Abstract
Effector cells derived from central memory CD8+ T cells were reported to engraft and survive better than those derived from effector memory populations, suggesting that they are superior for use in adoptive immunotherapy studies. However, previous studies did not evaluate the relative efficacy of effector cells derived from naïve T cells. We sought to investigate the efficacy of tumor-specific effector cells derived from naïve or central memory T-cell subsets using transgenic or retrovirally transduced T cells engineered to express a tumor-specific T-cell receptor. We found that naïve, rather than central memory T cells, gave rise to an effector population that mediated superior antitumor immunity upon adoptive transfer. Effector cells developed from naïve T cells lost the expression of CD62L more rapidly than those derived from central memory T cells, but did not acquire the expression of KLRG-1, a marker for terminal differentiation and replicative senescence. Consistent with this KLRG-1− phenotype, naïve-derived cells were capable of a greater proliferative burst and had enhanced cytokine production after adoptive transfer. These results indicate that insertion of genes that confer antitumor specificity into naïve rather than central memory CD8+ T cells may allow superior efficacy upon adoptive transfer.
Infusion of tumor-reactive T cells to treat cancer is transitioning from a promising possibility to a successful reality. Adoptive immunotherapy with T cells can effectively treat patients with EBV-associated malignancies and metastatic melanoma, and application of this treatment is broadening as our ability to generate T cells targeting diverse tumor antigens improves (1–10). Our expanding capacity to target novel antigens is driven, in part, by advances in genetic engineering that permit high efficiency transfer of genes encoding tumor specific T-cell receptors (TCR) into open repertoire mature T cells. These genetically modified T cells can specifically recognize tumor cells in vitro, and can induce objective tumor regression following infusion into patients (9).
The ability to engineer tumor recognition permits not only targeting of any antigen for which a specific TCR can be identified, but also selection of the CD8+ T-cell subset from which the cells for therapy will be generated. Resting CD8+ T cells exist as naïve (TN), central memory (TCM), and effector memory (TEM) populations, each with distinct phenotypic and functional characteristics (11). In vitro stimulation of these subsets induces their proliferation and differentiation into the cytolytic effector cells (TEFF) used for patient treatment. While the nature of CD8+ T-cell subsets is well defined (12), the heritable influence of those populations on the traits of their effector cell progeny is not well studied (13, 14). Understanding this relationship might be important for generating optimal effector cells for patient treatment.
The characteristics of CD8+ T-cell subsets have been elucidated primarily through study of viral infection (15–17). In this setting, memory cells are superior to naïve cells due to their increased precursor frequency (18), their rapid proliferation and their efficient acquisition of effector functions (12). However, these qualities might not be advantageous for adoptive immunotherapy where the precursor frequency is determined by the number of cells infused, and differentiation into effector cells occurs before cell infusion. Indeed, recent studies intimate this possibility; in nonhuman primates, induction of effector memory cells has been uniquely successful in protecting from simian immunodeficiency virus (19) yet, in another macaque study, adoptively transferred effector cells generated from effector memory cells rapidly perished (20).
Previous studies on the influence of CD8+ T cell differentiation states have not focused on the relative efficacy of naïve T cells (20–23). With the emergence of TCR gene therapy, naïve cells, which represent the most common CD8+ T-cell phenotype in many patients, have become an important potential source of effector cells. Herein we investigate the lineage relationship and therapeutic efficacy of effector cells of naïve or central memory origin, and we report the superior efficacy of effector cells derived directly from naïve T cells for adoptive immunotherapy of cancer.
Results
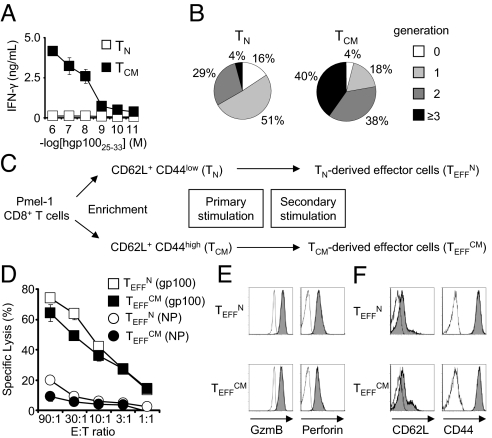
We used the pmel-1 TCR transgenic model of adoptive immunotherapy to study the development, function, and efficacy of tumor specific effector cells differentiated from naïve or central memory progenitors. This model reproduces the clinical challenge of breaking tolerance to a shared tumor/self antigen to induce regression of large, established tumors (24). Tumor-specific CD8+ T-cell populations enriched for TN or TCM phenotype cells were isolated from pmel-1 splenocytes (Fig. S1). These cells displayed not only the phenotypic but also the functional qualities ascribed to naïve and central memory cells as, in response to antigenic stimulation, IFN-γ production and proliferation were more efficient in the TCM subset (Fig. 1 A and B) (12). Emulating clinical protocol, effector CD8+ T cells were generated from each population by two stimulations (Fig. 1C) (9). For simplicity, effector cells of naïve or central memory origin were termed TEFFN and TEFFCM, respectively.
Fig. 1.
Effector CD8+ T cells derived from naïve or central memory cells acquire cytolytic phenotype and function. (A) Production of IFN-γ by freshly isolated cells in an overnight coculture assay. The error bars represent the standard error of the mean. (B) The number of cell divisions 2 days after peptide stimulation, as determined by CFSE dilution from a FACS histogram. (C) Schematic delineating generation of primary and secondary effector cells from naïve and central memory CD8+ T cells. (D) Cytolytic function of TEFFN and TEFFCM as determined by 51Cr release assay. The target peptide, gp10025–33 or NP366–374 is indicated in parenthesis. (E) Flow cytometric analysis indicating expression of granzyme B and perforin and (F) L-selectin and CD44. The open histograms indicate isotype antibody controls. All figures shown are representative of at least two independent experiments.
Effector Cells Generated from Naïve or Central Memory Cells Acquire Cytolytic Effector Cell Phenotype and Function.
Both TEFFN and TEFFCM demonstrated high levels of specific target killing consistent with effector CD8+ T-cell function (Fig. 1D). They also expressed the cytolytic granule proteins that typify effector cells, perforin 1, and granzyme (Fig. 1E). To characterize further their differentiation states, we determined expression of CD62L and CD44, the phenotypic markers by which the progenitor cell subsets were isolated. Both TEFFN and TEFFCM predominantly displayed the CD62L− CD44high phenotype of effector cells (Fig. 1F) (25). Taken together, these data indicated that effector cells were generated from both the TN and TCM populations.
Effector CD8+ T Cells of Naïve or Central Memory Origin Possess Distinct Gene Expression Signatures and Developmental Programs.
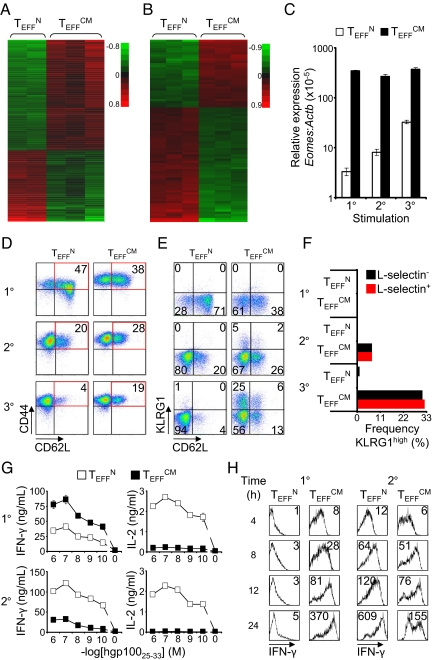
We sought to determine if, despite acquisition of similar effector phenotype and function, TEFFN and TEFFCM retained distinct gene expression signatures. Oligonucleotide microarrays were used to study the two effector cell groups in the resting condition and 4 h after restimulation. A false discovery rate (FDR) of 2% and 2-fold or greater difference identified 1,020 and 86 probe sets that were differentially expressed between TEFFN and TEFFCM in rested and restimulated cells, respectively (Fig. 2 A and B). An annotated list of selected genes with reported function in T cells and greater than 5-fold differential expression is shown in Table S1. Real time RT-PCR validation of selected differentially expressed genes is displayed in Fig. S2. These microarray data revealed unique gene expression signatures of effector cells of naïve or central memory origin. Thus, TN and TCM appeared to confer distinct programs to their effector cell progeny.
Fig. 2.
Naïve and central memory cells confer distinct genetic signatures and developmental programs to their effector cell progeny. Microarray analysis of the gene expression of (A) rested and (B) stimulated TEFFN and TEFFCM. Probe sets with FDR ≤2% and ≥2-fold differences in expression are shown. Red and green colors indicate increased and decreased expression respectively. The scales are log10. (C) Relative expression of Eomes by TEFFN or TEFFCM as determined by real time RT-PCR. The x axis indicates the number of times the cells were stimulated with antigen and IL-2. Error bars represent the standard error of the mean. (D) Flow cytometric determination of the expression of CD62L and CD44 by TEFFN and TEFFCM through serial stimulations. The numbers on the dot plots indicate the frequency of cells with central memory (CD62L+ CD44high) phenotype. (E) Expression of CD62L and KLRG1. Quadrant frequencies are shown. (F) Graph derived from panel E. Frequencies of cells from the CD62L+ or CD62L− fraction of TEFFN or TEFFCM with KLRG1high phenotype. (G) IFN-γ and IL-2 production, in coculture assays, by effector cells generated by primary or secondary stimulation of naïve or central memory cells. (H) Intracellular IFN-γ expression following peptide stimulation of TEFFN or TEFFCM. The mean fluorescence intensities are indicated. All figures shown are representative of at least two independent experiments.
To better understand the effector cell programs and lineage relationship of TEFFN and TEFFCM we studied changes in gene expression, phenotype, and function induced by antigen- and IL-2-driven differentiation. The T-box transcription factors T-bet and Eomesodermin (Eomes) control cytolytic development and function of CD8+ T cells (26). T-bet (encoded by Tbx21) was less than 2-fold differentially expressed in TEFFN and TEFFCM on microarray and real time RT-PCR. However, Eomes was the most highly overexpressed transcription factor in TEFFCM on microarray for both resting and stimulated cells. We examined expression of Eomes as effector differentiation was induced by serial stimulation with antigen and IL-2. Eomes mRNA transcripts were approximately 100-fold greater in TEFFCM than TEFFN following primary stimulation (Fig. 2C). The relatively high Eomes mRNA in TEFFCM was maintained through secondary and tertiary stimulation. Eomes expression by TEFFN increased with each stimulation, but did not attain the level observed in TEFFCM. Thus, effector cells of TN or TCM origin acquired and maintained different levels of Eomes expression despite repeated stimulation with the same developmental cues. These patterns of Eomes expression suggested differences in the development and functional programs that were conferred by TN or TCM.
To further study the lineage relationship between TEFFN and TEFFCM, we examined expression of CD62L, the adhesion molecule that distinguishes the central memory from the effector memory lineage (Fig. 2D). Following primary stimulation, TEFFN displayed greater frequency of CD62L+ CD44high cells than TEFFCM. However, this phenotype was transient as secondary and tertiary stimulation induced loss of CD62L. In contrast, TEFFCM maintained a more stable, albeit diminishing, subset of CD62L+ CD44high cells suggesting that expression of CD62L is a TCM lineage-specific trait that is not entirely lost with effector differentiation.
Because CD62L expression decreases with progressive differentiation of effector CD8+ T cells (25, 27), we hypothesized that the CD62L+ subpopulation of TEFFCM was less prone to terminal differentiation than the CD62L− subpopulation. To test for terminal differentiation of these subsets, we examined simultaneous expression of CD62L and killer cell lectin-like receptor subfamily G, member 1 (KLRG1), an inhibitory receptor that is expressed by senescent T cells (Fig. 2E) (28, 29). Serial stimulation did not induce KLRG1 expression in TEFFN. However, TEFFCM developed a subset of KLRG1high cells, suggesting a greater propensity to terminal maturation. Remarkably, the frequency of KLRG1high cells was equivalent in the CD62L− and CD62L+ subsets indicating that the CD62L+ subset was not protected from developing a senescent phenotype (Fig. 2F). This finding defies models of effector cell differentiation in which the CD62L+ CD44high TCM phenotype is mutually exclusive with the KLRG1high senescent TEFF phenotype (3, 13). These data suggest, rather, that effector cells of TCM origin might be a distinct lineage that can maintain the lineage-specific trait of CD62L expression even as they differentiate into senescent effector cells.
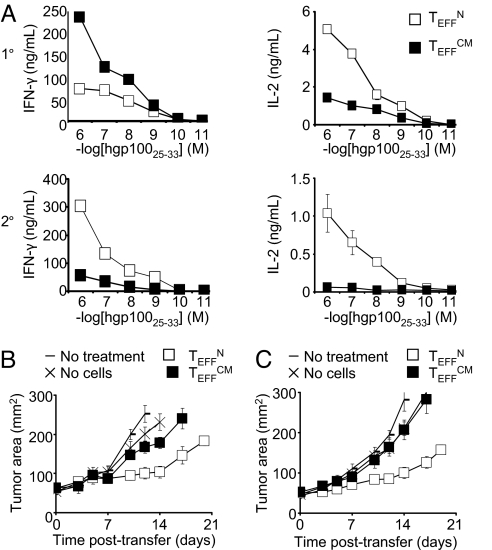
We further characterized TEFFN and TEFFCM by examining their secretion of cytokines. Effector CD8+ T cells gain the ability to elaborate IFN-γ but lose the capacity to produce IL-2 as they progressively differentiate (3, 13, 25, 30, 31). Following primary stimulation, TEFFCM were capable of greater IFN-γ and lesser IL-2 production than TEFFN, which suggested that TEFFCM were more differentiated (Fig. 2G). Remarkably, following secondary stimulation TEFFN elaborated greater quantities of both IFN-γ and IL-2 (Fig. 2G). The reversal between primary and secondary stimulation in the effector cells that produced greater IFN-γ was confirmed by flow cytometry (Fig. 2H). The enhanced production of both IFN-γ and IL-2 by TEFFN was somewhat paradoxical, but it might be explained by relative cellular senescence of TEFFCM. Taken together with their tendency to develop a KLRG1high phenotype, the data suggest that TEFFCM terminally differentiate and senesce more rapidly than TEFFN.
Effector CD8+ T Cells Generated Directly from Naïve Cells Mediate More Potent Antitumor Activity.
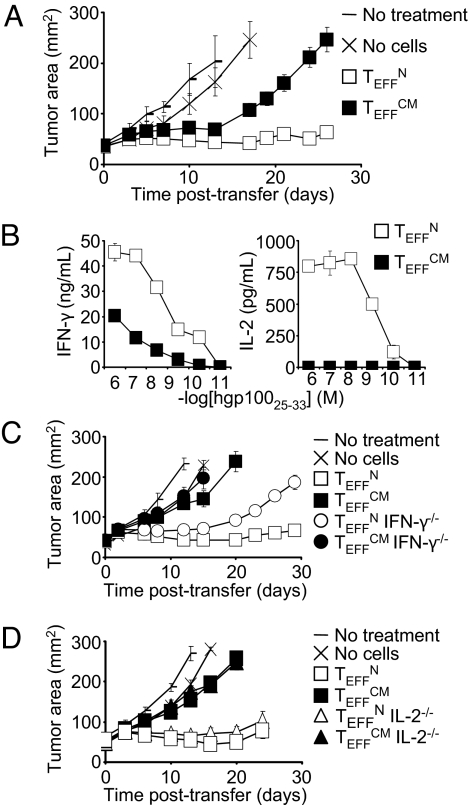
We tested the efficacy of effector cells derived from TN or TCM in tumor treatment. TEFFN and TEFFCM generated from a primary stimulation were adoptively transferred into nonlethally irradiated mice bearing established, vascularized tumors. Specific vaccination and IL-2 were coadministered to all treated mice. Both TEFFN and TEFFCM displayed significant antitumor activity. However, TEFFN were significantly more effective than TEFFCM (Fig. 3A). Greater efficacy of TEFFN was also observed with adoptive transfer of effector cells generated from a secondary stimulation (Fig. S3). However, it should be noted that higher numbers of secondary stimulation TEFFN were required to achieve antitumor activity similar to that seen with primary stimulation. These data are consistent with our previous findings indicating that T cells lose in vivo function with repeated in vitro stimulation (25).
Fig. 3.
Use of effector cells derived from naïve rather than central memory cells improves adoptive cell transfer therapy. (A) Tumor responses following treatment with 4 × 106 effector cells generated from naïve or central memory subsets. All groups except “No treatment” received adjuvant vaccine and IL-2. TEFFN versus TEFFCM, P < 0.01. (B) Production of IFN-γ and IL-2 by adoptively transferred cells reisolated from spleens 6 days following infusion. Cell numbers were equalized for the assay. (C) Tumor treatment with 7.65 × 105 wild-type or IFN-γ−/− effector cells. TEFFN versus IFN-γ−/− TEFFN, P < 0.05. (D) Tumor treatment with 1.05 × 106 wild-type or IL-2−/− effector cells. TEFFN versus IL-2−/− TEFFN, P > 0.05. All figures shown are representative of at least two independent experiments.
We next studied the mechanisms underlying the differences in efficacy of TEFFN and TEFFCM. We reisolated cells from recipient mice 6 days following infusion and tested their capacity to elaborate IFN-γ and IL-2. TEFFN produced greater quantities of both cytokines (Fig. 3B). Furthermore, TEFFCM appeared to lose the ability to produce IL-2 following infusion, as they secreted no detectable IL-2 in this assay (Fig. 3B). Results were similar with adoptive transfer of cells generated from secondary stimulation (Fig. S4).
To test the importance of IFN-γ and IL-2 production by effector cells, we treated tumor bearing mice with cells deficient in these cytokines. IFN-γ−/− TEFFN but not IL-2−/− TEFFN displayed impaired antitumor responses (Fig. 3 C and D). Thus, IFN-γ was a crucial effector cytokine. However, IL-2 appeared to mark effective cells, rather than mediate the antitumor response. The finding that IL-2 deficiency did not impair the function of effector cells was counterintuitive considering the capacity of IL-2 to potentiate T cell-based adoptive immunotherapy. However, this result was consistent with other work showing no benefit to overexpression of IL-2 by adoptively transferred T cells (32).
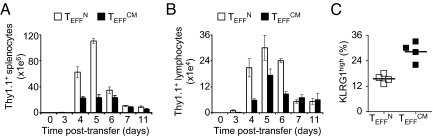
We next tested the capacity of the effector cells to expand following adoptive transfer. TEFFN displayed greater clonal expansion in both the spleen and the tumor draining lymph nodes (Fig. 4 A and B). Consistent with this enhanced expansion, TEFFN less frequently exhibited the KLRG1high phenotype of cells with limited proliferative potential (Fig. 4C). These findings were reproduced with adoptive transfer of effector cells generated from two simulations (Fig. S5). Taken together these data indicate the therapeutic superiority of effector cells generated from naïve cells, and they demonstrate greater capacity for cytokine production and expansion of these cells following infusion.
Fig. 4.
Effector cells derived directly from naïve cells possess greater potential for clonal expansion following infusion. (A and B) The number of cells present in spleens and lymph nodes, respectively, following adoptive transfer of TEFFN or TEFFCM. Error bars indicate the standard error of the mean. (n = 4 for each group and each time point). (C) The frequency of transferred cells in recipient spleens displaying KLRG1high phenotype 5 days following infusion. The transverse lines represent the means. P = 0.0024. All figures shown are representative of at least two independent experiments.
Application to Genetically Engineered Open Repertoire CD8+ T Cells.
Pmel-1 TCR-transgenic T cells develop in mice with a genetically restricted repertoire of self-specific precursors. The naturally occurring subsets of naïve and central memory cells in these mice might be artifactually induced by interactions with self-antigen or by differences in assembly and expression of the TCR. We sought to test if the principles elucidated in transgenic cells also applied to wild-type cells that developed with a full TCR repertoire. TN or TCM wild-type CD8+ T cells were stimulated and transduced with a retroviral vector encoding the pmel-1 TCR. This transduction model emulates clinical therapies, and permits study of T-cell subsets that develop in physiological conditions. Paralleling results with transgenic cells, TEFFN produced less IFN-γ following the primary stimulation, but more IFN-γ following the second stimulation. Also consistent with the transgenic cells, TEFFN produced more IL-2 after both stimulations (Fig. 5A). These data supported the similarity of transgenic cells to transduced wild-type cells, and they again demonstrated that as TEFFN differentiated they exceeded TEFFCM in production of both IFN-γ and IL-2.
Fig. 5.
Genetically engineered open repertoire cells reproduce the central findings of the transgenic mouse model. (A) Elaboration of IFN-γ and IL-2 by TCR transduced naïve- or central memory-derived effector cells. (B) Tumor growth following treatment with transduced effector cells generated by 4 × 106 primary or (C) 2 × 107 secondary stimulation of naïve or central memory cells. All figures shown are representative of at least two independent experiments.
The efficacy of transduced TEFFN and TEFFCM was studied by infusion of cells into tumor bearing mice. Expression of the TCR is weaker with gene transduction than with transgenic animals, and tumor therapy is less robust when transduced cells are used. Nevertheless, consistent with findings from transgenic cells, antitumor immunity was greater with infusion of TCR tranduced TEFFN (Fig. 5B). This finding was reproduced by effector cells generated from secondary stimulation (Fig. 5C). These data validated work in the transgenic mouse model, and they again indicated that the distinct program conferred by naïve progenitors to their effector cell progeny directed development of more effective cells for adoptive immunotherapy.
Discussion
Studies of viral infection have illuminated the distinct traits and niche roles of CD8+ T-cell subsets in physiological immunity. Adoptive immunotherapy is a nonphysiological setting in which lessons from the study of antiviral responses might not apply. In fact, the advantage of adoptive immunotherapy is its capacity to distort normal immunity by ablating the host immune system before cell infusion, administering massive numbers of antigen-specific T cells, and further activating transferred cells with immune adjuvants (1, 3, 13, 33). We found that the efficient physiological responses of central memory cells were not advantageous to their adoptively transferred effector cell progeny. Rather, effector cells derived from central memory cells rapidly differentiated, expediting senescence and resulting in diminished function following infusion. In contrast, effector cells of naïve origin displayed sustained effector development, with prolonged cytokine production, increased in vivo expansion, and, ultimately, more effective antitumor responses.
These data, showing effector cells with distinct traits determined by progenitor subsets, add to the increasing evidence that effector CD8+ T cells are not a homogenous population but rather an amalgamation of subsets with diverse functions and fates. CD8+ T cells can acquire Th1-, Th2-, Treg-, and Th17-type effector functions, which impact their abilities to combat infections and to mediate tumor regression (13, 34–36). Furthermore, effector cell subsets fated for either rapid extinction or for long-term survival as central memory or effector memory cells have been identified (37, 38). The effector cell subset destined for long-term survival tends to express IL-7Rα and lack expression of KLRG1 (15, 37). KLRG1, which identifies senescent effector cells with diminished replicative capacity, impairs proliferation through defective AKT (Ser-473) phosphorylation (28). In our studies, TEFFCM but not TEFFN acquired KLRG1 expression in vitro and in vivo reflecting a greater tendency of this effector cell lineage to develop phenotypic senescence and suggesting a mechanism for the diminished expansion of these cells that occurred following infusion. These findings further imply that the fate of short- or long-lived effector cells might be linked to their origin as naïve or memory T cells and underscore that the prevention of terminal differentiation, which can be accomplished using IL-21 or Wnt, is desirable in the generation of T-cell populations for adoptive immunotherapy (21, 31).
Naïve or memory progenitors developed into effector cells with distinct qualities, despite repeated stimulation with the same developmental cues. These differences could be due to development along separate pathways or to different positions along a common pathway. The tendency of both subsets to acquire Eomes, lose CD62L, and lose IL-2-production intimates that both subsets share a common developmental destination. However, the predilection of TEFFCM to retain a subset of CD62L positive cells indicates that effector cells could maintain certain lineage-specific traits. Furthermore, acquisition of KLRG1 by the CD62L+ subset of TEFFCM suggests that this lineage-specific trait was preserved even in terminal differentiation. Although these findings were from in vitro rather than in vivo studies, they support a model of separate but converging differentiation pathways for development of effector cells derived from naïve or central memory T cells. Thus, the present manuscript expands the scope of previous studies that did not distinguish between effector cells of naïve or central memory origin (3, 12, 27).
A report from study of nonhuman primates demonstrated that effector cells of central, rather than effector memory origin were capable of prolonged survival following infusion (20). However, naïve-derived effector cells were not studied, and a therapeutic endpoint was not used. Consistent with that work, we found that central memory-derived effector cells could indeed persist following adoptive transfer (Fig. S6), but that effector cells generated directly from naïve T cells were superior upon adoptive transfer for proliferation, cytokine release, and tumor treatment efficacy.
The clinical implication of this work is that the efficacy of adoptive immunotherapy might be improved by generating effector cells for therapy from naïve cells. Naïve viral antigen-specific T cells in cord blood can be expanded for adoptive transfer (39). Furthermore, in preliminary studies of melanoma patients, naïve cells (CD62L+ CD45RO−) represent 20 to 60% of the CD8+ T cells in leukapheresis samples and can be readily transduced with TCR-encoding vectors and expanded. The technique for isolating naïve cells for the treatment of patients with cancer in a Good Manufacturing Practices-compliant manner must be developed. However, this report lays the scientific groundwork for further study in human tissues, and it demonstrates the importance of selecting the optimal precursor cells for generation of effector cells for adoptive immunotherapy.
Methods
Mice and Cell Lines.
Pmel-1 Thy1.1 (24), pmel-1 Thy1.1 IFNγ−/− (40), pmel-1 Thy1.1 IL-2−/−, and C57BL/6 mice (The Jackson Laboratory) were bred and housed according to the guidelines of the Animal Care and Use Committee at the National Institutes of Health. Genotypes were confirmed by PCR analysis using The Jackson Laboratory protocols. B16 and MCA205 were obtained from the NCI Tumor Repository and grown in culture media.
CD8+ T-Cell Culture.
CD8+ T cells were isolated from splenocytes by magnetic bead negative selection (Miltenyi Biotec or Stemcell Technologies). CD44high and CD44low cells were separated using biotinylated anti-CD44 antibodies (BD Biosciences) and anti-biotin magnetic beads (Miltenyi Biotec) or by fluorescence activated cell sorting (FACS) on the basis of CD62L and CD44 expression. For experiments using IL-2−/− or IFN-γ−/− cells, only FACS sorting was used. Primary stimulation was accomplished using plate-bound anti-CD3 (2 μg/mL) and soluble anti-CD28 (1 μg/mL) (BD Biosciences). Cells were expanded in culture media (24) containing 30 to 60 IU IL-2 (Novartis) for 6–7 days. Secondary and tertiary stimulations were with irradiated splenocytes pulsed with hgp10025–33 (1 μM). Thus, the first ex vivo stimulation was always with CD3/CD28, and the second and third with peptide and IL-2.
CD8+ T-Cell Transduction.
Two days following initiation of stimulation as described above, cells were transduced with a MSGV1 retroviral vector with a codon optimized sequence encoding the pmel-1 TCR α- and β-chains linked by an internal ribosome entry site (41). Retrovirus was produced by transient transfection using the PlatE packaging line (Cell Biolabs Inc.) with cotransfection of the pCL-Eco (Imgenex) plasmid. A single transduction was performed using spinoculation on retronectin-coated plates.
In Vitro Assays.
Flow cytometry was performed by labeling cells with fluorescent antibodies specific for Thy1.1, CD62L, CD44, CD8 (all from BD Biosciences), KLRG1, granzyme B, or perforin (all from eBioscience). Intracellular staining was performed per manufacturer protocol (BD Biosciences). Carboxyfluorescein succinimidyl ester (CFSE) labeling was per manufacturer protocol (Invitrogen). Cytokine quantities were determined by ELISA (R&D Systems). 51Chromium release assays were performed as described (25). Real-time PCR was conducted using commercially available primer/probe sets (Applied Biosystems).
Oligonucleotide Microarrays.
Total RNA was isolated and processed using Expression 3′ amplification One-cycle Target Labeling and Control Reagents kit according to the manufacturer's directions (Affymetrix). Hybridization was performed according to the manufacturer's protocol. Laser detection (Affymetrix Scanner 3000 7G) was performed and signal intensities quantified (Affymetrix Genechip Command Console).
Microarray Analysis.
Gene expression was quantitated with the Affymetrix Expression Console (Affymetrix). Signal values from the Expression Console software were normalized with an adaptive variance-stabilizing, quantile-normalizing transformation (P. J. Munson, GeneLogic Workshop of Low Level Analysis of Affymetrix GeneChip Data, 2001, software available at http://abs.cit.nih.gov/geneexpression.html). The transform, termed S10, is scaled to match the logarithm transform (base 10).
Differential gene expression was assessed by analysis of variance (ANOVA). A one-factor ANOVA of four treatment levels was performed, followed by post hoc tests comparing naïve to memory cell populations in the unstimulated and stimulated states. A fold change of at least 2 and a false discovery rate of 2% (42) was required to declare a probeset differentially expressed. The array data were deposited in Gene Expression Omnibus (GEO), accession number GSE16522.
Adoptive Cell Transfer.
B16 melanoma tumors were initiated by s.c. injection of 5 × 105 cells. Cell transfer treatment occurred 7–9 days following tumor cell injection. Mice were pretreated with a conditioning regimen of 500 or 600 cGy total body irradiation the day of or the day before cell transfer. Cells were administered with 2 × 107 plaque forming units of recombinant vaccinia virus encoding gp100 and with 600,000 IU rhIL-2 (Novartis) given twice a day for a total of five or six doses (24). The number of cells administered ranged from 8 × 105 to 4 × 106 in primary stimulation experiments and from 1 × 107 to 4 × 107 in secondary stimulation experiments. Within any given experiment and for any head-to-head comparison, the number of T cells in each subset is normalized for the two groups. Greater numbers of secondary stimulation cells were required due to the loss of efficacy in vivo that accompanies repeated stimulation in vitro (25). Serial tumor measurements were performed by an investigator blinded to the treatment groups. Each treatment group consisted of five mice. Error bars reflect the standard error of the mean.
Statistics.
Tumor curves were assessed by one-way Analysis of Variance (ANOVA) with a Bonferroni multiple comparisons posttest. Single-measurement comparisons between two groups were tested using unpaired t-tests. Prism GraphPad software (GraphPad Software Inc.) was used for these analyses.
Supplementary Material
Acknowledgments.
The authors thank Lindsay Garvin, Arnold Mixon, Shawn Farid, and W. David Jones for their assistance with this project. We also thank Christopher A. Klebanoff for critically reading the paper and making numerous helpful suggestions. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research and the National Heart Lung and Blood Institute. This study was performed in partial fulfillment of a Ph.D. in Biochemistry (to D.C.P.) at the George Washington University, Washington, DC.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907448106/DCSupplemental.
References
- 1.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Childs R, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343:750–758. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 3.Gattinoni L, Powell DJ, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: Building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu C, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118:1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollard CM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pule MA, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lizee G, Cantu MA, Hwu P. Less yin, more yang: Confronting the barriers to cancer immunotherapy. Clin Cancer Res. 2007;13:5250–5255. doi: 10.1158/1078-0432.CCR-07-1722. [DOI] [PubMed] [Google Scholar]
- 8.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;32:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204–1212. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 12.Kalia V, Sarkar S, Gourley TS, Rouse BT, Ahmed R. Differentiation of memory B and T cells. Curr Opin Immunol. 2006;18:255–264. doi: 10.1016/j.coi.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Hinrichs CS, Gattinoni L, Restifo NP. Programming CD8+ T cells for effective immunotherapy. Curr Opin Immunol. 2006;18:363–370. doi: 10.1016/j.coi.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger C, Turtle CJ, Jensen MC, Riddell SR. Adoptive transfer of virus-specific and tumor-specific T cell immunity. Curr Opin Immunol. 2009;21:224–232. doi: 10.1016/j.coi.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- 17.MacLeod MKL, Clambey ET, Kappler JW, Marrack P. CD4 memory T cells: What are they and what can they do? Sem Immuno. 2009;21:53–61. doi: 10.1016/j.smim.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacLeod MKL, et al. CD4 memory T cells divide poorly in response to antigen because of their cytokine profile. Proc Natl Acad Sci. 2008;105:14521–14526. doi: 10.1073/pnas.0807449105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen SG, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger C, et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattinoni L, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klebanoff CA, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klebanoff CA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gattinoni L, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 27.Appay V, Rowland-Jones SL. Lessons from the study of T-cell differentiation in persistent human virus infection. Sem Immuno. 2004;16:205–212. doi: 10.1016/j.smim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Henson SM, et al. KLRG1 signaling induces defective Akt (Ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009;113:6619–6628. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 29.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100:3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 30.Darrah PA, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 31.Hinrichs CS, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heemskerk B, et al. Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2. Hum Gene Ther. 2008;19:496–510. doi: 10.1089/hum.2007.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immuno Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 34.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 35.Hinrichs CS, et al. Type 17 CD8+ T cells display enhanced anti-tumor immunity. Blood. 2009;114:596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu D, et al. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 37.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor α is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clambey ET, White J, Kappler JW, Marrack P. Identification of two major types of age-associated CD8 clonal expansions with highly divergent properties. Proc Natl Acad Sci. 2008;105:12997–13002. doi: 10.1073/pnas.0805465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanley PJ, et al. Functionally active virus-specific T-cells that target CMV, adenovirus and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114:1958–1967. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer DC, et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc Natl Acad Sci USA. 2008;105:8061–8066. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jorritsma A, et al. Selecting highly affine and well-expressed TCRs for gene therapy of melanoma. Blood. 2007;110:3564–3572. doi: 10.1182/blood-2007-02-075010. [DOI] [PubMed] [Google Scholar]
- 42.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royl Stat SocB. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.