Abstract
A biological mother’s movement appears necessary for optimal development in infant monkeys. However, nursery-reared monkeys are typically provided with inanimate surrogate mothers that move very little. The purpose of this study was to evaluate the effects of a novel, highly mobile surrogate mother on motor development, exploration, and reactions to novelty. Six infant rhesus macaques (Macaca mulatta) were reared on mobile hanging surrogates (MS) and compared to six infants reared on standard stationary rocking surrogates (RS) and to 9-15 infants reared with their biological mothers (MR) for early developmental outcome. We predicted that MS infants would develop more similarly to MR infants than RS infants. In neonatal assessments conducted at day 30, both MS and MR infants showed more highly developed motor activity than RS infants on measures of grasping (p=.009), coordination (p=.038), spontaneous crawl (p=.009), and balance (p=.003). At 2-3 months of age, both MS and MR infants displayed higher levels of exploration in the home cage than RS infants (p=.016). In a novel situation in which only MS and RS infants were tested, MS infants showed less of a stress response, spending less time near their surrogates in the first five minutes of the test session than RS infants (p=.05) and exhibiting a significantly lower rise in salivary cortisol after the test than RS infants (p=.018). Collectively, these results suggest that when nursery-rearing of infant monkeys is necessary, a mobile hanging surrogate may encourage more normative development of gross motor skills and exploratory behavior and may serve as a useful alternative to stationary or rocking surrogates.
Keywords: Rhesus macaque, surrogate, development, behavior, cortisol
INTRODUCTION
Nursery rearing of nonhuman primates may be employed to study certain aspects of infant primate development and becomes essential when the infant is born at high-risk due to prematurity or low birth weight or is subject to inadequate mothering. Many laboratories rear infants on inanimate surrogate mothers that are either stationary or produce simple rocking motions (Capitanio & Mason, 2000; Capitanio et al., 2005; Schneider & Suomi, 1992). Compared to the biological mother, this limited mobility may adversely impact infant motor development. Indeed, infants reared on simple rocking or highly mobile surrogates exhibit greater motor maturation than those reared with non-moving surrogates (Schneider & Suomi, 1992; Anderson, Kenney, & Mason, 1977; Mason & Berkson, 1975; Duijghuisen, Timmermans, Vochteloo, & Vossen, 1992).
Highly mobile inanimate surrogate mothers, which more closely mimic some of the movements of a biological mother, have previously been shown to elicit greater motor maturation, less fear of novelty, and greater exploration by infants than stationary surrogates. However, these surrogates required either large housing areas or cumbersome external machinery (Anderson et al., 1977; Mason & Berkson, 1975; Duijghuisen et al., 1992). Such requirements cannot typically be met in contemporary nurseries, which often require individual infant housing. Thus a small, non-mechanical surrogate that mimics some of a natural mother’s movements may produce more normal motor and exploratory behavior.
To examine the effects of surrogate mobility on motor and exploratory behavior, we compared a standard floor-mounted rocking surrogate (RS) to a novel “swinging” surrogate that provided significantly more movement and featured a more natural resting position for the infant than the rocking surrogate. The swinging, multidirectional surrogate (MS) hung from the ceiling of the cage, swung in multiple directions in the horizontal plane, and also showed some movement in the vertical plane. Additionally, this surrogate placed the infant in a vertical position at rest, similar to the way an infant rests while being held by its natural mother whereas the rocking surrogate required the infant to rest in a prone position at a 45° angle.
This study differs from previous reports of other kinds of surrogate mothers in several ways. First, we constructed a simple, easy to use, but highly mobile surrogate mother that did not require extensive space or equipment. Second, in contrast to previous studies employing mechanically-driven mobile surrogate mothers, we also included a comparison group of mother-reared (MR) infants to assess the efficacy of our surrogate in yielding normative development. Finally, unlike previous studies, we compared the two surrogate groups on their behavioral and physiological response to novelty as assessed by salivary cortisol (a measure that has not previously been utilized with respect to surrogate type).
We hypothesized that the orientation and degree of mobility of the surrogate mother would affect infant neurological development and behavior. Mother-reared infants must react and adapt to their mother’s movements, thereby receiving valuable proprioceptive and vestibular input and feedback that is not available to infants reared on inanimate surrogate mothers (Schneider, Champoux, and Moore, 2006). However, because the multidimensional surrogate produces motion that is somewhat unpredictable and requires effort to keep it under control, infants reared on these surrogates also have to react and adapt to its movements. As a result, we predicted that MS infants would exhibit more normative development of reflexive and motor skills, greater exploratory behavior, and less fear of novelty than RS infants, and that the MS infants would more closely resemble their MR infant counterparts.
METHODS
Subjects were 12 surrogate-reared infant rhesus macaques born and reared in 2005 at the Laboratory for Comparative Ethology (LCE), National Institutes of Health in Poolesville, MD. Infants were separated from their mothers within 72 hours postpartum and received their surrogates upon separation. All infants were nursery-reared according to the protocols established at the LCE, which followed previously published procedures (Ruppenthal, 1979). All 12 infants were individually housed and were divided into two surrogate groups of six animals each. One group of infants was reared with the LCE’s “standard” floor-mounted stationary rocking surrogates (RS) in their home cages. The surrogate was attached to a stationary base but the surrogate cylinder could move back and forth at the infant’s discretion. The rocking surrogate was 25-cm-high and was composed of a 16.5-cm-circumference polypropylene cylinder anchored to an 11.5-cm-wide circular metal base by a flexible metal coil that allowed the surrogate to rock. The second group of infants was reared with ceiling-mounted multidirectional surrogates (MS) that could move at the infant’s discretion in any direction in the horizontal plane and make small movements in the vertical plane. The MS surrogate was 50-cm long in total comprised of a 17-cm × 5-cm PVC cylinder attached to a 33-cm-long chain that was covered in the same-length PVC pipe of 1.5-cm wide and anchored to the cage ceiling. All surrogates were covered with a fleece material to provide contact comfort.
The standard protocol at the LCE insured that social groups composed of four animals each were formed once the youngest infant in the group was 37 days old. Group socialization lasted from 90-120 minutes per day in a large play cage equipped with various toys, climbing apparatus, and fleece cloths for contact comfort (Shannon et al., 1998).
Nursery-reared infants were compared to 9-15 mother-reared infants depending on the developmental measure. We assessed infants on three outcome measures: 1) gross motor skills and reflexes, 2) exploratory behavior, and 3) behavioral and physiological responses to novelty.
To assess gross motor skills and reflexes, we administered the Primate Neonatal Neurobehavioral Assessment (PNNA), modified from the human Brazelton Neonatal Behavioral Assessment Scale for use in monkeys (Schneider et al., 2006), to MR, MS and RS infants at 30 days of age. This test serves as a good predictor of neurological development and function. The six measures analyzed for this study were reaching, grasping, traction (a measure of upper body strength and tone in response to pulling on the arms), coordination, spontaneous crawl, and balance. Infants were given a score on each measure ranging from 0 (not present) to 2 (fully present) in half-point increments (see Schneider et al., 2006, for a full description of each category). For these analyses, six RS and six MS infants were compared to 15 MR infants. Inter-rater reliability was achieved with at least 90% agreement on these measures.
Exploratory behavior was assessed in the surrogate-reared infants’ home cages 2-3 times per week for neonatal days 30-90 of life. Because data were not available from the same birth cohort of MR infants, we compared surrogate infants to nine MR infants from the 2006 cohort. MR infants’ behavior was assessed 2-3 times per week during normal living conditions in their harem groups during the same 60-day period (Shannon et al., 1998). Five behaviors were analyzed: exploration (including locomotion, tactile/oral exploration of objects, and visual exploration), play (including toy play and movement play such as jumping), self-directed behaviors (including self-mouth, self-clasp, self-groom, and scratching), clinging to the surrogate or mother, and close social contact with the surrogate or mother. Using 5-minute focal animal sampling, data were collected with behavioral observation software that calculated durations and frequencies of each behavior (JWatcher; Blumstein, Daniel, & Evans, 2006), yielding a total of four measures for analyses.
Reactions to novelty were assessed on day 75. Each surrogate infant was given a 30-minute challenge on day 75 to measure behavioral and physiological reactivity to a novel environment. MR infants are not included in this analysis because the procedure would require either exposing both the mother and infant to the novel situation or separating the infant from its mother for the test. In either case, confounding variables would be present: in the first case, we would not be able to discern the infant’s reactions to novelty because the mother would control the infant’s actions, and in the second case the infant would become highly distressed upon separation and we would likely observe nothing more than this stress response.
Each surrogate infant was placed with its surrogate in a large novel cage that contained a variety of novel stimuli (objects, toys, and climbing apparatus) for 30 minutes. Infants were observed and their behavior coded for the first 5 minutes of the session, and then again from minutes 20-25. Coded behaviors included exploration and clinging to the surrogate.
Because no study to our knowledge has yet measured the effects of specific surrogate types on HPA activity in old-world monkeys, we sought to measure salivary cortisol in each infant immediately before and after the novelty test. At each time point a fruit-flavored 1-in long piece of dental rope was placed in the infant’s cheek pouch until it was adequately saturated with saliva. The dental ropes were then removed and centrifuged at 3000 rpm for 20 min, and the saliva was extracted and stored at -80°C (Roma, 2006). Salivary cortisol was analyzed via enzyme immunoassay (EIA, Salimetrics, State College, PA) according to the manufacturer’s directions. Intra-assay and inter-assay coefficients of variation (CVs) were 12.2 and 7.8%, respectively.
The PNNA and exploratory behavior data sets were each analyzed using a multivariate analysis of variance (MANOVA), with rearing condition as the between subjects variable and the measures as the dependent variables (six measures for PNNA, five measures for exploration). If a significant rearing × measure interaction was detected, we then conducted univariate analyses on each measure followed by trend analyses (i.e., difference or reverse Helmert contrasts) on the rearing factor. Since rearing had three factors, two contrasts were performed to test our specific hypothesis: the first compared MR to MS infants and the second compared RS infants to the average of MR and MS infants (see Suomi, Novak, & Well, 1996). For the novelty data, a mixed design analysis of variance (ANOVA) with surrogate type as the between subject variable and time point as the repeated measure was used to determine differences in behavior during novelty testing. A paired samples t-test was used to determine changes in salivary cortisol concentrations before and after the day 75 novelty test. An alpha level p<.05 was considered significant. SPSS statistical software was used for all analyses.
RESULTS
An overall mixed ANOVA for the PNNA revealed a significant main effect for rearing (F=3.06, p=.049) and measure (F=48.50, p<.001), as well as a significant interaction of rearing × measure (F=5.14, p<.001). Given the significant interaction, subsequent univariate ANOVAs and contrasts were run on each of the 6 measures. The statistical analysis revealed that both MR and MS infants scored significantly higher compared to RS infants on coordination, spontaneous crawl, and balance, and lower than RS infants on grasping (Figure 1). MS infants also scored significantly higher on reaching and lower on balance than MR infants. There was no group difference for traction.
Figure 1.
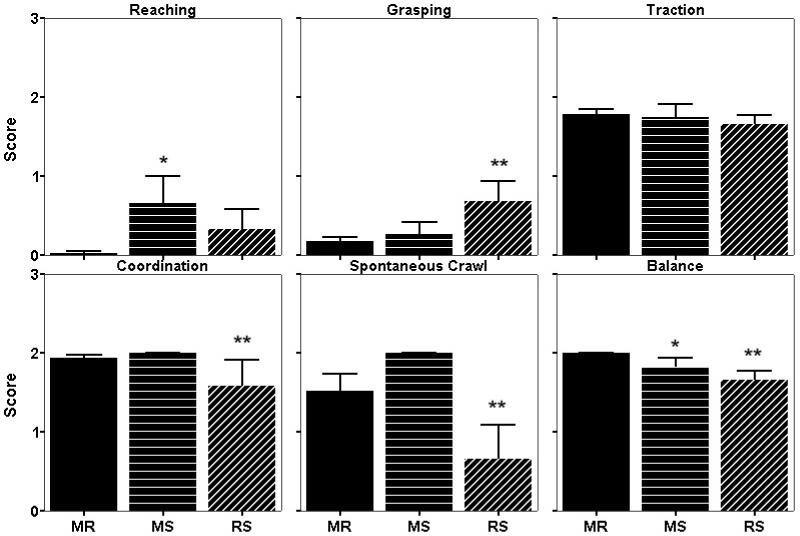
Average scores on gross motor reflexes and motor skills during the Primate Neonatal Neurobehavioral Assessment (PNNA). MR=mother-reared, MS=multidirectional surrogate, RS=rocking surrogate. *Differs from MR only; **Differs from both MR and MS.
An overall mixed ANOVA for exploratory behavior in the home cage revealed significant main effects of rearing (F=22.52, p<.001) and measure (F=171.53, p<.001) and a significant rearing × measure interaction (F=9.30, p<.001). Subsequent univariate ANOVAs with difference contrasts revealed that both MR and MS infants spent significantly more time exploring their environment than RS infants (Table 1). RS infants displayed more self-directed behaviors than MR and MS infants. The three groups did not differ in the time spent clinging to the mother or surrogate, but both MS and RS infants spent significantly less time in close proximity to their surrogates than MR infants did to their mothers (Table 1).
Table 1.
Mean duration of time spent in behaviors in the home cage (in seconds)
| Behavior | Rearing Condition | ||
|---|---|---|---|
| MR | MS | RS | |
| Explore | 127.77 | 112.70 | 81.17** |
| Self-directed | 32.13 | 53.39 | 78.60** |
| Cling mother/surrogate | 106.78 | 102.91 | 89.92 |
| Near mother/surrogate | 82.54 | 22.26* | 21.64* |
Note: MR=mother-reared, MS=multi-directional surrogate, RS=rocking surrogate.
Differs from MR only (p<.05).
Differs from MR and MS (p<.05).
On the day 75 novelty test, a repeated measures ANOVA revealed a significant time × group interaction for duration near the surrogate (F=4.59, p=.05) and a trend for frequency on the surrogate (F=3.27, p=.10). At the 0-5 minute time point, MS infants spent less time near their surrogate and showed lower frequencies on their surrogate than RS infants. No other behavioral differences were detected.
Regrettably, saliva samples taken at the time of the novelty test were not available for all infants because some failed to produce a sufficient volume of saliva for analysis. However, four RS subjects and three MS subjects produced sufficient amounts of saliva both before and immediately after the novelty test. Despite the loss of subjects, significant differences were detected such that RS infants showed a significant increase in salivary cortisol concentrations after the test compared to before the test (mean difference=1.99μg/dL, t=-4.67, p=.018), whereas MS infants showed no difference (mean difference=0.68μg/dL, t=-0.76, p=.526).
DISCUSSION
Our findings revealed that certain features of the surrogate mother, specifically mobility and orientation, can influence early motor development and exploratory behavior. Based on a subset of the subjects, surrogate type may also influence stress responsivity in a novel setting. Consistent with our predictions, at 1 month of age MS infants showed more normative motor development than did infants reared on a standard rocking surrogate. Specifically, MS infants developed similarly to MR infants on four out of six measures of the neonatal assessment whereas RS infants differed from both groups on these measures. Three of these four measures (coordination, spontaneous crawl, and balance) were qualitative measures of motor development which tested the infant’s ability to integrate vestibular and motor input and thus to synchronize its responses accordingly. On these three measures, MS infants displayed similar scores to MR infants, whereas RS infants lagged behind. The fourth measure, grasping, was not qualitative but rather reflexive in nature and merely tested the presence or absence of the movement. Grasping was not strongly present in MS or MR infants at day 30, but RS infants still showed a strong response indicating delayed development. Indeed, Schneider and Suomi (1992) demonstrated a stronger grasping reflex in infants reared on rocking surrogates than in mother-reared infants.
That exposure to motion can affect motor development was demonstrated by Schneider and Suomi (1992) when they showed that infant monkeys reared with a stationary rocking surrogate exhibited greater motor maturation than those reared with a non-moving surrogate. Here we modified the rocking surrogate to add even more motion in two planes and gave it an orientation that produced a more natural body resting position. Furthermore, the movements of the multidimensional surrogate were more unpredictable than a simple rocking motion, presumably providing increased proprioceptive and vestibular stimulation, and the infants had to learn to react appropriately to the surrogate’s movement to keep it under their control. The net result of this new surrogate was greater motor development on par with monkeys reared with a biological mother.
Also consistent with our predictions, at 2-3 months of age MR and MS infants spent similar amounts of time in exploratory behavior whereas RS infants spent significantly less time in these behaviors. Furthermore, MR and MS infants spent less time than RS infants in self-directed behaviors. We hypothesize that MS infants, like MR infants who have a protective mother nearby, are less wary of exploring their environment whereas RS infants may be more apprehensive as indicated by higher levels of self-mouthing and self-grasping. This hypothesis is supported by the reactions of the monkeys to a novel environment on day 75. In this situation, MS monkeys showed lower reactivity as indicated by fewer contacts with their surrogate, higher durations away from their surrogate, and lower cortisol rises than RS monkeys
Collectively, our results indicate that a surrogate which moves in multiple directions, and which places the infant in a more natural position similar to that of being held by a mother, encourages more normative development of motor skills and exploratory behavior and also appears to reduce emotional reactivity in a novel situation. Although at present these conclusions can only be made with respect to early infant life, it will be interesting to continue following these subjects to determine whether any effects of this surrogate type are long-lasting. Finally, for contemporary nurseries, which are often space-limited, the multidirectional surrogate provides a more practical and efficient means of nursery-rearing than mechanically-driven surrogates.
Table 2.
Day 75 Novelty Test Behavioral Results
| Behavior | Rearing Condition | |
|---|---|---|
| MS | RS | |
| Duration near Surrogate (s) | ||
| 0-5 min | 13.96 | 36.04* |
| 20-25 min | 30.12 | 12.60 |
| Frequency on Surrogate | ||
| 0-5 min | 7.00 | 11.17** |
| 20-25 min | 8.50 | 7.17 |
Note: MS=multi-directional surrogate, RS=rocking surrogate.
p=.05
p=.10
Acknowledgments
The studies described in this report were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the NICHD Animal Care and Use Committee. We thank Gerald Ruppenthal (1944-2005) for valuable insight and guidance during the early stages of this project. We acknowledge the assistance of Dr. Matthew FSX Novak, Caroline Kenney, Maggie Unkefer, and Erica Sheldon in data collection. This research was supported by funds from the Division of Intramural Research, National Institute of Child Health & Human Development, NIH, by NIH Grant RR11122 to MAN, and by NIH Predoctoral Training Grant T32NS007490. A preliminary report of this work was presented at the 29th annual meeting of the American Society of Primatologists in San Antonio, Texas, 2006.
REFERENCES
- Anderson CO, Kenney AM, Mason WA. Effects of maternal mobility, partner, and endocrine state on social responsiveness of adolescent rhesus monkeys. Developmental Psychobiology. 1977;10(5):421–434. doi: 10.1002/dev.420100503. [DOI] [PubMed] [Google Scholar]
- Blumstein DT, Daniel JC, Evans CS. JWatcher 1.0: An introductory user’s guide [Computer software] University of California; Los Angeles: 2006. Retrieved May 1, 2005 from. JWatcher Web site: http://www.jwatcher.ucla.edu. [Google Scholar]
- Capitanio JP, Mason WA. Cognitive style: problem solving by rhesus macaques (Macaca mulatta) reared with living or inanimate surrogate mothers. Journal of Comparative Psychology. 2000;114(2):115–225. doi: 10.1037/0735-7036.114.2.115. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Developmental Psychobiology. 2005;46:318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Duijghuisen JA, Timmermans PJ, Vochteloo JD, Vossen JM. Mobile surrogate mothers and the development of exploratory behavior and radius of action in infant long tailed macaques (Macaca fascicularis) Developmental Psychobiologoy. 1992;25(6):441–459. doi: 10.1002/dev.420250605. [DOI] [PubMed] [Google Scholar]
- Mason WA, Berkson G. Effects of maternal mobility on the development of rocking and other behaviors in rhesus monkeys: a study with artificial mothers. Developmental Psychobiology. 1975;8(3):197–211. doi: 10.1002/dev.420080305. [DOI] [PubMed] [Google Scholar]
- Roma P. The SPIT method for simultaneous and unobtrusive collection of salivary cortisol from individually housed infant monkeys. In: Sackett GP, Ruppenthal GC, Elias K, editors. Nursery Rearing of Nonhuman Primates in the 21st Century. Springer; New York: 2006. pp. 429–460. [Google Scholar]
- Ruppenthal GC. Survey of protocols for nursery rearing infant macaques. In: Ruppenthal GC, editor. Nursery Care of Nonhuman Primates. Plenum Press; New York: 1979. pp. 165–185. [Google Scholar]
- Schneider ML, Champoux M, Moore CF. Neurobehavioral Assessment of Nonhuman Primate Neonates. In: Sackett GP, Ruppenthal GC, Elias K, editors. Nursery Care of Nonhuman Primates in the 21st Century. Springer; New York: 2006. pp. 215–248. [Google Scholar]
- Schneider ML, Suomi SJ. Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): developmental changes, behavioral stability, and early experience. Infant Behavioral Development. 1992;15:155–177. [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. American.Journal of Primatologoy. 1998;46(4):311–21. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Novak MA, Well A. Aging in rhesus monkeys: different windows on behavioral continuity and change. Developmental Psychology. 1996;32(6):1116–1128. [Google Scholar]


