syndrome or isolated congenital anosmia that are characterized by dysgenesis of the OB and tract, the olfactory sulcus tends to be unusually shallow or even completely absent.82,83 Olfactory sulcal depth may, therefore, be a pathognomonic marker for aberrant development of both the forebrain and olfactory sensory afferents during the first half of pregnancy. We recently measured the depths of the olfactory sulci in 36 schizophrenia patients and 28 comparison subjects, matched for age and gender distribution.84 The olfactory sulci were shallower, bilaterally, in patients. A separate sample of 31 unaffected family members had sulcal depths that were intermediate between those of patients and controls but not significantly different from control subject values (figure 4). Interestingly, consistent with the right hemisphere bias for olfactory functioning and the larger volume of the right OB, noted above, olfactory sulcal depth was also significantly greater on the right side across groups. Most importantly, the depth of the “H-shaped” orbital sulcus, which sits adjacent to the olfactory sulcus on the ventral forebrain, was not shallower in patients. The orbital sulcus does not begin to develop until week 28 of gestation. Hence, it is relatively immune to the effects of early intrauterine insults that can influence development of the olfactory sulcus.85 Schizophrenia patients, therefore, are similar to individuals with other diagnoses who have known disruptions of early forebrain embryogenesis and exhibit congenital olfactory impairments, rather than individuals with postdevelopmental forebrain atrophy.82,83
Fig. 4.
Left: Coronal MRI scan indicating the olfactory bulbs (arrows) and the overlying olfactory sulci (white lines), through which the olfactory tracts project to the ventromedial temporal lobe. Right: Mean sulcal depth measurements for patients, controls, and family members, indicating bilateral depth reductions in patients.
Nasal Cavity
Macroscopic examination of the structures underlying olfaction can be extended even more peripherally to include evaluation of the nasal cavities. To this end, acoustic rhinometry can be employed. This simple bedside technique uses ultrasound technology to derive cross-sectional area measurements of the cavity from the tip of the nose posteriorly toward the nasopharynx. From these, volumes of individual segments of the left and right nasal cavities can be easily computed. Auxiliary measures include the minimum cross-sectional area and airflow resistance. These allow volume reductions resulting from mucosal engorgement or nasal congestion, which would compromise airflow, to be distinguished from deformations of the underlying cartilaginous structure.
There have been 2 studies of nasal cavity volume in schizophrenia. The initial investigation, which included only men, found that male patients had reduced posterior nasal cavity volumes relative to male controls and that this could not be explained by differences in stature, smoking, or vascular engorgement of the nasal tissue.86 The second larger study included both male and female patients (n = 85) and controls (n = 66), as well as unaffected first-degree relatives of both genders (n = 25).87 This study replicated the initial finding of reduced posterior nasal cavity volume in male schizophrenia patients. However, female patients did not differ from female controls and unaffected first-degree relatives of either sex did not differ from same-sex control subjects. These findings persisted after covarying for height and smoking history and were unrelated to clinical symptomatology or antipsychotic medication usage.
Embryologically, the nasal cavities develop through invagination of the nasal placodes, which are derived from neural crest tissue. This occurs between the 6th and 11th weeks of embryogenesis, in conjunction with palatal and nasal septal fusion. Although subsequent development results in overall enlargement, there is no substantial reshaping of the cavities.88 The time course of nasal cavity development thus coincides with the period of substantially increased embryological risk for schizophrenia.89–91 This suggests that posterior nasal cavity volume decrement may be a specific craniofacial dysmorphology marker of early embryological developmental disturbance in males with schizophrenia. Although the explanation for the gender specificity of this finding is unknown, the fact that schizophrenia manifests as a more severe illness in men is well documented.92 This measure may be an indicator of this increased male vulnerability.
Functional Abnormalities
Olfactory Cortex
Physiological studies of olfactory sensory processing support the findings from structural imaging by providing convergent evidence that the functional integrity of the afferent olfactory system is compromised at the level of the cortex. Evoked potentials (EPs), with their exquisite temporal resolution, have the ability to parse physiological responses into early obligate afferent responses and later contextual processes. Their use in olfactory research has been limited due to the technical difficulties associated with odor stimulus delivery. Recent advances in olfactometry equipment have alleviated this problem. It is now possible to present precisely timed pulses of odorants of specified concentrations, without transient changes in temperature, humidity, or pressure. This permits the olfactory nerve (cranial nerve I) to be stimulated without concomitant trigeminal nerve (cranial nerve V) stimulation. This has given rise to a growing body of chemosensory EP data.
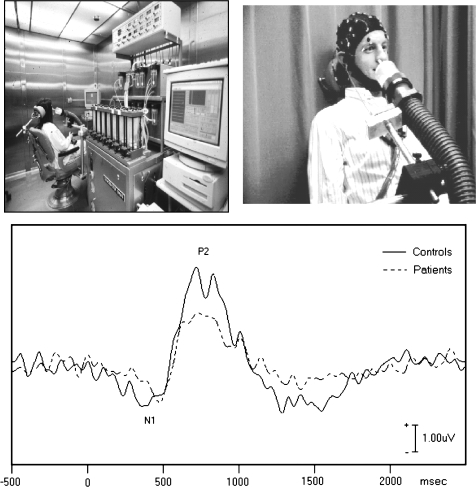
Initial studies employing this methodology confirmed the chemosensory specificity of the method and the absence of cross-modal contamination. Subjects with congenital anosmia, eg, had intact EP responses to chemosensory agents that stimulate the trigeminal nerve but no response to pure olfactory stimulants.93 Olfactory EPs were also found to be sensitive to both gender differences and the functional decline in olfactory ability that occurs with normal aging.94 Applying this methodology to the study of schizophrenia, Turetsky et al95 characterized the physiological EP response elicited by passive smelling of 3 different concentrations of hydrogen sulfide in 21 patients and 20 healthy controls. Figure 5 illustrates the characteristic biphasic olfactory EP waveform. Note that the N1 and P2 components seen here are delayed by 200–300 ms relative to typical auditory or visual EP waveforms. This reflects the additional time required in olfaction, compared with audition or vision, for molecules of an odorant to bind to chemoreceptors in the olfactory epithelium.
Fig. 5.
Top: Experimental setup for recording olfactory evoked potentials, illustrating the olfactometer (left) and hose used for odor delivery (right). Bottom: Biphasic olfactory chemosensory evoked potential elicited by an odorant. Amplitude of patient response is significantly reduced.
Patients exhibited dose-dependent reductions in the amplitudes of both N1 and P2, with associated P2 latency prolongation. Patient deficits were more prominent with increasing odor concentration, with the magnitude of the cortical response exhibiting an abnormal ceiling effect. The olfactory systems of these patients were essentially “overwhelmed” by the stronger stimuli, becoming more dysfunctional as they tried to respond to the increased stimulus load. These physiological measures were directly related to psychophysical performance measures: N1 amplitude, which specifically denotes primary olfactory cortex activity,96 was related to impaired odor detection threshold sensitivity; P2 amplitude, which reflects secondary sensory integrative processes, was related to impaired odor identification. These data provide the first direct evidence for a physiological impairment in the olfactory cortex in patients with schizophrenia. It is important to emphasize that this abnormality reflects an obligate physiological response elicited by repetitive stimulation of the first cranial nerve, in the absence of any attentional or cognitive processing demands. Although odor concentration varied, the stimuli were neither qualitatively distinctive nor task salient. It is unlikely, therefore, that this reflects other nonspecific factors that might alter brain function.
To assess the possible role of a genetic vulnerability factor in this physiological EP deficit, a second identical study was conducted in a sample of 15 healthy family members and matched controls.97 A profile of abnormalities was seen in the family members that precisely matched what was observed in patients. That is, there were dose-dependent deficits in N1 amplitude, P2 amplitude, and P2 latency. There are 2 important features to note concerning this physiological deficit. First, these family members were not impaired on psychophysical tests of olfactory ability. This likely reflects a threshold phenomenon, in which overt behavioral impairments may not be observed until the underlying anatomic substrate abnormality rises above some critical threshold. Such nonlinear structure-function relationships are well established in many organ systems. The underlying substrate was, nevertheless, compromised, as indicated by the abnormal EP responses. This confirms the expectation that such physiological measures are more sensitive to detecting subtle deficits. Second, contrary to what has been reported in virtually all previous studies of familial abnormalities in schizophrenia, the relatives in this study did not show an intermediate deficit (ie, a deficit whose magnitude was between that of patients and controls). Rather, the relatives’ deficit was equal to that of patients. This implies a much greater genetic contribution to, and less environmental modification of, this neural response—ie, greater heritability—indicating that it could be a more useful endophenotypic marker of neurodevelopmental vulnerability.
Olfactory Bulb and Tract
Unfortunately, the small size of the OBs, along with their location deep in the midline below the ventral forebrain, makes it extremely difficult to record isolated bulb responses in vivo in humans. To our knowledge, there are no electrophysiological or functional imaging studies of OB activity in humans, let alone in schizophrenia patients. However, high-resolution magnetic resonance spectroscopy techniques may, in the future, provide information concerning bulb composition and activity.
Nasal Epithelium
The functional integrity of the ORNs located in the epithelial lining of the posterior nasal cavity can also be assessed via the electro-olfactogram (EOG).98 The EOG represents the summed multicellular membrane depolarization of ORNs in response to a chemosensory odorant stimulus. This depolarization response can be recorded in vivo by a thin wire electrode inserted into the nasal cavity and positioned adjacent to the olfactory epithelium, allowing a direct examination of the physiological responsiveness of these peripheral sensory neurons. In contrast to scalp-recorded EPs, which have amplitudes on the order of 1–10 μv, the EOG response is several hundred microvolts in amplitude. This makes it possible to record responses to individual odor stimuli and to appreciate its exquisite sensitivity to odor concentration and exposure duration.
In a recent study of 21 patients and 18 controls—the only such study of its kind in schizophrenia—patients exhibited significantly larger EOG responses (figure 6).99 This increased excitability in patients was evident across all odor concentrations and durations. It was also evident following both left and right nostril stimulation, although the group difference was greater for the right nostril. The patient-control difference could not be explained by differences in age, gender, smoking, or use of antipsychotic medications. This study provides unequivocal evidence of a peripheral olfactory sensory impairment, although the explanation for this seemingly paradoxical finding remains unclear. There appear to be at least 3 different possibilities: (1) the absolute number of olfactory neurons is greater in schizophrenia patients, hence the observed EOG response is more robust; (2) there is a loss of specificity of olfactory receptor expression in schizophrenia, such that the number of neurons that respond to a particular odorant is increased even if the absolute number of neurons is not; and (3) the magnitude of the membrane depolarization current of individual ORNs is increased, so that the recorded EOG response is greater even if the number of responding neurons is unchanged. These alternative mechanisms should not be considered mutually exclusive. There is, in fact, some indirect evidence to support each of them.
Fig. 6.
Left: Experimental setup for recording electro-olfactogram (EOG). Wire recording electrode is inserted into the nasal cavity and held in position on the epithelial surface by an adjustable clip mechanism attached to a pair of glasses strapped to the head (reproduced from Turetsky et al99). Right: EOG response to a 100 ms odor pulse. Olfactory receptor neuron depolarization is significantly larger in patients.
As previously noted, ORNs undergo continuing neurogenesis, including proliferation and differentiation of neural precursors, axon growth, and synapse formation. At any given moment (including the instant of death), examination of the olfactory epithelium offers a snapshot of these regenerative processes, revealing cells in different stages of differentiation and migration. Using postmortem tissue, Arnold et al100 observed a decrease in p75-immunoreactive basal cells and an increase in the number of immature ORNs, suggesting dysregulated proliferation and differentiation of neuronal precursors in schizophrenia. In addition, in vitro olfactory epithelial cultures obtained through nasal epithelial biopsy of still-living patients exhibited increased rates of mitosis and greater cellular proliferation.101 Gene expression profiling of olfactory epithelial tissue also found increased expression of multiple genes related to cell proliferation, differentiation, and neurogenesis in schizophrenia.102 Collectively, these findings suggest dysregulated processes of olfactory epithelial neurodevelopment in schizophrenia. The observation of increased EOG amplitude could, therefore, reflect altered cellular composition secondary to dysregulated neurodevelopment.
This process may also be exacerbated by a loss of selectivity of olfactory neurons. Normally, a given olfactory neuron expresses only one olfactory receptor on its membrane surface, restricting its response to a specific odorant molecule configuration.103 There is evidence, however, that such selectivity can be altered, as in the case of the alteration of olfactory function with normal human aging.104 Perhaps more importantly, electrophysiological studies of olfactory development indicate that the olfactory epithelium of immature animals is highly nonselective, with individual olfactory neurons responding to many different odorants.105 Selectivity appears to be acquired only later in development. Because the basic finding of these cellular studies is an increase in the turnover rate and density of immature rather than mature neurons, this could translate into an increased number of neurons that respond more nonselectively to any given odorant.
Another possibility is that the magnitude of the EOG response is increased at the level of the individual neuron—ie, that there are alterations in the intracellular signal transduction pathways that lead to increased membrane depolarization. The binding of an odorant to an olfactory receptor results in increased levels of intracellular cyclic AMP (cAMP). cAMP functions as a second messenger, causing cyclic nucleotide-gated ion channels to open and cations to enter the cell. There is a strong correlation between the magnitude of this transmembrane current, which produces the observed EOG response, and the levels of adenylyl cyclase activation and cAMP accumulation within olfactory neurons.106 There is increasing evidence to suggest that this intracellular signaling cascade may be dysregulated in schizophrenia. An early study, using B-lymphocytes,107 found increased adenylyl cyclase activity and cAMP accumulation in cells from schizophrenia patients following stimulation with forskolin, which binds to a high-affinity site on the catalytic subunit of adenylyl cyclase. More recently it has been shown that DISC1, the schizophrenia susceptibility gene located on chromosome 1q42, acts intracellularly to sequester phosphodiesterase, the enzyme responsible for the degradation of cAMP and to release it in response to elevated levels of cAMP.108 Alterations of the quantity or function of the DISC1 protein will therefore necessarily alter the regulation of cAMP levels. Similarly, a polymorphism of the GNAS1 gene on chromosome 20q13, which codes for the α-subunit of the G-protein that stimulates adenylyl cyclase, has been associated with deficit syndrome schizophrenia.109 Alterations of this protein would affect the production, rather than the degradation, of cAMP following stimulation. Alterations in cAMP levels could also be a secondary effect of either glutamatergic110 or dopaminergic111 dysregulation, both of which have been implicated in schizophrenia pathophysiology. While these associations support the idea that cAMP signaling is disrupted in schizophrenia, the status of olfactory signal transduction in this disorder has yet to be examined.
A recent behavioral study112 tested the hypothesis that dysregulation of the cAMP-mediated signaling cascade contributed to olfactory dysfunction in schizophrenia. Turetsky et al measured odor detection threshold sensitivity to 2 different odors in 30 patients, 25 control subjects, and 19 unaffected family members. The specific odorants, citralva and lyral, were selected because, although they are qualitatively similar floral scents, they differed in the extent to which they activated intracellular adenylyl cyclase in the ORN to produce cAMP. Citralva produces a large increase in adenylyl cyclase and a robust EOG depoloarization response, while lyral elicits only a minimal response.106,113 On testing, control subjects did not exhibit any differences in their ability to detect the presence of these 2 odors. Both patients and family members, however, exhibited significant impairments in their ability to detect lyral but not citralva. This evidence of an odor-specific hyposmia indicates that the mechanisms underlying olfactory dysfunction in schizophrenia are not global ones that disrupt all aspects of sensory processing. It also implicates a genetically regulated disruption of cAMP-mediated signal transduction in peripheral ORNs as one specific mechanism underlying this dysfunction. While not dispositive in the absence of more direct physiological biochemical assessments, the results of this study provide strong inferential support for this hypothesis.
Olfactory Epithelial Biopsy
There is compelling preliminary evidence to suggest that the structural and functional impairments detailed above are mirrored by cellular and molecular abnormalities consistent with faulty cellular proliferation and innervation and/or dysregulated intracellular signaling.100–102 The fact that ORNs exhibit continuous neurodevelopmental proliferation and can be harvested, in living subjects, through olfactory epithelial biopsy offers the possibility of testing putative mechanisms underlying the developmental neuropathology of the disorder ex vivo. Such a model also offers the opportunity to examine tissues at specific stages of the illness and to capture neurobiological changes associated with different phases of the illness. Recent work using explant cultures derived from epithelial biopsy material has demonstrated that the course of neuronal differentiation and maturation can be tracked over time, from neuronal precursors through immature to fully mature functional olfactory neurons.114 A proportion of the cell lines derived from these explant cultures exhibit odor responsiveness and express neuronal protein markers.115 Cultured cells also express receptors for different neurotransmitters, including serotonin 5HT2A and 5HT2C, dopamine D2R, and N-methyl-D-aspartate (NMDA) NR1 and NR2A/2B.116 These receptors are functionally active and respond to appropriate ligands. The functional competence of different receptor classes can therefore be assessed by measuring G-protein activation116 and/or calcium influx following ligand stimulation (figure 7).117 This allows specific hypotheses, such as NMDA receptor hypofunction or altered cAMP-mediated signaling, to be tested directly using cultured neurons derived from clinically characterized patients or individuals at risk for disease. This work is still in its earliest stages. However, initial results suggest that there are robust disease-specific changes in both NMDA receptor function and ligand-induced G-protein activation in schizophrenia patients (C.G. Hahn, MD, PhD, unpublished data, 2009).
Fig. 7.
Ligand stimulation of dopamine receptors activates signaling pathways in human olfactory epithelial cultures. Cells were preincubated with either sch23390 (D1 receptor antagonist) or sulpiride (D2 receptor antagonist), followed by stimulation with 1 μM dopamine. Isoform specific activation of G-proteins was assessed for Gαs/olf, Gαi, Gαo, and Gαq/11. Asterisks indicate significant effects of dopamine antagonists (adapted from Borgmann-Winter et al116).
Making Sense of the Nonsense: An Attempt at Integration
Collectively, these research findings provide compelling evidence that schizophrenia patients exhibit structural and functional abnormalities of the olfactory system that extend from the cortex to the periphery. The presence of olfactory deficits in unaffected first-degree relatives of patients implicates, at least in part, a genetic etiology. Similarly, the presence of structural abnormalities associated with midline craniofacial and forebrain morphogenesis implicates an early embryonic developmental etiology. Importantly, there is an emerging pattern of relative dissociation between these genetic and developmental markers. Individuals who carry an increased genetic risk have functional olfactory deficits, as indicated by behavioral and electrophysiological measures. However, they do not exhibit the structural abnormalities that are most indicative of an early gestational disturbance (ie, reduced nasal cavity volume and shallow olfactory sulcus). Although data regarding other minor physical anomalies are somewhat contradictory,118–122 these olfactory findings are in agreement with studies indicating that physical anomalies can distinguish monozygotic twins discordant for schizophrenia,120 are prominent in sporadic but not familial schizophrenia,120 and are not found in unaffected family members.121,122 It thus appears that structural and functional deficits may represent 2 relatively independent sets of measures that can be used to track, respectively, intrauterine environmental and genetic contributions to schizophrenia vulnerability. Of course, this dissociation is not absolute and this is not to suggest that genetic factors do not contribute to morphological abnormalities. Rather, it suggests that these structural measures may be especially sensitive to the environmental factors that influence embryonic development, while functional olfactory deficits may be more sensitive endophenotypic markers of an underlying genetic vulnerability.
This has important implications for the use of these measures as potential biomarkers, particularly in the context of longitudinal studies of individuals who are either at genetic risk due to a family history of schizophrenia or exhibiting behavioral changes consistent with a schizophrenia prodrome. As noted above, olfactory identification impairments predicted subsequent conversion to schizophrenia among “ultra–high-risk” individuals who either were exhibiting subthreshold psychotic symptoms or had a family history of psychosis and a significant decline in functioning.49 While it still remains to be demonstrated, a composite measure that incorporates both structural and functional elements would presumably have greater sensitivity and specificity for predicting schizophrenia in this case than a functional measure alone. Also, the ultra–high-risk research strategy does not address the question of how to identify, prior to the onset of prodromal symptoms, those individuals with a family history of schizophrenia that are likely to develop the illness. In this case, the presence of both structural and functional olfactory abnormalities may be a critical distinguishing feature. Future longitudinal studies, therefore, should include the concurrent assessment of both sets of measures.
Although we cannot, at this point, identify the specific mechanisms that underlie these olfactory impairments, we can speculate about some of the broader processes that may be involved. Developmental abnormalities in midline craniofacial and forebrain morphogenesis suggest a disturbance in the cell-cell signaling mechanisms that regulate embryonic mesenchymal/epithelial tissue interactions.123 Mesenchymal/epithelial induction during the first trimester of embryogenesis gives rise to the entire frontonasal region, including the olfactory epithelium, OB and rudimentary forebrain.124 This set of complex tissue interactions is regulated by a group of molecules (including retinoic acid, sonic hedgehog, Notch4, and FGF) that control gene expression, and any dysregulation of this process could produce minor anomalies. Among these regulatory molecules, retinoic acid may be of particular interest. Retinoids have been previously implicated in the pathophysiology of schizophrenia125,126 and vitamin A deficiency is a prenatal nutritional factor that both increases the risk of illness and produces congenital malformations that resemble the stigmata of schizophrenia.127 Importantly, retinoic acid also regulates the expression of multiple schizophrenia candidate genes and target molecules, including dysbindin,128 dopamine and dopamine receptors, NMDA receptors, nicotine receptors, GABA receptors,129 and elements of the G-protein—cAMP—protein kinase A intracellular signaling pathway.130 The integrity of the olfactory system may also be maintained, in the adult brain, by retinoic acid-mediated regulation of neurogenesis.131 It remains to be determined whether retinoids are specifically implicated in the etiology of schizophrenia olfactory deficits. We would emphasize, though, that an epigenetic regulatory mechanism of this sort offers a plausible unifying explanation for this multitiered array of structural, functional, genetic, developmental, inter-, and intracellular abnormalities. We would also note, in this context, that there is a growing appreciation of the potential importance of epigenetic mechanisms in the pathophysiology of schizophrenia.132
Conclusions
The olfactory system offers an integrated translational research model that can bridge multiple levels and domains of inquiry. The fact that olfactory dysfunction precedes the development of acute schizophrenia symptomatology indicates that early developmental changes in other central nervous system regions are mirrored in this relatively simple sensory system. With the opportunity to obtain olfactory neural tissue from live patients through nasal epithelial biopsy, the peripheral olfactory system offers a uniquely accessible window through which to observe the pathophysiological antecedents and sequelae of schizophrenia. An in-depth longitudinal characterization of olfactory neuronal dysfunction utilizing convergent in vivo and in vitro methods may allow us to observe, in the context of ongoing brain development, the development of functional dysregulations that are correlated with molecular and cellular changes. Such an approach may not only facilitate the identification of preclinical biomarkers but also link these to specific disease mechanisms.
Funding
National Institutes of Health (MH59852 to B.I.T., MH63381 to P.J.M, MH80194 to C.G.H.); NARSAD Yound Investigator Award to K.B.W; Children’s Hospital of Philadelphia Institutional Development Funds to K.B.W.
References
- 1.Saykin AJ, Shtasel DL, Gur RE, et al. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 2.Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr Res. 2007;94:253–263. doi: 10.1016/j.schres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turetsky B, Cowell PE, Gur RC, Grossman RI, Shtasel DL, Gur RE. Frontal and temporal lobe brain volumes in schizophrenia. Relationship to symptoms and clinical subtype. Arch Gen Psychiatry. 1995;52:1061–1070. doi: 10.1001/archpsyc.1995.03950240079013. [DOI] [PubMed] [Google Scholar]
- 4.Schneider F, Habel U, Reske M, Toni I, Falkai P, Shah NJ. Neural substrates of olfactory processing in schizophrenia patients and their healthy relatives. Psychiatry Res. 2007;155:103–112. doi: 10.1016/j.pscychresns.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Seckinger RA, Goudsmit N, Coleman E, et al. Olfactory identification and WAIS-R performance in deficit and nondeficit schizophrenia. Schizophr Res. 2004;69:55–65. doi: 10.1016/S0920-9964(03)00124-5. [DOI] [PubMed] [Google Scholar]
- 6.Pause BM, Hellmann G, Göder R, Aldenhoff JB, Ferstl R. Increased processing speed for emotionally negative odors in schizophrenia. Int J Psychophysiol. 2008;70:16–22. doi: 10.1016/j.ijpsycho.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Sawa A, Cascella NG. Peripheral olfactory system for clinical and basic psychiatry: a promising entry point to the mystery of brain mechanism and biomarker identification in schizophrenia. Am J Psychiatry. 2009;166:137–139. doi: 10.1176/appi.ajp.2008.08111702. [DOI] [PubMed] [Google Scholar]
- 8.Price J. The central olfactory and accessory olfactory systems. In: Finger TE, Silver WL, editors. Neurobiology of Taste and Smell. New York, NY: Wiley; 1987. pp. 179–203. [Google Scholar]
- 9.Eslinger PJ, Damasio AR, Van Hoesen GW. Olfactory dysfunction in man: anatomical and behavioral aspects. Brain Cogn. 1982;1:259–285. doi: 10.1016/0278-2626(82)90028-8. [DOI] [PubMed] [Google Scholar]
- 10.Tanabe T, Yarita H, Iino M, Ooshima Y, Takagi SF. An olfactory projection area in orbitofrontal cortex of the monkey. J Neurophysiol. 1975;38:1269–1283. doi: 10.1152/jn.1975.38.5.1269. [DOI] [PubMed] [Google Scholar]
- 11.Takagi S. Degeneration and regeneration of the sensory neuron: studies on the olfactory epithelium. Shinkei Kenkyu No Shimpo. 1969;13:152–163. [PubMed] [Google Scholar]
- 12.Farbman A. Developmental Neurobiology of the Olfactory System. New York, NY: Raven Press; 1991. [Google Scholar]
- 13.Larsen WJ. Human Embryology. New York, NY: Churchill Livingstone; 2001. [Google Scholar]
- 14.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 15.Hollister JM, Laing P, Mednick SA. Rhesus incompatibility as a risk factor for schizophrenia in male adults. Arch Gen Psychiatry. 1996;53:19–24. doi: 10.1001/archpsyc.1996.01830010021004. [DOI] [PubMed] [Google Scholar]
- 16.Cannon TD, Rosso IM, Hollister JM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of genetic and perinatal influences in the etiology of schizophrenia. Schizophr Bull. 2000;26:351–366. doi: 10.1093/oxfordjournals.schbul.a033458. [DOI] [PubMed] [Google Scholar]
- 17.Gur RE, Mozley PD, Shtasel DL, et al. Clinical subtypes of schizophrenia: differences in brain and CSF volume. Am J Psychiatry. 1994;151:343–350. doi: 10.1176/ajp.151.3.343. [DOI] [PubMed] [Google Scholar]
- 18.O'Callaghan E, Larkin C, Kinsella A, Waddington JL. Familial, obstetric, and other clinical correlates of minor physical anomalies in schizophrenia. Am J Psychiatry. 1991;148:479–483. doi: 10.1176/ajp.148.4.479. [DOI] [PubMed] [Google Scholar]
- 19.Campbell I, Gregson RAM. Olfactory short term memory in normal, schizophrenic and brain-damaged cases. Aust J Psychol. 1972;24:179–185. [Google Scholar]
- 20.Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL. Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology. 1999;21:325–340. doi: 10.1016/S0893-133X(99)00019-6. [DOI] [PubMed] [Google Scholar]
- 21.Bradley EA. Olfactory acuity to a pheromonal substance and psychotic illness. Biol Psychiatry. 1984;19:899–905. [PubMed] [Google Scholar]
- 22.Geddes J, Huws R, Pratt P. Olfactory acuity in the positive and negative syndromes of schizophrenia. Biol Psychiatry. 1991;29:774–778. doi: 10.1016/0006-3223(91)90196-s. [DOI] [PubMed] [Google Scholar]
- 23.Kopala LC, Clark C, Hurwitz T. Olfactory deficits in neuroleptic naive patients with schizophrenia. Schizophr Res. 1993;8:245–250. doi: 10.1016/0920-9964(93)90022-b. [DOI] [PubMed] [Google Scholar]
- 24.Kopala L, Clark C, Hurwitz TA. Sex differences in olfactory function in schizophrenia. Am J Psychiatry. 1989;146:1320–1322. doi: 10.1176/ajp.146.10.1320. [DOI] [PubMed] [Google Scholar]
- 25.Isseroff R, Stoler M, Ophir D, Lancet D, Sirota P. Olfactory sensitivity to androstenone in schizophrenic patients. Biol Psychiatry. 1987;22:922–925. doi: 10.1016/0006-3223(87)90093-x. [DOI] [PubMed] [Google Scholar]
- 26.Serby M, Larson P, Kalkstein D. Olfactory sense in psychoses. Biol Psychiatry. 1990;28:830. doi: 10.1016/0006-3223(90)90520-c. [DOI] [PubMed] [Google Scholar]
- 27.Warner MD, Peabody CA, Csernansky JG. Olfactory functioning in schizophrenia and depression. Biol Psychiatry. 1990;27:457–458. doi: 10.1016/0006-3223(90)90557-i. [DOI] [PubMed] [Google Scholar]
- 28.Brewer WJ, Edwards J, Anderson V, Robinson T, Pantelis C. Neuropsychological, olfactory, and hygiene deficits in men with negative symptom schizophrenia. Biol Psychiatry. 1996;40:1021–1031. doi: 10.1016/0006-3223(95)00594-3. [DOI] [PubMed] [Google Scholar]
- 29.Houlihan DJ, Flaum M, Arnold SE, Keshavan M, Alliger R. Further evidence for olfactory identification deficits in schizophrenia. Schizophr Res. 1994;12:179–182. doi: 10.1016/0920-9964(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 30.Hurwitz T, Kopala L, Clark C, Jones B. Olfactory deficits in schizophrenia. Biol Psychiatry. 1988;23:123–128. doi: 10.1016/0006-3223(88)90081-9. [DOI] [PubMed] [Google Scholar]
- 31.Kopala L, Good K, Martzke J, Hurwitz T. Olfactory deficits in schizophrenia are not a function of task complexity. Schizophr Res. 1995;17:195–199. doi: 10.1016/0920-9964(94)00085-m. [DOI] [PubMed] [Google Scholar]
- 32.Kopala LC, Good K, Honer WG. Olfactory identification ability in pre- and postmenopausal women with schizophrenia. Biol Psychiatry. 1995;38:57–63. doi: 10.1016/0006-3223(94)00224-Q. [DOI] [PubMed] [Google Scholar]
- 33.Kopala LC, Good KP, Honer WG. Olfactory hallucinations and olfactory identification ability in patients with schizophrenia and other psychiatric disorders. Schizophr Res. 1994;12:205–211. doi: 10.1016/0920-9964(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 34.Malaspina D, Wray AD, Friedman JH, et al. Odor discrimination deficits in schizophrenia: association with eye movement dysfunction. J Neuropsychiatry Clin Neurosci. 1994;6:273–278. doi: 10.1176/jnp.6.3.273. [DOI] [PubMed] [Google Scholar]
- 35.Moberg PJ, Doty RL, Turetsky BI, et al. Olfactory identification deficits in schizophrenia: correlation with duration of illness. Am J Psychiatry. 1997;154:1016–1018. doi: 10.1176/ajp.154.7.1016. [DOI] [PubMed] [Google Scholar]
- 36.Seidman LJ, Oscar-Berman M, Kalinowski AG, et al. Experimental and clinical neuropsychological measures of prefrontal dysfunction in schizophrenia. Neuropsychology. 1995;9:481–490. [Google Scholar]
- 37.Seidman LJ, Goldstein JM, Goodman JM, et al. Sex differences in olfactory identification and Wisconsin Card Sorting performance in schizophrenia: relationship to attention and verbal ability. Biol Psychiatry. 1997;42:104–115. doi: 10.1016/S0006-3223(96)00300-9. [DOI] [PubMed] [Google Scholar]
- 38.Wu J, Buchsbaum MS, Moy K, et al. Olfactory memory in unmedicated schizophrenics. Schizophr Res. 1993;9:41–47. doi: 10.1016/0920-9964(93)90008-7. [DOI] [PubMed] [Google Scholar]
- 39.Dunn TP, Weller MP. Olfaction in schizophrenia. Percept Mot Skills. 1989;69:833–834. doi: 10.1177/00315125890693-121. [DOI] [PubMed] [Google Scholar]
- 40.Barinaga M. Olfaction. Smell's course is predetermined. Science. 2001;294:1269–1271. doi: 10.1126/science.294.5545.1269. [DOI] [PubMed] [Google Scholar]
- 41.Kopala LC, Good KP, Torrey EF, Honer WG. Olfactory function in monozygotic twins discordant for schizophrenia. Am J Psychiatry. 1998;155:134–136. doi: 10.1176/ajp.155.1.134. [DOI] [PubMed] [Google Scholar]
- 42.Kopala LC, Good KP, Morrison K, Bassett AS, Alda M, Honer WG. Impaired olfactory identification in relatives of patients with familial schizophrenia. Am J Psychiatry. 2001;158:1286–1290. doi: 10.1176/appi.ajp.158.8.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roalf DR, Turetsky BI, Owzar K, et al. Unirhinal olfactory function in schizophrenia patients and first-degree relatives. J Neuropsychiatry Clin Neurosci. 2006;18:389–396. doi: 10.1176/jnp.2006.18.3.389. [DOI] [PubMed] [Google Scholar]
- 44.Park S, Schoppe S. Olfactory identification deficit in relation to schizotypy. Schizophr Res. 1997;26:191–197. doi: 10.1016/s0920-9964(97)00045-5. [DOI] [PubMed] [Google Scholar]
- 45.Mohr C, Rohrenbach CM, Laska M, Brugger P. Unilateral olfactory perception and magical ideation. Schizophr Res. 2001;47:255–264. doi: 10.1016/s0920-9964(00)00113-4. [DOI] [PubMed] [Google Scholar]
- 46.Mohr C, Hubener F, Laska M. Deviant olfactory experiences, magical ideation, and olfactory sensitivity: a study with healthy German and Japanese subjects. Psychiatry Res. 2002;111:21–33. doi: 10.1016/s0165-1781(02)00132-4. [DOI] [PubMed] [Google Scholar]
- 47.Becker E, Hummel T, Piel E, Pauli E, Kobal G, Hautzinger M. Olfactory event-related potentials in psychosis-prone subjects. Int J Psychophysiol. 1993;15:51–58. doi: 10.1016/0167-8760(93)90095-7. [DOI] [PubMed] [Google Scholar]
- 48.Kwapil TR, Chapman JP, Chapman LJ, Miller MB. Deviant olfactory experiences as indicators of risk for psychosis. Schizophr Bull. 1996;22:371–382. doi: 10.1093/schbul/22.2.371. [DOI] [PubMed] [Google Scholar]
- 49.Brewer WJ, Wood SJ, McGorry PD, et al. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry. 2003;160:1790–1794. doi: 10.1176/appi.ajp.160.10.1790. [DOI] [PubMed] [Google Scholar]
- 50.Corcoran C, Whitaker A, Coleman E, et al. Olfactory deficits, cognition and negative symptoms in early onset psychosis. Schizophr Res. 2005;80:283–293. doi: 10.1016/j.schres.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doty RL. Olfactory system. In: Getchell TV, Bartoshuk LM, Doty RL, Snow JB, editors. Smell and Taste in Health and Disease. New York, NY: Raven Press; 1991. pp. 175–203. [Google Scholar]
- 52.Postolache TT, Wehr TA, Doty RL, et al. Patients with seasonal affective disorder have lower odor detection thresholds than control subjects. Arch Gen Psychiatry. 2002;59:1119–1122. doi: 10.1001/archpsyc.59.12.1119. [DOI] [PubMed] [Google Scholar]
- 53.Gross-Isseroff R, Luca-Haimovici K, Sasson Y, Kindler S, Kotler M, Zohar J. Olfactory sensitivity in major depressive disorder and obsessive compulsive disorder. Biol Psychiatry. 1994;35:798–802. doi: 10.1016/0006-3223(94)91142-8. [DOI] [PubMed] [Google Scholar]
- 54.Pause BM, Miranda A, Goder R, Aldenhoff JB, Ferstl R. Reduced olfactory performance in patients with major depression. J Psychiatr Res. 2001;35:271–277. doi: 10.1016/s0022-3956(01)00029-2. [DOI] [PubMed] [Google Scholar]
- 55.Amsterdam JD, Settle RG, Doty RL, Abelman E, Winokur A. Taste and smell perception in depression. Biol Psychiatry. 1987;22:1481–1485. doi: 10.1016/0006-3223(87)90108-9. [DOI] [PubMed] [Google Scholar]
- 56.Striebel KM, Beyerstein B, Remick RA, Kopala L, Honer WG. Olfactory identification and psychosis. Biol Psychiatry. 1999;45:1419–1425. doi: 10.1016/s0006-3223(98)00245-5. [DOI] [PubMed] [Google Scholar]
- 57.Brewer WJ, Pantelis C, Anderson V, et al. Stability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosis. Am J Psychiatry. 2001;158:107–115. doi: 10.1176/appi.ajp.158.1.107. [DOI] [PubMed] [Google Scholar]
- 58.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pearlson GD, Barta PE, Powers RE, et al. Ziskind-Somerfeld Research Award 1996. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry. 1997;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- 60.Nasrallah HA, Sharma S, Olson SC. The volume of the entorhinal cortex in schizophrenia: a controlled MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1317–1322. doi: 10.1016/s0278-5846(97)00166-8. [DOI] [PubMed] [Google Scholar]
- 61.Turetsky BI, Moberg PJ, Roalf DR, Arnold SE, Gur RE. Decrements in volume of anterior ventromedial temporal lobe and olfactory dysfunction in schizophrenia. Arch Gen Psychiatry. 2003;60:1193–1200. doi: 10.1001/archpsyc.60.12.1193. [DOI] [PubMed] [Google Scholar]
- 62.Baiano M, Perlini C, Rambaldelli G, et al. Decreased entorhinal cortex volumes in schizophrenia. Schizophr Res. 2008;102:171–180. doi: 10.1016/j.schres.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 63.Prasad KM, Patel AR, Muddasani S, Sweeney J, Keshavan MS. The entorhinal cortex in first-episode psychotic disorders: a structural magnetic resonance imaging study. Am J Psychiatry. 2004;161:1612–1619. doi: 10.1176/appi.ajp.161.9.1612. [DOI] [PubMed] [Google Scholar]
- 64.Joyal CC, Laakso MP, Tiihonen J, et al. A volumetric MRI study of the entorhinal cortex in first episode neuroleptic-naive schizophrenia. Biol Psychiatry. 2002;51:1005–1007. doi: 10.1016/s0006-3223(01)01368-3. [DOI] [PubMed] [Google Scholar]
- 65.Sim K, DeWitt I, Ditman T, et al. Hippocampal and parahippocampal volumes in schizophrenia: a structural MRI study. Schizophr Bull. 2006;32:332–340. doi: 10.1093/schbul/sbj030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crespo-Facorro B, Nopoulos PC, Chemerinski E, Kim JJ, Andreasen NC, Magnotta V. Temporal pole morphology and psychopathology in males with schizophrenia. Psychiatry Res. 2004;132:107–115. doi: 10.1016/j.pscychresns.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Fogliarini C, Chaumoitre K, Chapon F, et al. Assessment of cortical maturation with prenatal MRI. Part I: normal cortical maturation. Eur Radiol. 2005;15:1671–1685. doi: 10.1007/s00330-005-2782-1. [DOI] [PubMed] [Google Scholar]
- 68.Azoulay R, Fallet-Bianco C, Garel C, Grabar S, Kalifa G, Adamsbaum C. MRI of the olfactory bulbs and sulci in human fetuses. Pediatr Radiol. 2006;36:97–107. doi: 10.1007/s00247-005-0030-0. [DOI] [PubMed] [Google Scholar]
- 69.Mueller A, Rodewald A, Reden J, Gerber J, von Kummer R, Hummel T. Reduced olfactory bulb volume in post-traumatic and post-infectious olfactory dysfunction. Neuroreport. 2005;16:475–478. doi: 10.1097/00001756-200504040-00011. [DOI] [PubMed] [Google Scholar]
- 70.Mueller A, Abolmaali ND, Hakimi AR, et al. Olfactory bulb volumes in patients with idiopathic Parkinson's disease a pilot study. J Neural Transm. 2005;112:1363–1370. doi: 10.1007/s00702-005-0280-x. [DOI] [PubMed] [Google Scholar]
- 71.Buschhüter D, Smitka M, Puschmann S, et al. Correlation between olfactory bulb volume and olfactory function. Neuroimage. 2008;42:498–502. doi: 10.1016/j.neuroimage.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 72.Rombaux P, Mouraux A, Bertrand B, Nicolas G, Duprez T, Hummel T. Retronasal and orthonasal olfactory function in relation to olfactory bulb volume in patients with posttraumatic loss of smell. Laryngoscope. 2006;116:901–905. doi: 10.1097/01.mlg.0000217533.60311.e7. [DOI] [PubMed] [Google Scholar]
- 73.Rombaux P, Mouraux A, Bertrand B, Nicolas G, Duprez T, Hummel T. Olfactory function and olfactory bulb volume in patients with postinfectious olfactory loss. Laryngoscope. 2006;116:436–439. doi: 10.1097/01.MLG.0000195291.36641.1E. [DOI] [PubMed] [Google Scholar]
- 74.Rombaux P, Duprez T, Hummel T. Olfactory bulb volume in the clinical assessment of olfactory dysfunction. Rhinology. 2009;47:3–9. [PubMed] [Google Scholar]
- 75.Turetsky BI, Moberg PJ, Yousem D, Arnold SE, Doty RL, Gur RE. Olfactory bulb volume is reduced in patients with schizophrenia. Am J Psychiatry. 2000;157:828–830. doi: 10.1176/appi.ajp.157.5.828. [DOI] [PubMed] [Google Scholar]
- 76.Turetsky BI, Moberg PJ, Arnold SA, Doty RL, Gur RE. Low olfactory bulb volume in 1st-degree relatives of patients with schizophrenia. Am J Psychiatry. 2003;160:703–708. doi: 10.1176/appi.ajp.160.4.703. [DOI] [PubMed] [Google Scholar]
- 77.Doty RL, Bromley SM, Moberg PJ, Hummel T. Laterality in human nasal chemoreception. In: Christman S, editor. Cerebral Asymmetries in Sensory and Perceptual Processing. Amsterdam, The Netherlands: North Holland Publishing; 1997. pp. 497–542. [Google Scholar]
- 78.Heine O, Galaburda AM. Olfactory asymmetry in the rat brain. Exp Neurol. 1986;91:392–398. doi: 10.1016/0014-4886(86)90078-6. [DOI] [PubMed] [Google Scholar]
- 79.Prasada Rao PD, Finger TE. Asymmetry of the olfactory system in the brain of the winter flounder, Pseudopleuronectes americanus. J Comp Neurol. 1984;225:492–510. doi: 10.1002/cne.902250403. [DOI] [PubMed] [Google Scholar]
- 80.Scherfler C, Schocke MF, Seppi K, et al. Voxel-wise analysis of diffusion weighted imaging reveals disruption of the olfactory tract in Parkinson's disease. Brain. 2006;129:538–542. doi: 10.1093/brain/awh674. [DOI] [PubMed] [Google Scholar]
- 81.Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977;1:86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- 82.Vogl TJ, Stemmler J, Heye B, et al. Kallman syndrome versus idiopathic hypogonadotropic hypogonadism at MR imaging. Radiology. 1994;191:53–57. doi: 10.1148/radiology.191.1.8134597. [DOI] [PubMed] [Google Scholar]
- 83.Abolmaali ND, Hietschold V, Vogl TJ, Hüttenbrink KB, Hummel T. MR evaluation in patients with isolated anosmia since birth or early childhood. AJNR AM J Neuroradiol. 2002;23:157–163. [PMC free article] [PubMed] [Google Scholar]
- 84.Turetsky BI, Crutchley P, Walker J, Gur RE, Moberg PJ. Depth of the olfactory sulcus: A marker of early embryonic disruption in schizophrenia? Schizophr Res. 2009 doi: 10.1016/j.schres.2009.09.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giménez M, Junqué C, Vendrell P, et al. Abnormal orbitofrontal development due to prematurity. Neurology. 2006;67:1818–1822. doi: 10.1212/01.wnl.0000244485.51898.93. [DOI] [PubMed] [Google Scholar]
- 86.Moberg PJ, Roalf DR, Gur RE, Turetsky BI. Smaller nasal volumes as stigmata of aberrant neurodevelopment in schizophrenia. Am J Psychiatry. 2004;161:2314–2316. doi: 10.1176/appi.ajp.161.12.2314. [DOI] [PubMed] [Google Scholar]
- 87.Turetsky BI, Glass CA, Abbazia J, Kohler CG, Gur RE, Moberg PJ. Reduced posterior nasal cavity volume: a gender-specific neurodevelopmental abnormality in schizophrenia. Schizophr Res. 2007;93:237–244. doi: 10.1016/j.schres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larsen WJ. Human Embryology. 3rd ed. New York, NY: Churchill Livingstone; 2001. [Google Scholar]
- 89.Cantor-Graae E, McNeil TF, Torrey EF, et al. Link between pregnancy complications and minor physical anomalies in monozygotic twins discordant for schizophrenia. Am J Psychiatry. 1994;151:1188–1193. doi: 10.1176/ajp.151.8.1188. [DOI] [PubMed] [Google Scholar]
- 90.Gallagher BJ, McFalls JA, Jones BJ, Pisa AM. Prenatal illness and subtypes of schizophrenia: the winter pregnancy phenomenon. J Clinical Psychol. 1999;55:915–922. doi: 10.1002/(sici)1097-4679(199907)55:7<915::aid-jclp12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 91.Hulshoff Pol HE, Hoek HW, Susser E, et al. Prenatal exposure to famine and brain morphology in schizophrenia. Am J Psychiatry. 2000;157:1170–1172. doi: 10.1176/appi.ajp.157.7.1170. [DOI] [PubMed] [Google Scholar]
- 92.Shtasel DL, Gur RE, Gallacher F, Heimberg C, Gur RC. Gender differences in the clinical expression of schizophrenia. Schizophr Res. 1992;7:225–231. doi: 10.1016/0920-9964(92)90016-x. [DOI] [PubMed] [Google Scholar]
- 93.Kobal G. Electrophysiological measurement of olfactory function. In: Doty R, editor. Handbook of Olfaction and Gustation. 2nd ed. New York, NY: Informa Health Care; 2003. pp. 382–416. [Google Scholar]
- 94.Evans WJ, Cui L, Starr A. Olfactory event-related potentials in normal human subjects: effects of age and gender. Electroencephalogr Clin Neurophysiol. 1995;95:293–301. doi: 10.1016/0013-4694(95)00055-4. [DOI] [PubMed] [Google Scholar]
- 95.Turetsky BI, Moberg PJ, Owzar K, Johnson SA, Doty RL, Gur RE. Physiological impairment of olfactory stimulus processing in schizophrenia. Biol Psychiatry. 2003;53:403–411. doi: 10.1016/s0006-3223(02)01865-6. [DOI] [PubMed] [Google Scholar]
- 96.Kettenmann B, Hummel C, Stefan H, Kobal G. Multiple olfactory activity in the human neocortex identified by magnetic source imaging. Chem Senses. 1997;22:493–502. doi: 10.1093/chemse/22.5.493. [DOI] [PubMed] [Google Scholar]
- 97.Turetsky BI, Kohler CG, Gur RE, Moberg PJ. Olfactory physiological impairment in first-degree relatives of schizophrenia patients. Schizophr Res. 2008;102:220–229. doi: 10.1016/j.schres.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Knecht M, Hummel T. Recording of the human electro-olfactogram. Physiol Behav. 2004;83:13–19. doi: 10.1016/j.physbeh.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 99.Turetsky BI, Hahn CG, Arnold SE, Moberg PJ. Olfactory receptor neuron dysfunction in schizophrenia. Neuropsychopharmacology. 2009;34:767–774. doi: 10.1038/npp.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arnold SE, Han LY, Moberg PJ, et al. Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch Gen Psychiatry. 2001;58:829–835. doi: 10.1001/archpsyc.58.9.829. [DOI] [PubMed] [Google Scholar]
- 101.Féron F, Perry C, Hirning MH, McGrath J, Mackay-Sim A. Altered adhesion, proliferation and death in neural cultures from adults with schizophrenia. Schizophr Res. 1999;40:211–218. doi: 10.1016/s0920-9964(99)00055-9. [DOI] [PubMed] [Google Scholar]
- 102.McCurdy RD, Féron F, Perry C, et al. Cell cycle alterations in biopsied olfactory neuroepithelium in schizophrenia and bipolar I disorder using cell culture and gene expression analyses. Schizophr Res. 2006;82:163–173. doi: 10.1016/j.schres.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 103.Ronnett GV, Moon C. G proteins and olfactory signal transduction. Annu Rev Physiol. 2002;64:189–222. doi: 10.1146/annurev.physiol.64.082701.102219. [DOI] [PubMed] [Google Scholar]
- 104.Rawson NE, Gomez G, Cowart B, Restrepo D. The use of olfactory receptor neurons (ORNs) from biopsies to study changes in aging and neurodegenerative diseases. Ann N Y Acad Sci. 1998;855:701–707. doi: 10.1111/j.1749-6632.1998.tb10648.x. [DOI] [PubMed] [Google Scholar]
- 105.Gesteland RC, Yancey RA, Farbman AI. Development of olfactory receptor neuron selectivity in the rat fetus. Neuroscience. 1982;7:3127–3136. doi: 10.1016/0306-4522(82)90235-4. [DOI] [PubMed] [Google Scholar]
- 106.Lowe G, Nakamura T, Gold GH. Adenylate cyclase mediates olfactory transduction for a wide variety of odorants. Proc Natl Acad Sci U S A. 1989;86:5641–5645. doi: 10.1073/pnas.86.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Natsukari N, Kulaga H, Baker I, Wyatt RJ, Masserano JM. Increased cyclic AMP response to forskolin in Epstein-Barr virus-transformed human B-lymphocytes derived from schizophrenics. Psychopharmacology (Berl) 1997;130:235–241. doi: 10.1007/s002130050234. [DOI] [PubMed] [Google Scholar]
- 108.Millar JK, Pickard BS, Mackie S, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 109.Minoretti P, Politi P, Coen E, et al. The T393C polymorphism of the GNAS1 gene is associated with deficit schizophrenia in an Italian population sample. Neurosci Lett. 2006;397:159–163. doi: 10.1016/j.neulet.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 110.Chetkovich DM, Sweatt JD. NMDA receptor activation increases cyclic AMP in area CA1 of the hippocampus via calcium/calmodulin stimulation of adenylyl cyclase. J Neurochem. 1993;61:1933–1942. doi: 10.1111/j.1471-4159.1993.tb09836.x. [DOI] [PubMed] [Google Scholar]
- 111.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 112.Turetsky BI, Moberg PJ. An odor-specific detection threshold sensitivity deficit implicates abnormal intracellular cyclic AMP signaling in schizophrenia. Am J Psychiatry. 2009;166:226–233. doi: 10.1176/appi.ajp.2008.07071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sklar PB, Anholt RRH, Snyder SH. The odorant-sensitive adenylate cyclase of olfactory receptor cells. J Biol Chem. 1986;261:15538–15543. [PubMed] [Google Scholar]
- 114.Hahn CG, Han LY, Rawson NE, et al. In vivo and in vitro neurogenesis in human olfactory epithelium. J Comp Neurol. 2005;483:154–163. doi: 10.1002/cne.20424. [DOI] [PubMed] [Google Scholar]
- 115.Gomez G, Rawson NE, Hahn CG, Michaels R, Restrepo D. Characteristics of odorant elicited calcium changes in cultured human olfactory neurons. J Neurosci Res. 2000;62:737–749. doi: 10.1002/1097-4547(20001201)62:5<737::AID-JNR14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 116.Borgmann-Winter KE, Rawson NE, Wang HY, et al. Human olfactory epithelial cells generated in vitro express diverse neuronal characteristics. Neuroscience. 2009;158:642–653. doi: 10.1016/j.neuroscience.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hahn CG, Gomez G, Restrepo D, et al. Aberrant intracellular calcium signaling in olfactory neurons from patients with bipolar disorder. Am J Psychiatry. 2005;162:616–618. doi: 10.1176/appi.ajp.162.3.616. [DOI] [PubMed] [Google Scholar]
- 118.Gourion D, Goldberger C, Bourdel MC, Jean Bayle F, Lôo H, Krebs MO. Minor physical anomalies in patients with schizophrenia and their parents: prevalence and pattern of craniofacial abnormalities. Psychiatry Res. 2004;125:21–28. doi: 10.1016/j.psychres.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 119.Ismail B, Cantor-Graae E, McNeil TF. Minor physical anomalies in schizophrenic patients and their siblings. Am J Psychiatry. 1998;155:1695–1702. doi: 10.1176/ajp.155.12.1695. [DOI] [PubMed] [Google Scholar]
- 120.Kelly BD, Cotter D, Denihan C, et al. Neurological soft signs and dermatoglyphic anomalies in twins with schizophrenia. Eur Psychiatry. 2004;19:159–163. doi: 10.1016/j.eurpsy.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 121.Griffiths TD, Sigmundsson T, Takei N, et al. Minor physical anomalies in familial and sporadic schizophrenia; the Maudsley family study. J Neurol Neurosurg Psychiatry. 1998;64:56–60. doi: 10.1136/jnnp.64.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Compton MT, Bollini AM, McKenzie Mack L, et al. Neurological soft signs and minor physical anomalies in patients with schizophrenia and related disorders, their first-degree relatives, and non-psychiatric controls. Schizophr Res. 2007;94:64–73. doi: 10.1016/j.schres.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 123.Maynard TM, Sikich L, Lieberman JA, LaMantia AS. Neural development, cell-cell signaling, and the “two hit” hypothesis of schizophrenia. Schizophr Bull. 2001:457–476. doi: 10.1093/oxfordjournals.schbul.a006887. [DOI] [PubMed] [Google Scholar]
- 124.LaMantia AS, Bhasin N, Rhodes K, Heemskerk J. Mesenchymal/epithelial induction mediates olfactory pathway formation. Neuron. 2000:411–425. doi: 10.1016/s0896-6273(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 125.Goodman AB. Microarray results suggest altered transport and lowered synthesis of retinoic acid in schizophrenia. Mol Psychiatry. 2005;10:620–621. doi: 10.1038/sj.mp.4001668. [DOI] [PubMed] [Google Scholar]
- 126.Rioux L, Arnold SE. The expression of retinoic acid receptor alpha is increased in the granule cells of the dentate gyrus in schizophrenia. Psychiatry Res. 2005;133:13–21. doi: 10.1016/j.psychres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 127.Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull. 2008;34:1054–1063. doi: 10.1093/schbul/sbn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guo AY, Riley BP, Thiselton DL, Kendler KS, Zhao Z. The dystrobrevin-binding protein 1 gene: features and networks. Mol Psychiatry. 2009;14:18–29. doi: 10.1038/mp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goodman AB. Three independent lines of evidence suggest retinoids as causal to schizophrenia. Proc Natl Acad Sci U S A. 1998;95:7240–7244. doi: 10.1073/pnas.95.13.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kurie JM, Allopenna J, Dmitrovsky E. Retinoic acid stimulates protein kinase A-associated G proteins during human teratocarcinoma differentiation. Biochim Biophys Acta. 1994;1222:88–94. doi: 10.1016/0167-4889(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 131.Rawson NE, LaMantia A-S. A speculative essay on retinoic acid regulation of neural stem cells in the developing and aging olfactory system. Exp Gerontol. 2007;42:46–53. doi: 10.1016/j.exger.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 132.Roth TL, Lubin FD, Sodhi M, Kleinman JE. Epigenetic mechanisms in schizophrenia. Biochim Biophys Acta. 2009;1790:869–877. doi: 10.1016/j.bbagen.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]