Contents
New reproductive technologies based on stem cells offer several potential benefits to carnivore species. For example, development of lines of embryonic stem cells in cats and dogs would allow for the generation of transgenic animal models, which could be used to advance both veterinary and human health. Techniques such as spermatogonial stem cell transplantation and testis xenografting offer new approaches to propagate genetically valuable individual males, even if they should die before producing sperm. These techniques might therefore have application to the conservation of endangered species of carnivores, as well as to biomedical research. Recently, our laboratory has successfully performed spermatogonial stem cell transplantation in the dog, with a recipient dog producing sperm of donor genetic origin. Testis xenografting has been used to produce sperm from pre-pubertal testis tissue from both cats and ferrets. These early steps reinforce the need not only for research on stem cell technologies, but also for additional research into complementary technologies of assisted reproduction in carnivores, so the widest array of research and clinical benefits can be realized.
Introduction
Stem cells are defined by their abilities to self-renew and to produce progeny cells of one or more tissue types. Although somatic stem cells can be used for a number of research and clinical applications, this manuscript will concentrate solely on stem cells that originate from the germline and can be used to propagate it and/or modify it—embryonic stem cells and spermatogonial stem cells. Some controversy exists regarding the presence of ovarian and extra-ovarian stem cells capable of contributing to the germline in adult females (Hutt and Albertini, 2006), but this debate is outside the scope of this report. Technologies of assisted reproduction based on stem cells have the potential to be used for the preservation of genetically valuable animals, such as endangered species of wildlife or animal models of diseases. In addition, the opportunities provided by these techniques for germline modification can greatly expand the utilization of canine and feline biomedical models of disease, thereby benefiting both human and veterinary medicine. Because reproductive research in cats and dogs lags behind that in humans, laboratory rodents, and agricultural species, this manuscript will review the current state of knowledge and describe key points and technological advancements that will be needed to promote applications of stem cell-based technologies to canine and feline reproduction.
Embryonic Stem Cells: Current State of Knowledge
Embryonic stem cells (ESC) are pluripotent, with the ability to differentiate into multiple cell types representing all three embryonic tissue lineages. Of practical importance, they can maintain this undifferentiated nature despite repeated passages in culture. ESC are typically derived from the inner cell mass of a blastocyst, and were first generated in mice (Evans and Kaufman, 1981; Martin, 1981), then non-human primates (Thomson et al., 1995; Thomson et al., 1996), and then in humans (Thomson et al., 1998; Reubinoff et al., 2000). The ability to maintain ESC in culture provides access to modify the genome of these cells, which can then be injected into a blastocyst to generate chimeric offspring. Should the transduced ESC contribute to the germline, then this approach would allow the generation of transgenic offspring. When grown in vitro, the plasticity displayed by ESC in being able to be induced to differentiate into various cell types makes them an attractive resource for the generation of cells that could be used for transplantation-based, regenerative therapies. Dogs and cats are already important biomedical models for transplantation studies and for the study of a number of heritable diseases that have direct homology to human conditions. For these reasons, derivation of canine and feline ESC would be of great significance for basic scientific/ pre-clinical studies that would benefit both human and veterinary medicine.
Verification that a cell line represents actual ESC involves multiple levels of evidence. First, the line should express specific markers, such as alkaline phosphatase and transcription factors such as Oct4 and Nanog. Next, it should be demonstrated that the cell line can differentiate into tissues representing all three embryonic lineages (endoderm, mesoderm, and ectoderm). For this purpose, generation of embryonic bodies or injection of putative ESC into immunodeficient mouse models to produce teratomas are often used. Yet, the “gold standard” for demonstration of true ESC nature in non-human animal models is demonstration of the ability of the cells to contribute to the germline in chimeric animals.
No cell lines have been described for the dog or cat that fulfill all these criteria; only one report of ESC-like cells has been made in the cat (Yu et al., 2008). These authors noted that after 7 passages, evidence of differentiation appeared and alkaline phosphatase activity was lost (Yu et al., 2008). Similar results have been obtained by another group, who also found that enzymatic dissociation induced differentiation in this species (James Kehler, personal communication). Reports have been made regarding 3 putative ESC, or ESC-like, lines in dogs. The first described ESC-like cells that maintained an undifferentiated state for 8 passages (Hatoya et al., 2006). The second brief report described only differentiation into hematopoietic cells (Schneider et al., 2007). The most recent report has been the most complete, with evidence of multiple markers consistent with ESC, as well as clear evidence of differentiation into tissues from all three embryonic lineages, and demonstration of a stable karyotype through 15 passages (Hayes et al., 2008). Reflecting species-specific difficulties inherent to the dog, the line has not yet successfully been used for teratoma formation nor for contribution to a chimera.
Embryonic Stem Cells: Future Directions
The current inability for this last cell line to form teratomas or to show germline transmission might not represent a shortcoming in these cells. As will be described below, support of canine germ cells in murine recipients appears to be less robust than for other species. Demonstration of contribution to a chimera would also require the ability to harvest canine blastocysts at precise times, inject them with the ESC, and then transfer them to synchronized recipient females. Although successful embryo transfer in the dog has been described several times [(Tsutsui et al., 2001a; Tsutsui et al., 2001b; Lee et al., 2005) and Kim, Meyers-Wallen and Travis unpublished observations], manipulation of canine embryos at the blastocyst stage to form chimeras would be a novel and significant development. One key requirement for these studies in the dog is the synchronization of bitches that would be used for embryo donor and recipient. Several methods have been described and are in use with varying degrees of efficacy (Kutzler, 2007). Embryo flushing from one female, injection of ESC into the blastocysts, and then transplantation of them back into that same female, would dispense with the need for synchronization. However, recovery of embryos by flushing as a surgical procedure has been shown to have significantly lower yield than flushing after removal of the tract (Tsutsui et al., 2001b). An alternative to strict synchronization (i.e. having two bitches experience the LH surge within a day of each other) would be provided by the ability to cryopreserve dog embryos. This would allow for collection and manipulation of embryos to occur at any interval before the date of transfer. However, this has never been performed successfully in the canine model and represents a critical enabling technology in need of study.
As noted, feline ESC have to this date not been reported. In some respects, this model is more amenable to immediate use of ESC to generate chimeric or transgenic individuals because the required downstream technologies of assisted reproduction are more advanced in the cat. Feline oocytes can be matured in vitro; in vitro fertilization (IVF) and intra-cytoplasmic sperm injection (ICSI) have both been performed successfully in cats; feline embryos have been successfully cryopreserved and thawed; and embryo transfer has been performed in several felid species. In their own right, these technologies have the ability to contribute to the propagation of genetically valuable individuals (Pope et al., 2006; Pukazhenthi et al., 2006; Swanson, 2006). Should feline ESC be generated, then experience with these downstream technologies will provide a foundation for a rapid translation to clinical and research applications.
Although somewhat outside the scope of this paper, it is worth noting that transgenic cats expressing red fluorescent protein have been generated through the technique of somatic cell nuclear transfer (SCNT) (Yin et al., 2008a). Since successful cloning was first reported in cats (Shin et al., 2002), the SCNT technology has been applied to African Wildcats (Gomez et al., 2004), and has received much study. Indeed, feline SCNT has advanced to the point where second generation clones have been produced (Yin et al., 2008b), and the fertility of cloned individuals by normal mating has been documented (Choi et al., 2007). However, despite this progress, feline SCNT remains a relatively low yield, labor and cost-intensive process. For example, only 2 live, apparently normal African Wildcat kittens resulted from the transfer of 1552 embryos into domestic cat recipients (Gomez et al., 2004). Of 176 cloned embryos expressing the red fluorescent protein transgene that were transferred, only 2 transgenic kittens were produced (Yin et al., 2008a). Should they be developed, feline and canine ESC might provide a more practical approach for the generation of transgenic cats and dogs.
In this regard, one finding of potentially great practical importance for non-rodent animal species was the recent demonstration that expression of 4 genes was sufficient to give murine and human fibroblasts the essential characteristics of ESC (Takahashi and Yamanaka, 2006; Okita et al., 2007). Referred to as “iPS” cells (for “induced pluripotent stem” cells), this technology is highly deserving of study in carnivores in that it might greatly reduce usage of laboratory animals and the associated costs, while simultaneously advancing the rate of progress. But caution should be exercised when contemplating clinical application until more research can be performed. For example, tumors have been noted in 20% of resultant murine offspring, and these were associated with reactivation of the c-myc transgene (Okita et al., 2007). Recently, iPS cells have been described that avoided the induction of this transcription factor (Nakagawa et al., 2008). The derivation and characteristics of iPS cells, and the potential applications of this technology, are highly deserving of concentrated study.
Spermatogonial Stem Cells: Current State of Knowledge
Thus far, most work on technologies of assisted reproduction in males has focused on mature spermatozoa. Successful semen collection, cryopreservation, and thawing techniques have been determined for a number of species. Advancements in the handling and storage of mature sperm have revolutionized the practice of both human and veterinary clinical reproductive medicine. In addition, these innovations have changed the nature of agriculture and agricultural economics, as artificial insemination has often supplanted natural breeding in intensive production regimens. Research on mature sperm is limited in that there are no cell culture systems that support spermatogenesis in vitro; therefore, sperm can only be obtained as primary cells from reproductively mature individuals. The stem cells that will produce sperm, on the other hand, are present in early neonates.
Soon after birth in most species, the gonocytes migrate to the basement membrane of the seminiferous cords; at this point in time the gonocytes transition into spermatogonia. One must be careful to distinguish between spermatogonial stem cells (SSC) and spermatogonia in general. The term “spermatogonia” is used collectively to refer to a continuum of cells ranging from those resting on the basement membrane (known as either Aisolated or Asingle), to proliferating sub-populations (Apaired to Aaligned), to differentiating sub-populations [A1-4, Intermediate and Type B]. The differentiating stages are committed to enter spermatogenesis, but whether the Aisolated cells represent the only spermatogonia with a true stem cell nature is not yet fully clear [Please see (Russell et al., 1990) for a review of spermatogenesis.].
The development of reproductive technologies based on SSC could preserve the breeding potential of males that die prior to puberty. This can be of great importance when the genetic contribution of a single individual could have significant impact on the long-term viability of a population. Examples of this would include attempts to preserve the genetic information represented in offspring of founder individuals in captive breeding programs, or attempts to propagate individuals with diseases that preclude natural mating. In addition, because SSC can be maintained in culture, these cells are similar to ESC in providing an opportunity for genetic manipulation that is not present in the terminally-differentiated spermatozoa. Efforts to take advantage of the attributes of SSC have thus far focused on two technologies: spermatogonial stem cell transplantation (SSCT) and testis xenografting.
Spermatogonial stem cell transplantation
Before describing this technique, it bears mentioning that several different terms have been used for this technology, including “spermatogonial stem cell transplantation” (SSCT) and “germ cell transplantation,” and both names are somewhat unsatisfactory. First, both terms imply that only germ cells are being transplanted, when in most cases using fresh cells, a mixed population including Sertoli cells is actually transplanted (though only the spermatogonia appear to colonize). “Germ cell transplantation” implies that transplantation of several stages of germ cells might be equally efficacious, and this is not the case for the later stages of spermatogenesis. Conversely, the term “SSCT” implies that only SSC are transplanted (which is not true), and makes an assumption that the only germ cells that are colonizing and eventually producing the sperm are the true SSC. This point is unclear given that the “stemness” of the early proliferating spermatogonia still needs to be determined, as is knowledge of the specific stages of spermatogonia that can be transplanted successfully. Identification of surface and expression markers for the individual stages of spermatogonia for the different species will be needed to determine the most precise name for this technology.
Regardless of terminology, this procedure entails the transplantation of spermatogonia from a donor into the seminiferous tubules of a recipient, where they will move to their appropriate niche. At least 4 distinct steps are typically involved: 1). Preparation of the recipient's testes, 2). Isolation of the donor spermatogonia, 3). Transplantation of the donor cell suspension, often enriched in spermatogonia, and 4). Collection of sperm and determination of their genetic origin. First performed in the mouse (Brinster and Avarbock, 1994; Brinster and Zimmermann, 1994), SSCT has been attempted using germ cells isolated from a variety of donor species. Transplanted rat and hamster spermatogonia were able to produce sperm in the testes of recipient mice (Clouthier et al., 1996; Ogawa et al., 1999). However, donor cells from rabbits and dogs (Dobrinski et al., 1999), primates (Nagano et al., 2001), bulls (Oatley et al., 2002), and cats (Kim et al., 2006) have been able to colonize the testes of recipient mice, but have not been able to undergo spermatogenesis. These results directed scientists to reduce the phylogenetic distance between the donor and recipient species, requiring recipient preparation in non-mouse models. Allogeneic transplantation has been performed successfully in the pig (Honaramooz et al., 2002a) and goat (Honaramooz et al., 2003). Recently, we have performed this technique successfully in the dog (Kim, et al., 2008), and will use our work in the dog as the primary example for discussion of the steps of this technology, which has recently been the subject of a more thorough review (Dobrinski and Travis, 2007).
The recipient testes should have their endogenous germ cells depleted prior to transplantation. This would facilitate access of the donor spermatogonia to their appropriate niche on the basement membrane of the seminiferous tubules, allowing more room for proliferation of donor spermatogonia and ultimately providing a higher yield of sperm of donor origin (Brinster et al., 2003). Although a number of techniques have been described for this purpose, in practice, chemotherapeutic drugs or external beam irradiation are the methods most commonly used for depletion of endogenous male germ cells. In cats and dogs, we prefer focal irradiation because of the lack of systemic effects (Kim et al., 2006; Kim et al., 2008) . Germ cells are known to be highly radiosensitive (Dym and Clermont, 1970; Huckins, 1978), whereas Sertoli cells and Leydig cells are relatively radioresistant (Dym and Clermont, 1970; Joshi et al., 1990; van der Meer et al., 1992; Vergouwen et al., 1994). Thus, protocols to deplete the germ cells can be optimized that leave the supporting somatic cell components functional.
Typically, donor cells are isolated by decapsulation of the testes, followed by enzymatic dissociation of the seminiferous tubules with collagenase. Trypsin is then used to separate the individual cells (Bellvé et al. 1977; Bellvé et al. 1977; Dobrinski et al. 1999; Kim et al., 2006; Kim et al., 2008). In some species, surface markers and specific culture conditions can be utilized to enrich the relative percentage of SSC in the donor cells, but such markers and conditions have not yet been identified or optimized for carnivores. Once the donor cell population is obtained, then transplantation can be performed. In the mouse, the efferent ducts can easily be observed because the testes are readily separated from the epididymides (Ogawa et al., 1997). However, in larger species, dense connective tissue makes visualization of the efferent ducts technically challenging. In these species, ultrasonography can be used to guide injection into the rete testis (Honaramooz et al., 2002a; Honaramooz et al., 2003; Izadyar et al., 2003; Herrid et al., 2006). In both cases, retrograde injection is performed to fill as many seminiferous tubules as possible with the donor cells. In our experience, ultrasonographic guidance is of high importance to achieve injection into the rete testis of dogs (Kim et al., 2008).
Once the transplantation is performed, it is necessary to give the introduced cells a chance to occupy their niche on the basement membrane, to expand, and to produce progeny cells that undergo spermatogenesis. Allowing time for several spermatogenic cycles is advisable to maximize the likelihood of identifying sperm of donor origin. In laboratory species for which transgenic technology is already available, observation of fluorescent, enzymatic, or genetic markers can help identify and quantify the relative amount of donor versus endogenous recipient spermatogenesis. In carnivore species for which transgenesis is not available, we have utilized a genetic approach. In the dog, we identified microsatellite markers that differed between donor and recipient. Testing the recipient's sperm in comparison with the known profiles of the donor and the recipient allowed us to identify the presence of donor alleles in the recipient's sperm. However, this approach is not quantitative. Therefore, we next performed quantitative PCR using TaqMan probes for single-nucleotide polymorphisms (SNPs) that differed between donor and recipient to determine the relative production of sperm of donor origin. In one recipient dog in which SSCT was successful, we quantified that 19.5% of the sperm were of donor origin (Kim et al., 2008).
Interestingly, we did not need to suppress the recipient's immune system for this procedure to be successful between unrelated dogs. In xenogeneic transplantation into mice, immunodeficient strains have been used to avoid rejection (Clouthier et al., 1996; Dobrinski et al., 1999; Dobrinski et al., 2000; Kim et al., 2006). But in several species, there are reports of heterologous transplantation without immunosuppression of the recipient (Honaramooz et al., 2002a; Honaramooz et al., 2003; Herrid et al., 2006). It is currently unclear how the donor spermatogonia avoid rejection by the recipient, given that their position on the basement membrane lies on the blood side of the Sertoli cell tight junctions that are considered a major component of the blood-testis barrier.
Testis xenografting
Unlike SSCT, testis xenografting is technically very straightforward, involving only the placement of 1-2 mm3 pieces of testis tissue into an immunodeficient mouse host. Because the tubule and interstitial cell architecture is maintained inside the xenografts, there is little preparation that needs to be performed in the donor tissue or host. One step that is usually performed is castration of the immunodeficient host so that local testosterone production in the xenografts is stimulated. However, xenografts can also be placed under the host's testicular capsule (Shinohara et al., 2002). Because the mouse cannot recognize the xenografts as foreign, they are not rejected, but rather are nurtured. The individual xenografts can then support spermatogenesis. Mature testicular sperm can be collected by dissection of part or all of a xenograft, after a period of time that varies with donor species. However, because of the absence of epididymal maturation, these sperm can only be used for ICSI to generate offspring.
For most studies, neonatal or early pre-pubertal donor tissue has been used (Oatley et al., 2005). Using the domestic cat as donor, we demonstrated that pre-pubertal xenografts would support spermatogenesis, but once meiosis began in the donor tissue, then testis xenografts would not produce sperm (Kim et al., 2007). Although xenografts from adult tissue uniformly degenerated, the xenografts from pubertal donors often survived and produced physiologically relevant levels of testosterone (Kim et al., 2007). Thus, xenograft failure with pubertal donor tissue was not primarily due to inadequate testosterone production by the xenograft Leydig cells. Why xenografts of pubertal and adult tissue uniformly fail is currently unknown.
Testis xenografting has been performed successfully using donor tissue from a number of species including the mouse, pig, and goat (Honaramooz et al., 2002b), Djungarian hamster (Schlatt et al., 2002), bull (Oatley et al., 2005), rabbit (Shinohara et al., 2002), and cat (Snedaker et al., 2004; Kim et al., 2007). Interestingly, in some species such as the rhesus monkey, the onset of spermatogenesis is accelerated in comparison to when tissue had just been left within the donor (Honaramooz et al., 2004). In agricultural species, the time needed for spermatogenesis in the xenografts to produce mature testicular sperm is more or less the same as if the tissue had been left in the donor. However, two reports have shown that in feline xenografts, production of mature sperm is greatly delayed versus age-matched controls left within the testis (Snedaker et al., 2004; Kim et al., 2007). Moreover, spermatogenesis seems to be somewhat abnormal in the feline xenografts (Kim et al., 2007). We are currently investigating whether these xenograft-derived feline sperm are functional, using ICSI as an assay. Live offspring have been derived using sperm produced from mouse and rabbit xenografts (Shinohara et al., 2002).
We have also performed testis xenografting using canine donor tissue. Interestingly, although some xenografts were supported and survived, we observed successful sperm production in <1% of the total xenografts (Gourdon, Kim, Meyers-Wallen, and Travis, unpublished observations). Cats and dogs therefore seem to differ from the majority of other species. To determine whether this represented a broad incompatibility between carnivores and the mouse hosts, we next performed xenografting using the domestic ferret as a source of donor tissue. Notably, xenograft sperm production was outstanding in terms of timing, the number of sperm produced, and the histological appearance of the xenograft seminiferous tubules (Gordoun, Kim, Meyers-Wallen, and Travis unpublished observations). Thus, there appears to be a species-specific incompatibility in the dog, and to a lesser extent, the cat. A somewhat similar situation was observed in the marmoset (Callithrix jacchus) (Wistuba et al., 2004). This was ascribed to a deletion in the luteinizing hormone receptor of this species, which makes it insensitive to that hormone (Wistuba et al., 2004). It will be interesting to determine the underlying incompatibility between the mouse host and canine spermatogenesis.
Spermatogonial Stem Cells: Future Directions
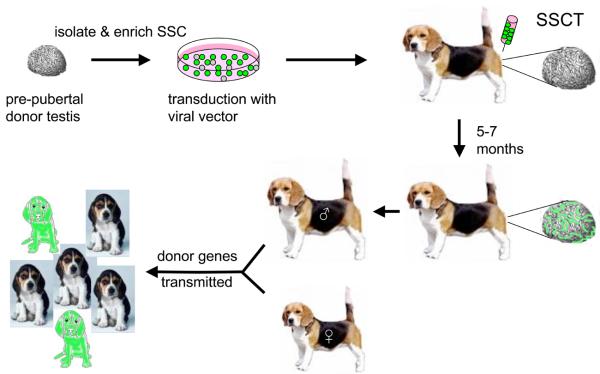
Both SSCT and testis xenografting offer different relative advantages and disadvantages. Additional research on SSCT might ultimately improve our understanding of testis immunology as well as of SSC niches and the nature of “stemness” in the continuum of cells that are spermatogonia. SSCT might also provide a practical means of developing transgenesis in carnivore species in which ESC are not yet available (Figure 1). To facilitate this, research to identify surface markers for the various stages of canine and feline spermatogonia is needed. Such markers would allow enrichment of the appropriate stem cell population(s), and therefore increase the relative efficiency of sperm of donor origin. In addition, enrichment of the SSC would improve the efficiency of transduction, facilitating the generation of transgenic offspring.
Figure 1.
Method of using SSCT to produce transgenic dogs. This diagram presents a schematic approach of how SSCT could be used to achieve transgenesis in a model carnivore species. SSC would be collected from pre-pubertal donor testes to maximize the relative percentage of SSC in the mixed cells collected from the seminiferous tubules. If surface markers for canine SSC are identified, these could be used to enrich the SSC population. The cells would then be incubated with a viral vector carrying a gene of interest. For the purposes of this diagram, the color green denotes a marker such as green fluorescent protein. The transduced cells would be washed and then injected into the rete testis of a recipient that already had its endogenous SSC population depleted (not shown in diagram). After waiting a period of time sufficient to allow the injected SSC to colonize and undergo spermatogenesis, the recipient's semen would be collected and evaluated for the presence of the transgene. Depending on the percentage of sperm carrying the transgene, the recipient could be bred to pass that gene on to offspring. Assuming that the transgene had no impact on either male reproductive fitness or embryo survival, the relative percentage of offspring expressing the transgene would approximate the percentage of sperm carrying that gene.
From a conservation perspective, xenogeneic SSCT might also be useful to generate sperm from genetically valuable individuals that die prior to gamete production. However, for this latter application, appropriate recipient species must be identified that can support donor spermatogenesis. It is not yet known whether the taxonomic distances between domestic cats and dogs and wild felids and canids will prevent the use of the domestic species as recipients for conservation efforts. A potential advantage of SSC relative to testis xenografting is that epididymal maturation of sperm can also take place. This would theoretically allow the sperm produced to be used for a number of downstream applications ranging from artificial insemination to IVF to ICSI. But again, caution should be exercised because species-specific differences might exist in epididymal conditions that prevent extra-testicular maturation of any xenogeneic sperm produced.
As noted above, testis xenografting is technically easier than SSCT, although there is an absolute requirement for maintenance of a colony of immunodeficient mice. Although this technique is currently limited regarding donor age, it would have utility upon occurrences of neonatal or pre-pubertal mortality, which can be a problem in captive breeding programs. Research into the cause of pubertal/adult xenograft failure might identify interventions that could overcome this limitation. In any case, one must have the downstream technology of ICSI developed for each donor species for the xenograft-derived sperm to be of practical use. Logistically, development of good cryopreservation protocols for the 1-2 mm3 pieces of donor testis tissue would greatly enable incorporation of xenografting technology into conservation efforts. This would allow immediate preservation of the testicular tissue, and give time for procurement of immunodeficient mice of young age to serve as hosts. It would also allow the preservation of genetic information of species for which ICSI has yet to be optimized. Therefore, tissue that is currently being discarded could be banked for future use.
Summary
Currently, there are no definitive feline or canine ESC lines. iPS technology might provide an alternative technical approach and is highly deserving of study because of the multitude of potential applications for ESC. SSCT in the dog has recently been performed by our lab, and represents another approach to transgenesis in this species. Although SCNT has been used to produce transgenic cats, SSCT might be more technically accessible to a wider range of investigators who wish to generate transgenic cats. Although SSCT can also be used to preserve the genetic information of genetically valuable donors, the relative technical ease of testis xenografting makes this approach of greater practical interest for the conservation of threatened and endangered species being bred in captivity. Testis xenografting has yet to be successful using canine donor tissue, although it has been used to produce morphologically mature cat and ferret sperm. Should neonatal or pre-pubertal male animals of high genetic value die, cryopreservation of small pieces of their testis tissue should be considered. If an ICSI protocol is one day optimized for their species, then xenografting of the thawed donor testis material might allow for the future introduction of their genetic diversity into their population.
Acknowledgements
This research was supported in part by an Empire State Stem Cell Board grant through the New York State Department of Health (to A.J.T.), the Baker Institute for Animal Health, Morris Animal Foundation (to A.J.T.), and the National Institutes of Health HD-045664 (to A.J.T.).
References
- Bellvé AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellvé AR, Millette CF, Bhatnagar YM, O'Brien D. Dissociation of the mouse testis and characterization of isolated spermatogenic cells. J.Histochem.Cytochem. 1977;25:480–494. doi: 10.1177/25.7.893996. [DOI] [PubMed] [Google Scholar]
- Brinster CJ, Ryu BY, Avarbock MR, Karagenc L, Brinster RL, Orwig KE. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol Reprod. 2003;69:412–20. doi: 10.1095/biolreprod.103.016519. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc.Nat'l.Acad.Sci.,U.S.A. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc.Nat'l.Acad.Sci.,U.S.A. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EG, Yin XJ, Lee HS, Kim LH, Shin HD, Kim NH, Kong IK. Reproductive fertility of cloned male cats derived from adult somatic cell nuclear transfer. Cloning Stem Cells. 2007;9:281–90. doi: 10.1089/clo.2006.0069. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Avarbock MR, Maika SD, Hammer RE, Brinster RL. Rat spermatogenesis in mouse testis. Nature. 1996;381:418–21. doi: 10.1038/381418a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrinski I, Avarbock MR, Brinster RL. Transplantation of germ cells from rabbits and dogs into mouse testes. Biol Reprod. 1999;61:1331–9. doi: 10.1095/biolreprod61.5.1331. [DOI] [PubMed] [Google Scholar]
- Dobrinski I, Avarbock MR, Brinster RL. Germ cell transplantation from large domestic animals into mouse testes. Mol. Reprod. Dev. 2000;57:270–9. doi: 10.1002/1098-2795(200011)57:3<270::AID-MRD9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Dobrinski I, Travis AJ. Germ cell transplantation for the propagation of companion animals, non-domestic and endangered species. Reprod Fertil Dev. 2007;19:732–9. doi: 10.1071/rd07036. [DOI] [PubMed] [Google Scholar]
- Dym M, Clermont Y. Role of spermatogonia in the repair of the seminiferous epithelium following x-irradiation of the rat testis. Am J Anat. 1970;128:265–82. doi: 10.1002/aja.1001280302. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Gomez MC, Pope CE, Giraldo A, Lyons LA, Harris RF, King AL, Cole A, Godke RA, Dresser BL. Birth of African Wildcat cloned kittens born from domestic cats. Cloning Stem Cells. 2004;6:247–58. doi: 10.1089/clo.2004.6.247. [DOI] [PubMed] [Google Scholar]
- Hatoya S, Torii R, Kondo Y, Okuno T, Kobayashi K, Wijewardana V, Kawate N, Tamada H, Sawada T, Kumagai D, Sugiura K, Inaba T. Isolation and characterization of embryonic stem-like cells from canine blastocysts. Mol Reprod Dev. 2006;73:298–305. doi: 10.1002/mrd.20392. [DOI] [PubMed] [Google Scholar]
- Hayes B, Fagerlie SR, Ramakrishnan A, Baran S, Harkey M, Graf L, Bar M, Bendoraite A, Tewari M, Torok-Storb B. Derivation, characterization, and in vitro differentiation of canine embryonic stem cells. Stem Cells. 2008;26:465–73. doi: 10.1634/stemcells.2007-0640. [DOI] [PubMed] [Google Scholar]
- Herrid M, Vignarajan S, Davey R, Dobrinski I, Hill JR. Successful transplantation of bovine testicular cells to heterologous recipients. Reproduction. 2006;132:617–24. doi: 10.1530/rep.1.01125. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Behboodi E, Blash S, Megee SO, Dobrinski I. Germ cell transplantation in goats. Mol Reprod Dev. 2003;64:422–8. doi: 10.1002/mrd.10205. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Li MW, Penedo MC, Meyers S, Dobrinski I. Accelerated Maturation of Primate Testis by Xenografting into Mice. Biol Reprod. 2004 doi: 10.1095/biolreprod.103.025536. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Megee SO, Dobrinski I. Germ cell transplantation in pigs. Biol Reprod. 2002a;66:21–8. doi: 10.1095/biolreprod66.1.21. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002b;418:778–81. doi: 10.1038/nature00918. [DOI] [PubMed] [Google Scholar]
- Huckins C. Behavior of stem cell spermatogonia in the adult rat irradiated testis. Biol Reprod. 1978;19:747–60. doi: 10.1095/biolreprod19.4.747. [DOI] [PubMed] [Google Scholar]
- Hutt KJ, Albertini DF. Clinical applications and limitations of current ovarian stem cell research: a review. J Exp Clin Assist Reprod. 2006;3:6. doi: 10.1186/1743-1050-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadyar F, Den Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, Spoormakers TJ, Colenbrander B, Oldenbroek JK, Van der Ploeg KD, Woelders H, Kal HB, De Rooij DG. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003;126:765–74. [PubMed] [Google Scholar]
- Joshi DS, Yick J, Murray D, Meistrich ML. Stage-dependent variation in the radiosensitivity of DNA in developing male germ cells. Radiat Res. 1990;121:274–81. [PubMed] [Google Scholar]
- Kim Y, Selvaraj V, Dobrinski I, Lee H, McEntee MC, Travis AJ. Recipient preparation and mixed germ cell isolation for spermatogonial stem cell transplantation in domestic cats. J Androl. 2006;27:248–56. doi: 10.2164/jandrol.05034. [DOI] [PubMed] [Google Scholar]
- Kim Y, Selvaraj V, Pukazhenthi B, Travis AJ. Effect of donor age on success of spermatogenesis in feline testis xenografts. Reprod Fertil Dev. 2007;19:869–76. doi: 10.1071/rd07056. [DOI] [PubMed] [Google Scholar]
- Kim Y, Turner D, Nelson J, Dobrinski I, McEntee M, Travis AJ. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction. 2008 doi: 10.1530/REP-08-0226. epub 5 September 20081. DOI: 10.1530/REP-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzler MA. Estrus induction and synchronization in canids and felids. Theriogenology. 2007;68:354–74. doi: 10.1016/j.theriogenology.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Lee BC, Kim MK, Jang G, Oh HJ, Yuda F, Kim HJ, Hossein MS, Kim JJ, Kang SK, Schatten G, Hwang WS. Dogs cloned from adult somatic cells. Nature. 2005;436:641. doi: 10.1038/436641a. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, McCarrey JR, Brinster RL. Primate Spermatogonial Stem Cells Colonize Mouse Testes. Biol Reprod. 2001;64:1409–1416. doi: 10.1095/biolreprod64.5.1409. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Oatley JM, de Avila DM, McLean DJ, Griswold MD, Reeves JJ. Transplantation of bovine germinal cells into mouse testes. J Anim Sci. 2002;80:1925–31. doi: 10.2527/2002.8071925x. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Reeves JJ, McLean DJ. Establishment of spermatogenesis in neonatal bovine testicular tissue following ectopic xenografting varies with donor age. Biol Reprod. 2005;72:358–64. doi: 10.1095/biolreprod.104.030783. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Arechaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol. 1997;41:111–22. [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Xenogeneic spermatogenesis following transplantation of hamster germ cells to mouse testes. Biol Reprod. 1999;60:515–21. doi: 10.1095/biolreprod60.2.515. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Pope CE, Gomez MC, Dresser BL. In vitro production and transfer of cat embryos in the 21st century. Theriogenology. 2006;66:59–71. doi: 10.1016/j.theriogenology.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Pukazhenthi B, Comizzoli P, Travis AJ, Wildt DE. Applications of emerging technologies to the study and conservation of threatened and endangered species. Reprod Fertil Dev. 2006;18:77–90. doi: 10.1071/rd05117. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. Cache River Press, Inc.; St. Louis, MO: 1990. [Google Scholar]
- Schlatt S, Kim SS, Gosden R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction. 2002;124:339–46. doi: 10.1530/rep.0.1240339. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Adler H, Braun J, Kienzle B, Wolf E, Kolb HJ. Canine embryo-derived stem cells--toward clinically relevant animal models for evaluating efficacy and safety of cell therapies. Stem Cells. 2007;25:1850–1. doi: 10.1634/stemcells.2006-0357. [DOI] [PubMed] [Google Scholar]
- Shin T, Kraemer D, Pryor J, Liu L, Rugila J, Howe L, Buck S, Murphy K, Lyons L, Westhusin M. A cat cloned by nuclear transplantation. Nature. 2002;415:859. doi: 10.1038/nature723. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Inoue K, Ogonuki N, Kanatsu-Shinohara M, Miki H, Nakata K, Kurome M, Nagashima H, Toyokuni S, Kogishi K, Honjo T, Ogura A. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum Reprod. 2002;17:3039–45. doi: 10.1093/humrep/17.12.3039. [DOI] [PubMed] [Google Scholar]
- Snedaker AK, Honaramooz A, Dobrinski I. A game of cat and mouse: xenografting of testis tissue from domestic kittens results in complete cat spermatogenesis in a mouse host. J Androl. 2004;25:926–30. doi: 10.1002/j.1939-4640.2004.tb03163.x. [DOI] [PubMed] [Google Scholar]
- Swanson WF. Application of assisted reproduction for population management in felids: the potential and reality for conservation of small cats. Theriogenology. 2006;66:49–58. doi: 10.1016/j.theriogenology.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci U S A. 1995;92:7844–8. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Hearn JP. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol Reprod. 1996;55:254–9. doi: 10.1095/biolreprod55.2.254. [DOI] [PubMed] [Google Scholar]
- Tsutsui T, Hori T, Kawakami E. Intratubal transplantation of early canine embryos. J Reprod Fertil Suppl. 2001a;57:309–14. [PubMed] [Google Scholar]
- Tsutsui T, Hori T, Okazaki H, Tanaka A, Shiono M, Yokosuka M, Kawakami E. Transfer of canine embryos at various developmental stages recovered by hysterectomy or surgical uterine flushing. J Vet Med Sci. 2001b;63:401–5. doi: 10.1292/jvms.63.401. [DOI] [PubMed] [Google Scholar]
- van der Meer Y, Huiskamp R, Davids JA, van der Tweel I, de Rooij DG. The sensitivity to X rays of mouse spermatogonia that are committed to differentiate and of differentiating spermatogonia. Radiat Res. 1992;130:296–302. [PubMed] [Google Scholar]
- Vergouwen RP, Huiskamp R, Bas RJ, Roepers-Gajadien HL, de Jong FH, van Eerdenburg FJ, Davids JA, de Rooij DG. Radiosensitivity of testicular cells in the prepubertal mouse. Radiat Res. 1994;139:316–26. [PubMed] [Google Scholar]
- Wistuba J, Mundry M, Luetjens CM, Schlatt S. Cografting of hamster (Phodopus sungorus) and marmoset (Callithrix jacchus) testicular tissues into nude mice does not overcome blockade of early spermatogenic differentiation in primate grafts. Biol Reprod. 2004;71:2087–91. doi: 10.1095/biolreprod.104.033431. [DOI] [PubMed] [Google Scholar]
- Yin XJ, Lee HS, Yu XF, Choi E, Koo BC, Kwon MS, Lee YS, Cho SJ, Jin GZ, Kim LH, Shin HD, Kim T, Kim NH, Kong IK. Generation of cloned transgenic cats expressing red fluorescence protein. Biol Reprod. 2008a;78:425–31. doi: 10.1095/biolreprod.107.065185. [DOI] [PubMed] [Google Scholar]
- Yin XJ, Lee HS, Yu XF, Kim LH, Shin HD, Cho SJ, Choi EG, Kong IK. Production of second-generation cloned cats by somatic cell nuclear transfer. Theriogenology. 2008b;69:1001–6. doi: 10.1016/j.theriogenology.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Jin G, Yin X, Cho S, Jeon J, Lee S, Kong I. Isolation and characterization of embryonic stem-like cells derived from in vivo-produced cat blastocysts. Mol Reprod Dev. 2008 doi: 10.1002/mrd.20867. [DOI] [PubMed] [Google Scholar]