Abstract
We describe here a coordinated brain imaging and animal models approach in which we have shown that the hippocampal CA1 region is a principal node in schizophrenia pathogenesis and have identified a novel treatment approach to the disorder based on inhibition of glutamate release. To identify biomarkers, we have focused on the putative prodromal period, typically lasting a few years, preceding the first onset of psychosis. About one-third of a high-risk cohort followed prospectively for 2.5 years will progress to threshold psychosis, making it possible to perform a relatively short prospective study. We have utilized a technological development in functional imaging techniques in which we measure cerebral blood volume (CBV), which allows for interrogation of subregions of the brain in the basal state at submillimeter resolution. Measurements of CBV in schizophrenia as well as in high-risk or prodromal stages can then pinpoint brain subregions differentially targeted during the earliest stages of the disorder. Our data suggest that the CA1 subfield of the hippocampal formation is most consistently implicated across disease stages, identifying a putative biomarker suitable for guiding drug development. Our studies in transgenic mice mutant in the glutamate synthetic enzyme glutaminase support the hypothesis that CA1 hyperfunction is due to altered glutamatergic neurotransmission. As a proof of principle, the glutaminase-deficient mice suggest that pharmacotherapies that reduce glutamatergic neurotransmission in the CA1 subfield may be a uniquely effective therapeutic strategy in schizophrenia and preventative in prodromal stages of the disorder.
Keywords: glutaminase, GLS1, hippocampus, CA1, cerebral blood volume, fMRI
Introduction
Schizophrenia and related psychotic disorders involve a series of initiating abnormalities during gestation that engender frank disease years later in early adulthood.1,2 When patients present with first-episode psychosis, multiple brain circuits are affected,3 making the task of dissociating primary from secondary sites of brain dysfunction challenging. We describe here advances in brain imaging using a high-resolution functional variant of magnetic resonance imaging (fMRI) that provides sufficient spatial resolution to identify brain subregions implicated in the disorder, in particular in the hippocampal formation. We describe how this has been applied in patients with schizophrenia—as well as patients at high risk or showing prodromal signs and symptoms—in order to pinpoint brain subregions differentially targeted in the early stages of the disorder. In recent clinical studies, we have seen that hyperfunction of the CA1 subfield of the hippocampal formation is most consistently implicated across disease stages. Our parallel studies in transgenic mice suggest that this hyperfunction reflects excessive glutamatergic synaptic transmission and identify a novel pathway by which hippocampal glutamate transmission can be reduced in CA1 with relative selectivity, providing a new direction for the pharmacotherapy of schizophrenia.
Hippocampal Circuitry and Schizophrenia
Over the past 2 decades, a range of postmortem studies have implicated the hippocampal formation in schizophrenia.4–6 There are subtle measures reflective of abnormal neuronal functioning or connectivity, such as reductions in dendritic spine density,7 altered interneuronal GABAergic function,8 and smaller subregional CA1 and CA3 neuronal size9,10 as well as smaller neuronal size in the subiculum and entorhinal layer II regions.11 Several recent structural studies of the hippocampal formation have implicated the CA1 subfield and the subiculum in schizophrenia.12–15 While a clear neuropathology of schizophrenia has yet to emerge, the evidence is unified in suggesting that hippocampal abnormalities do exist in schizophrenia, do not involve profound cell loss, and likely reflect alterations in synaptic function.16
Postmortem findings have informed a number of models that propose a mechanism to account for disease-related changes in the functional state of the hippocampal formation. For example, observations of postmortem changes in GABAergic interneurons17,18 predict increased excitatory activity. The physiological properties of the trisynaptic hippocampal circuit suggest that the CA1 subfield may exhibit the greatest increase in excitatory activity.8,19 Animal modeling approaches have relied on the observation that NMDA antagonists phenocopy many key clinical features of schizophrenia.20 One view predicts that NMDA antagonists would reduce activity of inhibitory interneurons—leading to increased excitation21—and other models predict that NMDA antagonists act on primary neurons, leading to decreased CA1 excitation.22 Recently, using the NMDA antagonist model in orbitofrontal cortex, Homayoun and Moghaddam23 have shown a selective suppression of interneuron activity, resulting in pyramidal cell activation, and that both atypical antipsychotic drugs and metabotropic glutamate receptor agonists (mGluR2/3 agonists) decrease pyramidal neuron activity. However, because of the complex distribution of interneurons throughout the hippocampal subregions, and because interneurons can either inhibit or indirectly excite primary neurons, the relationship between altered synaptic connectivity and overall functional activity in a given brain subregion must be determined empirically.
Functional Imaging of the Hippocampus
Brain disorders, no matter how complex or chronic, target the brain with differential regional vulnerability. Specifically, because each subregion of the brain expresses a unique molecular profile—detected at the mRNA or protein level—a pathogenic mechanism is likely to differentially target 1 brain region over another. Recent hippocampal gene expression maps provide a mechanistic basis for this empirical observation.24,25 Although many diseases involve hippocampal dysfunction, most if not all of them target the hippocampal formation differentially. For example, hippocampal dysfunction subsequent to transient ischemia differentially affects the CA1 subfield due to its higher expression of NMDA receptors. In contrast, adrenalectomy differentially affects the dentate gyrus due to its higher expression of aldosterone receptors. Similarly, early stages of Alzheimer’s disease and normal aging differentially impact the hippocampal subregions.26–28
Because hippocampal subregions are highly interconnected, the hippocampal formation also illustrates the challenge of pinpointing differentially targeted subregions when dealing with chronic disorders with relatively subtle histopathology, such as schizophrenia. Specifically, primary dysfunction in 1 hippocampal subregion will over time cause secondary dysfunction in connected subregions, making it challenging to differentiate primary from secondary sites of dysfunction. It is therefore important to assess the functional integrity of multiple hippocampal subregions, individually and simultaneously, and during the earliest stages of disease.
The hippocampus functions as an integrated circuit, in which each hippocampal subregion has a unique computational role. Thus, any cognitive task used may differentially activate individual hippocampal subregions and thus bias the results to findings in that particular subregion. There have now been numerous studies that have shown that if there is a difference in the basal state, this will confound activation patterns, leading to potentially false conclusions.26,27 Because most disease states, including schizophrenia, affect basal metabolic properties of dysfunctional regions, examining the basal state without a cognitive activation task is notably free of this confound. While it is possible that disease-associated basal-state alterations may be subtle, and so require a cognitive task to “bring out the lesion,” as a sort of stress test, it should first be determined whether an effect can be detected in the basal state.
In addition to the theoretical arguments for functional imaging of the basal state, this approach has important technical advantages. In contrast to activation in fMRI studies, where high temporal resolution is required to capture a brief and transient effect, when imaging disease-associated changes in the basal state, one can slow down image acquisition and dramatically improve spatial resolution. Submillimeter resolution of brain function can be obtained by applying gadolinium-based contrast agents to obtain estimates of cerebral blood volume (CBV), providing sufficient spatial resolution to visualize individual hippocampal subregions simultaneously with other brain regions implicated in schizophrenia, such as the amygdala and prefrontal cortex.28
Imaging the Schizophrenia Basal State
Specifically, we have shown that imaging CBV with magnetic resonance imaging (MRI) can generate high-resolution functional maps of the brain.28 Previous studies have shown that MRI-based measures of basal CBV correlate tightly with Positron-Emission Tomography (PET)-based measures of glucose uptake,29 and both imaging variables successfully localize brain dysfunction. We have now optimized the generation of basal CBV maps of the hippocampal formation28,30,31 and used this MRI approach to interrogate the hippocampal circuit.
Basal metabolic increases in the medial temporal lobe or hippocampal formation, which are associated with symptoms of psychosis, have been shown in the majority of published Single Photon Emission Computed Tomography (SPECT)-32,33 and PET-based studies of schizophrenia using basal cerebral blood flow,26,34,35 a correlate of CBV.36 Other results argue that the disease itself, not the use of psychotropic medications, is the dominant cause of hippocampal hyperfunction.35 However, because of the poorer spatial resolution of these techniques, the functional state of individual subregions of the hippocampus was not resolved.
In order to identify brain subregions uniquely vulnerable to schizophrenia pathophysiology, we performed 3 clinical studies of brain areas implicated in the disorder,37 including subregions of the hippocampus, prefrontal cortex, ventral striatum, and amygdala. First, to identify brain regions abnormal in the established illness, we compared patients with schizophrenia with control subjects, matched for age and sex. Second, using the findings from this first study as a guide, we obtained baseline images of subjects with prodromal features who were at high risk for psychotic disorders. We followed these patients prospectively over 2 years to identify if brain subregions would differentially predict illness progression to syndromal psychosis. Third, we tested the association of abnormal brain subregions found in the first 2 analyses with the positive and negative symptoms of psychosis in both patient groups.
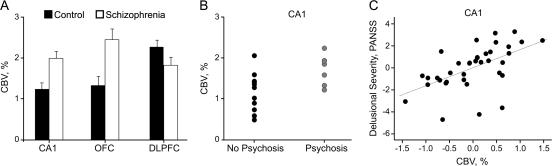
We found that several brain subregions were abnormal in schizophrenia, including the CA1 subfield, orbitofrontal cortex, and dorsolateral cortex, with a trend to abnormality in the subiculum. Notably, the pattern of dysfunction found showed abnormal hyperfunction within the CA1 subfield, orbitofrontal cortex, and subiculum, whereas abnormal hypofunction was found in the dorsolateral cortex (figure 1A). Imaging high-risk individuals revealed that hyperactivity in the CA1 subfield of the hippocampal formation uniquely predicted clinical progression to syndromal psychosis (figure 1B and figure 2A). Dysfunction of the CA1 subfield was selectively associated with both the positive and the negative symptoms of psychosis. Specifically, progressive CA1 subfield hyperfunction was related to delusional severity in both patient groups and was additionally associated with social withdrawal and avolition in the high-risk group. CA1 subfield dysfunction associated with total negative symptoms in the schizophrenia group at a trend level but did not associate with individual subitems of the positive and negative symptom scale. Collapsing delusional severity across the 2 clinical scales used in prodromal and schizophrenia research revealed a continuum of severity through the high-risk to the schizophrenia patient groups, with an association of progressive CA1 subfield hyperfunction with worsening delusional severity (figure 1C).
Fig. 1.
Clinical CBV Imaging. (A) An increase in CBV between control and schizophrenia groups was observed selectively in hippocampal CA1 and the orbitofrontal cortex (OFC), while a CBV decrease was observed in the dorsolateral prefrontal cortex (DLPFC). (B) Initial baseline CBV measurements in CA1 in the prodromal subjects who progressed clinically to psychosis (after 2 y) were significantly higher than those who did not. (C) CA1 CBV correlated with delusional severity (measured on the positive and negative symptom scale, PANSS). Source: Redrawn from Schobel et al,37 with permission of the American Medical Association (Archives of General Psychiatry). Copyright 2009 American Medical Association. All rights reserved. CBV, cerebral blood volume; CA1, hippocampal subregion.
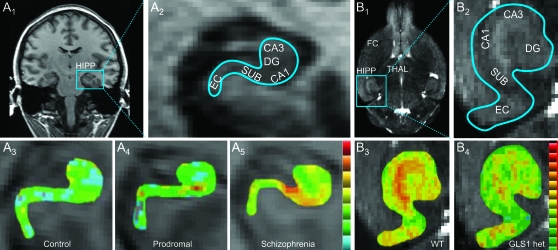
Fig. 2.
CBV Imaging of the Hippocampus in Clinical Subjects and Mice. (A) In clinical subjects (A1), high-resolution T1-weighted images in a coronal section can resolve hippocampal subregions (A2). Individual CBV maps of the hippocampal formation are shown from a healthy control (A3), a prodromal subject (A4), and a schizophrenia subject (A5). Maps are color coded such that warmer colors reflect higher CBV values. CBV increased from the control to the high-risk subject in CA1 and to the schizophrenia subject in CA1 and SUB. (B) In mouse, high-resolution images in a horizontal section (B1) resolve hippocampal subregions (B2). In WT mice, regional CBV is fairly uniform across hippocampal subregions (B3), while in GLS1 het mice, CA1 and SUB show a selective reduction (B4). A similar comparison in FC revealed no difference between WT and GLS1 het mice (images not shown). CBV, cerebral blood volume, HIPP, hippocampus; CA1 and CA3, hippocampal subregions; SUB, subiculum; DG, dentate gyrus; THAL, thalamus; EC, entorhinal cortex; FC, frontal cortex; WT, wild-type mice; GLS1 het, GLS1 heterozygous mice. Source: Panel A is redrawn from Schobel et al,37 with permission of the American Medical Association (Archives of General Psychiatry). Copyright 2009 American Medical Association. All rights reserved. Panel B is redrawn from Gaisler-Salomon et al38 with permission of the authors.
Our clinical findings argue that dysfunction of the CA1 subfield best fulfills clinical criteria for a differentially targeted brain region across illness stages: First, it is found in the established illness; second, it predicts clinical progression to threshold or full-blown psychosis from the high-risk state; and third, it is differentially associated with both the positive and the negative symptoms of psychosis. By including the high-risk subjects, we minimized issues associated with disease chronicity and eliminated medication effects. Treatment of mice with risperidone did not affect hippocampal CBV, arguing that drug treatment did not confound the imaging data. The observed pattern of dysfunction found in our study, characterized by abnormal elevations of CBV, is suggestive of a basal hypermetabolic state29,39 within the hippocampus, particularly in CA1 in schizophrenia.
Modeling Schizophrenia in Mice
There is growing recognition of the value of mouse models in the elucidation of mechanisms inherent to schizophrenia and other psychiatric disease-related pathology.40–45 Animal studies make possible the translation of observations in humans, often marred by the potential confounds of disease chronicity and drug treatment, into putative mechanisms of disease origin and progression. Often, putative mechanisms evolve into theories, which in turn allow for the identification of novel treatment venues.46–48 Considering the diversity of schizophrenia symptoms, animal models are faced with the difficult task of assaying multiple aspects of the disorder. Moreover, many of these aspects are poorly defined and are limited by the imperfect correspondence of animal behavioral indices to human signs and symptoms. CBV imaging, which can be equally well applied to assessing the basal in vivo function of diminutive brain structures in mice as in humans, provides a way to bridge between the animal models and endophenotypes.
Specifically, a cross-species imaging approach can be used to identify whether mouse models are characterized by abnormalities in similar brain subregions and networks as those found in humans with schizophrenia. Modeling schizophrenia in mice may potentially identify novel treatment directions. If a particular interference with normal function leads to a schizophrenia-relevant phenotype, it is biologically plausible that the inverse would be beneficial in treating schizophrenia symptoms. Thus, if exploring novel treatment venues is a central goal of modeling the disease state in animals, an inverse model—a model that imitates the effect of treatment rather than the disease state itself—could in fact provide a more direct pathway to novel therapeutics, while being equally informative as to the etiology of the disorder.
One such model was generated by Yee et al,49 who showed that mice with reduced expression of the glycine transporter in the forebrain—to enhance NMDA receptor function—demonstrate a procognitive behavioral profile and an attenuated response to psychosis-inducing drugs. Indeed, pharmacological reduction of glycine transporter function in humans has been shown to be effective in treating negative and cognitive symptoms in some clinical studies48 and bolstered the idea that glutamate dysfunction plays an important pathogenic role in schizophrenia.
Pharmacotherapy of Schizophrenia via Presynaptic Inhibition of Glutamate Release
Dysfunction in glutamatergic synaptic transmission has been repeatedly implicated in the etiology of schizophrenia. In light of the well-documented pro-psychotic effects of phencyclidine (PCP), ketamine, and other NMDA receptor antagonists observed in human and animal studies, it has been proposed that schizophrenia involves NMDA-type glutamate receptor hypofunction.20,50 The glutamatergic hypothesis has evolved further to suggest that schizophrenia involves excess glutamate release, which may paradoxically be induced by blockade of NMDA-type glutamate receptors. Consistent with this, elevated levels of glutamate and glutamatergic markers have been seen in first-episode drug-naive schizophrenia patients and in schizophrenia-prone individuals.51
In further support of the glutamatergic hypothesis, a growing body of evidence indicates that presynaptic reductions in glutamate transmission have broad potential in the treatment of schizophrenia as well as other neuropsychiatric disorders.52 Moghaddam and Adams46 made the seminal observation that mGluR2/3 agonists attenuate both PCP-induced glutamate release and PCP-induced motoric stimulation. This preclinical work culminated in the recent report that reducing glutamate release via mGluR2/3-mediated presynaptic inhibition with LY2140023 showed significant promise in early clinical trials.47 Other means of reducing glutamate release could thus offer new treatment possibilities.
Glutaminase as a Novel Drug Target
One possible novel target is glutaminase, the neuronal enzyme that recycles glutamine into glutamate. Once glutamate is released into the synapse, it is taken up by astrocytes, which convert it to glutamine.53,54 Glutamine is then transported back into neurons via specific transporters.55 Although there are mechanisms for de novo synthesis of glutamate in neurons, most neurotransmitter glutamate is recycled through the glutamate-glutamine cycle.56 While it has been identified in kidney, intestinal endothelium, fetal liver, lymphocytes, adipocytes, and tumor cells,57 glutaminase is expressed with high baseline activity in brain. Crucially, the expression levels of glutaminase in the mouse brain appear to be particularly high in the hippocampus, especially in the Schaeffer collateral projections to CA1.58
To explore the therapeutic potential of targeting glutaminase, we phenotyped mice mutant in the gene encoding brain-kidney glutaminase GLS1. While GLS1 knockouts die shortly after birth,59 mice heterozygous for the GLS1 (GLS1 hets) are fully viable despite a mild yet significant reduction in glutamate levels in several brain regions including the hippocampus.38 In light of our clinical imaging findings, we hypothesized that if schizophrenia is associated with increased CBV in CA1 and this was due to excessive glutamate release, then the reduction in glutamate in GLS1 hets should have the opposite effect on hippocampal activity and reduce CBV in the CA1 subfield.
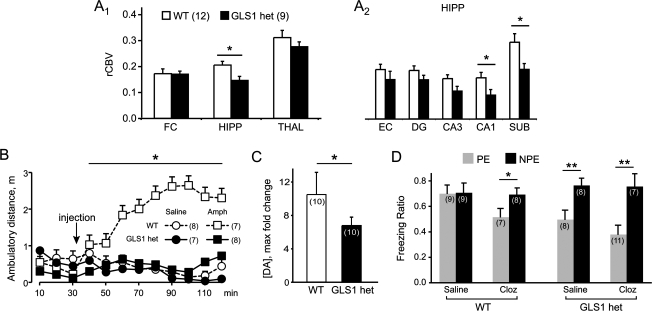
Strikingly, relative CBV analysis of GLS1 hets revealed an imaging phenotype that was the inverse of that seen in our clinical data (figure 2B), namely hippocampal hypoactivity, principally in CA1 and subiculum (figure 3A). Electrophysiological studies (data not shown) demonstrated that GLS1 hets show reduced hippocampal glutamatergic synaptic transmission in the same subregions, indicating that the pathological processes that lead to increased metabolic activity in the hippocampus in schizophrenia may be absent in GLS1 hets and, by extension, that decreased presynaptic glutamate may afford protection from these processes.
Fig. 3.
Schizophrenia Resilience Phenotypes of GLS1 hets. (A) GLS1 hets have reduced rCBV in the HIPP but not in the FC or THAL; WT > GLS1 hets (A1). Within the HIPP subregions, rCBV was significantly reduced in CA1 and SUB (A2). WT > GLS1 hets, *P < .05, numbers per group are given in parentheses. (B) GLS1 het mice show an attenuated response to amphetamine (Amph). Ambulatory distance was measured prior to and after intraperitoneal injection of amphetamine (2 mg/kg) or saline (injection, arrow). WT mice showed a robust increase in activity following Amph, while GLS1 hets, as well as saline-treated mice of both genotypes, showed no increase in activity. WT-Amph > GLS1 het-Amph, GLS1 het-saline, WT-saline, *P < .05. GLS1 hets do respond to higher-dose Amph. (C) While microdialysis measurements showed no genotypic difference in baseline DA recovery, the DA surge following Amph was attenuated in the GLS1 hets. WT-Amph > GLS1-Amph, *P < .05. (D) GLS1 hets displayed clozapine (Cloz)-like potentiation of LI. Low freezing levels were observed in WT and GLS1 het mice under saline- or Cloz-treated conditions prior to testing. Freezing ratios during the tone test showed LI. LIis seen as a lower suppression ratio in pre-exposed (PE) compared with not pre-exposed (NPE) subjects. We observed LI in Cloz-treated WT mice and in saline-and Cloz-treated GLS1 hets but not in saline-treated WT mice. NPE > PE, *P < .05, **P < .01. Cloz-treated GLS1 hets did not show enhanced LI. Thus, GLS1 het mice appear similar to WT mice treated with Cloz. GLS1 het, GLS1 heterozygous mice; HIPP, hippocampus; FC, frontal cortex; THAL, thalamus; WT, wild-type mice; CA1 and CA3, hippocampal subregion; SUB, subiculum; DG, dentate gyrus; EC, entorhinal cortex; RCBV, regional CBV; LI, latent inhibition. Source: Redrawn from Gaisler-Salomon et al38 with permission of the authors.
We assayed the GLS1 het mice on several well-characterized behavioral and neurochemical dimensions of schizophrenia-related pathology. GLS1 hets displayed a strikingly attenuated sensitivity to the behavioral (figure 3B) and neurochemical (figure 3C) effects of the dopamine releaser amphetamine, which induces hyperlocomotion in animals and enhanced dopamine (DA) release in patients with schizophrenia. Similar to the findings in the glycine transporter mutant mice,49 GLS1 hets displayed a procognitive, antipsychotic drug-like action in the latent inhibition assay (figure 3D). We also assayed GLS1 hets for abnormalities in motor, sensorimotor, and cognitive functions and found that save for a mild deficit in contextual fear conditioning, GLS1 hets demonstrated a remarkably normal behavioral profile, arguing for an acceptable side effect profile for glutaminase inhibition.38
While variations in the GLS1 gene have not been associated with schizophrenia,60,61 postmortem studies have shown increased GLS1 expression62 and activity.63 Interestingly, there has been the suggestion that antipsychotic drugs inhibit glutaminase activity.64,65 So, while there is evidence for involvement of glutaminase in schizophrenia, the lack of association argues against a primary pathogenic role.
The assembled findings indicate that GLS1 het mice may be seen as a proof of concept that glutaminase inhibition should prove therapeutic in schizophrenia and related psychotic disorders, with a benign side effect profile. Furthermore, the relatively specific effects on hippocampal circuitry observed in GLS1 hets potentially validate a key site of action for pharmacotherapy of schizophrenia and support further the insight that the hippocampus—and in particular the CA1 subregion—may be the crucial node in the pathological circuitry underlying syndromal psychosis.
Conclusions
Our recent clinical studies provide empirical evidence that hyperfunction of the CA1 subfield, present in prodromal stages of the disorder, predicts clinical phenotypes across the transition to schizophrenia and related psychotic disorders. Concurrently, our preclinical studies suggest that inhibition of glutaminase is a promising way to reduce hyperfunction of the CA1 subfield of the hippocampus based upon a presynaptic glutamate reduction mechanism. Convergent with our clinical and preclinical studies, several studies have implicated hyperfunction of glutamate transmission in the pathophysiology of schizophrenia and shown the promise of glutamate-limiting therapies to treat primary symptoms of the illness based on nondopaminergic mechanisms. These advances promise to move therapies beyond treatments that are primarily based on D2 dopamine receptor blockade, with their well-recognized limited clinical efficacy. More broadly, our convergent imaging data pointing to dysfunction of hippocampal subregions should have immediate clinical potential for biomarker discovery and development of more effective therapeutics targeted at preclinical stages of schizophrenia, with the ultimate aim of prevention through valid risk identification and targeted treatment.
Funding
National Institute on Drug Abuse (T32 DA016224 to I.G.-S., K02 DA000356 to S.R.); NARSAD (to S.A. Schobel); National Institute on Aging (R01 AG025161 to S.A. Small); National Institute of Mental Health (P50 MH066171 to S.R.); the Irving Institute for Clinical and Translational Research; and the Lieber Center for Schizophrenia Research.
Acknowledgments
S.A. Schobel is a Janssen Scholar in Translational Neuroscience.
References
- 1.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 2.Jarskog LF, Miyamoto S, Lieberman JA. Schizophrenia: new pathological insights and therapies. Annu Rev Med. 2007;58:49–61. doi: 10.1146/annurev.med.58.060904.084114. [DOI] [PubMed] [Google Scholar]
- 3.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 5.Dwork AJ. Postmortem studies of the hippocampal formation in schizophrenia. Schizophr Bull. 1997;23:385–402. doi: 10.1093/schbul/23.3.385. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger DR. Cell biology of the hippocampal formation in schizophrenia. Biol Psychiatry. 1999;45:395–402. doi: 10.1016/s0006-3223(98)00331-x. [DOI] [PubMed] [Google Scholar]
- 7.Rosoklija G, Toomayan G, Ellis SP, et al. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- 8.Benes FM, Lim B, Matzilevich D, Subburaju S, Walsh JP. Circuitry-based gene expression profiles in GABA cells of the trisynaptic pathway in schizophrenics versus bipolars. Proc Natl Acad Sci U S A. 2008;105:20935–20940. doi: 10.1073/pnas.0810153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benes FM, Vincent SL. Changes in dendritic spine morphology in response to increased availability of monoamines in rat medial prefrontal cortex. Synapse. 1991;9:235–237. doi: 10.1002/syn.890090311. [DOI] [PubMed] [Google Scholar]
- 10.Zaidel DW, Esiri MM, Harrison PJ. The hippocampus in schizophrenia: lateralized increase in neuronal density and altered cytoarchitectural asymmetry. Psychol Med. 1997;27:703–713. doi: 10.1017/s0033291796004618. [DOI] [PubMed] [Google Scholar]
- 11.Arnold SE, Franz BR, Gur RC, et al. Smaller neuron size in schizophrenia in hippocampal subfields that mediate cortical-hippocampal interactions. Am J Psychiatry. 1995;152:738–748. doi: 10.1176/ajp.152.5.738. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Lee JM, Kim HP, et al. Asymmetry analysis of deformable hippocampal model using the principal component in schizophrenia. Hum Brain Mapp. 2005;25:361–369. doi: 10.1002/hbm.20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csernansky JG, Wang L, Jones D, et al. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry. 2002;159:2000–2006. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- 14.Narr KL, Thompson PM, Szeszko P, et al. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Schobel SA, Kelly MA, Corcoran CM, et al. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophr Res. 2009;114:110–118. doi: 10.1016/j.schres.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison PJ, Eastwood SL. Neuropathological studies of synaptic connectivity in the hippocampal formation in schizophrenia. Hippocampus. 2001;11:508–519. doi: 10.1002/hipo.1067. [DOI] [PubMed] [Google Scholar]
- 17.Benes FM, Lim B, Matzilevich D, Walsh JP. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci U S A. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heckers S, Konradi C. Hippocampal neurons in schizophrenia. J Neural Transm. 2002;109:891–905. doi: 10.1007/s007020200073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benes FM. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol Psychiatry. 1999;46:589–599. doi: 10.1016/s0006-3223(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 20.Javitt DC. Glutamate and schizophrenia: phencyclidine, n-methyl-d-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 21.Greene R. Circuit analysis of NMDAR hypofunction in the hippocampus, in vitro, and psychosis of schizophrenia. Hippocampus. 2001;11:569–577. doi: 10.1002/hipo.1072. [DOI] [PubMed] [Google Scholar]
- 22.Siekmeier PJ, Hasselmo ME, Howard MW, Coyle J. Modeling of context-dependent retrieval in hippocampal region CA1: implications for cognitive function in schizophrenia. Schizophr Res. 2007;89:177–190. doi: 10.1016/j.schres.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Homayoun H, Moghaddam B. Orbitofrontal cortex neurons as a common target for classic and glutamatergic antipsychotic drugs. Proc Natl Acad Sci U S A. 2008;105:18041–18046. doi: 10.1073/pnas.0806669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X, Lein ES, He A, et al. Transcriptional profiling reveals strict boundaries between hippocampal subregions. J Comp Neurol. 2001;441:187–196. doi: 10.1002/cne.1406. [DOI] [PubMed] [Google Scholar]
- 25.Leonardo ED, Richardson-Jones JW, Sibille E, Kottman A, Hen R. Molecular heterogeneity along the dorsal-ventral axis of the murine hippocampal CA1 field: a microarray analysis of gene expression. Neuroscience. 2006;137:177–186. doi: 10.1016/j.neuroscience.2005.08.082. [DOI] [PubMed] [Google Scholar]
- 26.Heckers S, Rauch SL, Goff D, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 27.Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23(suppl 1):S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Small SA. Measuring correlates of brain metabolism with high-resolution MRI: a promising approach for diagnosing Alzheimer disease and mapping its course. Alzheimer Dis Assoc Disord. 2003;17:154–161. doi: 10.1097/00002093-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez RG, Fischman AJ, Guimaraes AR, et al. Functional MR in the evaluation of dementia: correlation of abnormal dynamic cerebral blood volume measurements with changes in cerebral metabolism on positron emission tomography with fludeoxyglucose F 18. AJNR Am J Neuroradiol. 1995;16:1763–1770. [PMC free article] [PubMed] [Google Scholar]
- 30.Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci U S A. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno H, Wu WE, Lee T, et al. Imaging the Aβ-related neurotoxicity of Alzheimer disease. Arch Neurol. 2007;64:1467–1477. doi: 10.1001/archneur.64.10.1467. [DOI] [PubMed] [Google Scholar]
- 32.Malaspina D, Harkavy-Friedman J, Corcoran C, et al. Resting neural activity distinguishes subgroups of schizophrenia patients. Biol Psychiatry. 2004;56:931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawasaki Y, Suzuki M, Maeda Y, et al. Regional cerebral blood flow in patients with schizophrenia. A preliminary report. Eur Arch Psychiatry Clin Neurosci. 1992;241:195–200. doi: 10.1007/BF02190252. [DOI] [PubMed] [Google Scholar]
- 34.Friston KJ, Liddle PF, Frith CD, Hirsch SR, Frackowiak RS. The left medial temporal region and schizophrenia. A PET study. Brain. 1992;115:367–382. doi: 10.1093/brain/115.2.367. [DOI] [PubMed] [Google Scholar]
- 35.Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- 36.Grubb RL, Jr, Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5:630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- 37.Schobel SA, Lewandowski NM, Corcoran C, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaisler-Salomon I, Miller GM, Chuhma N, et al. Glutaminase-deficient mice display hippocampal hypoactivity, insensitivity to pro-psychotic drugs and potentiated latent inhibition: relevance to schizophrenia. Neuropsychopharmacology. 2009;34:2305–2322. doi: 10.1038/npp.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gado MH, Phelps ME, Hoffman EJ, Raichle ME. Changes in cerebral blood volume and vascular mean transit time during induced cerebral seizures. Radiology. 1976;121:105–109. doi: 10.1148/121.1.105. [DOI] [PubMed] [Google Scholar]
- 40.Gingrich JA, Hen R. The broken mouse: the role of development, plasticity and environment in the interpretation of phenotypic changes in knockout mice. Curr Opin Neurobiol. 2000;10:146–152. doi: 10.1016/s0959-4388(99)00061-6. [DOI] [PubMed] [Google Scholar]
- 41.Gainetdinov RR, Mohn AR, Caron MG. Genetic animal models: focus on schizophrenia. Trends Neurosci. 2001;24:527–533. doi: 10.1016/s0166-2236(00)01886-5. [DOI] [PubMed] [Google Scholar]
- 42.Jentsch JD, Olausson P, Moore H. Animal models of psychosis. In: Soares JC, Gershon S, editors. Handbook of Medical Psychiatry. Vol 20. New York, NY: Marcel Dekker; 2003. pp. 317–333. [Google Scholar]
- 43.Chen J, Lipska BK, Weinberger DR. Genetic mouse models of schizophrenia: from hypothesis-based to susceptibility gene-based models. Biol Psychiatry. 2006;59:1180–1188. doi: 10.1016/j.biopsych.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 44.Flint J, Shifman S. Animal models of psychiatric disease. Curr Opin Genet Dev. 2008;18:235–240. doi: 10.1016/j.gde.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Kellendonk C, Simpson EH, Kandel ER. Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci. 2009;32:347–358. doi: 10.1016/j.tins.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 47.Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 48.Javitt DC. Glycine transport inhibitors and the treatment of schizophrenia. Biol Psychiatry. 2008;63:6–8. doi: 10.1016/j.biopsych.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 49.Yee BK, Balic E, Singer P, et al. Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. J Neurosci. 2006;26:3169–3181. doi: 10.1523/JNEUROSCI.5120-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 51.van Elst LT, Valerius G, Büchert M, et al. Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:724–730. doi: 10.1016/j.biopsych.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 52.Conn P, Lindsley C, Jones C. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 54.Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27:735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 55.Chaudhry FA, Reimer RJ, Edwards RH. The glutamine commute: take the N line and transfer to the A. J Cell Biol. 2002;157:349–355. doi: 10.1083/jcb.200201070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maciejewski PK, Rothman DL. Proposed cycles for functional glutamate trafficking in synaptic neurotransmission. Neurochem Int. 2008;52:809–825. doi: 10.1016/j.neuint.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr. 1995;15:133–159. doi: 10.1146/annurev.nu.15.070195.001025. [DOI] [PubMed] [Google Scholar]
- 58.Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 59.Masson J, Darmon M, Conjard A, et al. Mice lacking brain/kidney phosphate-activated glutaminase (GLS1) have impaired glutamatergic synaptic transmission, altered breathing, disorganized goal-directed behavior and die shortly after birth. J Neurosci. 2006;26:4660–4671. doi: 10.1523/JNEUROSCI.4241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maeshima H, Ohnuma T, Sakai Y, et al. Increased plasma glutamate by antipsychotic medication and its relationship to glutaminase 1 and 2 genotypes in schizophrenia—Juntendo University Schizophrenia Projects (JUSP) Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1410–1418. doi: 10.1016/j.pnpbp.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 61.Zhang B, Yuan Y, Jia Y, et al. An association study between polymorphisms in five genes in glutamate and GABA pathway and paranoid schizophrenia. Eur Psychiatry. 2005;20:45–49. doi: 10.1016/j.eurpsy.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 62.Bruneau EG, McCullumsmith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Increased expression of glutaminase and glutamine synthetase mRNA in the thalamus in schizophrenia. Schizophr Res. 2005;75:27–34. doi: 10.1016/j.schres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 63.Gluck MR, Thomas RG, Davis KL, Haroutunian V. Implications for altered glutamate and GABA metabolism in the dorsolateral prefrontal cortex of aged schizophrenic patients. Am J Psychiatry. 2002;159:1165–1173. doi: 10.1176/appi.ajp.159.7.1165. [DOI] [PubMed] [Google Scholar]
- 64.Sherman AD, Mott J. Amphetamine stimulation of glutaminase is blocked by neuroleptics. Life Sci. 1985;36:1163–1167. doi: 10.1016/0024-3205(85)90233-4. [DOI] [PubMed] [Google Scholar]
- 65.Sherman AD, Hamrah M, Mott J. Effects of neuroleptics on glutaminase from rat synaptosomes. Neurochem Res. 1988;13:535–538. doi: 10.1007/BF00973293. [DOI] [PubMed] [Google Scholar]