Abstract
Background
Dynamic mitral regurgitation (MR) contributes to decompensation in chronic dilated heart failure. Reduction of MR was the primary physiologic endpoint in the ESCAPE trial, which compared acute therapy guided by JVP, edema, and weight (CLIN) to therapy guided additionally by pulmonary artery catheters (PAC) toward pulmonary wedge pressure ≤15 and right atrial pressure ≤8 mmHg.
Methods and Results
Patients were randomized to PAC or CLIN during hospitalization with chronic HF and mean LVEF 20%, and at least 1 symptom and 1 sign of congestion. MR and mitral flow patterns, measured blinded to therapy and timepoint, were available at baseline and discharge in 133 patients, and at 3 months in 104 patients. Changes in MR and related transmitral flow patterns were compared between PAC and CLIN patients. Jugular venous pressure, edema, and weights decreased similarly during therapy in the hospital for both groups. In PAC but not in CLIN patients, MR jet area, MR/LAA ratio, and E velocity were each significantly reduced and deceleration time increased by discharge. By 3 months, patients had clinical evidence of increased JVP, edema, and weight since discharge, reaching significance in the PAC arm, and the change in MR was no longer different between the 2 groups, although the change in E velocity remained greater in PAC patients.
Conclusions
During hospitalization, therapy guided by PAC to reduce left-sided pressures improved MR and related filling patterns more than therapy guided clinically by evidence of systemic venous congestion. This early reduction did not translate into improved outcomes out of the hospital, where volume status reverted toward baseline.
Keywords: hemodynamics, heart failure, cardiomyopathy, mitral regurgitation
Introduction
Mitral regurgitation (MR) is a central feature of progression of dilated left ventricular failure, in which it plays a role as both cause and effect[1;2]. From the spectrum of asymptomatic left ventricular dysfunction through evaluation for transplantation, the severity of mitral regurgitation carries strong prognostic weight[3–6].
Acute therapy tailored to reduce measured left-ventricular filling pressures in decompensated heart failure has been shown to cause marked reduction in mitral regurgitation[7]. In the absence of inotropic therapy, the increase in forward stroke volume results primarily from redistribution of regurgitant volume[8;9]. Echocardiographic measurements focusing precisely on the mechanics of mitral regurgitation in dilated heart failure have demonstrated that the major change during therapy with vasodilators and diuretics is attributable to reduction of effective regurgitant orifice area[9]. This reduction in regurgitant orifice area is related in part to decrease in mitral annular distension with improved leaflet coaptation[9]. While therapy in these studies was targeted toward pulmonary capillary wedge pressure ≤15 mmHg, it was not known whether similar reduction of mitral regurgitation would result from therapy guided by clinical examination, which is dominated by evidence of right-sided pressures as approximated from jugular vein inspection[10].
The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization was designed to compare the impact of therapy guided by clinical assessment of filling pressures to therapy guided additionally by pulmonary artery catheterization for patients hospitalized with an exacerbation of advanced heart failure[11]. The primary clinical outcome variable of days alive out of hospital during the 6 months was neutral. The primary physiologic variable was pre-specified to be mitral regurgitation, selected for its importance in prognosis, its sensitivity to filling pressures, and the ability to be measured blindly without potential influence from patient or physician knowledge of treatment arm. The hypothesis was that mitral regurgitation would be more effectively reduced when therapy to relieve congestion was guided by filling pressure goals of pulmonary capillary wedge pressure ≤15 and right atrial pressure ≤8 mm Hg in addition to clinical assessment of volume status, which reflects predominantly right-sided filling pressures.
Methods
Trial design
The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial randomized 433 patients at 26 sites between January 18, 2000 and November 17, 2003. Inclusion criteria included hospitalization with chronic advanced heart failure despite recommended therapies. Patients were randomly assigned to therapy guided by pulmonary artery catheterization (PAC group) or by clinical assessment (CLIN group), as previously described[11]. The goals in both groups were reduction of filling pressures, assessed by jugular venous pressure (JVP), edema, and symptoms in the CLIN group and additionally with goals of pulmonary capillary wedge pressure ≤15 and right atrial pressure ≤8 mmHg in the PAC group. After discharge, the protocol specified that patients return for clinical assessment and adjustment of medications at 2 weeks, 1 month, 2 months, and 3 months after hospital discharge.
Clinical Data
Changes in weight, estimated JVP, and edema, as assessed on the 0–4 scale, were determined between baseline and discharge. In those patients randomized to PAC, differences between RA pressure and pulmonary capillary wedge pressure at the time of insertion and removal were also described.
Echocardiographic examinations
All sites provided the echocardiographic core laboratory with a validation echocardiogram according to defined standardized views for two-dimensional and Doppler measurements, as pre-specified for review prior to initiation. The protocol specified that echocardiograms be obtained in patients at the time of randomization (baseline), hospital discharge (DC), and at 3 months. However, to maximize enrollment, it was emphasized that neither randomization nor discharge should be delayed due to difficulties in scheduling echocardiograms.
Echocardiograms were analyzed at the core laboratory at the University of Texas Southwestern in Dallas. All studies were analyzed blinded to randomization and study order. The studies were analyzed offline by a single sonographer using a calibrated ultrasound measuring system. A portion of the studies was re-measured to evaluate intra-observer variability. Two-dimensional and Doppler echocardiographic parameters were measured in accordance with American Society of Echocardiography criteria. Available measurements included: mitral regurgitant color jet area from the apical 4 chamber view, left ventricular end-diastolic dimension, left ventricular end-systolic dimension, left atrial diameter, left ventricular ejection fraction using apical single plane disc method, left atrial area (LAA) measured from the apical 4 chamber view. The protocol included calculation of mitral regurgitant volume and effective regurgitant orifice from color proximal isovelocity surface area on the apical projection, but these measurements could not be included in the analysis since too few recorded tapes provided adequate data on the majority of patients. The mitral regurgitant color jet area was measured and the ratio of this area to left atrial area determined. These were considered to be adequate for measurement if the origin of the jet was seen at the level of the mitral valve and the borders of the jet were clearly delineated. The left atrial area was measured if the left atrial borders were clearly identified.
Mitral filling patterns included measurement of early mitral inflow velocity (E wave), and deceleration time (Decel time). The peak systolic and diastolic pulmonary venous flow velocities were measured and the ratio of systolic to diastolic peak velocities calculated (PV S/D).
Changes in echocardiographic variables for each patient were determined from baseline to discharge and baseline to three months. The number of paired samples that could be analyzed for each variable varied, depending on the number of adequate measurements from each echocardiographic study. Echocardiographic results were calculated both for mean changes and for median relative changes compared to individual baseline values.
Statistics
Continuous variables were summarized by mean and standard deviation (SD) statistics. Paired T-tests were used to assess significance of changes between baseline and discharge and baseline and 3 months. Standard t-tests were used to compare changes in the PAC group to changes in the CLIN group. A p-value of <0.05 was statistically significant. The question of whether the two strategies led acutely to different reduction of mitral regurgitation was considered to be separate from the question of whether there were differences sustained at 3 months, for which the outpatient therapies and compliance would be important factors. Thus the strategies were compared separately at the two time points. No correction was made for multiple comparisons.
Results
Study population
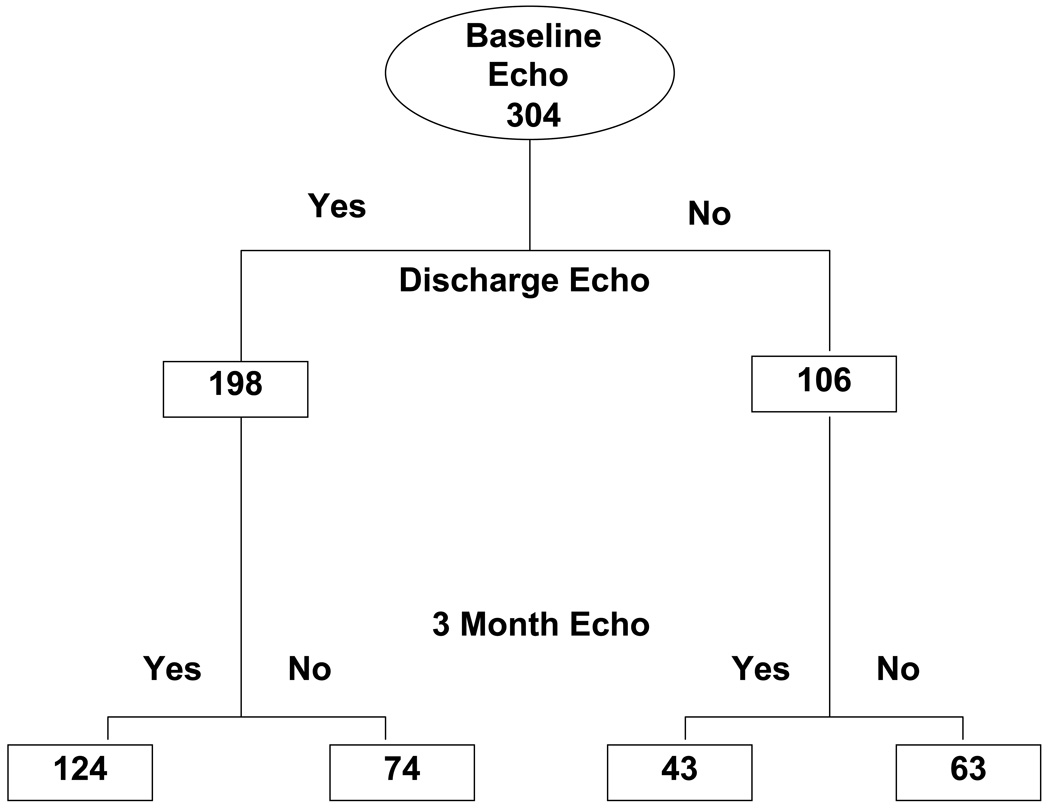
Both baseline and discharge echocardiograms were available for analysis in 198 patients (Figure 1). Measurements of MR were adequate for comparison between baseline and discharge for 133 patients, in whom demographics and hemodynamics confirmed severe heart failure with reduced ejection fraction (average 20%), low systolic blood pressure compared to most hospitalized HF populations (average 104 mmHg), and markedly elevated jugular venous pressures (Table 1). Baseline profiles were not different between the 133 patients with paired MR measurements, the 106 patients who had an echocardiogram at baseline but not at discharge, and the 65 for whom both echocardiograms were obtained but change in MR could not be assessed from the views provided (Table 1). Estimated JVP elevation and edema were comparable in the two randomized groups CLIN and PAC, and the resting pulmonary capillary wedge pressure was 25 ±9 mmHg and RA pressure 13 ±9 mm Hg in the PAC group. LV dilation, LVEF, mitral regurgitation and flow patterns were comparable in the two groups at baseline (Table 2).
Figure 1.
Consort diagram indicating the number of patients with echocardiograms submitted at the 3 timepoints: baseline in hospital prior to randomization, discharge, and three months after discharge.
Table 1.
Clinical Characteristics of Patients With and Without Echocardiograms and MR Measurements
| Baseline and Discharge MR Measurement (SD) |
Baseline without Discharge Echo |
Echoes Without MR Measurement |
|||
|---|---|---|---|---|---|
| CLIN | PAC | Total | |||
| N | 66 | 67 | 133 | 106 | 65 |
| Age | 58 (15) | 56 (13) | 57 (14) | 56 (14) | 54 (14) |
| Gender | 74% | 77% | 76% | 72% | 78% |
| Minority | 27% | 37% | 32% | 37% | 46% |
| CAD | 44% | 51% | 48% | 48% | 51% |
| Baseline LVEF | 20 (6) | 20 (5) | 20 (7) | 19 (6) | 21 (6) |
| SBP mmHg | 104 (12) | 104 (15) | 104 (14) | 106 (14) | 108 (15) |
| Est JVP mmHg | 13 (4) | 13 (4) | 13 (4) | 13 (4) | 13 (4) |
| Creatinine mg/dl | 1.5 (0.6) | 1.6 (0.6) | 1.5 (0.6) | 1.5 (0.5) | 1.3 (0.5) |
| RAP* mmHg | 14 (11) | 14 (11) | 12 (6) | 14 (8) | |
| PCW* mmHg | 25 (10) | 25 (10) | 24 (10) | 24 (10) | |
Obtained only in patients randomized to PAC-guided therapy
CAD = coronary artery disease
Est JVP = clinician-estimated jugular venous pressure
PCW = pulmonary capillary wedge pressure
RAP = right atrial pressure
Table 2.
Mitral Regurgitation and Flow Patterns: Baseline and Changes During Hospitalization
| CLIN | PAC- Guided Therapy | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Change | P-value* within Group |
Baseline | Change | P-value* Within Group |
P-value* Between Groups |
|
| MR area cm2 (n =133) | 10.3 ±6.7 | 0.3 ±4.2 | 9.3 ±6.7 | −2.0 ±5.4 | 0.004 | 0.01 | |
| MR area / LAA (n =132) | 0.3 ±0.2 | 0.0 ±0.1 | 0.3 ±0.2 | −0.1 ±0.2 | 0.01 | 0.02 | |
| E Velocity cm/sec (n= 143) | 97 ±29 | 7 ±41 | 0.16 | 100 ±30 | −8 ±25 | 0.01 | 0.01 |
| Decel Time msec (n= 116) | 147 ±63 | 7 ±54 | 135 ±37 | 19 ±67 | 0.05 | ||
| Pulm Vein Sys/Dias (n= 61) | 0.5 ±1.1 | −0.25 ±.5 | 0.5 ±0.4 | 0.24 ±1.0 | 0.20 | 0.14 | |
| LVEDI cm (n= 175) | 3.3 ±0.7 | 0.02 ±0.28 | 3.4 ±0.6 | −0.02 ±0.28 | |||
| LVEF % (n= 130) | 20 ±9 | −0.4 ±8.0 | 20 ±10 | −0.2 ±9.7 | |||
P-values listed if ≤0.20
Decel = deceleration
LAA = left atrial area
LVEDD = left ventricular end-diastolic dimension
LVEDI = left ventricular end-diastolic dimension index
LVEF = left ventricular ejection fraction
MR = mitral regurgitation
Sys/Dias = systole/diastole
Changes from baseline to discharge
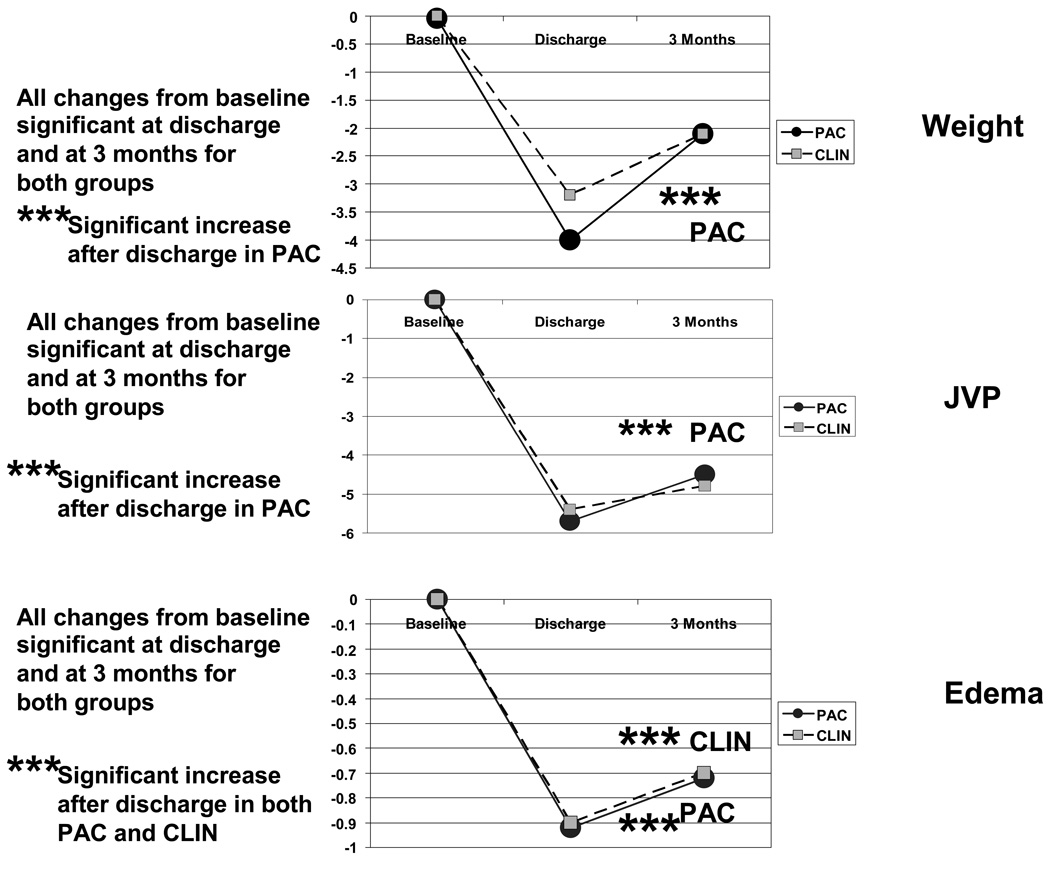
Patient weight decreased significantly in both groups during therapy in hospital, by 3.2 ±3.8 Kg in the CLIN group and 4.1 ±5.7 Kg in the PAC group, without significant difference between groups (p =0.24). Estimated JVP and edema decreased comparably in both groups (Figure 2). For the PAC group, measured PCW decreased by average 8 ±9 mmHg and RA pressure by 5 ±12 mmHg.
Figure 2.
Changes in clinical evidence of fluid retention between baseline and discharge and between discharge and 3 months. These graphs include only patients who had echocardiograms and the clinical measurements recorded at all 3 times points: 74 CLIN patients and 65 PAC patients with weights, 75 CLIN patients and 63 PAC patients with jugular venous pressures, and 79 CLIN patients and 66 PAC patients with recorded assessment of edema. All changes were significantly different from baseline both at discharge and at 3 months. For PAC patients, all 3 parameters increased significantly between discharge and 3 months, during which time the edema increased significantly in the CLIN group as well.
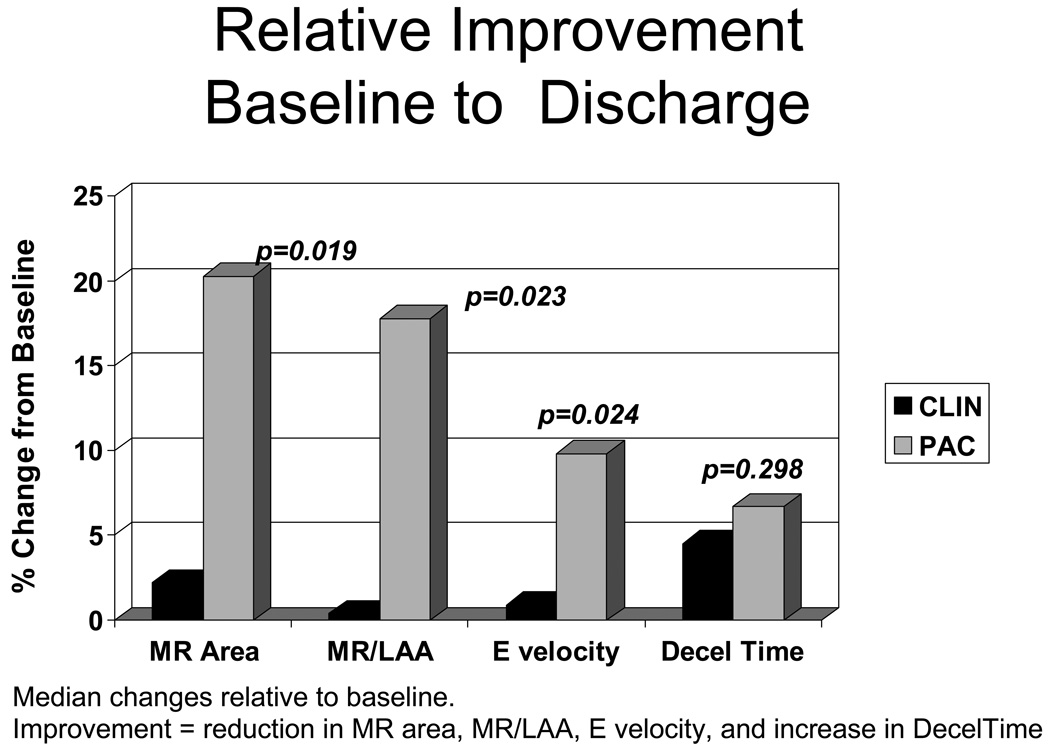
The MR color jet area decreased by 2 cm2 in the PAC group (p <0.004), but did not changed in the CLIN group (between group difference for change p <0.01, Table 2). The median % reduction relative to baseline was 2% in CLIN and 20% in PAC (Figure 3). The ratio of MR/LAA decreased significantly from 0.3 to 0.2 in the PAC group (18% relative reduction compared to individual baseline), but not in the CLIN Group (between group p =0.02). Doppler patterns representing left heart filling patterns also demonstrated greater improvement in PAC patients (Table 2, Figure 3). There was a significant decrease in E wave velocity in the PAC group but not in the CLIN group (between group p =0.01). Deceleration time increased significantly only in PAC patients (p =0.05), without significant between-group difference. Changes in pulmonary vein inflow patterns could be measured at baseline and discharge in only 61 patients, but there was a trend for increasing systolic/diastolic ratio only in the PAC group (difference between PAC and CLIN p =0.14). Left ventricular dimensions and ejection fraction did not change significantly from randomization to the time of discharge.
Figure 3.
Relative improvement between baseline and discharge in mitral regurgitation (MR area), mitral regurgitation area expressed as a proportion of left atrial area (MR/LAA) and E velocity, for all of which a decrease is an improvement. For deceleration (Decel) time, an increase represents an improvement. Changes are expressed here as the median of individual % changes between baseline and discharge divided by baseline values (contrast to Table 2, where they are expressed as mean absolute change from baseline). CLIN is patients who were randomized to therapy guided by clinical assessment of filling pressures, and PAC is patients randomized to therapy guided additionally by a pulmonary artery catheter.
Changes from baseline to 3 months
Increase in Evidence of Fluid Retention After Discharge
By 3 months after discharge, JVP and weights had increased back toward baseline in both groups, changes which were significant in the PAC group. Peripheral edema increased significantly in both groups (Figure 2).
Of the 198 patients with echoes at baseline and discharge, 124 had echoes also at 3 months. An additional 43 patients had paired echoes at baseline and 3 months but not at discharge (Figure 1). After 3 months, there was no longer a difference seen between the PAC and CLIN groups for either MR area or MR/LAA area (Table 3). MR/LAA area was reduced compared to baseline in both groups, and MR area was reduced compared to baseline in the CLIN group. Mitral flow patterns continued to show trends for improved filling for patients after initial tailored therapy with PAC. The reduction in E velocity in PAC patients was less than at discharge, but remained significantly reduced compared to the CLIN patients at 3 months. At 3 months, Decel time remained longer than at baseline for PAC (p =0.02), but not for CLIN. LV end-diastolic dimensions remained unchanged for all groups. In the absence of detectable change in LV dimension, the 5 point increase in LV ejection fraction in the PAC group at 3 months compared to baseline (p =0.01) may be a chance finding and is of unclear clinical significance.
Table 3.
Changes at 3 Months After Discharge
| CLIN | P-value Change Within CLIN |
PAC during hosp |
P-value Change Within PAC |
P-value Change in CLIN vs. PAC |
|
|---|---|---|---|---|---|
| MR area cm2 (n =104) | −2.4 ±5.3 | < 0.001 | −1.0 ±6.0 | 0.17 | |
| MR area / LAA (n =103) | −0.1 ±0.2 | < 0.01 | −0.1 ±0.2 | 0.01 | |
| E Velocity cm/sec (n =129) | 5.7 ±2.8 | 0.16 | −8 ±25 | 0.09 | 0.02 |
| Decel Time msec (n =113) | 13 ±69 | 27 ±86 | 0.05 | ||
| Pulm Vein Sys/Dias (n =47) | −0.2 ±1.6 | 0.1 ±0.4 | |||
| LVEDI cm (n =158) | 0.01 ±0.44 | −0.02 ±0.37 | |||
| LVEF % (n =110) | 2.5 ±11 | 0.11 | 5.1 ±10 | <0.01 | 0.21 |
Same as in Table 2.
Discussion
The primary physiologic endpoint of the ESCAPE trial was mitral regurgitation, which was more effectively reduced when therapy to relieve congestion was guided by pulmonary artery catheter goals than by clinical assessment alone during hospitalization. The reduction of mitral regurgitation was accompanied by parallel improvement seen in the inflow E velocity and in the deceleration time, which both reflect left ventricular filling and may be surrogates for left ventricular filling pressures. The differences seen between the strategies at hospital discharge were largely lost after three months of outpatient management, during which therapy was guided in both groups only by clinical assessment, which revealed increased fluid retention after discharge.
Assessment of Filling Pressures
Serial assessment of filling pressures is a Level I recommendation accompanying adjustment of diuretics to treat fluid retention in the outpatient setting[12]. Inpatient hospitalization is associated with excess volume in 83% patients admitted in the ADHERE registry[13], and it is recommended that optimal volume status be restored prior to discharge, based on clinical assessment. The most helpful clinical sign used to detect elevated left-sided filling pressures in chronic heart failure remains the elevated jugular venous pressure, as confirmed recently by the analysis of physician estimates of hemodynamics for the ESCAPE trial[10]. However, the jugular venous pressure actually reflects right-sided filling pressures. For most patients with chronic heart failure, right and left-sided filling pressures track together[14], but it is not known whether the degree of elevation is sufficiently mirrored to optimize the left side consistently during serial assessment of the right side. Furthermore, clinical examination of jugular veins can be done well by experts, but is still less reliable than direct measurement[10;15]. Reduction of JVP during therapy in the hospital was similar in both the CLIN and PAC arms, as was reduction of peripheral edema. The overall fluid loss, as estimated by acute change in weight, was similar in the two groups, although numerically greater in the PAC arm. Nonetheless, there was slightly but significantly less renal dysfunction in the PAC arm than the CLIN arm[16]. It is plausible but cannot be proven from existing data that monitoring of the left-sided filling pressures guided adjustment of the “optimal” amount of diuresis and vasodilation for individual patients, achieving more normal left-sided filling pressures than when they were assessed indirectly from the jugular venous pressures and other clinical signs reflecting the right side of the heart.
Reduction of Mitral Regurgitation
Mitral regurgitation is at least moderate in almost all patients at the time of hospital admission with decompensated heart failure and dilated left ventricular failure[17–19]. Therapy tailored to reduce measured filling pressures to near-normal levels has been shown to decrease valvular regurgitation [17;20]. This decrease in mitral regurgitant volume during unloading therapy was recognized initially with nitroprusside, and has been shown to be the major component of increased stroke volume during vasodilator and diuretic therapy[8;17;21]. This redistribution of flow can be attributed to reduction of effective regurgitant orifice area[9]. Most of the studies quantitating reduction of mitral regurgitation were performed during monitoring with a pulmonary artery catheter to guide reduction of filling pressures, but lessons learned from these studies have been translated into therapy without invasive monitoring. Thus it was not known whether similar results could be achieved during contemporary therapy guided by refined clinical assessment alone. The clinical importance of mitral regurgitation, its relationship to elevated filling pressures, and the ability to isolate its measurement from knowledge of treatment strategy were key factors in the decision to define mitral regurgitation as the primary physiologic variable in the ESCAPE trial, comparing an invasive hemodynamic strategy to clinical assessment.
The reduction of MR in the PAC arm between baseline and discharge appears to be a robust finding. The improvements between baseline and discharge for PAC patients, and the difference between CLIN and PAC strategies are evident whether analyzing the absolute changes in MR measurements, or the relative changes expressed as a percentage of baseline values (Table 2 and Figure 3). The clinical significance of this change in MR is emphasized by concordant improvements in other measurements. The E velocity declined only in the PAC arm, consistent with a meaningful decrease in left ventricular filling pressure, reflecting MR reduction[22;23]. E velocity has been correlated with MR regurgitant fraction severity[22;23]. Deceleration time, which increased significantly only in the PAC patients, has correlated inversely with pulmonary capillary wedge pressures[24]. Left ventricular end-diastolic dimension is generally insensitive to acute changes, particularly without inotropic therapy, and did not change in this study. Pulmonary vein profiles could be measured at baseline and discharge in only 61 patients, with a trend toward improvement only in the PAC group.
Outcomes After Hospital Discharge
Although MR at the time of discharge was significantly reduced in the PAC arm compared to the CLIN arm at discharge, six-month outcomes were no different between the groups[11]. The failure of acute reduction of MR to improve outcomes in this trial could indicate that 1) MR is an epi-phenomenon that is not itself important to outcomes, 2) any benefit of reduced MR can be outweighed by deleterious effects of the therapies used, or 3) reduced MR is associated with improved outcomes only if it can be sustained after discharge.
The presence and severity of MR have consistently been associated with worse prognosis throughout the spectrum of heart failure, from asymptomatic patients early post-infarction through candidates for cardiac transplantation [3;4;6;25]. Changes in prognostic factors do not necessarily translate into changes in prognosis, although the contribution of mitral regurgitation to elevate left-sided filling pressures and to reduce effective forward stroke volume, make it attractive as a therapeutic target. Surgery to repair mitral regurgitation in heart failure has not led to better outcomes[26], in part because mitral regurgitation often recurs or is replaced by mitral stenosis[27]. For the related measurement of deceleration time, better outcome has been associated with restrictive pattern reversibility and prolongation of the deceleration time in patients during HF therapy[28–31], shown by Xie et al. in 1994[32] and recently by Grayburn et al. in the Beta-blocker Evaluation of Survival Trial[33].
With regard to the therapies used in hospital to decrease volume, the overall use of diuretics was similar in the two arms, with a trend towards slightly lower diuretic use at discharge in the PAC arm[16]. Inotropic therapy was associated with a worse outcome in the ESCAPE trial, and there was slightly higher use of inotropic therapy in the PAC arm than the CLIN arm, but there was still no difference in outcomes when patients receiving inotropic therapy were excluded from analysis[11].
With regard to maintenance of early improvement in MR, the changes observed in the hospital were less evident at 3 months in the PAC group, although improvements in the E velocity and deceleration time that occurred in the PAC group were still significant at 3 months. Once ventricular remodeling is advanced, perhaps a large acute reduction in MR cannot easily be maintained. On the other hand, there was a significant decrease in MR after discharge in the CLIN group during HF management such that the CLIN and PAC groups converged by 3 months of outpatient HF management.
It is known from similar populations that most of the interventions during the 3 months after hospital discharge involve telephone-directed adjustments in diuretic regimens, most commonly based on changes in weight at home[34]. Weight changes at home are less reliable than previously thought for changes in ventricular filling pressures[35]. The ESCAPE protocol specified that patients return for clinical assessment and adjustment of medications at 2 weeks, 1 month, 2 months, and 3 months after hospital discharge. The physicians assessing fluid status during these visits were the same as those whose assessments correlated well with measured right-sided pressures at the time of randomization[10]. Despite these frequent opportunities for adjustment of medications, the current study reveals that jugular venous pressures and weights increased between discharge and 3 months, changes which were significant in the PAC patients. This may also relate to the decrement reported between one month and 3 months in the symptomatic improvement seen in the PAC patients (as reflected in Minnesota Living with Heart Failure scores)[11]. It is not clear whether these changes could have been prevented by even greater vigilance, or whether physiologic factors in advanced heart failure limit prolonged maintenance of lower volume status achieved during hospitalization.
There was a late increase in left ventricular ejection fraction in both CLIN and PAC, with an improvement of 5 percentage points in the PAC arm that reached significance. Multiple mechanisms could be invoked to explain such an improvement, including lower wall stress and reduced myocardial oxygen demand, better coronary perfusion and coronary venous drainage. However, the absence of demonstrable changes in ventricular dimensions may render this more likely to be a chance finding.
Limitations
Multiple protocol issues complicate the interpretation of these results. The substantial amount of missing data confounds analysis, but comparison of groups with and without echoes and without and without measurable MR does not suggest any systematic bias of this missing data. The echoes that were obtained did not have consistently adequate views for measurement. Thus, the requirement that each site provide a validation echocardiographic tape with all of the key views did not ensure that subsequent tapes provided similar image quality.
At best, echocardiographic evaluation at multiple sites remains technically challenging in patients with low ejection fraction, and changes are affected by multiple factors as the pressure gradient between LV and left atria can vary widely, influenced by systemic blood pressure, contractile reserve and diastolic pressure, not solely on the size of the abnormal regurgitant orifice. The ability to index the color jet of mitral regurgitation to left atrial area does provide some improvement in correlation with angiographic evaluation of MR[36]. The measurement of E velocity and deceleration time provided more quantitative evaluation and clearly supported the significance of the changes observed in mitral regurgitation.
It is unfortunate that the data obtained did not allow more quantitative estimation in a larger number of patients. However, the data obtained are consistent with more rigorous prospective data that was obtained previously from several non-randomized experiences in single center investigations, demonstrating significant reduction of mitral regurgitation for therapy guided by a strategy using invasive monitoring. For this larger study in 26 centers, the use of a core lab to perform all measurements blinded to therapy and timepoint increases the confidence in the results obtained.
With all the limitations, this is a unique dataset in which to assess the impact of current HF therapy guided with and without a PAC, as well as the limited influence of acute hemodynamic improvement on outcomes after hospital discharge.
CONCLUSIONS
This study of strategies for decompensated heart failure demonstrates that mitral regurgitation was more effectively reduced when measured right and left-sided filling pressures were used to guide therapy in hospital than when estimated jugular venous pressure and edema were reduced to a similar level during clinical assessment alone. Current management of volume status after discharge may not be adequate to maintain improvements that can be achieved in hospital. The limitations of outpatient therapy may reflect disparity between visible right and occult left-sided filling pressure elevations, difficulty in assessing right-sided filling pressures, and more relaxed goals for volume status in patients who appear stable in the non-acute setting. It is not known whether other strategies would better sustain the acute reduction in mitral regurgitation or whether chronic reduction in mitral regurgitation would translate into improved clinical outcomes with advanced heart failure.
Clinical Summary.
Therapy during heart failure hospitalization focuses on relief of elevated filling pressures, which can currentlybe guided by clinical assessment with or without invasive monitoring. In the randomized ESCAPE study in decompensated chronic dilated heart failure, comparison of the clinical and invasive strategies showed that mitral regurgitation was effectively reduced in hospital only with the invasive strategy, targeting right atrial pressure < 8 mmHg and pulmonary capillary wedge pressure < 15 mmHg, not with the clinical assessment strategy, which targeted and achieved similar reduction of elevated jugular venous pressure, edema, and orthopnea. It has been shown earlier that renal function was also slightly better with the invasive strategy, as was symptomatic improvement in the first month after discharge. However, these differences between strategies were lost by 3 months, during which there was evidence of recurrent increases in filling pressures. As there was no difference in 6 month re-hospitalization or survival between the clinical and invasive strategies, there is currently no rationale for routine use of invasive monitoring to adjust therapy or to reduce mitral regurgitation. The key components of outpatient therapy for advanced heart failure remains optimal tolerated doses of neurohormonal antagonists and diuretic therapy to maintain fluid balance. It is not clear whether future strategies to decrease recurrent heart failure events should select different targets during hospitalization, or develop better strategies to maintain acute reductions in filling pressures and mitral regurgitation during chronic heart failure management.
Acknowledgments
Sources of Funding
The ESCAPE study was supported by contract N01-HV-98177 from the National Heart, Lung, and Blood Institute.
ABBREVIATIONS USED
- CLIN
clinical assessment of filling pressures from symptoms and physical examination and the group of patients randomized to therapy guided by clinical assessment alone
- Decel
deceleration time
- E
early mitral inflow velocity
- JVP
jugular venous pressure
- LAA
left atrial area
- LV
left ventricle
- LVEDD
left ventricular end-diastolic dimension
- LVEDI
left ventricular end-diastolic dimension index
- LVEF
left ventricular ejection fraction
- MR
mitral regurgitation
- PAC
pulmonary artery catheter and the group of patients randomized to therapy guided by a pulmonary artery catheter in addition to clinical assessment
- PCW
pulmonary capillary wedge pressure
- PV S/D
pulmonary vein systolic/diastolic flow ratio
- RAP
right atrial pressures
Footnotes
Conflict of Interest Disclosures
Dr. Stevenson serves as a consultant and has received research support from Medtronics, Inc. The other authors have no conflicts of interest to disclose in relation to this manuscript.
Reference List
- 1.Karagiannis SE, Karatasakis GT, Koutsogiannis N, Athanasopoulos GD, Cokkinos DV. Increased distance between mitral valve coaptation point and mitral annular plane: significance and correlations in patients with heart failure. Heart. 2003;89:1174–1178. doi: 10.1136/heart.89.10.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otsuji Y, Handschumacher MD, Schwammenthal E, Jiang L, Song JK, Guerrero JL, Vlahakes GJ, Levine RA. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997 Sep 16;96:1999–2008. doi: 10.1161/01.cir.96.6.1999. [DOI] [PubMed] [Google Scholar]
- 3.Bruch C, Klem I, Breithardt G, Wichter T, Gradaus R. Diagnostic usefulness and prognostic implications of the mitral E/E' ratio in patients with heart failure and severe secondary mitral regurgitation. Am J Cardiol. 2007 Sep 1;100:860–865. doi: 10.1016/j.amjcard.2007.03.108. [DOI] [PubMed] [Google Scholar]
- 4.Okura H, Takada Y, Kubo T, Asawa K, Taguchi H, Toda I, Yoshiyama M, Yoshikawa J, Yoshida K. Functional mitral regurgitation predicts prognosis independent of left ventricular systolic and diastolic indices in patients with ischemic heart disease. J Am Soc Echocardiogr. 2008;21:355–360. doi: 10.1016/j.echo.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Amigoni M, Meris A, Thune JJ, Mangalat D, Skali H, Bourgoun M, Warnica JW, Barvik S, Arnold JM, Velazquez EJ, Van de WF, Ghali J, McMurray JJ, Kober L, Pfeffer MA, Solomon SD. Mitral regurgitation in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both:prognostic significance and relation to ventricular size and function. Eur Heart J. 2007;28:326–333. doi: 10.1093/eurheartj/ehl464. [DOI] [PubMed] [Google Scholar]
- 6.Cioffi G, Tarantini L, De FS, Pulignano G, Del SD, Stefenelli C, Di LA, Opasich C. Functional mitral regurgitation predicts 1-year mortality in elderly patients with systolic chronic heart failure. Eur J Heart Fail. 2005;7:1112–1117. doi: 10.1016/j.ejheart.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton MA, Stevenson LW, Child JS, Moriguchi JD, Walden J, Woo M. Sustained reduction in valvular regurgitation and atrial volumes with tailored vasodilator therapy in advanced congestive heart failure secondary to dilated (ischemic or idiopathic) cardiomyopathy. Am J Cardiol. 1991 Feb 1;67:259–263. doi: 10.1016/0002-9149(91)90556-z. [DOI] [PubMed] [Google Scholar]
- 8.Weiland DS, Konstam MA, Salem DN, Martin TT, Cohen SR, Zile MR, Das D. Contribution of reduced mitral regurgitant volume to vasodilator effect in severe left ventricular failure secondary to coronary artery disease or idiopathic dilated cardiomyopathy. Am J Cardiol. 1986 Nov 1;58:1046–1050. doi: 10.1016/s0002-9149(86)80036-4. [DOI] [PubMed] [Google Scholar]
- 9.Rosario LB, Stevenson LW, Solomon SD, Lee RT, Reimold SC. The mechanism of decrease in dynamic mitral regurgitation during heart failure treatment: importance of reduction in the regurgitant orifice size. J Am Coll Cardiol. 1998;32:1819–1824. doi: 10.1016/s0735-1097(98)00461-6. [DOI] [PubMed] [Google Scholar]
- 10.Drazner MH, Hellkamp AS, Leier CV, Shah MR, Miller LW, Russell SD, Young JB, Califf RM, Nohria A. Value of Clinician Assessment of Hemodynamics in Advanced Heart Failure: The ESCAPE Trial. Circ Heart Fail. 2008;1:170–177. doi: 10.1161/CIRCHEARTFAILURE.108.769778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ESCAPE Investigators and Coordinators: Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005 Oct 5;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 12.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005 Sep 20;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Filippatos G. Reassessing treatment of acute heart failure syndromes: the ADHERE Registry. Eur Heart J. 2005;7:B13–B19. [Google Scholar]
- 14.Drazner MH, Hamilton MA, Fonarow G, Creaser J, Flavell C, Stevenson LW. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant. 1999;18:1126–1132. doi: 10.1016/s1053-2498(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 15.McGee SR. Physical examination of venous pressure: a critical review. Am Heart J. 1998;136:10–18. doi: 10.1016/s0002-8703(98)70175-9. [DOI] [PubMed] [Google Scholar]
- 16.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008 Apr 1;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson LW, Bellil D, Grover-McKay M, Brunken RC, Schwaiger M, Tillisch JH, Schelbert HR. Effects of afterload reduction (diuretics and vasodilators) on left ventricular volume and mitral regurgitation in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1987 Sep 15;60:654–658. doi: 10.1016/0002-9149(87)90376-6. [DOI] [PubMed] [Google Scholar]
- 18.Strauss RH, Stevenson LW, Dadourian BA, Child JS. Predictability of mitral regurgitation detected by Doppler echocardiography in patients referred for cardiac transplantation. Am J Cardiol. 1987 Apr 1;59:892–894. doi: 10.1016/0002-9149(87)91114-3. [DOI] [PubMed] [Google Scholar]
- 19.Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez-Sendon JL, Ponikowski P, Tavazzi L. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 20.Stevenson LW, Tillisch JH. Maintenance of cardiac output with normal filling pressures in patients with dilated heart failure. Circulation. 1986;74:1303–1308. doi: 10.1161/01.cir.74.6.1303. [DOI] [PubMed] [Google Scholar]
- 21.Guiha NH, Cohn JN, Mikulic E, Franciosa JA, Limas CJ. Treatment of refractory heart failure with infusion of nitroprusside. N Engl J Med. 1974;291:587–592. doi: 10.1056/NEJM197409192911201. [DOI] [PubMed] [Google Scholar]
- 22.Ozdemir K, Altunkeser BB, Sokmen G, Tokac M, Gok H. Usefulness of peak mitral inflow velocity to predict severe mitral regurgitation in patients with normal or impaired left ventricular systolic function. Am Heart J. 2001;142:1065–1071. doi: 10.1067/mhj.2001.118465. [DOI] [PubMed] [Google Scholar]
- 23.Thomas L, Foster E, Schiller NB. Peak mitral inflow velocity predicts mitral regurgitation severity. J Am Coll Cardiol. 1998;31:174–179. doi: 10.1016/s0735-1097(97)00454-3. [DOI] [PubMed] [Google Scholar]
- 24.Temporelli PL, Scapellato F, Corra U, Eleuteri E, Firstenberg MS, Thomas JD, Giannuzzi P. Chronic mitral regurgitation and Doppler estimation of left ventricular filling pressures in patients with heart failure. J Am Soc Echocardiogr. 2001;14:1094–1099. doi: 10.1067/mje.2001.114846. [DOI] [PubMed] [Google Scholar]
- 25.Feinberg MS, Schwammenthal E, Shlizerman L, Porter A, Hod H, Friemark D, Matezky S, Boyko V, Mandelzweig L, Vered Z, Behar S, Sagie A. Prognostic significance of mild mitral regurgitation by color Doppler echocardiography in acute myocardial infarction. Am J Cardiol. 2000 Nov 1;86:903–907. doi: 10.1016/s0002-9149(00)01119-x. [DOI] [PubMed] [Google Scholar]
- 26.Wu AH, Aaronson KD, Bolling SF, Pagani FD, Welch K, Koelling TM. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol. 2005 Feb 1;45:381–387. doi: 10.1016/j.jacc.2004.09.073. [DOI] [PubMed] [Google Scholar]
- 27.Magne J, Senechal M, Mathieu P, Dumesnil JG, Dagenais F, Pibarot P. Restrictive annuloplasty for ischemic mitral regurgitation may induce functional mitral stenosis. J Am Coll Cardiol. 2008 Apr 29;51:1692–1701. doi: 10.1016/j.jacc.2007.11.082. [DOI] [PubMed] [Google Scholar]
- 28.Yong Y, Nagueh SF, Shimoni S, Shan K, He ZX, Reardon MJ, Letsou GV, Howell JF, Verani MS, Quinones MA, Zoghbi WA. Deceleration time in ischemic cardiomyopathy: relation to echocardiographic and scintigraphic indices of myocardial viability and functional recovery after revascularization. Circulation. 2001 Mar 6;103:1232–1237. doi: 10.1161/01.cir.103.9.1232. [DOI] [PubMed] [Google Scholar]
- 29.Temporelli PL, Corra U, Imparato A, Bosimini E, Scapellato F, Giannuzzi P. Reversible restrictive left ventricular diastolic filling with optimized oral therapy predicts a more favorable prognosis in patients with chronic heart failure. J Am Coll Cardiol. 1998;31:1591–1597. doi: 10.1016/s0735-1097(98)00165-x. [DOI] [PubMed] [Google Scholar]
- 30.Pozzoli M, Traversi E, Cioffi G, Stenner R, Sanarico M, Tavazzi L. Loading manipulations improve the prognostic value of Doppler evaluation of mitral flow in patients with chronic heart failure. Circulation. 1997 Mar 4;95:1222–1230. doi: 10.1161/01.cir.95.5.1222. [DOI] [PubMed] [Google Scholar]
- 31.Giannuzzi P, Temporelli PL, Bosimini E, Silva P, Imparato A, Corra U, Galli M, Giordano A. Independent and incremental prognostic value of Doppler-derived mitral deceleration time of early filling in both symptomatic and asymptomatic patients with left ventricular dysfunction. J Am Coll Cardiol. 1996;28:383–390. doi: 10.1016/0735-1097(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 32.Xie GY, Berk MR, Smith MD, Gurley JC, DeMaria AN. Prognostic value of Doppler transmitral flow patterns in patients with congestive heart failure. J Am Coll Cardiol. 1994;24:132–139. doi: 10.1016/0735-1097(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 33.Grayburn PA, Appleton CP, DeMaria AN, Greenberg B, Lowes B, Oh J, Plehn JF, Rahko P, St John SM, Eichhorn EJ. Echocardiographic predictors of morbidity and mortality in patients with advanced heart failure: the Beta-blocker Evaluation of Survival Trial (BEST) J Am Coll Cardiol. 2005 Apr 5;45:1064–1071. doi: 10.1016/j.jacc.2004.12.069. [DOI] [PubMed] [Google Scholar]
- 34.Shah MR, Flavell CM, Weintraub JR, Young MA, Hasselblad V, Fang JC, Nohria A, Lewis EF, Givertz MM, Mudge G, Jr, Stevenson LW. Intensity and focus of heart failure disease management after hospital discharge. Am Heart J. 2005;149:715–721. doi: 10.1016/j.ahj.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 35.Zile MR, Bennett TD, St John SM, Cho YK, Adamson PB, Aaron MF, Aranda JM, Jr, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008 Sep 30;118:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 36.Spain MG, Smith MD, Grayburn PA, Harlamert EA, DeMaria AN. Quantitative assessment of mitral regurgitation by Doppler color flow imaging: angiographic and hemodynamic correlations. J Am Coll Cardiol. 1989 Mar 1;13:585–590. doi: 10.1016/0735-1097(89)90597-4. [DOI] [PubMed] [Google Scholar]