Abstract
Blood vessel formation during ischemia and wound healing requires coordination of the inflammatory response with genes that regulate blood vessel assembly. Here we show that the reticulon family member 4B, aka Nogo-B, is upregulated in response to ischemia and is necessary for blood flow recovery secondary to ischemia and wound healing. Mice lacking Nogo-B exhibit reduced arteriogenesis and angiogenesis that are linked to a decrease in macrophage infiltration and inflammatory gene expression in vivo. Bone marrow-derived macrophages isolated from Nogo knock-out mice have reduced spreading and chemotaxis due to impaired Rac activation. Bone marrow reconstitution experiments show that Nogo in myeloid cells is necessary to promote macrophage homing and functional recovery after limb ischemia. Thus, endogenous Nogo coordinates macrophage-mediated inflammation with arteriogenesis, wound healing, and blood flow control.
Keywords: inflammation, ischemia, vascular
Reticulons (Rtn) are a family of proteins that are localized primarily to the endoplasmic reticulon (ER) of most cells by virtue of an ER targeting motif in the carboxy terminal tail of their reticulon homology domains (1, 2). In mammals, there are four family members: Rtn 1, 2, 3, and 4, with each gene giving rise to multiple isoforms. Insights into Rtn functions have been dissected using overexpression, knockdown, or knockout strategies, and a clear role for these proteins in tubulogenesis of the peripheral ER and membrane curvature has emerged (3–5). However, despite the similarities of these proteins, there is evidence that different isoforms of each Rtn subclass may exert additional roles in mammalian cell function other than establishing the ER membrane curvature.
In mammalian cells, the Rtn 4 family has three isoforms, named Nogo-A, -B, and -C. Nogo-A is highly expressed in the nervous system and is implicated in controlling axonal regeneration pathways, Nogo-B is expressed in vascular cells and cardiac myocytes in vivo and multiple cell-types in vitro, and Nogo-C is expressed in the nervous system and skeletal muscle cells (1, 6). Previous work has identified Nogo-B as a regulator of vascular remodeling in vivo (7) and cardiac function in mice and humans (8, 9). In mice and rabbits, neointimal expansion of injured blood vessels is associated with a marked reduction in endogenous Nogo-B levels suggesting that Nogo-B negatively regulates the extent of vascular injury, and, in humans, the loss of Nogo-B strongly correlates with stenotic lesions and plaque rupture (7, 10, 11). This phenotype is most clearly observed in mice lacking Nogo-A and -B (Nogo−/− mice), where there is no overt developmental vascular phenotype, however, a clear postnatal occlusive vascular remodeling is observed after vascular injury, a phenotype rescued by local gene transfer of Nogo-B into the vessel wall (7) proving that this phenotype is Nogo-B-dependent. However, the endogenous role of Nogo-B in inflammatory tissue repair and scope of Nogo function in non-neural cells is virtually unexplored. In the present study, we show an unanticipated role for Nogo-B in determining the degree of tissue revascularization by regulating the extent of macrophage recruitment to sites of ischemia or wounds.
Results
Nogo-B Levels Are Induced During Tissue Ischemia, and Mice Deficient in Nogo-A/B Exhibit Impaired Responses to Tissue Injury.
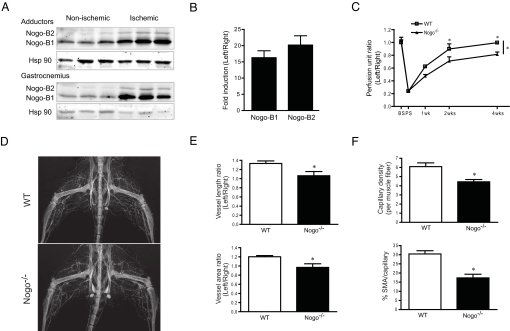
Since the role of Nogo in regulating the tissue repair are not known, we examined if the levels of endogenous Nogo are regulated during ischemia-provoked injury in vivo. Hindlimb ischemia was surgically induced as previously described in C57Bl6 mice (12, 13), and the levels of Nogo-B were examined by Western blotting in tissues extracts from nonischemic and ischemic tissues 3 days postischemia. As seen in Fig. 1A, Nogo-B1 (≈45 kDa on 10% SDS-PAGE gel) is expressed in nonischemic tissue, and an additional splice-variant, likely Nogo-B2 (≈48 kDa), is more clearly observed in ischemic tissue. Both isoforms are markedly upregulated in extracts prepared from adductor and gastrocnemius muscles, postischemia. The increase in Nogo-B levels are associated with an increase in Nogo-B1 and -B2 mRNA levels via qRT-PCR (Fig. 1B). Thus, tissue ischemia induces Nogo-B expression.
Fig. 1.
Nogo-B is induced with ischemia and is necessary for arteriogenesis and angiogenesis, thus functional recovery after ischemia. (A) Nogo-B1 and -B2 proteins were induced in both adductor and gastrocnemius muscles by ischemia, and Hsp 90 was used as a loading control. (B) qPCR of RNA isolated from WT gastrocnemius muscle (n = 3) showed induction of Nogo expression 3 days after ischemia. Fold changes of ischemic (Left) versus nonischemic (Right) are shown. Ribosomal RNA (18s) was used as internal control. (C) Gastrocnemius blood flow in WT (n = 9) and Nogo−/− (n = 10) mice BS, PS, 1 week, 2 weeks, and 4 weeks after arteriectomy. (D and E) Representative arteriograms (D) and quantification (E) of arteriogenesis after 2 weeks of ischemia in WT and Nogo−/− mice (n = 7). (F) Quantification of capillary density (PECAM-1) and pericyte recruitment (smooth muscle α-actin) in gastrocnemius muscles before and 2 weeks after ischemia (n = 5). Data are expressed as mean ± SEM. Two-way ANOVA; *, P < 0.05.
To examine if the upregulation of Nogo contributes to tissue remodeling postischemia, WT and Nogo−/− mice (14) were exposed to limb ischemia, and gastrocnemius blood flow was assessed via directly measurement in the surgically manipulated left limb compared to the contralateral right limb, using a deep penetrating Laser Doppler probe. As seen in Fig. 1C, before surgery (BS), the ratio of blood flow between the left limb and right limb is 1, and blood flow postsurgery (PS) is reduced to the same extent in WT and Nogo−/− mice. However, the time-dependent recovery of blood flow over a 4-week period, is reduced in Nogo−/− mice. Identical results were obtained in a different source of Nogo−/− mice (Nogo−/−lacZ; Fig. S1) (15) demonstrating that this effect is independent of the source of Nogo−/− mice (14, 15). The impaired flow recovery in the Nogo−/− mice suggests that perhaps Nogo may influence vascular patterning, thus we examined neonatal vascular patterning via whole-mount staining and quantification of the mouse ear vasculature in 3-week-old WT and Nogo−/− mice. As seen in Fig. S2 A and B, the loss of Nogo does not influence patterning of this circulation. It is well accepted that severe limb ischemia triggers flow and macrophage-dependent collateral arterial remodeling and/or growth (arteriogenesis) in the thigh and increases capillary density (angiogenesis) in the calf (16–18). As seen in Fig. 1 D and E, the loss of Nogo reduces arteriogenesis in the adductor muscle groups (representative angiogram in Fig. 1D and quantitative angiography in Fig. 1E), and angiogenesis in the gastrocnemius muscle (Fig. 1F, upper panel via PECAM-1 staining quantified as capillary/muscle fiber ratios), secondary to limb ischemia. In addition, the recruitment of stabilizing mural cells to the angiogenic vessels (Fig. 1F, lower panel, quantified as smooth muscle α-actin positive/PECAM-1 positive capillaries) is also reduced in Nogo−/− mice. Thus, mice deficient in Nogo exhibit impaired recovery after ischemia that may be due to defective arteriogenesis/angiogenesis.
Nogo−/− Mice Exhibit Defects in Macrophage Recruitment After Injury.
The recruitment of monocytes/macrophages and associated macrophage-derived cytokines are necessary for arteriogenesis secondary to limb ischemia (19), thus, we examined the presence of F4/80 positive macrophages recruited to the adductor and gastrocnemius muscle groups after 3 days of ischemia. As seen in Fig. 2A, and quantified in Fig. 2B, the number of F4/80 positive macrophages were markedly reduced in tissues from Nogo−/− mice postischemia. This difference in F4/80 positive macrophages in tissue was not due to differences in circulating monocytes at baseline or postischemia (side scatter low/CD11b+ population in Fig. 2C and quantified in Fig. 2D). These findings suggest that the reduction in monocyte/macrophage homing to injured tissue but not mobilization of monocytes may explain, in part, the reduced arteriogenesis and blood flow recovery in Nogo−/− mice. Next, we examined a different model of macrophage-dependent tissue remodeling after full-thickness wounding of the skin. As seen in Fig. S3 A and B, Nogo−/− mice exhibited a delayed wound healing response compared to WT mice. Macrophage infiltration was also impaired in Nogo−/− mice during wound healing compared to WT mice (Fig. S3C and quantified in Fig. S3D). Next, we examined the expression of 92 proinflammatory and angiogenic genes by qPCR arrays in total RNA extracted from the gastrocnemius muscle group from WT and Nogo−/− mice after 3 days of limb ischemia (Fig. S4). Nogo−/− mice showed a marked decrease in the expression of genes implicated in inflammation [CCL2, CCL11, CCR5, CSF-1, IL-1β, TNFα, TNFRSF1B (TNF-R1)], macrophage homing (CCR2, MSR-1), and angiogenesis/vascular remodeling (angiopoetin 2) consistent with lower numbers of macrophages detected immunochemically and reduced recovery of blood flow postischemia. Analysis of blood chemistry demonstrated no differences in total blood cell populations or leukocyte differentials between the strains (Table S1). To examine if Nogo regulates the pool of circulating progenitor cells released into the circulation secondary to ischemia, true CD34+ cells (Fig. S5 A and B) and early outgrowth endothelial progenitor cells (EPC) isolated from blood (Fig. S5 C and D) were examined in WT and Nogo−/− mice before and 7 days after ischemia. Ischemia increased the number of CD34+, and early outgrowth EPCs in WT and Nogo−/− mice showing that ischemia induced mobilization of endothelium progenitor cells is essentially normal in Nogo−/− mice.
Fig. 2.
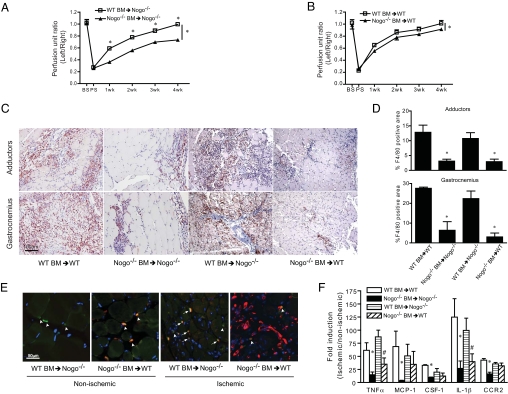
Loss of Nogo impairs macrophage homing but not activation. (A) Representative images of macrophage (F4/80) staining of adductor and gastrocnemius muscles 3 days after ischemia and (B) quantification of F4/80 staining indicating impaired macrophage recruitment in Nogo−/− compared to WT mice (n = 5). (C and D) FACS analysis of circulating monocytes before and 3 days after ischemia (n = 3). Blood monocytes (green population) were defined by CD11bhigh/side scatterlow (SSClow) in CD45+ leukocytes. Data are expressed as mean ± SEM. One-way ANOVA analysis is used; *, P < 0.05.
Bone Marrow Transfer Experiments Document that Nogo in Circulating and in Resident Tissue Monocytes/Macrophages Contributes to Impaired Tissue Recovery in Nogo−/− Mice.
To delineate if Nogo in circulating monocytes influences functional recovery of blood flow postischemia, bone marrow transplantation (BMT) experiments were performed. In this experiment, Nogo−/− mice were lethally irradiated, and reconstituted with WT or Nogo−/− bone marrow (BM) cells for 6 weeks, followed by hindlimb ischemia. BM reconstitution was confirmed by complete blood counts (Table S1) and PCR from whole blood. Reconstitution of WT BM into Nogo−/− mice improves blood flow recovery postischemia close to that seen in WT mice, suggesting that Nogo-B in circulating cells is sufficient for functional recovery after ischemia (Fig. 3A). Next, lethally irradiated WT mice were reconstituted with Nogo−/− or WT BM, and blood flow recovery was assessed after hindlimb ischemia. As seen in Fig. 3B, transfer of Nogo−/− BM into WT mice significantly reduces blood flow recovery, albeit to a lesser extent then WT marrow correcting the Nogo−/− defect. Examination of macrophage infiltration (via F4/80) postischemia documents that transfer of WT BM into Nogo−/− mice rescues the defect in macrophage homing as quantified in the adductor and gastrocnemius muscles, respectively (Fig. 3 C and D). Nogo−/− BM failed to fully home in Nogo−/− and WT mice. Next, we studied whether resident tissue macrophages in lethally irradiated WT mice were present and if they could produce cytokines to partially explain why the Nogo−/− BM only partially promoted ischemic disease. As seen in Fig. 3F, resident macrophages in WT mice transplanted with Nogo−/− BM can generate proangiogenic cytokine/chemokines (i.e., MCP-1, CSF-1, and CCR2, but not TNFα and IL-1β), that may contribute to partial functional recovery in WT mice transplanted with Nogo−/− BM. Collectively, these results suggest that Nogo-B in BM-derived monocytes (BMM) or in resident tissue macrophages is critical for functional recovery after ischemia.
Fig. 3.
WT BM can rescue the impairment of blood flow recovery and macrophage homing in Nogo−/− mice. (A) Hindlimb ischemia was performed on Nogo−/− mice reconstituted with BM from WT or Nogo−/− mice (n = 6 of each group), and gastrocnemius blood flow was measured. (B) Hindlimb ischemia was performed on WT mice reconsituted with BM from WT or Nogo−/− mice (n = 6 of each group), and gastrocnemius blood flow was measured. (C) Representative images of macrophage (F4/80) staining of adductor and gastrocnemius muscles 3 days after ischemia. (D) Quantification of F4/80 staining indicating WT but not Nogo−/− BM (n = 3 of each group) rescued the defect of macrophage recruitment in Nogo−/−. (E) Representative merged IF images of macrophage (F4/80 in green) and Nogo (in red) staining in nonischemic and ischemic gastrocnemius muscles after BM transplantation. Arrow heads indicate resident macrophage, and arrows indicate macrophage from circulation. (F) qRT-PCR analysis of cytokine/chemokine gene expression in gastrocnemius muscles 3 days after ischemia in each BM transplantation groups (n = 3 of each group). Data are expressed as mean ± SEM. One-way ANOVA; *, P < 0.05 compare to WT mice reconstituted with WT BM; #, P < 0.05 compare to WT mice reconstituted with Nogo−/− BM.
Nogo-B Is Highly Expressed in Monocytes/Macrophages and Nogo−/− Monocytes Are Defective in Cell Migration and Spreading.
Western blot analysis of human blood-borne monocytes and murine BMM demonstrates that Nogo-B, but not Nogo-A (not shown), is highly expressed (Fig. S6A), consistent with a previous report (20). To examine if endogenous Nogo-B directly influences monocyte/macrophage function, BMM were isolated from WT and Nogo−/− mice, and their migration and spreading were assessed. As seen in Fig. 6B, using a modified Boyden chamber, the loss of Nogo did not affect BMM chemokinesis, but reduced migration in response to a gradient of the murine chemokines, CSF-1 and MCP-1. The loss of Nogo-B reduced BMM spreading onto glass or fibronectin-coated slides and decreased the adhesive area of BMM to glass and fibronectin (Fig. S6 C and D), suggesting that endogenous Nogo-B regulates chemotaxis and spreading, which may explain, in part, the reduced number of macrophages trafficking into the ischemic limbs or wounds from Nogo−/− mice.
BMM Isolated from Nogo−/− Mice Show Defects in Rac Activity, Morphology, and Chemokine Production.
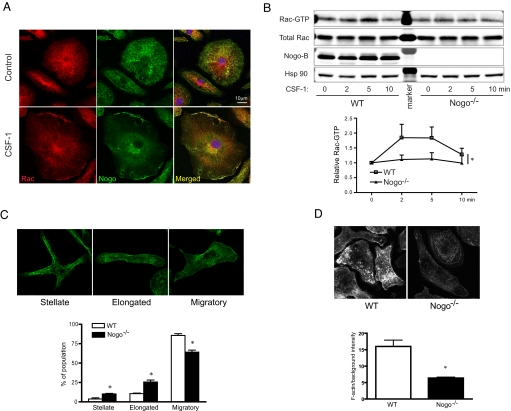
The reduction in chemokine-mediated migration in Nogo−/− BMM is reminiscent of cells exhibiting impaired Rac-mediated cytoskeletal changes (21–23). Thus, we examined Rac localization, activation, and cytoskeletal dynamics in WT and Nogo−/− BMM. As seen in Fig. 4A, in nonstimulated BMM, Rac (red) localizes mainly in a diffuse pattern in the cytosol (upper panel) and stimulation with CSF-1 promotes Rac localization to the plasma membrane (lower panel). Endogenous Nogo-B (green) also partially colocalizes with Rac under control conditions and colocalizes with Rac after stimulation. CSF-1 increases Rac activity, an effect significantly reduced in BMM isolated from Nogo−/− mice, (Fig. 4B). We also explored other signaling pathways activated by CSF-1, such as Akt and mitogen-activated protein kinases (p42/44 ERK and p38 MAPK), which are known pathways that influence BMM motility and morphology. As seen in Fig. S7, these pathways are not different in WT versus Nogo−/− BMM.
Fig. 4.
Nogo colocalizes with Rac; loss of Nogo impairs Rac activation and F-actin assembly in BMM. (A) Representative confocal images showing Rac localization in WT BMM under quiescent (Upper) and CSF-1-stimulated (5 min, Lower) condition. Rac conpartially colocalizes with Nogo-B in plasma membrane upon stimulation in WT BMM. (B) Western blotting for active Rac indicates impaired kinetics of Rac activation upon CSF-1 stimulation. Lower panel show densitometric analysis from four individual Rac activity assays. (C) Confocal images illustrating stellate, elongated, and migratory morphology of BMM in vitro (Upper); quantification of BMM morphology in WT and Nogo−/−. More than 100 cells from three individual experiments in each group were quantified (Lower). (D) Confocal images of phalloidin staining of BMM stimulated with CSF-1 (Upper); quantification of F-actin intensity of the basal plane of BMM. Data are expressed as mean ± SEM; *, P < 0.05.
Primary BMM isolated from mice can exhibit multiple morphologies when studied in vitro. For example, BMM can exhibit stellate, elongated, or migratory phenotypes as previously described (23). Next, we quantified these morphological end points in BMM isolated from WT and Nogo−/− mice. As seen in Fig. 5C, BMM isolated from WT mice exhibited a prominent migratory phenotype, with less elongated and stellate-like cells. In contrast, BMM cells isolated from Nogo−/− mice were less migratory and more elongated and stellate shape consistent with the reduced spreading and chemotaxis in these cells. F-actin levels upon stimulation of CSF-1 were also accessed by phalloidin staining. As seen in Fig. 5D, the amount of F-actin was significantly decreased in Nogo−/− BMM.
To further examine the effect of Nogo-B on BMM gene expression function, we WT and Nogo−/− BMM with LPS (10 ng/mL). LPS induction of most genes were not different between the strains, however BMM from Nogo−/− mice showed a marked decrease in the expression of genes implicated in inflammation (TNF, MMP12, IL-1β, IL-6ra) and the macrophage chemokine CCL2 (aka MCP-1) as shown in Fig. S8. Collectively, these data show that the loss of Nogo impairs several mononcyte/macrophages functions including migration, spreading, Rac activation, actin reorganization, and cytokine/chemokine gene expression, all of which may explain the defective tissue repair processes in the Nogo−/− mice.
Discussion
This paper documents an unanticipated role of Rtn 4 in inflammation and tissue repair. Here we show that Rtn 4, aka Nogo, is an endogenous regulator of inflammatory tissue remodeling and wound healing that is mediated, in part, via impaired macrophage homing to ischemic tissue and wounds in vivo. Since the Nogo-deficient mice used in this study lack Nogo isoforms -A and -B (14), and only Nogo-B is detectable in vasculature and macrophages, we interpret these results supporting a critical role of Nogo-B. The relative importance of host versus inflammatory cell Nogo-B is skewed toward the role of Nogo in circulating inflammatory cells, since transfer of WT BM into irradiated Nogo-deficient mice rescues the impaired ischemic vascular response and homing of macrophages to ischemic tissue. Mechanistically, the loss of Nogo-B in BMM reduces the migratory phenotypes of isolated cells in vitro. Previous work has shown that Nogo-B in macrophages is a substrate for the protein kinase MAPKAP-K2, however, the loss of Nogo in BMM did not influence their chemotactic response to C5a (24). Since the specific functional roles of the Rtn family members in vivo are still virtually unexplored data showing a marked reduction in macrophage infiltration and macrophage-mediated tissue remodeling in Nogo−/− mice combined with defects in macrophage function in vitro are striking and suggests an important Nogo-B function during inflammation in vivo.
Little is known regarding the expression of Nogo-B in vivo and in vitro. In vivo, using mice that express LacZ in the Nogo-A/B locus, the expression of Nogo is found in neurons (15, 25), atrial myocytes, arteries, and veins (7), whereas in vitro, neural cells express Nogo-A and primary cultures of EC and VSM and cancer cell lines express Nogo-B (26, 27). In the model of hindlimb ischemia, there was a marked increase in Nogo-B protein levels in tissue extracts and gene expression in the ischemic limb. The increase in Nogo-B expression in tissue is likely due to increased gene expression in vascular cells as well as the recruitment of monocytes/macrophages into tissue. In this model of severe limb ischemia, it is believed that ischemia triggers the redistribution of blood flow into preformed collaterals, and the increase in shear stress concomitant with the recruitment of macrophages and macrophage-derived cytokines such as MCP-1, TNFα, or VEGF participate in arteriogenesis in the adductor muscles, blood flow recovery, and angiogenesis in the gastrocnemius muscle (16, 17, 28). Our data support this model since Nogo−/− mice had defects in macrophage recruitment and arteriogenesis, blood flow recovery, and angiogenesis. Similar results were obtained in a model of full-thickness wounds, where Nogo−/− mice exhibit delayed macrophage infiltration and wound healing. A causal role for defective macrophages in the hindlimb ischemia model is suggested via the BMT experiments since WT BMM almost completely rescued the defective macrophage homing and impaired function in Nogo−/− recipients. However, since transfer of Nogo−/− BM in WT recipients did not fully recapitulate the same degree of impaired limb function as in Nogo−/− mice, we cannot unequivocally exclude a role for host Nogo in this response until tissue-specific knockout mice are used in similar experiments. Tissue macrophages are notoriously difficult to completely eliminate from tissues, have different kinetics of repopulating (29) and local macrophages may proliferate in tissue after ischemia (30), thus, the presence of residual Nogo-positive macrophages after irradiation in WT mice may contribute to the recovery of function postischemia in mice transplanted with Nogo−/− BMM. Indeed our data suggests that skeletal muscle resident macrophages exist in irradiated mice before and after ischemia, and they contribute to cytokine gene expression.
During inflammation, monocytes are actively recruited to sites of inflammation where they differentiate into tissue macrophages, processes critically dependent on cytoskeletal remodeling. Since our in vivo data suggested that the impaired responses to ischemia and wounding may be due, in part, to defective macrophage recruitment, we focused on aspects of macrophage function in vitro. Indeed, BMM lacking Nogo did not spread well and exhibited attenuated migratory responses to the chemoattractants, CSF-1 and MCP-1. Since Rac is necessary for the responses to these chemokines, we examined Rac localization and activation in WT and Nogo-deficient cells. We found that Rac partially colocolizes with Nogo in the peripheral ER and plasma membrane and loss of Nogo significantly reduced Rac activation. Nogo−/− BMM has altered cell morphology and reduced F-actin clustering, consistent with reduced activation of Rac. Thus, we surmise that Nogo deficiency influences the activation of Rac, which in turn reduces F-actin polarization and cell migration. Precisely how Nogo-B regulates Rac activation is not known, however, there is precedence showing that other Rtn family members can modulate ER function and protein trafficking. Rtns are critical for assembly of the tubulated ER in yeast and mammalian cells (3, 31). In addition, Rtn 2B regulates the trafficking of the glutamate transporter (32), Rtn 1B negatively regulates the localization of the ER associated GTP activation protein, TBC1D20, a GAP for the small GTPase Rab1 (33), and Rtn 3 overexpression blocks ER-Golgi trafficking (34). Our data showing an impairment of Rac activation can explain the spreading/migratiory and actin defects in Nogo-deficient BMM. This effect, in turn, limits the number of macrophages infiltrating into tissue, thereby reducing arteriogenesis and delaying healing. Thus, understanding the endogenous roles of Rtns and Nogo may provide insights into pathways that regulate vascular inflammation associated with atherosclerosis, wound healing, and tumor progression.
Experimental Procedures
All animal studies were approved by the institutional animal care and use committees of Yale University. Two strains of Nogo−/− mice used were from Marc Tessier-Lavigne (MTL) (14) and Steven Strittmatter (SS) (15) mice.
Supplementary Material
Acknowledgments.
We thank Stephen Strittmatter and Marc Tessier-Lavigne for Nogo−/− mice used throughout the studies; Dan Wu, Anthony Koleske, and Martin Schwartz for helpful discussions; and Zhenwu Zhuang for technical assistance. This work was supported in part by National Institutes of Health Grants R01 HL 064793, RO1 HL 061371, R01 HL 081190, and PO1 HL 70295; Yale Proteomics Contract N01-HV-28186 (to W.C.S.); awards from the American Heart Association (to J.Y. and Y.S.); and Program 3 + 3 Fellowship from the Centro Nacional de Investigaciones Cardiovasculares.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907359106/DCSupplemental.
References
- 1.Oertle T, Schwab ME. Nogo and its paRTNers. Trends Cell Biol. 2003;13:187–194. doi: 10.1016/s0962-8924(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 2.Teng FY, Tang BL. Cell autonomous function of Nogo and reticulons: The emerging story at the endoplasmic reticulum. J Cell Physiol. 2008;216:303–308. doi: 10.1002/jcp.21434. [DOI] [PubMed] [Google Scholar]
- 3.Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 4.Shibata Y, et al. The reticulon and DP1/Yop1p proteins form immobile oligomers in the tubular endoplasmic reticulum. J Biol Chem. 2008;283:18892–18904. doi: 10.1074/jbc.M800986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shnyrova A, Frolov VA, Zimmerberg J. ER biogenesis: Self-assembly of tubular topology by protein hairpins. Curr Biol. 2008;18:R474–R476. doi: 10.1016/j.cub.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Yang YS, Strittmatter SM. The reticulons: A family of proteins with diverse functions. Genome Biol. 2007;8:234. doi: 10.1186/gb-2007-8-12-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acevedo L, et al. A new role for Nogo as a regulator of vascular remodeling. Nat Med. 2004;10:382–388. doi: 10.1038/nm1020. [DOI] [PubMed] [Google Scholar]
- 8.Gramolini AO, et al. Comparative proteomics profiling of a phospholamban mutant mouse model of dilated cardiomyopathy reveals progressive intracellular stress responses. Mol Cell Proteomics. 2008;7:519–533. doi: 10.1074/mcp.M700245-MCP200. [DOI] [PubMed] [Google Scholar]
- 9.Bullard TA, et al. Identification of Nogo as a novel indicator of heart failure. Physiol Genomics. 2008;32:182–189. doi: 10.1152/physiolgenomics.00200.2007. [DOI] [PubMed] [Google Scholar]
- 10.Paszkowiak JJ, et al. Evidence supporting changes in Nogo-B levels as a marker of neointimal expansion but not adaptive arterial remodeling. Vascul Pharmacol. 2007;46:293–301. doi: 10.1016/j.vph.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Feo JA, et al. Low levels of Nogo-B in human carotid atherosclerotic plaques are associated with an atheromatous phenotype, restenosis, and stenosis severity. Arterioscler Thromb Vasc Biol. 2007;27:1354–1360. doi: 10.1161/ATVBAHA.107.140913. [DOI] [PubMed] [Google Scholar]
- 12.Ackah E, et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu J, et al. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci USA. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng B, et al. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 15.Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 16.Heil M, et al. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ Res. 2004;94:671–677. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 17.Weihrauch D, Arras M, Zimmermann R, Schaper J. Importance of monocytes/macrophages and fibroblasts for healing of micronecroses in porcine myocardium. Mol Cell Biochem. 1995;147:13–19. doi: 10.1007/BF00944778. [DOI] [PubMed] [Google Scholar]
- 18.Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: Similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helisch A, Schaper W. Arteriogenesis: The development and growth of collateral arteries. Microcirculation. 2003;10:83–97. doi: 10.1038/sj.mn.7800173. [DOI] [PubMed] [Google Scholar]
- 20.Rousseau S, Peggie M, Campbell DG, Nebreda AR, Cohen P. Nogo-B is a new physiological substrate for MAPKAP-K2. Biochem J. 2005;391:433–440. doi: 10.1042/BJ20050935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen WE, Jones GE, Pollard JW, Ridley AJ. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci. 1997;110:707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- 22.Wells CM, Walmsley M, Ooi S, Tybulewicz V, Ridley AJ. Rac1-deficient macrophages exhibit defects in cell spreading and membrane ruffling but not migration. J Cell Sci. 2004;117:1259–1268. doi: 10.1242/jcs.00997. [DOI] [PubMed] [Google Scholar]
- 23.Wheeler AP, et al. Rac1 and Rac2 regulate macrophage morphology but are not essential for migration. J Cell Sci. 2006;119:2749–2757. doi: 10.1242/jcs.03024. [DOI] [PubMed] [Google Scholar]
- 24.Rousseau S, et al. CXCL12 and C5a trigger cell migration via a PAK1/2-p38alpha MAPK-MAPKAP-K2-HSP27 pathway. Cell Signal. 2006;18:1897–1905. doi: 10.1016/j.cellsig.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Huber AB, Schwab ME. Nogo-A, a potent inhibitor of neurite outgrowth and regeneration. Biol Chem. 2000;381:407–419. doi: 10.1515/BC.2000.053. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, et al. Link of a new type of apoptosis-inducing gene ASY/Nogo-B to human cancer. Oncogene. 2001;20:3929–3936. doi: 10.1038/sj.onc.1204536. [DOI] [PubMed] [Google Scholar]
- 27.Oertle T, Merkler D, Schwab ME. Do cancer cells die because of Nogo-B? Oncogene. 2003;22:1390–1399. doi: 10.1038/sj.onc.1206278. [DOI] [PubMed] [Google Scholar]
- 28.Arras M, et al. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: Analysis using a transgenic bone marrow transplantation model. Blood. 1997;90:986–993. [PubMed] [Google Scholar]
- 30.Khmelewski E, Becker A, Meinertz T, Ito WD. Tissue resident cells play a dominant role in arteriogenesis and concomitant macrophage accumulation. Circ Res. 2004;95:E56–E64. doi: 10.1161/01.RES.0000143013.04985.E7. [DOI] [PubMed] [Google Scholar]
- 31.De Craene JO, et al. Rtn1p is involved in structuring the cortical endoplasmic reticulum. Mol Biol Cell. 2006;17:3009–3020. doi: 10.1091/mbc.E06-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, et al. Reticulon RTN2B regulates trafficking and function of neuronal glutamate transporter EAAC1. J Biol Chem. 2008;283:6561–6571. doi: 10.1074/jbc.M708096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haas AK, et al. Analysis of GTPase-activating proteins: Rab1 and Rab43 are key Rabs required to maintain a functional Golgi complex in human cells. J Cell Sci. 2007;120:2997–3010. doi: 10.1242/jcs.014225. [DOI] [PubMed] [Google Scholar]
- 34.Wakana Y, et al. Reticulon 3 is involved in membrane trafficking between the endoplasmic reticulum and Golgi. Biochem Biophys Res Commun. 2005;334:1198–1205. doi: 10.1016/j.bbrc.2005.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.