Abstract
Background
Inflammation markers and MetS are associated with risk of CHF. We evaluated whether combining inflammation markers and metabolic syndrome (MetS) provided additive information for incident congestive heart failure (CHF), and if incorporating inflammation markers to the MetS definition added prognostic information.
Methods and Results
We studied 4017 men and women ≥ 65 years old, without baseline CHF or diabetes, participating in the Cardiovascular Health Study, an observational study with 12.2 years follow-up and 966 cases of incident CHF. Baseline “C-reactive protein (CRP)-MetS” or “interleukin-6 (IL-6)-MetS” were defined as presence of 3 out of 6 components, with elevated CRP (≥3 mg/L) or IL-6 (≥2.21 pg/mL) as a 6th component added to ATPIII criteria. Cox models adjusted for CHF risk factors and incident coronary disease, were used to calculate HRs for CHF. MetS and elevated inflammation markers were independently associated with CHF risk (HRs, 95 % CI: 1.32, 1.16–1.51 for MetS; 1.53, 1.34–1.75 for CRP; 1.37, 1.19–1.55 for IL-6). There was a 20% relative excess risk attributed to the combination of MetS and CRP (95% CI −44% to 88%). CRP-MetS and IL-6-MetS definitions reclassified 18% and 13%, respectively of participants as MetS. Both CRP-MetS and IL-6-MetS increased risk of CHF by 60% compared to those without MetS.
Conclusion
MetS and inflammation markers provided additive information on CHF risk in this elderly cohort. Inflammation-incorporated MetS definitions identified more participants with the same risk level as ATPIII MetS. Considering inflammation markers and MetS together may be useful in clinical and research settings.
Keywords: heart failure, metabolism, inflammation
Congestive heart failure (CHF) is a major public health problem. Approximately 5 million people in the United States suffer from it and an additional half million are newly diagnosed annually.1 While CHF occurs most often as a result of coronary heart disease (CHD), a large percentage of cases occurs in its absence.2 CHF is associated with insulin resistance independent of diabetes mellitus (DM).3 More recently it has been recognized that inflammation factors are associated with the development and progression of CHF with and without CHD.4–13
The metabolic syndrome (MetS) is a constellation of risk factors that co-segregate14–16 and is associated with increased cardiovascular events.17, 18 Insulin resistance and inflammation are postulated among the important underlying pathophysiologies of the syndrome.19 Increased levels of inflammation markers such as C-reactive protein (CRP) and interleukin-6 (IL-6) are observed in subjects with MetS20–22 as well as in people with components of MetS.23–26 The traditional definition of MetS incorporates measures of insulin resistance but not measures of inflammation. It has been proposed that inflammation be incorporated into the MetS definition.27
Little is known about the interrelationships between MetS, inflammation, and incident CHF at the population level. In this study, we tested the hypothesis that the combination of MetS and elevated levels of inflammation markers is associated with increased risk of CHF in the elderly. Further, we incorporated elevated CRP or IL-6 levels into the MetS definition and evaluated this modified MetS definition for its prognostic information on CHF risk.
Methods
Subjects
The Cardiovascular Health Study (CHS) is a prospective population-based observational cohort study of people ≥ 65 years old at baseline initiated to evaluate risk factors for the development and progression of cardiovascular disease. The design, rationale and examination details have been described elsewhere.28 Briefly, participants were randomly selected from Medicare eligibility lists from four U.S. field counties. An initial cohort of 5,201 was recruited between 1989 and 1990 (“original cohort”) and an additional 687 African-Americans were recruited between 1992 and 1993 (“new cohort”). Exclusion criteria included active treatment for cancer, being wheelchair-bound or institutionalized at baseline, or inability to participate in the examination.29 Comprehensive examinations and interviews were performed annually. The study was approved by institutional review boards at each site. Informed consent was obtained from all subjects. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Self-reported health behaviors, medical history, anthropometric measures, current medication use, seated blood-pressure readings, and fasting blood chemistry measures were obtained at baseline. Internal carotid intima-media thickness (IMT) was measured at baseline in a standard manner as previously described.30 All subjects in the original and new cohort were included in the present study, except for those with a baseline history of CHF (n= 275), known valvular heart disease (on the baseline questionnaire or aortic stenosis on study echocardiography, n = 65), hemodialysis or serum creatinine ≥ 3.5 mg/dL (n = 4), or missing values for key variables (n = 599). There were 277 missing components of MetS, 17 CRP, and 305 IL-6. Subjects with DM (n = 928) were excluded given our intention to examine the independent influence of MetS on CHF incidence without the confounding strong effect of DM on CHF.7
Laboratory Methods
Phlebotomy was performed on the morning of enrollment after an 8–12 hour fast.28 Serum total cholesterol, high-density lipoproteins (HDL), triglycerides, glucose, insulin and creatinine were measured at a central laboratory.31 Low-density lipoprotein cholesterol (LDL) was calculated for those with triglycerides <400 mg/dL. CRP was measured by an in-house validated high-sensitivity enzyme-linked immunosorbent assay (ELISA).32 Interleukin-6 (IL-6) was measured by high-sensitivity ELISA (R&D Systems, Minneapolis, MN).33 The interassay coefficients of variation were 6% for CRP and 7% for IL-6.32, 33 Elevated CRP was defined as ≥3 mg/L corresponding to the “high risk” category in the 2003 AHA/CDC consensus statement (corresponding to approximately the top tertile of the population distribution).34 CRP <3 mg/L was defined as normal in this study, corresponding to the “low to average risk” category in the AHA/CDC statement. Elevated IL-6 was defined as values in the top tertile of the distribution (≥ 2.21 pg/mL).
Definitions
MetS was defined using the modified ATP III criteria.15 Subjects were classified as having MetS if they had 3 or more of the following 5 characteristics: 1) waist circumference ≥102 cm for men and ≥ 88 cm for women; 2) triglycerides ≥ 150 mg/dL; 3) HDL <40 mg/dL in men or <50 mg/dL in women; 4) systolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 85 mmHg or hypertension medication use in participants with diagnosed hypertension; 5) fasting glucose >100mg/dL or on drug treatment for elevated blood glucose. Use of fibrates and niacin/nicotinic acid was rare (1.8%) and was not included in the definition of MetS. Since subjects with DM, defined as a fasting glucose of ≥ 126 mg/dl or the use of hypoglycemic agents or insulin, were excluded from the analysis, our definition of MetS included subjects with fasting glucose levels between 100 and 125 mg/dl. Inflammation-MetS (“CRP-MetS” or “IL-6-MetS”) was defined as 3 or more of 6 components, including elevated CRP or IL-6 as a 6th component.
Baseline and incident CHD was defined as a history of myocardial infarction (MI) or a non-MI event, specifically, angina pectoris or a revascularization procedure (coronary artery bypass grafting or percutaneous coronary intervention).5 All cases were adjudicated by a committee that reviewed relevant medical documents.
Adjudication of Incident Congestive Heart Failure Events
Our outcome was incident CHF through June 30, 2005. Methods used to assess CHF events have been reported previously.35, 36 Subjects were interviewed every 6 months and follow-up examinations were conducted annually at each study center until May 31, 1998, after which telephone follow up continued. Self-report of a physician diagnosis of CHF was confirmed by review of medical records by a committee for index CHF events. Presence of CHF was determined by cardiomegaly and pulmonary edema on chest x-ray; or dilated ventricle/wall-motion abnormalities by echocardiography or contrast ventriculography; or a physician diagnosis of CHF which included administration of medical treatment (diuretic plus either digitalis, vasodilator, or angiotensin-converting enzyme inhibitor).28 Pertinent data for hospitalization or outpatient visits including history, symptoms (shortness of breath, fatigue, orthopnea, paroxysmal nocturnal dyspnea), physical signs (edema, pulmonary rales, gallop rhythm, displaced left ventricular apical impulse), chest x-ray findings and medication use were considered.
Statistical Analysis
Baseline characteristics were compared between those with or without MetS using chi-square tests for discrete variables and t tests for continuous data. The incidence rate of CHF in groups based on MetS or inflammation status is presented as events per 1000 person-years. Cox proportional hazards models were used to examine the association of MetS, its components and each inflammation marker with incident CHF. To control for confounding factors, the following variables were included in multivariable models: age, sex, race, field center, cardiovascular disease risk factors [baseline CHD, LDL cholesterol, current smoking) and incident CHD during follow up (time-dependent covariate). A model further adjusting for fasting insulin was used to evaluate the independent role of MetS from insulin levels, as insulin resistance is one of the underlying causes of the syndrome.19 To further account for mediation by subclinical atherosclerosis, baseline internal carotid IMT was added to the models. Kaplan-Meier curves with the endpoint of CHF were constructed based on inflammation status (low or elevated CRP or IL-6 considered separately) and MetS status, with participants cross classified by both exposures. A log-rank test was performed to examine the difference among the four groups. To determine whether MetS and an inflammation marker increased the incidence of CHF in an additive way, hazard ratios of CHF were calculated for each group and the effect modification between MetS and inflammation was evaluated by using Rothman’s synergy index (SI).37, 38 SI is the ratio of the observed effect of the joint exposure divided by the sum of the effects of each factor acting separately and defined as follows:
where RR indicates relative risk.37 The SI is a means of evaluating additive interaction; a value of 1 indicates no interaction and a value > 1 indicates a positive interaction between the two variables.
To evaluate the combined association of CRP and IL-6 with CHF, the correlation of CRP and IL-6 was examined by using Spearman’s coefficient. Then, subjects were divided into four groups based on the levels of the two inflammation markers (elevated CRP plus elevated IL-6, elevated CRP plus low IL-6, low CRP plus elevated IL-6, and low CRP plus low IL-6). Hazard ratios were calculated for each group and compared to those for low values for both markers.
Last, we incorporated CRP or IL-6 into the MetS definition and assessed whether addition of CRP or IL-6 to the ATP III definition of MetS improved risk prediction of CHF (CRP-MetS, IL-6-MetS). The population attributable risk of the modified MetS for CHF was calculated to evaluate the impact of adding inflammation markers to MetS definition.
95% confidence intervals (CIs) were constructed; a P value of <0.05 was considered significant. Statistical analyses were performed using SPSS 14.0.2 software for Windows (SPSS Inc., Chicago, Illinois), S-Plus (release 6.1, Insightful Inc, Seattle, WA) and Stata 8.0 for Windows (Stata Co, College Station, Texas).
Results
Baseline characteristics of participants with and without MetS at baseline are shown in Table 1. Compared to subjects without MetS, subjects with MetS were more likely to be women, hypertensive, and have higher body mass index and waist circumference and worse lipid profiles. CRP, IL-6, insulin concentrations, and internal carotid IMT were also higher in the MetS group. Age, race, and smoking status were similar between the two groups.
Table 1.
Baseline Characteristics of the Cohort*
| Metabolic Syndrome absent (n=2481) | Metabolic Syndrome present (n=1536) | P | |
|---|---|---|---|
| Age (years) | 72.5 (5.5) | 72.5 (5.3) | 0.882 |
| Women (%) | 57 | 65 | <0.001 |
| African-American (%) | 15 | 13 | 0.582 |
| Body mass index (kg/m2) | 24.9 (3.9) | 28.6 (4.6) | <0.001 |
| Waist Circumference (cm) | 88.8 (11.7) | 100.0 (11.6) | <0.001 |
| Hypertension (%) | 46 | 72 | <0.001 |
| Systolic blood pressure (mm Hg) | 133 (21) | 140 (21) | <0.001 |
| Diastolic blood pressure (mm Hg) | 70 (11) | 72 (11) | <0.001 |
| Current smoking (%) | 13 | 12 | 0.444 |
| Total cholesterol (mg/dl) | 210 (36) | 218.1 (41) | <0.001 |
| Triglyceride (mg/dl) | 107 (41) | 173.6 (79) | <0.001 |
| HDL (mg/dl) | 61 (16) | 49 (13) | <0.001 |
| LDL (mg/dl) | 128 (34) | 136 (36) | <0.001 |
| Glucose (mg/dl) | 96 (9) | 105 (9) | <0.001 |
| CRP (mg/L)† | 1.89 [0.96, 3.59] | 3.00 [1.72, 4.94] | <0.001 |
| IL-6 (pg/mL)† | 1.44 [1.02, 2.14] | 1.85 [1.31, 2.72] | <0.001 |
| Insulin (pmol/L)† | 10 [8, 13] | 15 [12, 20] | <0.001 |
| Internal carotid IMT (mm)† | 1.21 [0.94, 1.64] | 1.35 [1.01, 1.78] | <0.001 |
HDL indicates high-density lipoprotein; LDL, low-density lipoprotein; CRP, C-reactive protein; IL-6, interleukin-6; IMT, intima-media thickness.
for continuous variables mean (SD) is shown. For categorical variables percent is shown.
median [IQR]
There were 966 incident cases of CHF among the 4017 participants over a median follow-up of 12.2 years (incidence rate of 21.7 per 1000 person-years). Table 2 shows the hazard ratios for incident CHF by baseline MetS, each MetS component and elevated CRP or IL-6 level. MetS, high waist circumference, low HDL, high blood pressure and elevated CRP or IL-6 were each associated with incident CHF after adjustment for age, gender, race, field center, prevalent CHD, LDL, smoking, and incident CHD. When insulin level was added to the model, hazard ratios were attenuated slightly for MetS, waist circumference, low HDL and elevated blood pressure, but not for CRP or IL-6. When internal carotid IMT was added to the model, hazard ratios were slightly further attenuated. The association of waist circumference with CHF did not retain statistical significance with full adjustment.
Table 2.
Hazard Ratios for Incident CHF by Baseline Metabolic Syndrome, it’s Components and Inflammation Status
| Incidence rate with risk factor (per 1,000 person-years) | Incidence rate without risk factor (per 1,000 person-years) | Unadjusted HR (95% CI) | Model 1 HR* (95% CI) | Model 2 HR* (95% CI) | Model 3 HR* (95% CI) | |
|---|---|---|---|---|---|---|
| MetS (n=1536) | 26 | 19.2 | 1.42 (1.25, 1.61) | 1.32 (1.16, 1.51) | 1.24 (1.07, 1.43) | 1.20 (1.04, 1.38) |
| MetS Components | ||||||
| Elevated waist circumference (n = 1727) | 22.6 | 21.1 | 1.23 (1.08, 1.41) | 1.19 (1.04, 1.36) | 1.11 (0.96, 1.28) | 1.10 (0.95, 1.26) |
| Elevated triglycerides (n=1146) | 22.7 | 21.3 | 1.06 (0.92, 1.22) | 1.07 (0.92, 1.13) | 0.99 (0.86, 1.15) | 0.97 (0.84, 1.12) |
| Low HDL (n=1004) | 26.2 | 20.3 | 1.32 (1.15, 1.52) | 1.27 (1.10, 1.46) | 1.21 (1.04, 1.40) | 1.18 (1.02, 1.37) |
| Elevated blood pressure (n=2917) | 25.9 | 12.1 | 2.17 (1.83, 2.57) | 1.82 (1.53, 2.16) | 1.76 (1.48, 2.10) | 1.69 (1.42, 2.01) |
| Elevated fasting glucose (n=1881) | 24.1 | 19.8 | 1.20 (1.05, 1.36) | 1.14 (1.00, 1.29) | 1.06 (0.93, 1.22) | 1.06 (0.93, 1.22) |
| Inflammation Markers | ||||||
| CRP ≥ 3mg/L (n=1560) | 28.1 | 18.1 | 1.68 (1.48, 1.91) | 1.53 (1.34, 1.75) | 1.51 (1.32, 1.73) | 1.48 (1.29, 1.69) |
| IL-6 ≥ 2.21 pg/mL (n=1152) | 31.7 | 18.5 | 1.77 (1.55, 2.02) | 1.37 (1.19, 1.58) | 1.34 (1.16, 1.55) | 1.32 (1.14, 1.53) |
HR indicates hazard ratio; CI, confidence interval; MetS, metabolic syndrome; CRP, C-reactive protein; IL-6, interleukin-6.
Model 1 adjusted for age, gender, race, field center, prevalent CHD, LDL, smoking, MetS (for CRP and IL-6), CRP ≥ 3mg/L (for MetS, MetS components, and IL-6), IL-6 ≥ 2.21 pg/mL (for MetS, MetS components, and CRP), and incident CHD as a time-dependent covariate
Model 2 adjusted for Model 1 variables plus, incident CHD as a time-dependent covariate and fasting insulin
Model 3 adjusted for Model 2 variables plus internal carotid IMT.
Metabolic Syndrome, Inflammation, and Incident CHF
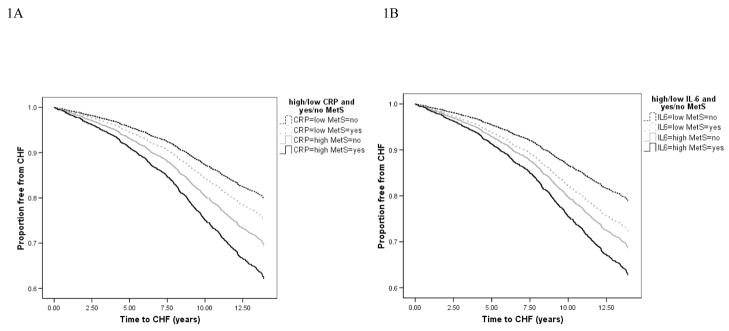
Kaplan-Meier curves categorizing participants on the basis of MetS and CRP or IL-6 values are shown in Figure 1. Participants with both MetS and an elevated inflammation marker had a lower time free from CHF compared to participants with neither or either of MetS or an elevated inflammation marker (P <0.001). Incidence rates and Cox proportional hazards models corresponding to this analysis of joint associations of MetS and an inflammation marker are shown in Table 3. The incidence of CHF ranged from 16.7 per 1000 person-years among those without MetS or elevated CRP to 31.1 per 1000 in those with MetS and elevated CRP. Rates were similar for MetS and IL-6. In adjusted analysis, compared to those with neither risk factor, presence of MetS without elevated CRP was associated with a 1.27-fold increased risk of CHF, while elevated CRP in the absence of MetS increased the risk 1.57-fold. With both factors together, this hazard ratio was 2.05 (95% CI 1.73–2.43). Results were similar with IL-6, with corresponding hazard ratios of 1.38, 1.59 and 1.95. The synergy index (SI) between MetS and CRP was 1.22 (95% CI 0.56–1.88), indicating a 22% relative excess risk than expected under an additive model. The SI for IL-6 and MetS was 0.80 (95% CI 0.40–1.20). The Spearman’s rank correlation coefficient between CRP and IL-6 was 0.48 (P<0.001). When two inflammation markers, CRP and IL-6, were combined, subjects in whom both IL-6 and CRP were elevated had twice the risk of CHF compared with those in whom both IL-6 and CRP were not elevated (HR 1.96, 95% CI 1.62–2.36). The synergy index (SI) between CRP and IL-6 was 1.30 (95% CI 0.49–2.11). There were no significant gender differences in associations of CRP or IL-6 and incident CHF.
Figure 1.
Time free from Congestive Heart Failure based on MetS and inflammation status (1A: CRP, 1B: IL-6). Solid black line indicates subjects with MetS and an elevated inflammation marker (1A: CRP, 1B: IL-6). Solid gray line indicates subjects without MetS but with an elevated inflammation marker. Dashed line indicates subjects with MetS but without an inflammation marker. Dotted line indicates subjects with neither of them. MetS indicates metabolic syndrome; CRP, C-reactive protein; IL-6, interleukin-6.
Table 3.
Hazard Ratio of Incident CHF by the Combination of MetS and Elevated Inflammation Markers
| C-reactive protein | Interleukin-6 | ||||||
|---|---|---|---|---|---|---|---|
| Elevated inflammation marker | MetS | Incidence rate per 1,000 person-years | Number | Adjusted HR (95% CI) of CHF* | Incidence rate per 1,000 person-years | Number | Adjusted HR (95% CI) of CHF* |
| (−) | (−) | 16.7 | 1695 | 1.00 (ref) | 16.5 | 1899 | 1.00 (ref) |
| (−) | (+) | 21.2 | 762 | 1.27 (1.06, 1.53) | 22.4 | 966 | 1.38 (1.17, 1.62) |
| (+) | (−) | 25.2 | 786 | 1.57 (1.32, 1.88) | 30.0 | 582 | 1.59 (1.32, 1.92) |
| (+) | (+) | 31.1 | 774 | 2.05 (1.73, 2.43) | 33.5 | 570 | 1.95 (1.63, 2.34) |
HR indicates hazard ratio; CI, confidence interval; CHF, congestive heart failure; MetS, metabolic syndrome.
adjusted for age, gender, race, field center, prevalent CHD, LDL, smoking, and incident CHD as a time-dependent covariate.
Inflammation-incorporated Metabolic Syndrome
To test whether the inclusion of CRP or IL-6 in the definition of MetS increased the predictive value of MetS for incident CHF, we created two new definitions of MetS: CRP-MetS and IL-6-MetS. The prevalence of CRP-MetS was 49.2% (1978/4017), with 442 of 2481 (17.8%) subjects without ATP III MetS reclassified as CRP-MetS. CRP-MetS was associated with an adjusted hazard ratio of 1.58 (95% CI 1.39–1.81) for CHF (Table 4). Compared to those without MetS by either definition, those subjects with newly reclassified CRP-MetS had an adjusted hazard ratio of CHF of 1.73 (95% CI: 1.41–2.11), which was similar to the hazard ratio of those with the MetS and CRP-MetS (Table 5).
Table 4.
Hazard Ratios of Incident CHF with CRP-MetS and IL-6-MetS
| Reference group | Incidence rate (per 1,000 person-years) | Incidence rate in the reference group (per 1,000 person-years) | Unadjusted HR (95% CI) | Adjusted HR* (95% CI) | Adjusted HR† (95% CI) | Adjusted HR‡ (95% CI) | |
|---|---|---|---|---|---|---|---|
| CRP-MetS§ (n = 1978) | No CRP-MetS | 26.5 | 17.4 | 1.65 (1.45, 1.87) | 1.58 (1.39, 1.81) | 1.50 (1.30, 1.73) | 1.44 (1.25, 1.66) |
| IL-6-MetS§ (n = 1861) | No IL-6-MetS | 27.7 | 17.1 | 1.71 (1.50, 1.94) | 1.61 (1.41, 1.83) | 1.53 (1.33, 1.76) | 1.47 (1.28, 1.69) |
HR indicates hazard ratio; CI, confidence interval; CHF, congestive heart failure; MetS, metabolic syndrome.
adjusted for age, gender, race, field center, prevalent CHD, LDL, smoking, and incident CHD as a time-dependent covariate.
adjusted for age, gender, race, field center, prevalent CHD, LDL, smoking, and incident CHD as a time-dependent covariate and fasting insulin level.
adjusted for age, gender, race, field center, prevalent CHD, LDL, smoking, and incident CHD as a time-dependent covariate, fasting insulin level, and internal carotid IMT.
CRP-MetS and IL6-MetS are defined as 3 or more of 6 components (5 components plus elevated CRP or IL-6, respectively).
Table 5.
Hazard Ratios of Incident CHF based on MetS and MetS with Inflammation Included as a Component (CRP-MetS and IL6-MetS*)
| MetS | CRP- MetS | Number | Adjusted HR† (95% CI) | MetS | IL6- MetS | Number | Adjusted HR† (95% CI) |
|---|---|---|---|---|---|---|---|
| (−) | (−) | 2039 | 1.00 (ref) | (−) | (−) | 2156 | 1.00 (ref) |
| (−) | (+) | 442 | 1.73 (1.41, 2.11) | (−) | (+) | 325 | 1.96 (1.58, 2.42) |
| (+) | (+) | 1536 | 1.56 (1.36, 1.79) | (+) | (+) | 1536 | 1.55 (1.36, 1.78) |
HR indicates hazard ratio; CI, confidence interval; CHF, congestive heart failure; MetS, metabolic syndrome.
CRP-MetS and IL6-MetS are defined as 3 or more of 6 components (5 components plus elevated CRP or IL-6, respectively).
adjusted for age, gender, race, field center, prevalent CHD, LDL, smoking, and incident CHD as a time-dependent covariate. All participants with MetS are diagnosed as CRP-MetS or IL-6-MetS since they have at least 3 components. Therefore, the fourth group with MetS and without inflammation-MetS doesn’t exist.
There were 1861/4017 subjects (46.3%) with IL6-MetS, with 325 of 2481 (13.1%) without the MetS by the ATP III definition reclassified as IL-6-MetS. The adjusted hazard ratio of CHF in those with versus without IL6-MetS was 1.61 (95% CI 1.41–1.83, Table 4). Compared to those without MetS by either definition, the adjusted hazard ratio of IL-6-MetS without ATP III MetS was 1.96 (95% CI 1.58–2.42), which was similar to the hazard ratio of those with the MetS and IL6-MetS (Table 5). Hazard ratios for CRP-MetS and IL-6-MetS were slightly lower when insulin level and internal carotid IMT were added to the models (Table 4), but were still higher than for MetS adjusted for insulin and internal carotid IMT (Table 2). The population attributable risk of the modified MetS definitions for CHF was higher than ATP III MetS (17.7% [95% CI 10.9–24.4%] for CRP-MetS, 17.8% [95% CI 11.4–24.1%] for IL6-MetS, and 7.1% [95% CI 1.5–12.6%] for ATP III MetS). The population attributable risks of elevated CRP or IL-6 alone were 15.8% (95% CI 10.2–21.2%) and 8.5% (95% CI 3.9–13.3%), respectively.
Discussion
In this study of older community-living adults, MetS was associated with development of CHF over a median of 12.2 years of follow up. Using the inflammation markers CRP or IL-6 together with ATP III defined MetS provided additive information to MetS in predicting incident CHF. Moreover, modified definitions of MetS that incorporated CRP or IL-6 detected more subjects at risk of CHF, based on a higher population attributable risk. Observed associations were independent of prevalent and incident CHD, and internal carotid IMT, a measure of subclinical vascular disease. To our knowledge, this is the first prospective study to evaluate the incorporation of inflammation markers into MetS definition with CHF as an outcome.
Three population-based studies have investigated the link between ATP III defined MetS and incident CHF.17, 39, 40 In these studies, the hazard ratios of MetS for incident CHF were approximately 1.5, similar to our findings. When each component of MetS was examined, abdominal obesity and high plasma glucose (≥110 mg/dL) were associated with incident CHF in the Multi-Ethnic Study of Atherosclerosis.41 In our study, low HDL and hypertension were associated with incident CHF. Differences in finding may relate to population ethnicity differences and the older age and much longer follow up in CHS. Several other studies have also examined the effects of inflammation on the development of CHF.5–8, 12, 41 In the Health Aging and Body Composition study6 and the Framingham Heart study12, CRP and IL-6 were both risk factors for CHF, but IL-6 had a stronger predictive value than CRP, unlike our findings. In the Rotterdam Study of older subjects, CRP was strongly and independently associated with incident CHF in men, but, unlike our findings the association was weak and not independent of other risk factors in women.8 In a previous CHS paper, CRP in the highest quintile was associated with incident CHF over 5.5 years in the full cohort.7 In the current analysis with 12.2 years of follow-up, inflammation measured by either CRP or IL-6 was associated with incident CHF in non-diabetic men and women, and elevated levels of the two inflammation markers had similar hazard ratios.
In this study MetS and inflammation status provided additive predictive information, although synergy indexes were not statistically significant. This finding is in keeping with a previous cross-sectional study of another cohort which showed that the presence of both CRP and insulin resistance was related to a history of heart failure.42 Further, higher population attributable risks for inflammation-modified definitions than in the ATP III definition alone appeared to be due to a high population attributable risk of inflammation markers.
How MetS and inflammation are associated with incident CHF is uncertain. Our models were adjusted for baseline and interval coronary heart disease events, carotid IMT, and insulin levels, suggesting that the associations were independent of atherosclerosis and hyperinsulinemia. One possible mechanism is that MetS and inflammation are associated with factors that lead to changes in the myocardium. It has been noted that markers of increased collagen production are associated with increased inflammation factors.43 Increased production of collagen in the heart can lead to a stiff noncompliant heart with resulting diastolic dysfunction and heart failure. Prior analyses of CHS has shown that approximately 50% of heart failure was associated with diastolic dysfunction.5, 7 Another possible mechanism linking MetS, inflammation and CHF are the activation of the renin-angiotensin system and the sympathetic nervous system. Activation of the renin-angiotensin system may inhibit the metabolic actions of insulin.44 Increased sympathetic tone increases peripheral vascular resistance.45
The strengths of this study include its prospective community-based design, large sample size, long follow-up, and large number of incident cases. All CHF cases were adjudicated by an expert panel, limiting the inclusion of false-positive cases. The careful adjudication of prebaseline and incident CHD events, and adjustment for carotid IMT allowed us to comprehensively evaluate whether observed associations were independent of atherosclerosis. Limitations of this study also merit consideration. First, categorization of continuous measures can result in misclassification of exposures such as inflammation and MetS. However, we believe that use of cut points recommended by the AHA/CDC statement34 and the present definition of MetS15 is practical for clinical interpretation. Second, we only evaluated a single measurement of biomarkers at baseline. We believe that our large sample size compensated partly for this weakness. Lastly, for most participants with CHF we did not have echocardiogram data available on systolic function at the time of their diagnosis. This information would have allowed us to examine the differences in associations of inflammation-MetS with CHF with depressed systolic function and CHF with preserved systolic function.
Clinical Implications
In conclusion, in this study MetS and inflammation markers were each independently associated with risk of developing CHF. Incorporation of inflammation markers into the definition of ATP III-defined MetS increased the number of people at risk for CHF and improved risk stratification for people at risk for CHF.
Clinical Summary
Inflammation markers and the metabolic syndrome (MetS) are associated with risk of CHF. We evaluated whether combining inflammation markers and MetS provided additive information for predicting future heart failure (HF), and if incorporating inflammation markers to the MetS definition added prognostic information. We studied 4017 men and women ≥ 65 years old, without baseline HF or diabetes, participating in the Cardiovascular Health Study, an observational study with 12.2 years follow-up. Baseline “C-reactive protein (CRP)-MetS” or “interleukin-6 (IL-6)-MetS” were defined as presence of 3 out of 6 components, with elevated CRP (≥3 mg/L) or IL-6 (≥2.21 pg/mL) as a 6th component added to ATPIII criteria. Cox models, adjusted for HF risk factors and incident coronary disease, were used to calculate HRs for HF. MetS and elevated inflammation markers were independently associated with HF risk (HRs, 95 % CI: 1.32, 1.16–1.51 for MetS; 1.53, 1.34–1.75 for CRP; 1.37, 1.19–1.55 for IL-6). There was a 20% relative excess risk attributed to the combination of MetS and CRP (95% CI −44% to 88%). CRP-MetS and IL-6-MetS definitions reclassified 18% and 13%, respectively of participants as MetS. Both CRP-MetS and IL-6-MetS increased risk of HF by 60% compared to those without MetS. MetS and inflammation markers provided additive information on HF risk in this elderly cohort. Inflammation-incorporated MetS definitions identified more participants with the same risk level as ATPIII MetS. Considering inflammation markers and MetS together may be useful in clinical and research settings.
Acknowledgments
The authors thank the staff and participants in the Cardiovascular Health Study. A full list of participating CHS investigators and institutions can be found at http://www.chs-nhlbi.org. We thank Josef Coresh, MD, PhD for critical reading of the manuscript.
Funding Sources
This research was supported by contracts N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 through N01-HC-85086, N01 HC-15103, N01 HC-55222, and U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke.
Footnotes
Disclosures
The sponsor was involved in the design and conduct of the study and approval of the final manuscript.
References
- 1.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(4 Suppl A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 3.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294(3):334–41. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 4.Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart. 2004;90(4):464–70. doi: 10.1136/hrt.2002.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barzilay JI, Kronmal RA, Gottdiener JS, Smith NL, Burke GL, Tracy R, Savage PJ, Carlson M. The association of fasting glucose levels with congestive heart failure in diabetic adults > or =65 years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43(12):2236–41. doi: 10.1016/j.jacc.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 6.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108(19):2317–22. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 7.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35(6):1628–37. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 8.Kardys I, Knetsch AM, Bleumink GS, Deckers JW, Hofman A, Stricker BH, Witteman JC. C-reactive protein and risk of heart failure. The Rotterdam Study. Am Heart J. 2006;152(3):514–20. doi: 10.1016/j.ahj.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91(11):988–98. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 10.Mueller C, Laule-Kilian K, Christ A, Brunner-La Rocca HP, Perruchoud AP. Inflammation and long-term mortality in acute congestive heart failure. Am Heart J. 2006;151(4):845–50. doi: 10.1016/j.ahj.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 11.Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, Mabuchi N, Sawaki M, Kinoshita M. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31(2):391–8. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- 12.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D'Agostino RB. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107(11):1486–91. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 13.Yndestad A, Kristian DJ, Oie E, Ueland T, Gullestad L, Aukrust P. Systemic inflammation in heart failure--the whys and wherefores. Heart Fail Rev. 2006;11(1):83–92. doi: 10.1007/s10741-006-9196-2. [DOI] [PubMed] [Google Scholar]
- 14.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 15.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PW, Kannel WB, Silbershatz H, D'Agostino RB. Clustering of metabolic factors and coronary heart disease. Arch Intern Med. 1999;159(10):1104–9. doi: 10.1001/archinte.159.10.1104. [DOI] [PubMed] [Google Scholar]
- 17.McNeill AM, Katz R, Girman CJ, Rosamond WD, Wagenknecht LE, Barzilay JI, Tracy RP, Savage PJ, Jackson SA. Metabolic syndrome and cardiovascular disease in older people: The cardiovascular health study. J Am Geriatr Soc. 2006;54(9):1317–24. doi: 10.1111/j.1532-5415.2006.00862.x. [DOI] [PubMed] [Google Scholar]
- 18.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112(20):3066–72. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 19.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 20.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 22.Wannamethee SG, Lowe GDO, Shaper AG, Rumley A, Lennon L, Whincup PH. The metabolic syndrome and insulin resistance: relationship to haemostatic and inflammatory markers in older non-diabetic men. Atherosclerosis. 2005;181(1):101–8. doi: 10.1016/j.atherosclerosis.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 23.Frohlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing H, Muche R, Brenner H, Koenig W. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23(12):1835–9. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- 24.Lakoski SG, Cushman M, Palmas W, Blumenthal R, D'Agostino RB, Jr, Herrington DM. The relationship between blood pressure and C-reactive protein in the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2005;46(10):1869–74. doi: 10.1016/j.jacc.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 25.Piche ME, Lemieux S, Weisnagel SJ, Corneau L, Nadeau A, Bergeron J. Relation of high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-alpha, and fibrinogen to abdominal adipose tissue, blood pressure, and cholesterol and triglyceride levels in healthy postmenopausal women. Am J Cardiol. 2005;96(1):92–7. doi: 10.1016/j.amjcard.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 26.Sesso HD, Wang L, Buring JE, Ridker PM, Gaziano JM. Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension. 2007;49(2):304–10. doi: 10.1161/01.HYP.0000252664.24294.ff. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109(23):2818–25. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 28.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 29.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3(4):358–66. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 30.O'Leary DH, Polak JF, Wolfson SK, Jr, Bond MG, Bommer W, Sheth S, Psaty BM, Sharrett AR, Manolio TA. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22(9):1155–63. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 31.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41(2):264–70. [PubMed] [Google Scholar]
- 32.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43(1):52–8. [PubMed] [Google Scholar]
- 33.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 34.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 35.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 36.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):270–7. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 37.Hallqvist J, Ahlbom A, Diderichsen F, Reuterwall C. How to evaluate interaction between causes: a review of practices in cardiovascular epidemiology. J Intern Med. 1996;239(5):377–82. doi: 10.1046/j.1365-2796.1996.431782000.x. [DOI] [PubMed] [Google Scholar]
- 38.Rothman K. Modern Epidemiology. Boston: Little, Brown; 1986. [Google Scholar]
- 39.Butler J, Rodondi N, Zhu Y, Figaro K, Fazio S, Vaughan DE, Satterfield S, Newman AB, Goodpaster B, Bauer DC, Holvoet P, Harris TB, de Rekeneire N, Rubin S, Ding J, Kritchevsky SB. Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol. 2006;47(8):1595–602. doi: 10.1016/j.jacc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 40.Ingelsson E, Arnlov J, Lind L, Sundstrom J. The metabolic syndrome and risk for heart failure in middle-aged men. Heart. 2006;92(10):1409–13. doi: 10.1136/hrt.2006.089011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51(18):1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 42.Cummings DM, King DE, Mainous AG, Geesey ME. Combining serum biomarkers: the association of C-reactive protein, insulin sensitivity, and homocysteine with cardiovascular disease history in the general US population. Eur J Cardiovasc Prev Rehabil. 2006;13(2):180–5. doi: 10.1097/01.hjr.0000185973.59512.d3. [DOI] [PubMed] [Google Scholar]
- 43.Rutschow S, Li J, Schultheiss HP, Pauschinger M. Myocardial proteases and matrix remodeling in inflammatory heart disease. Cardiovasc Res. 2006;69(3):646–56. doi: 10.1016/j.cardiores.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Prasad A, Quyyumi AA. Renin-angiotensin system and angiotensin receptor blockers in the metabolic syndrome. Circulation. 2004;110(11):1507–12. doi: 10.1161/01.CIR.0000141736.76561.78. [DOI] [PubMed] [Google Scholar]
- 45.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007–18. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]