Abstract
The evolution of insecticide resistance in mosquitoes is threatening the effectiveness and sustainability of malaria control programs in various parts of the world. Through their unique mode of action, entomopathogenic fungi provide promising alternatives to chemical control. However, potential interactions between fungal infection and insecticide resistance, such as cross-resistance, have not been investigated. We show that insecticide-resistant Anopheles mosquitoes remain susceptible to infection with the fungus Beauveria bassiana. Four different mosquito strains with high resistance levels against pyrethroids, organochlorines, or carbamates were equally susceptible to B. bassiana infection as their baseline counterparts, showing significantly reduced mosquito survival. Moreover, fungal infection reduced the expression of resistance to the key public health insecticides permethrin and dichlorodiphenyltrichloroethane. Mosquitoes preinfected with B. bassiana or Metarhizium anisopliae showed a significant increase in mortality after insecticide exposure compared with uninfected control mosquitoes. Our results show a high potential utility of fungal biopesticides for complementing existing vector control measures and provide products for use in resistance management strategies.
Keywords: biopesticide, DDT, pyrethoids, resistance management, vector control
Increasing incidences of insecticide resistance are reported in the major African malaria vector species Anopheles gambiae s.s. (1–3), Anopheles funestus (4, 5), and Anopheles arabiensis (6, 7), thereby threatening the efficiency of insecticides approved for malaria vector control. As the use of indoor residual spraying and insecticide-treated nets is scaling up (ref. 8 and http://www.rollbackmalaria.org/gmap/), so will the selection pressure for insecticide resistance. Resistance management strategies, such as the use of chemical mixtures or rotations, are suggested to improve the sustainability of these approaches (9, 10), but with significant cross-resistance between the currently approved classes of insecticide (9–11) and no new public health insecticides introduced in the last 20 years (12), practical options are few. For sustainable malaria vector control, the requirement for new products with unique modes of action is becoming increasingly evident.
Previous work has suggested that entomopathogenic fungi could play this role (13–15). A range of isolates belonging to the fungal species Metarhizium anisopliae and Beauveria bassiana have been shown to infect and significantly reduce the longevity of adult Anopheles mosquitoes, killing them within 14 days (13, 14, 16). This slow speed of kill is considered to dramatically reduce the selection pressure for fungal resistance development (15, 17); yet, because Plasmodium maturation within the mosquito also takes ≈14 days (18) delayed kill is considered to be sufficient to significantly reduce the mosquito's vectorial capacity. Prelethal effects of fungal infection, including reductions in Plasmodium sporozoite formation (13), feeding propensity (13, 19), and fecundity (19), increase the potential impact on malaria transmission even further.
Under appropriate conditions (humidity and pH) and adequate nutrient availability, fungal spores can infect insects via attachment to the epicuticle and subsequent germination (15, 20). Spores penetrate the cuticle via formation of germ tubes and cuticle-degrading enzymes (21) and grow in the host hemocoel where they use nutrients, destroy host cells, and eventually kill the insect (20). For fungus-based biopesticides to play a prominent role in malaria control, an important criterion is that fungal susceptibility will remain unaffected by resistance to insecticides. The mode of action of entomopathogenic fungi (i.e., via external contact and proliferation through the hemocoel) makes direct cross-reactions with insecticides unlikely. However, the indirect effects of insecticide resistance mechanisms have been shown to reduce pathogen proliferation. Enhanced concentrations of esterases in organophosphate-resistant Culex mosquitoes have been implicated in limiting growth of filarial worms (22, 23), and enhanced monooxygenase levels in pyrethroid-resistant Anopheles species increase oxidative stress to the detriment of Plasmodium survival (23). Because enzymatic detoxification of insecticides is also an important resistance mechanism in Anopheles mosquitoes (11), enhanced detoxification may interact with fungal metabolites, such as cyclic peptide toxins (15, 20), and could reduce the effect of these virulence factors.
The compatibility of fungus- and insecticide-based control methods also will depend on the effect of fungal infection on insecticide-resistant mosquito mortality and resistance levels. Studies on insect hosts other than mosquitoes have indicated that fungal infection can act synergistically with insecticides, increasing the impact of otherwise sublethal insecticide doses (24–26). Mixtures of M. anisopliae and deltamethrin were shown to enhance virulence of both components when tested against ticks, indicating synergistic effects that would enhance the effectiveness of low fungi and insecticide concentrations (27). In contradiction to these findings, however, studies on the wax moth Galleria mellonella have indicated that the elevation of detoxifying enzymes in response to infection with M. anisopliae increases host resistance to organophosphate insecticides (28). To ensure the compatibility of fungal biopesticides and chemical control tools, such potential adverse effects on resistance levels will have to be excluded for anophelines.
So far, there have been no reports on the effects of insecticide resistance mechanisms on mosquito susceptibility to fungal infection or the effects of fungal infection on mosquito insecticide resistance levels. In this study, therefore, we investigated the effectiveness of two potential fungal biocontrol pathogens, M. anisopliae and B. bassiana, against insecticide-resistant Anopheles mosquitoes. Effects of insecticide resistance status on fungal susceptibility and effects of fungal infection on insecticide resistance levels were tested in a diverse suite of insecticide-resistant Anopheles colonies.
Results
Mosquito Susceptibility to Fungus Infection.
We studied the infectivity and virulence of B. bassiana in a permethrin-resistant A. funestus colony (AfPerm), two dichlorodiphenyltrichloroethane (DDT)-resistant A. arabiensis colonies (Aa1DDT and Aa2DDT), one bendiocarb-resistant A. gambiae s.s. colony (AgBend), and the corresponding baseline colonies from which each was selected. Replicate samples of mosquitoes were infected with the fungus by using a standard exposure bioassay previously shown to provide reliable infection in the laboratory (16). B. bassiana caused 100% mortality in all eight colonies within 8–20 days (Fig. 1), with levels of infection exceeding 95% [confirmed by fungal sporulation on mosquito cadavers (16)]. Cox regression analyses showed the effect of Beauveria infection on mosquito survival to be significant in all tested colonies (P < 0.001). Additionally, a highly significant interaction between fungus treatment and insecticide resistance was found in A. funestus (P = 0.002) and A. gambiae s.s. (P < 0.001), indicating quantitative differences in the effect of fungus infection on mosquito survival between the baseline and insecticide-resistant colonies. Beauveria infection reduced survival more strongly in permethrin-resistant A. funestus mosquitoes [hazard ratio (HR) = 47,241.42; P < 0.001] than in its baseline colony (HR = 28.22; P < 0.001) yet reduced survival less strongly in bendiocarb-resistant A. gambiae s.s. (HR = 5.70; P < 0.001) than in the baseline mosquitoes (HR = 71.70; P < 0.001). However, similar differences in HRs were observed in the corresponding uninfected control mosquitoes, a significantly lower daily mortality rate in permethrin-resistant A. funestus mosquitoes (HR = 0.003; P = 0.005) and higher mortality rate in bendiocarb-selected A. gambiae s.s. mosquitoes (HR = 28.20; P < 0.001) compared with those of their baseline colonies, suggesting that these quantitative differences in the effect of fungus infection on survival are caused by inherent differences in laboratory colony longevity rather than any effect of resistance per se.
Fig. 1.
Effect of fungus infection on baseline (susceptible) and insecticide-resistant mosquito survival. Mean (±SEM) cumulative proportional survival of B. bassiana-infected (red) and uninfected control mosquitoes (black) of four different mosquito strains, A. funestus (Af), two strains of A. arabiensis (Aa1 and Aa2), and A. gambiae s.s. (Ag). Survival of baseline colonies is shown on the left and colonies selected for resistance to either permethrin (Perm), DDT, or bendiocarb (Bend) on the right. Data represent nine replicates, each containing ≈30 females, except for the baseline Af colony where n = 6 for both curves.
Effect of Fungal Infection on Insecticide Resistance.
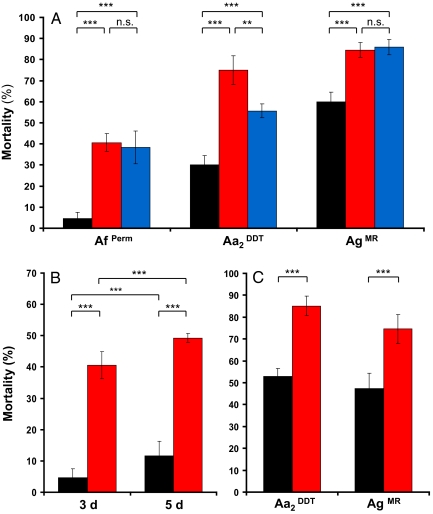
To investigate whether fungal infection affects the expression of insecticide resistance, we conducted a series of experiments to examine prelethal effects of fungal infection on insecticide sensitivity in resistant mosquitoes. First, we assessed the effect of B. bassiana or M. anisopliae infection on the expression of permethrin resistance in three mosquito strains with known levels of resistance to this insecticide, permethrin-resistant A. funestus (AfPerm), DDT-resistant A. arabiensis (Aa2DDT), and a recently established, unselected, multiple-resistant strain of A. gambiae s.s. (AgMR). Replicate sets of mosquitoes were infected with either Beauveria or Metarhizium and exposed to permethrin 3 days later, using standard World Health Organization (WHO) insecticide assay protocols. Mortality was recorded 24 h after insecticide exposure and compared with permethrin-induced mortality in uninfected controls. In all three mosquito strains, permethrin induced significantly higher mortality in mosquitoes preinfected with Beauveria (P < 0.001) or Metarhizium (P < 0.001) than that in the uninfected groups (Fig. 2A). Both fungi induced similar increases in susceptibility to permethrin. In A. arabiensis, we found significantly higher mortality levels induced by Beauveria infection compared with Metarhizium infection (χ2 = 36.04, P < 0.001), which may be caused by differences in pathogenicity of the two fungal species or a different insecticide resistance mechanism in this mosquito species.
Fig. 2.
Effect of fungus infection on mosquito insecticide-resistance levels. (A) Corrected mean (±SEM) percentage mortality of uninfected (black), Beauveria-infected (red), and Metarhizium-infected (blue) mosquitoes after exposure to permethrin 3 days after fungal infection. The tested mosquito strains A. funestus (AfPerm), A. arabiensis from Sudan (Aa2DDT), and A. gambiae s.s. (AgMR) exhibit high baseline levels of permethrin resistance. Data represent mosquito mortality of five replicates, each containing 25 females. (B) Effect of permethrin exposure on Beauveria-infected (red) and uninfected (black) permethrin-resistant A. funestus (AfPerm) mosquitoes at 3 days (Left) and 5 days (Right) after fungal infection. Data represent corrected mean (±SEM) percentage mortality from five replicates, each containing 25 females. (C) Corrected mean (±SEM) percentage mortality of Beauveria-infected (red) and uninfected (black) DDT-resistant mosquitoes [A. arabiensis (Aa2DDT) and A. gambiae s.s. (AgMR)]. Data represent five replicates, each containing 25 females. For each treatment, mortality of mosquitoes exposed to insecticide was corrected for mortality of counterparts exposed to control papers, using Abbott's formula (39). Asterisks indicate significant differences determined by χ2 test. **, P < 0.01; ***, P < 0.001.
Using the AfPerm line, we also examined the effects of B. bassiana infection on permethrin resistance levels 5 days after fungal exposure. This revealed a significant (χ2 = 18.38, P < 0.001) increase in permethrin-induced mortality compared with that of the 3-day group (Fig. 2B). However, mortality of the uninfected control group was also significantly higher at 5 days (χ2 = 57.22, P < 0.001), which is consistent with age-dependent responses to insecticide exposure found in several mosquito species (29, 30) and suggests equivalent relative effects of fungus, at least over the initial time course of infection.
The effect of fungus infection on DDT resistance was assessed by using DDT-resistant colonies of A. arabiensis (Aa2DDT) and A. gambiae s.s. (AgMR). We preinfected replicate samples of mosquitoes with B. bassiana spores and compared susceptibility to DDT between the infected and uninfected groups 3 days later. For both tested species, infection with B. bassiana led to significantly higher mortality after DDT exposure compared with that of the uninfected controls (P < 0.001) (Fig. 2C). This increase in the mosquito's susceptibility to DDT shows that fungal infection negatively affects the expression of DDT resistance.
Discussion
Our results show that resistance against three of the four classes of public health insecticides does not confer enhanced resistance to infection by B. bassiana. The fungus was highly infective and virulent to a diverse suite of resistant Anopheles mosquito strains. Furthermore, infection with either B. bassiana or M. anisopliae prelethally interferes with the expression of permethrin and DDT resistance in genetically resistant mosquitoes, increasing their susceptibility to these insecticides. The exact mechanisms involved in the interactions between insecticide resistance and fungal infection remain unclear. In the tested A. funestus (AfPerm) colony, resistance is mediated by elevated levels of monooxygenases (5, 31, 32). In the A. arabiensis (Aa2DDT) colony, the West African kdr target site mutation is present but does not correlate with the resistance phenotype (6, 30), and resistance is conferred metabolically through elevated GST and esterase concentrations (30). Resistance in the A. gambiae s.s. (AgMR) colony may be mediated by the West African kdr target site mutation and metabolic detoxification. Because metabolic resistance mechanisms are present in all species tested, a reallocation of insecticide-detoxifying enzymes toward fungal toxins possibly reduced the quantity of enzymes available to target insecticides and resulted in the observed postfungus infection decrease in resistance. However, as is the case for wild-type resistant mosquitoes (33), there are diverse, potentially interacting mechanisms conferring resistance in our tested mosquito species, and the lack of a clear correlation between resistance genotype and phenotype complicates assessing the exact interactions between insecticide resistance mechanisms and fungal infection.
Direct effects of the neurotoxic insecticides on the fungus and its proliferation inside the mosquito were not studied. The fungus was allowed to proliferate for 3 days in the insect before being exposed to the insecticide. There were no differences in infection percentages between insecticide-exposed and nonexposed groups, but that could be a result of already extensive fungus growth at day 3. Especially for testing the efficacy of fungus–insecticide combinations, testing the effect of neurotoxic and other classes of insecticides on fungal infectivity and virulence would be interesting.
Increased knowledge on fungus–insect interactions will augment options for improving fungus-based applications against mosquitoes. For example, modification of fungal spores to enhance their virulence can be used to improve the commercial effectiveness of fungus-based control methods. Genetic alterations that caused overproduction of a cuticle-degrading protease have been shown to effectively increase the speed of kill by the fungus (34). Furthermore, exploring the effectiveness of fungi against mosquito strains with other resistance mechanisms, such as resistance to microbial agents such as Bacillus thuringiensis var. israelensis (B.t.i.) or insect growth regulators such as methoprene, would substantiate further the usefulness of fungus-based biological control tools against mosquitoes where other current control measures are failing.
Overall, the significant reductions in mosquito survival and insecticide resistance levels induced by fungal infection support the potential use of fungal biopesticides against mosquito vectors in areas where insecticide resistance levels are increasing, potentially adding new product options to the very limited selection of chemicals currently available. With their relatively slow speed of kill, considered to dramatically reduce the selection pressure for resistance development while killing mosquitoes before being able to transmit the malaria parasite (15), fungal biopesticides may provide a sustainable vector control tool. The susceptibility of insecticide-resistant mosquitoes to fungal pathogens adds weight to the possibility of using biopesticides within insecticide resistance management strategies, such as rotations or mosaics (10), to slow the spread of resistance (15, 17). The use of oil-formulated spores in point sources such as black cotton cloths (14) or African water storage pots (35) have shown potential for field implementation and would allow for the integration of fungi in existing control measures. Like protozoan (36) or nematode infections (37), fungal pathogen infection exerts an additional fitness cost for the insect. Because these costs are associated with a slower spread of resistance (38), the additional burden of a fungal infection may reduce the speed of insecticide resistance formation in anopheline vectors. Moreover, with fungal infection reducing the expression of permethrin and DDT resistance, developing “combination treatments” may enhance the efficacy and effective lifespan of key insecticides where resistance has reached high levels. Together, these findings provide a compelling case for viewing biopesticides and chemical insecticides not as mutually exclusive but as complementary technologies that may improve the efficiency and sustainability of integrated malaria vector control programs.
Materials and Methods
Mosquitoes.
An overview of mosquito colony names, abbreviations, resistance selection, and origins is given in Table S1. The A. funestus colonies (AfPerm) originated from collections in southern Mozambique. Mosquitoes from the baseline colony (FUMOZ) were selected for high levels of resistance to the pyrethroid permethrin in the insectary of the National Institute for Communicable Diseases (Johannesburg, South Africa) for 2 years, resulting in the colony FUMOZ-R, of which adults show 0–1% mortality when exposed to 1% lambda-cyhalothrin for 1 h (31). The two A. arabiensis colonies used (Aa1DDT and Aa2DDT) originated from Mamfene, KwaZulu-Natal, South Africa (MBN) and from Sennar, south-central Sudan (SENN), respectively. The SENN baseline colony was selected for DDT resistance for 16 generations, after which SENN-DDT adults showed 12.1% mortality when exposed to 4% DDT and 0% mortality when exposed to 0.75% permethrin for 1 h (30). For this study, adults of the F50–F54 generation were used, which were shown to have lower baseline resistance levels to DDT and permethrin (Fig. 2 A and C). The A. gambiae s.s. colony used in survival assays (AgBend) originated from Obuasi, Ghana (SOG), of which a bendiocarb-resistant colony (BENROG) was selected in the laboratory. The A. gambiae s.s. colony used in insecticide resistance assays (AgMR) originated from Ahafo, Ghana (GAH). This colony has not been selected for resistance to insecticides in the laboratory but carries quantified levels of resistance to all four classes of insecticides. Both SOG and FUMOZ baseline colonies exhibit low levels of resistance, which is increased by orders of magnitude in the selected lines. A summary of mosquito insecticide resistance or susceptibility to insecticides approved by WHO is given in Table S2.
Larvae were reared in plastic bowls filled with distilled water. For A. funestus, the water was supplemented with green algae. Larval food was supplied daily and contained a mixture of finely crushed dog biscuit and brewer's yeast (31). Adults were collected daily from the bowls and transferred to holding cages in which cotton wool soaked in 10% glucose solution was provided. All species were maintained at 25 °C and 80% relative humidity with a 12-h day/night photoperiod and artificial 45-min dusk/dawn cycles. For experiments, 2- to 5-day-old mosquitoes were used.
Fungus.
M. anisopliae var. anisopliae (Metsch.) Sorokin, isolate ICIPE-30 (14) (courtesy N. Maniania, International Centre of Insect Physiology and Ecology, Nairobi, Kenya) and B. bassiana isolate IMI 391510 (13) were used. Both fungi were produced through solid-state fermentation with glucose-impregnated hemp (courtesy F. van Breukelen and M. Jumbe, Wageningen University and Research Centre, Wageningen, The Netherlands) of which conidia were dried and stored in the dark at 4 °C.
The viability of the conidia was assessed by mixing some dry spores of each stock in oil (Shell Ondina Oil 917) and plating a drop on Sabouraud dextrose agar. After 20–26 h of incubation at 27 °C, the proportion of germinated conidia was determined with a light microscope at a magnification of 400×. Stocks showing 85% or higher sporulation were used for experiments.
Fungal Exposures.
Adult mosquitoes were exposed to 100 mg of dry conidia by using the suspensor setup previously shown to give reliable infections, as described by Scholte et al. (16). Female mosquitoes were exposed to one suspensor in a holding cage for 24 h, after which the suspensor was replaced with clean cotton wool soaked in 10% glucose solution. Control mosquitoes were exposed in the same way but to a suspensor without fungus.
Survival Bioassays.
The effect of fungal infection on mosquito survival was tested in baseline and insecticide-resistant colonies of A. funestus (AfPerm), A. arabiensis from South Africa (Aa1DDT), A. arabiensis from Sudan (Aa2DDT), and A. gambiae s.s. from Obuasi, Ghana (AgBend). For each colony, nine test and nine control replicates were performed on 3 consecutive days, exposing ≈30 mosquitoes per replicate to dry spores of B. bassiana or control suspensors for 24 h. For the baseline A. funestus colony, six replicates were performed. Mosquito mortality was recorded daily, and cadavers were removed from each holding cage, dipped in 70% ethanol, and placed on moist filter paper in sealed Petri dishes. To verify infection, these were incubated at 25 °C for 3 or more days and assessed for fungus sporulation (i.e., emerging hyphae) by using a dissection microscope (16).
Permethrin Resistance Assays.
The resistant colonies of A. funestus (AfPerm), A. arabiensis from Sudan (Aa2DDT), and A. gambiae s.s. from Ahafo, Ghana (AgMR) were used to test the effect of fungal infection on permethrin resistance. A 3-day waiting period was chosen between fungal exposures and assessments for insecticide resistance to allow for some progression of the fungal infection while not losing large numbers through death. Mosquitoes from the same cohort received either a control, Beauveria, or Metarhizium treatment, of which 25 females per treatment were exposed 3 days later to a control paper and 25 females to a filter paper treated with 0.75% permethrin for 1 h, according to WHO protocol (39). Mosquitoes were then transferred to clean holding tubes and provided with 10% glucose solution by using cotton balls, and the proportion of dead mosquitoes was scored 24 h after insecticide exposure. Five replicates were performed per mosquito species and for each group; mortality per replicate exposed to insecticide was corrected by using mortality data of counterparts exposed to control papers, according to Abbott's formula (39). After mortality measurements, mosquitoes were removed from the exposure tubes with an aspirator, killed through drowning in 70% alcohol, and checked for fungal infection as described above.
With the same methods, the effect of a more advanced Beauveria infection was tested on A. funestus (AfPerm) by measuring the permethrin-resistance levels of control and Beauveria-infected mosquitoes 5 days after fungus exposure. Five replicates of 25 mosquitoes each were performed, and mortality of permethrin-exposed mosquitoes was corrected for control mosquito mortality.
DDT Resistance Assays.
The effect of Beauveria infection on DDT resistance was tested in five separate experiments in DDT-resistant A. arabiensis from Sudan (Aa2DDT) and A. gambiae s.s. from Ghana (AgMR). Three days after Beauveria exposure, control and infected mosquitoes (25 females per group) were exposed to 4% DDT papers or untreated papers, and mortality was measured 24 h later and corrected for control mortality as described for the permethrin assays.
Data Analysis.
Differences in the computed survival curves of treated and control mosquitoes were analyzed by using Cox regression analyses (40) with SPSS 15.0 software. Mortality data were analyzed separately for each tested mosquito species, including all replicates of the control and fungus-infected groups for both the baseline and the insecticide-resistant colony. A full model, with all main effects and possible interactions included, was calculated to estimate the effects of Beauveria on the hazard ratio. Beauveria-infected mosquito survival was analyzed separately to quantify and compare the effect of fungus infection between the resistant and the baseline colonies.
Mortality of the permethrin- and DDT-exposed groups was corrected for the mortality of their corresponding control groups by using Abbott's formula (39). Corrected mosquito mortality was compared by using a χ2 goodness-of-fit test with GenStat 9.0 software.
Supplementary Material
Acknowledgments.
We thank S. Koenraadt for assistance with statistical analyses and J. Hemingway and A. F. Read for comments and advice. This work was supported by the Adessium Foundation (Reeuwijk, The Netherlands), Dutch Scientific Organization VIDI Grant 864.03.004 (to B.G.J.K.), and the South African Research Chairs Initiative of the Department of Science and Technology with the National Research Foundation (M.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908530106/DCSupplemental.
References
- 1.Awolola TS, Brooke BD, Hunt RH, Coetzee M. Resistance of the malaria vector Anopheles gambiae s. s. to pyrethroid insecticides, in south-western Nigeria. Ann Trop Med Parasitol. 2002;96:849–852. doi: 10.1179/000349802125002581. [DOI] [PubMed] [Google Scholar]
- 2.Diabate A, et al. The spread of the Leu-Phe kdr mutation through Anopheles gambiae complex in Burkina Faso: Genetic introgression and de novo phenomena. Trop Med Int Health. 2004;9:1267–1273. doi: 10.1111/j.1365-3156.2004.01336.x. [DOI] [PubMed] [Google Scholar]
- 3.Tia E, et al. Pyrethroid and DDT resistance of Anopheles gambiae s.s. (Diptera: Culicidae) in five agricultural ecosystems from Cote-d'Ivoire. Bull Soc Pathol Exot. 2006;99:278–282. [PubMed] [Google Scholar]
- 4.Hargreaves K, et al. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;14:181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 5.Brooke BD, et al. Bioassay and biochemical analyses of insecticide resistance in southern African Anopheles funestus (Diptera: Culicidae) Bull Entomol Res. 2001;91:265–272. doi: 10.1079/ber2001108. [DOI] [PubMed] [Google Scholar]
- 6.Abdalla H, et al. Insecticide susceptibility and vector status of natural populations of Anopheles arabiensis from Sudan. Trans R Soc Trop Med Hyg. 2007;102:263–271. doi: 10.1016/j.trstmh.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Hargreaves K, et al. Anopheles arabiensis and An. quadriannulatus resistance to DDT in South Africa. Med Vet Entomol. 2003;17:417–422. doi: 10.1111/j.1365-2915.2003.00460.x. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. The Global Malaria Action Plan, Roll Back Malaria Partnership. Geneva: WHO; 2008. [Google Scholar]
- 9.Kelly-Hope L, Ranson H, Hemingway J. Lessons from the past: Managing insecticide resistance in malaria control and eradication programmes. Lancet Infect Dis. 2008;8:387–389. doi: 10.1016/S1473-3099(08)70045-8. [DOI] [PubMed] [Google Scholar]
- 10.Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Manag Sci. 2007;63:628–633. doi: 10.1002/ps.1406. [DOI] [PubMed] [Google Scholar]
- 11.Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 12.Zaim M, Guillet P. Alternative insecticides: An urgent need. Trends Parasitol. 2002;18:161–163. doi: 10.1016/s1471-4922(01)02220-6. [DOI] [PubMed] [Google Scholar]
- 13.Blanford S, et al. Fungal pathogen reduces potential for malaria transmission. Science. 2005;308:1638–1641. doi: 10.1126/science.1108423. [DOI] [PubMed] [Google Scholar]
- 14.Scholte E-J, et al. An entomopathogenic fungus for control of adult African malaria mosquitoes. Science. 2005;308:1641–1642. doi: 10.1126/science.1108639. [DOI] [PubMed] [Google Scholar]
- 15.Thomas MB, Read AF. Can fungal biopesticides control malaria? Nat Rev Microbiol. 2007;5:377–383. doi: 10.1038/nrmicro1638. [DOI] [PubMed] [Google Scholar]
- 16.Scholte EJ, Njiru BN, Smallegange RC, Takken W, Knols BGJ. Infection of malaria (Anopheles gambiae s. s.) and filariasis (Culex quinquefasciatus) vectors with the entomopathogenic fungus Metarhizium anisopliae. Malar J. 2003;2:29. doi: 10.1186/1475-2875-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Read AF, Lynch PA, Thomas MB. How to make evolution-proof insecticides for malaria control. PLoS Biol. 2009;7:e1000058. doi: 10.1371/journal.pbio.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beier JC. Malaria parasite development in mosquitoes. Annu Rev Entomol. 1998;43:519–543. doi: 10.1146/annurev.ento.43.1.519. [DOI] [PubMed] [Google Scholar]
- 19.Scholte E-J, Knols BGJ, Takken W. Infection of the malaria mosquito Anopheles gambiae with the entomopathogenic fungus Metarhizium anisopliae reduces blood feeding and fecundity. J Invertebr Pathol. 2006;91:43–49. doi: 10.1016/j.jip.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Clarkson JM, Charnley AK. New insights to the mechanisms of fungal pathogenesis in insects. Trends Microbiol. 1996;4:197–203. doi: 10.1016/0966-842x(96)10022-6. [DOI] [PubMed] [Google Scholar]
- 21.St. Leger RJ, Joshi L, Roberts D. Ambient pH is a major determinant in the expression of cuticle-degrading enzymes and hydrophobin by Metarhizium anisopliae. Appl Environ Microbiol. 1998;64:709–713. doi: 10.1128/aem.64.2.709-713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarroll L, et al. Insecticides and mosquito-borne disease. Nature. 2000;407:961–962. doi: 10.1038/35039671. [DOI] [PubMed] [Google Scholar]
- 23.McCarroll L, Hemingway J. Can insecticide resistance status effect parasite transmission in mosquitoes? Insect Biochem Mol Biol. 2002;32:1345–1351. doi: 10.1016/s0965-1748(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 24.Ericsson JD, Kabaluk JT, Goettel MS, Myers JH. Spinosad interacts synergistically with the insect pathogen Metarhizium anisopliae against two exotic wireworms, Agriotes lineatus and Agriotes obscurus (Coleoptera: Elateridae) J Econ Entomol. 2007;100:31–38. doi: 10.1603/0022-0493(2007)100[31:siswti]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Furlong MJ, Groden E. Evaluation of synergistic interactions between the Colorado potato beetle (Coleoptera: Chrysomelidae) pathogen Beauveria bassiana and the insecticides, imidacloprid, and cyromazine. J Econ Entomol. 2001;94:344–356. doi: 10.1603/0022-0493-94.2.344. [DOI] [PubMed] [Google Scholar]
- 26.Pachamuthu P, Kamble ST. In vivo study on combined toxicity of Metarhizium anisopliae 0 (Deuteromycotina: Hyphomycetes) strain ESC-1 with sublethal doses of chlorpyrifos, propetamphos, and cyfluthrin against German cockroach (Dictyoptera: Blattellidae) J Econ Entomol. 2000;93:60–70. doi: 10.1603/0022-0493-93.1.60. [DOI] [PubMed] [Google Scholar]
- 27.Bahiense TC, Fernandes EKK, Bittencourt VREP. Compatibility of the fungus Metarhizium anisopliae and deltamethrin to control a resistant strain of Boophilus microplus tick. Vet Parasitol. 2006;141:319–324. doi: 10.1016/j.vetpar.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Serebrov VV, et al. Effect of entomopathogenic fungi on detoxification enzyme activity in greater wax moth Galleria mellonella L. (Lepidoptera, Pyralidae) and role of detoxification enzymes in development of insect resistance to entomopathogenic fungi. Biol Bull. 2006;33:581–586. [Google Scholar]
- 29.Lines JD, Nassor NS. DDT resistance in Anopheles gambiae declines with mosquito age. Med Vet Entomol. 1991;5:261–265. doi: 10.1111/j.1365-2915.1991.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 30.Matambo TS, et al. Insecticide resistance in the malarial mosquito Anopheles arabiensis and association with the kdr mutation. Med Vet Entomol. 2007;21:97–102. doi: 10.1111/j.1365-2915.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- 31.Hunt RH, Brooke BD, Pillay C, Koekemoer LL, Coetzee M. Laboratory selection for and characteristics of pyrethroid resistance in the malaria vector Anopheles funestus. Med Vet Entomol. 2005;19:271–275. doi: 10.1111/j.1365-2915.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- 32.Amenya DA, et al. Isolation and sequence analysis of P450 genes from a pyrethroid-resistant colony of the major malaria vector Anopheles funestus. DNA Seq. 2005;16:437–445. doi: 10.1080/10425170500356727. [DOI] [PubMed] [Google Scholar]
- 33.Djouaka RF, et al. Expression of the cytochrome P450's, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s. s. from Southern Benin and Nigeria. BMC Genomics. 2008;9:538. doi: 10.1186/1471-2164-9-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St. Leger RJ, Joshi L, Bidochka MJ, Roberts DW. Construction of an improved mycoinsecticide overexpressing a toxic protease. Proc Natl Acad Sci USA. 1996;93:6349–6354. doi: 10.1073/pnas.93.13.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farenhorst M, et al. African water storage pots for the delivery of the entomopathogenic fungus Metarhizium anisopliae to the African malaria vectors Anopheles gambiae s. s. and Anopheles funestus. Am J Trop Med Hyg. 2008;78:910–916. [PubMed] [Google Scholar]
- 36.Hogg JC, Hurd H. Malaria-induced reduction of fecundity during the first gonotrophic cycle of Anopheles stephensi mosquitoes. Med Vet Entomol. 1995;9:176–180. doi: 10.1111/j.1365-2915.1995.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 37.Gassmann AJ, Stock SP, Carriere Y, Tabashnik BE. Effect of entomopathogenic nematodes on the fitness cost of resistance to Bt toxin Cry1Ac in pink bollworm (Lepidoptera: Gelechiidae) J Econ Entomol. 2006;99:920–926. doi: 10.1603/0022-0493-99.3.920. [DOI] [PubMed] [Google Scholar]
- 38.Gassmann AJ, Carriere Y, Tabashnik BE. Fitness costs of insect resistance to Bacillus thuringiensis. Annu Rev Entomol. 2009;54:147–163. doi: 10.1146/annurev.ento.54.110807.090518. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors, Bio-Efficacy and Persistence of Insecticides on Treated Surfaces. Geneva: WHO; 1998. [Google Scholar]
- 40.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.