Abstract
Group B Streptococcus (GBS) causes serious infection in neonates and is an important target of vaccine development. Zwitterionic polysaccharides (ZPS), obtained through chemical introduction of positive charges into anionic polysaccharides (PS) from GBS, have the ability to activate human and mouse antigen presenting cells (APCs) through toll-like receptor 2 (TLR2). To generate a polysaccharide vaccine with antigen (Ag) and adjuvant properties in one molecule, we have conjugated ZPS with a carrier protein. ZPS-glycoconjugates induce higher T-cell and Ab responses to carrier and PS, respectively, compared to control PS-glycoconjugates made with the native polysaccharide form. The increased immunogenicity of ZPS-conjugates correlates with their ability to activate dendritic cells (DCs). Moreover, protection of mothers or neonate offspring from lethal GBS challenge is better when mothers are immunized with ZPS-conjugates compared to immunization with PS-conjugates. In TLR2 knockout mice, ZPS-conjugates lose both their increased immunogenicity and protective effect after vaccination. When ZPS are coadministered as adjuvants with unconjugated tetanus toxoid (TT), they have the ability to increase the TT-specific antibody titer. In conclusion, glycoconjugates containing ZPS are potent vaccines. They target Ag to TLR2-expressing APCs and activate these APCs, leading to better T-cell priming and ultimately to higher protective Ab titers. Thus, rational chemical design can generate potent PS-adjuvants with wide application, including glycoconjugates and coadministration with unrelated protein Ags.
Keywords: adjuvant, dendritic cell, Group B Streptococcus
Classically, capsular polysaccharides (PS) from bacteria are considered to be T-independent Ags (1) able to activate B cells directly, without a contribution of help by CD4+ T cells. Therefore, the immune response to PS fails to induce significant and prolonged titers of antibody with high avidity in infants and persistent Ag-specific memory B cells in adults. For this reason, in vaccines, PS are mostly used conjugated with a carrier protein that provides T-cell help and consequently immunological memory (2). Glycoconjugate vaccines against the Gram-positive bacterium Group B Streptococcus (GBS) have been shown to confer serotype specific protection in mice and have been tested in clinical trials (3). In humans, addition of alum to the vaccine formulation did not further increase the immune response induced (4). In contrast, in animal models, adsorption to Al(OH)3 (alum) enhances the immunogenicity of the glycoconjugate (5, 6) which may be explained by the possibility that animals are more naive to GBS than humans. Moreover, a number of commercially available glycoconjugate vaccines, such as those against Meningococcus C, Haemophilus influenza, and the 7-valent Pneumococcus vaccine, use alum or aluminium phosphate as an adjuvant. Thus, the combination of glycoconjugates with adjuvants likely generates potent vaccines, able to activate APCs and induce strong Ag-specific T and B cell responses.
It has been shown that capsular PS that naturally carry a zwitterionic charge motif (ZPS) in their repeating structure activate T cells in coculture with monocytes. This activation is MHC class II-dependent (7), and upregulation of costimulatory molecules on APCs contributes to this stimulation (8). The structures of the natural ZPS, polysaccharide A (PSA) extracted from the capsule of Bacteroides fragilis, and Sp1 from type 1 Streptococcus pneumoniae, have been resolved (9, 10). Recently, Toll-like receptor 2 (TLR2) has been identified as the receptor implicated in the activation of APCs induced by PSA (11). Because PSA injected in a rat abscess model induces protection against the bacterium (12), the use of ZPS that combine Ag and adjuvant properties in the same molecule may represent a strategy for vaccine design. Moreover, a number of vaccine candidates consisting of protein Ag fused to synthetic lipopeptides targeting TLR2 have been proposed in the past. For instance, a vaccine against tuberculosis composed of a protein Ag linked to a TLR2 agonist has been generated (13) and MHC class I-restricted peptide epitopes linked to lipopeptides recognized by TLR2 have been developed (14, 15). We have applied the same strategy to PS Ags and selected for this purpose the PS extracted from the capsule of GBS serotypes Ib and V.
Native PS of the GBS capsule are anionic and T-independent Ags. We have demonstrated that the chemical introduction of zwitterionic motifs into PS from GBS generates ZPS that are able to directly activate mouse and human APCs through a TLR2-dependent mechanism (16). We reasoned that first, GBS-derived ZPS alone should be able to act as adjuvants, as do other TLR2 agonists. Here, we show that injection of these ZPS together with unconjugated Ag tetanus toxoid (TT) increases the TT-specific antibody titer, clearly demonstrating their adjuvant activity in vivo. Second, if unconjugated ZPS are true T-cell-dependent Ags, they should be able to induce a strong IgG response on their own. We injected ZPS alone into mice but were not able to detect increased Ab titers to the ZPS or the maternal PS. Therefore, we conjugated ZPS prepared from GBS capsular PS with a carrier protein to provide a canonical T-cell antigen. We tested if conjugated ZPS maintain the adjuvant potential in vivo that the unconjugated forms have shown in vitro (16) and whether or not ZPS-containing glycoconjugate vaccines are more immunogenic than the corresponding native PS-conjugates. We also assessed whether the increased immunogenicity found in vivo is TLR2-dependent.
The purpose of this study is to rationally design a glycoconjugate both able to activate T cells and to target and activate cells expressing TLR2 to unite in the same molecule Ag and adjuvant activities. This approach represents a promising strategy for the use of chemical modification in glycoconjugate vaccine design.
Results
ZPS-Conjugates Are More Immunogenic Than the Corresponding PS-Conjugates.
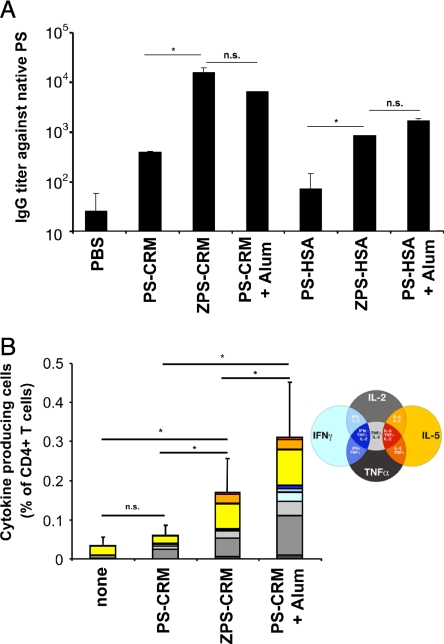
Given the ability of chemically derived ZPS to activate APCs in vitro (16), we attempted to test whether this biological activity would also confer adjuvant function in vivo. Therefore, we conjugated ZPS with a carrier protein and compared them to the glycoconjugates containing the native form of the corresponding PS. The zwitterionic PS were obtained as described by the introduction of a positive charge in the aliphatic chain of the terminal N-acetylneuraminic acid (NeuNAc) residue. The covalent attachment of the carrier protein CRM197 or HSA to the zwitterionic PS was performed according to the protocol used for the conjugation of the native CPS (17). Experimental details and the final structure of the conjugates are explained in the SI Text. We conjugated ZPS to the widely used and highly immunogenic carrier protein CRM197 or to HSA (SI Text). NMR spectra confirmed the successful modification of the native serotype Ib polysaccharide that generated the zwitterionic motif (Fig. S1, Ib-ZPS), and the integrity of Ib ZPS polysaccharide after conjugation to CRM197 (Fig. S1, Ib-ZPS-CRM) or HSA. The PS-to-protein ratio of all glycoconjugates is 1:1 (w:w). We immunized mice with ZPS-conjugates or those made with the native form of the PS and compared their immunogenicity. As positive control we used the PS-conjugate formulated with alum as adjuvant. ZPS from both serotypes Ib and V were used to generate glycoconjugates, and the results obtained after immunization were similar for serotype Ib (shown throughout this study) and V. After three immunizations, sera were tested in ELISAs by using the native PS conjugated to an unrelated carrier for detection (PS-HSA for CRM197-containing glycoconjugates and vice versa). By this method, we assessed for IgG antibody titers specific for the native PS, which is the relevant form present in the GBS capsule. As shown in Fig. 1A, the ZPS conjugated with CRM197 was considerably more efficient in inducing antibodies against the bacterial PS than the corresponding native PS-conjugate. The titers induced by ZPS-conjugate reached or exceeded those induced by the positive control, alum-adjuvanted PS-conjugate. As expected, HSA turned out to be a less immunogenic carrier protein, because native PS conjugated to HSA did not induce antibodies significantly above background. In contrast, ZPS-HSA did induce a detectable titer that was comparable to that found after vaccination with the positive control containing alum. We also tested whether Ag-specific titers were induced more rapidly, as described for TLR2 agonists (18), and whether the Ig subclass distribution was altered by the ZPS. ZPS-conjugates induced high titers already after two injections (Fig. S2) and did not alter the relative contributions of different IgG subclasses (Fig. S3). Thus, we have generated glycoconjugates that show accelerated and increased immunogenicity in the absence of an additional adjuvant.
Fig. 1.
ZPS-conjugate immunogenicity. (A) Balb/C mice were immunized thrice as indicated. Two weeks after the third immunization, sera were tested by ELISA for PS-specific IgG titer. Results shown are pooled from two experiments using Balb/C mice out of a total of five experiments using Balb/C, CD1 or C57BL/6 strains with similar results. (B) Mice were vaccinated with glycoconjugates as indicated in (A) and spleen CD4+ T-cell cytokine responses to CRM197 at 2 weeks post-third dose were evaluated. T cells producing one, two, or three cytokines are represented as annotated and add up to the bars shown. Therefore, the bar height indicates the total of all cytokine positive CD4+ cells as percentage of total CD4+ cells. Error bars indicate SD of total percentage of all cytokines of six mice. This experiment was performed at least three times with similar results. Statistical significance was analyzed by using unpaired Student's t test. *, P < 0,01; n.s., not significant.
To show that higher IgG production is due to a better anti-carrier T-cell response, we evaluated the ex vivo T-cell response in mice immunized with glycoconjugates. Splenocytes were cultured with CRM197, and after 4 h of stimulation, Brefeldin A was added overnight to block secretion and retain cytokines in the T cells, which allows detection of cytokines produced by individual CD4 positive T cells through intracellular staining. The total height of bars in Fig. 1B shows the overall percentage of T cells responding to CRM197 by cytokine production, and the color-coding indicates which cytokines or combinations are produced by individual T cells. We find that ZPS-CRM induce a higher overall percentage of cytokine-producing CRM197-specific CD4 T cells than the corresponding PS-CRM (P < 0.01). In all groups, vaccinated with or without adjuvant, the dominant cytokines induced are IL-5, IL-2, and TNF-α, a combination that is expected for effector and memory T-cell populations in the Th2 prone Balb/C mice used here. We conclude that the ZPS conjugation to CRM197 leads to enhanced CRM197-specific T-cell responses compared to glycoconjugates containing native PS. In contrast, the cytokine profile is unaltered by ZPS-conjugates compared to that induced by PS-conjugates, suggesting that the adjuvant effect increases the magnitude but does not alter the quality of the specific T-cell response. We also tested the T-cell response to the whole glycoconjugate or single components of it and found that the response is directed against the protein, not the PS part of the glycoconjugate (Fig. S4). We conclude that ZPS act as adjuvants for increased Ab production through increased T-cell responses to the protein part of the glycoconjugate.
We next tested whether the adjuvant effect of ZPS enhances the Ab responses to conjugated or unconjugated proteins. After injection of ZPS-CRM or ZPS-HSA as described above, we found the ELISA titers to these proteins were strongly enhanced compared to native PS-CRM or PS-HSA injection (Fig. S5A). We also coadministered ZPS with the unconjugated protein Ag TT and found strongly increased antiTT titers (Fig. S5B). In conclusion, ZPS are able to act as adjuvant both for conjugated and unconjugated proteins. In contrast, ZPS used alone, without carrier, are not able to induce PS-specific IgG antibody titers (Fig. S2), suggesting that a protein component is required and that the combination of B cell epitopes and TLR2 agonist activity is not sufficient to increase immunogenicity of the PS part.
ZPS-Conjugates Activate Bone-Marrow-Derived DCs (BM-DCs).
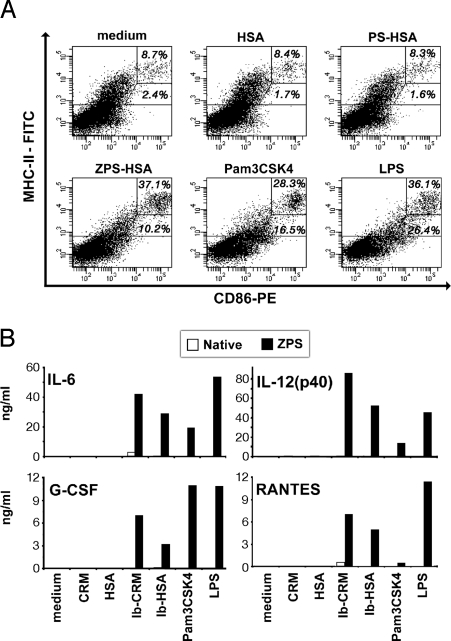
Because glycoconjugates containing ZPS are more efficient than the corresponding native ones at inducing Ab and T-cell responses, we tested whether this correlates with their ability to activate APCs. The dot plots in Fig. 2A show that ZPS conjugated to HSA are able to induce the upregulation of MHC class II and the costimulatory molecule CD86, whereas HSA alone and PS-HSA do not activate BM-DCs. Similar results were obtained also with CRM197 as carrier protein (Fig. S6). Pam3CSK4, a TLR2 agonist, and LPS, a TLR4 agonist, are used as positive controls. ZPS-conjugates induced in BM-DCs the production of cytokines like IL-6 and IL-12, chemokines like regulated upon activation, normal T cells expressed and secreted (RANTES) and the stimulating factor G-CSF (Fig. 2B). Taken together, these results strongly suggest that the ability of ZPS to activate DCs will improve the priming of carrier-specific T cells and as a consequence the T-cell help given to PS-specific B cells, ultimately leading to higher antibody titers. Thus, we most likely have generated a glycoconjugate that contains B-cell epitopes, T-cell epitopes and adjuvant properties, leading to an overall better immunogenicity.
Fig. 2.
BM-DCs are activated by ZPS-conjugates. (A) Mouse BM-DCs were incubated for 20 h with ZPS and PS conjugates (10 μg/mL), CRM197 and HSA alone (10 μg/mL), and Pam3CSK4 or LPS (1 μg/mL). The upregulation of CD86 and MHC class II was evaluated by flow cytometry. Data represent mean + SD of triplicates and are representative of three experiments. (B) After 20 h of incubation, cytokine and chemokine presence in the supernatants was tested through Bio-plex analysis. Data represent mean + SD of triplicates and are representative of two experiments with similar outcome.
ZPS-Conjugates Confer Enhanced Protection Against GBS Infection.
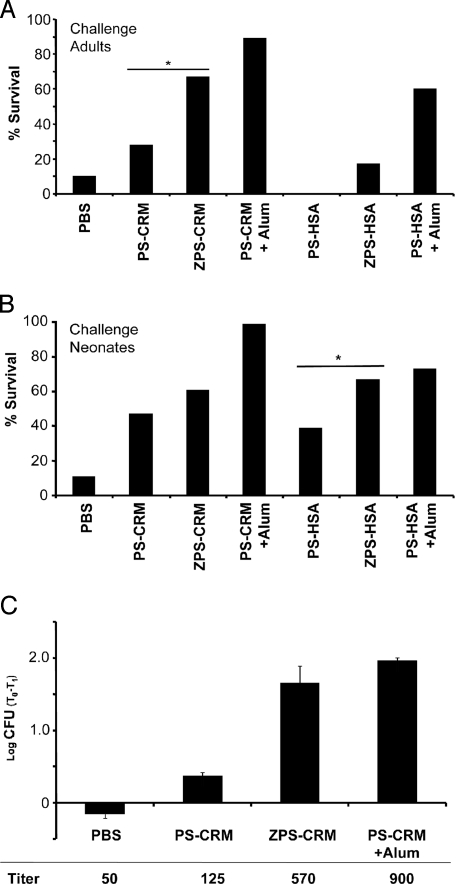
To verify whether antibodies induced by ZPS-conjugates were also protective against bacterial infection, we immunized CD1 or Balb/C mice, and after three immunizations we performed a challenge with GBS strain H36B. The percentage of survival after 2 days post challenge was evaluated and the statistical significance was estimated by Fisher's exact test. As shown in Fig. 3A, protection conferred by ZPS-conjugates is significantly increased compared to that given by PS-conjugates and similar to that induced by alum-adjuvanted PS-conjugates, the positive control. We also used a neonatal mouse model of group B streptococcal infection (19) to evaluate the protection after challenge in the offspring of immunized mothers. Neonates from mothers immunized with ZPS-conjugates are better protected from GBS infection than those born from mice immunized with PS-conjugates. This is particularly visible in the context of the less immunogenic carrier HSA, and ZPS-HSA induce a level of protection in neonates that is similar to that of alum-adjuvanted PS-HSA, as determined by Fisher's exact test (Fig. 3B). By using an opsonophagocytosis assay, we confirmed that sera from mice immunized with ZPS-conjugates were more efficient to promote killing of GBS by differentiated HL60 cells than sera from mice immunized with PS-conjugates (Fig. 3C), consistent with the above protection data. The half-maximal titers were also calculated from the opsonophagocytosis data (Fig. 3C bottom), and ZPS-CRM induces clearly higher titers than PS-CRM, reaching levels comparable to those of the Alum-adjuvanted positive control. We were also interested to see if Alum could be used in combination with the ZPS-conjugates and whether this combination would confer a further increase in immunogencity. Results in Fig. S7 A and B show that the ZPS adjuvant activity synergizes with alum to increase further the PS-specific IgG antibody titer and to induce better CRM197-specific CD4+ T-cell responses.
Fig. 3.
ZPS-conjugates confer protection against GBS challenge. (A) CD1 mice were immunized i.p. with ZPS and PS conjugates (1 μg). PS-conjugate plus alum was used as a positive and PBS as negative control. Two weeks after the third immunization, adult mice received a lethal challenge of GBS, and the percentage of survival 2 days after the injection was evaluated. Pooled data from two experiments are shown. (B) A lethal dose of GBS was administered s.c. to the offspring of mothers immunized as in (A). The challenge was performed within 48 h after birth, and the percentage of survival was estimated 2 days post-injection. Each experiment was repeated at least three times by using CD1 or Balb/C strains with similar outcome. Statistical significance was calculated with Fisher's Exact Test. *, P < 0.01. (C) Opsonophagocytosis assays were performed to evaluate the capacity of sera from mice immunized as in (A) to induce GBS killing by differentiated HL60 cells in presence of rabbit complement. Triplicate results from sera diluted 1:100 were expressed as the mean log10 reduction in GBS colony-forming units before (T0) and after (T1) 60 min of incubation at 37 °C. From the whole titration curves of the same assay, we also calculated opsonophagocytosis titers that are indicated below the graph.
TLR2 Is Critical for ZPS-Conjugate Adjuvant Activity In Vivo.
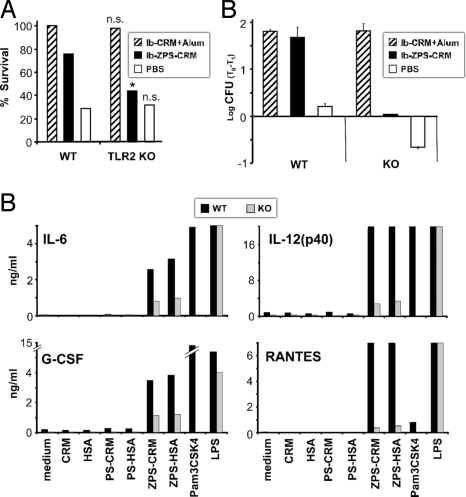
We and others demonstrated that the ability of ZPS to activate APCs is TLR2-dependent (11, 16). To test if this receptor is also required for the adjuvant activity of ZPS-conjugates in vivo, we immunized female TLR2−/− mice with glycoconjugates and paired them after three immunizations with wt males. The offspring received a challenge with a lethal dose of GBS and the percentage of survival was evaluated. Following this protocol, the offspring are genotypically heterozygous for TLR2 and therefore express this receptor, but they have acquired the antibody repertoire from mothers immunized in the absence of this receptor. In this experiment we compared the Alum-adjuvanted PS-conjugate, our positive control, to the ZPS-conjugate in wt versus ko mice. Fig. 4A shows that at challenge doses leading to 20–30% survival in the negative control groups, alum-adjuvanted native glycoconjugate induces full protection in litters born from vaccinated wt and TLR2−/− mothers. In contrast, ZPS-conjugate induced protection is significantly reduced in litters from TLR2−/− mothers, clearly indicating that TLR2 is crucial for the protection induced by ZPS-conjugates. Similarly, the opsonophagocytosis titers induced by ZPS-conjugates were abolished in the absence of TLR2, whereas those induced by PS-conjugate plus alum were not (Fig. 4B). To see whether the observed differences between wt and TLR2−/− mice are also reflected in differential DC activation by ZPS-conjugates, we assessed cytokine production by BM-DCs from both genotypes in response to ZPS-conjugates. As shown in Fig. 4C, the production of IL-6, IL-12, G-CSF, and RANTES induced by ZPS-conjugate or Pam3CSK4 is strongly reduced when BM-DCs were generated from TLR2−/− mice. No difference is observed when using the TLR4 agonist LPS.
Fig. 4.
ZPS-conjugate activity in vivo is TLR2-dependent. (A) C57BL/6 wt and TLR2−/− mice on a C57BL/6 background were treated i.p. as indicated. WT and TLR2−/− female mice received three doses at day 1, 21, 35, and at day 38 all mice were coupled with wt males. The offspring from wt and TLR2−/− mice were injected s.c. with a lethal dose of GBS. The challenge doses were chosen to have the same percentage of survival for neonates born from wt or TLR2−/− mice treated with PBS. Significance was calculated by using Fisher's Exact Test. *, P < 0,05; n.s., not significant compared with the respective wt group. Data pooled from two independent experiments are shown. (B) Opsonophagocytosis assays were performed by using sera from wt and TLR2−/− mothers of (A). Triplicate results from sera diluted 1:100 were expressed as the mean log10 reduction in GBS colony-forming units before (T0) and after (T1) 60 min of incubation at 37 °C. (C) BM-DCs generated from the bone-morrow of wt or TLR2−/− mice were incubated for 20 h with ZPS and PS conjugates (10 μg/mL), CRM197 and HSA alone (10 μg/mL), and Pam3CSK4 or LPS (1 μg/mL). After 20 h of incubation the supernatants were assessed by Bio-plex analysis for cytokine and chemokine content. The production of IL-6, IL-12, G-CSF, and RANTES induced by ZPS-conjugates or Pam3CSK4 was considerably reduced in supernatants of BM-DCs generated from TLR2−/− mice. LPS induce the same cytokine and chemokine production in both genotypes. Data represent mean + SD of triplicates and are representative of two experiments.
Thus, TLR2 has a crucial role in the in vivo activity of ZPS-conjugates and is required for the induction of higher functional Ab titers and consequently for higher protection. Since we observed in vitro that activation of BM-DCs by ZPS-conjugate is TLR2 dependent, we conclude that TLR2-dependent DC activation is the most likely mechanism of in vivo adjuvanticity of ZPS.
Discussion
Adjuvants represent an important component of many modern vaccines as they increase the immunogenicity of coadministered Ags such as purified, soluble recombinant proteins, which are per se less immunogenic than whole or split, killed or attenuated pathogens used in the past. Although a number of glycoconjugate vaccines induce high antibody titers without adjuvants, in other cases adjuvants are used to induce a protective immune response. Here, we show that rational chemical modification can be used to produce a glycoconjugate vaccine in which the PS Ag has acquired additional adjuvant properties. This work is based on previous findings showing that natural ZPS, such as PSA, activate T cells and APCs (7, 8, 12). Because this biological activity depends on the zwitterionic structure of this capsular PS (20–22), we generated a similar charge motif in a vaccine candidate PS by the chemical introduction of positive charges into the naturally anionic capsular PS of GBS (16). The resulting ZPS activate APCs through a TLR2-dependent mechanism, and this effect depends on the integrity of the zwitterionic motif (16), similar to what was found for natural ZPS.
Here, we show that in vivo, ZPS enhance IgG titers specific for a coadministered protein Ag, clearly demonstrating the adjuvant activity of the ZPS. Adjuvanticity is maintained also when the ZPS is used in place of the native PS as Ag in a glycoconjugate vaccine against GBS. In fact, ZPS-conjugates are more immunogenic than the corresponding PS-conjugates, and mice immunized with ZPS-conjugates are better protected from GBS infection than mice immunized with the PS-conjugates. Increased induction of specific Ab titers is associated with increased T-cell responses to the carrier protein. The fact that unconjugated ZPS are unable to induce Ab responses strongly suggests that T-cell help is required for ZPS adjuvanticity. The observation that the increased immunogenicity of ZPS-conjugates is TLR2 dependent further suggests that TLR2-expressing APCs may be involved. We find that ZPS-conjugates activate BM-DCs in vitro inducing surface molecules for T-cell priming and production of cytokines, chemokines and growth factors. BM-DC activation induced by ZPS-conjugates is TLR2 dependent because BM-DCs generated from TLR2−/− mice are largely unresponsive. Taking together these data, we hypothesize that the increased immunogenicity of ZPS-glycoconjugates is based on the TLR2 agonist properties of ZPS that allow these conjugates to target TLR2-expressing DCs and activate them. This in turn leads to better T-cell priming, increased T-cell help and ultimately to higher specific Ab titers (Fig. 5). This model also explains why conjugation to a protein carrier is still required for the immunogenicity of ZPS; the TLR2-dependent adjuvant effect on DCs can be transmitted to B cells only via T-cell help, and in fact we find increased T-cell responses to the carrier protein when conjugated to ZPS. In contrast, we were not able to find ZPS- or PS-specific T-cell responses.
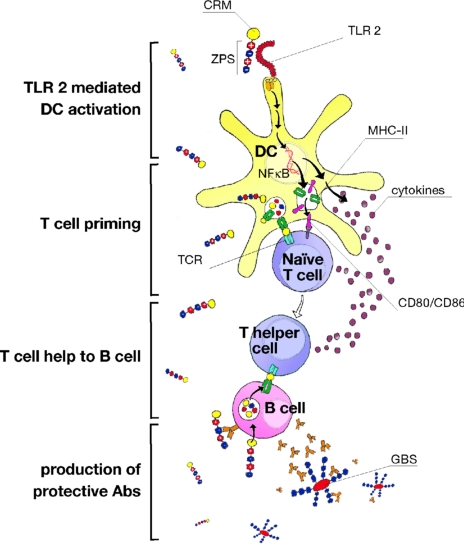
Fig. 5.
Proposed mechanism of increased immunogenicity of ZPS-conjugates. ZPS-conjugates are targeted to TLR2 positive DCs and activate them through receptor engagement. Increased DC activation leads to improved priming of naive T cells specific for the conjugated protein. Enhanced T-cell help to B cells will lead to higher Ab titers and thus improved protection against infection by GBS.
Compared to other Ag presenting cells, DCs are critical for the full activation of naive T cells, and their maturation is essential for this (23, 24). DC maturation can be triggered by Toll-like receptor agonists that induce the increase in surface MHC class II and costimulatory molecules and thereby link innate to adaptive immune responses (25, 26). TLR2 has already been demonstrated to be the receptor implicated in the ability of PSA to link innate and adaptive immunity (11). Moreover the Haemophilus influenzae type b-outer membrane protein complex glycoconjugate has an optimal immunogenicity thanks to the TLR2 agonist properties of the carrier protein (18). Other examples of vaccines owing their potency at least in part to the sometimes fortuitous presence of TLR2 agonists are the yellow fever vaccine (27) and PS vaccines against Streptococcus pneumoniae (28). Thus, TLR2 is a receptor implicated in the strong immunogenicity of natural ZPS but also of vaccines based on PS, attenuated virus or glycoconjugates.
Efforts have also been made to generate synthetic vaccines in which a peptide Ag was covalently linked to a TLR2 agonist (13, 15). Such linked peptides are better internalized by DCs than the peptide alone or mixed with the TLR2 agonist, and this appears to depend on the TLR2 agonist internalization that carries along the linked peptide (29). Therefore, we speculate that the ZPS as TLR2 agonist may increase T-cell priming through the internalization of ZPS-conjugates by DCs and targeting the carrier protein to endolysosomes. It has also been shown for vaccine formulations containing agonists to TLRs other than TLR2 that the physical coupling of Ag and agonist is far more effective than simple coadministration. We show here that ZPS are effective in both coupled and uncoupled form, and experiments are underway to compare directly the potency of these formulations.
When TLR2-deficient BM-DCs were stimulated with ZPS-conjugates, cytokine production was strongly reduced but not entirely abolished, at difference to the complete DC unresponsiveness to Pam3CSK4. This may be due to a different usage of the TLR2/1 or TLR2/6 heterodimers as compared to Pam3CSK4, or to an additional receptor. A synergy between TLRs and C-type lectin receptors recognizing PS structures has been described (30–32), and we speculate that similar mechanisms may contribute to the strong DC activation by ZPS.
It has been proposed that optimal vaccine formulations are able to target the Ag to DCs to allow more efficient Ag processing and presentation, and moreover provide the stimuli to induce DC maturation to enhance the adaptive immune response (33, 34). The ZPS-conjugates have both properties: a PS Ag that targets itself to TLR2-expressing DCs and activates, through TLR2, DC maturation, thereby increasing processing and presentation of the conjugated protein to naive T cells. Here, we have used this strategy first to provide increased T-cell help to B cells recognizing the B cell epitopes of the PS, and second to increase the immune response to an unrelated protein, either conjugated or coadministered.
Over the years, chemical tools have proven essential to make progress in the generation of synthetic oligosaccharides and glycoconjugates (35). Chemical modifications in PS structure have been performed to enhance the PS-specific IgG antibodies or to eliminate epitopes that produce antibodies cross-reactive with host tissue (36, 37). More recently, as an example of rational design of the carbohydrate, the capsular PS serotype V GBS has been chemically desialylated to generate a glycoconjugate able to induce the IgM-to-IgG switching (38). In the present work, we have exploited existing structural knowledge of the charge motif in natural ZPS to generate chemically a similar structure in a vaccine PS and thereby to obtain the TLR2 agonist properties conferred by this peculiar charge motif. The modified PS acts as an adjuvant and has been used to generate a glycoconjugate vaccine that is more immunogenic and more protective. Our approach adds, through rational chemical modification, biological function to vaccine Ags. This strategy may be applied to many other polysaccharides and represents a path for rational chemical design of adjuvants and glycoconjugate vaccines.
Materials and Methods
Mice and Immunizations.
Groups of six to eight female, 6-week-old Balb/C, C57BL/6, CD1 outbred mice (Charles River), or C57BL/6 TLR2−/− (39) (kindly provided by Giuseppe Teti, Messina, Italy) were used for experiments reviewed and approved by the institutional review committees. Animals were immunized i.p. at days 0, 21, and 35 with 1 μg glycoconjugates made as indicated. Where indicated, alum was used at 0.4 mg AlOH3/dose. Serum and spleen samples were collected at 2 weeks after the third immunization. In adult mice, the challenges were performed injecting i.p. strain H36B (serotype Ib) at 1 × 108 CFU at 2 weeks after the third immunization. For the neonatal challenge experiments, we first determined the 80% lethal doses (LD80) by titration in both wt and ko mice. H36B was administered at 1× LD80 to the pups s.c. between 24 and 48 h after birth. Mortality was recorded daily for the 2 days after challenge. Statistical significance was estimated by Fisher's exact test.
Ag-Specific T-Cell Cytokine Responses.
Three mice per treatment were killed, spleens were collected, and single cell suspensions were obtained. Red blood cells were lysed and splenocytes cultured in RPMI (Gibco) containing 2.5% FCS (HyClone), beta-mercaptoethanol and antibiotics. Splenocytes were stimulated in the presence of anti-CD28 (1 μg/mL) (Becton-Dickinson) and the carrier protein CRM197 (30 μg/mL), or with anti-CD28 alone (unstimulated, <0.1% total cytokine-positive cells), or with anti-CD28 plus anti-CD3 (0.1 μg/mL) (Becton-Dickinson). After 4 h of stimulation, Brefeldin A (2.5 μg/mL)(Sigma–Aldrich) was added for additional 12 h. Cells were washed and stained with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit for 405 nm excitation (Invitrogen). Cells were fixed, permeabilized, and stained with the following mAbs: allophycocyanin-Alexa750-conjugated anti-CD4 (Caltag), Pacific Blue-conjugated anti-CD3, Alexa700-conjugated anti-TNF-α, peridinin chlorophyll protein cyanine5.5-conjugated anti-IFNγ, PE-conjugated anti-IL-5, and Alexa488-conjugated anti-IL-2 (Becton-Dickinson). Cells were acquired on a LSR-II (Becton-Dickinson) and analyzed by using FlowJo software (Tree Star). For each individual mouse, percentages of unstimulated samples were subtracted from the Ag-stimulated sample.
Determination of Ag-Specific Antibody by ELISA.
For titration of IgG specific for the native polysaccharides, Maxisorp plates (Nunc) were coated with 1 μg/mL (in PBS) of the glycoconjugate that contains a different carrier protein to that used for the immunization, to detect only the antibodies specific for the polysaccharide. Antibody titers are those dilutions that gave an OD higher than the mean plus 5× the SD of the average OD obtained in the preimmune sera. The titers were normalized with respect to the reference serum assayed in parallel.
Determination of Cytokine and Chemokine Production.
Cytokine and chemokine secretion in supernatants was assessed by Bio-Plex analysis (Bio-Rad), according to the manufacturer's instructions by using the mouse 23-Plex panel. The following soluble proteins are assayed: IL-1α, IL1-β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-17, Eotaxin, G-CSF, GM-CSF, IFN-γ, KC, MCP-1, macrophage inflammatory protein (MIP)-1α, MIP-1β, RANTES, and TNF-α.
Opsonophagocytosis Assay.
Serum samples from mice immunized with serotype Ib glycoconjugates were tested for their in vitro ability to promote the opsonization of type Ib GBS strain H36B for phagocytosis and killing by differentiated HL60 cells in the presence of rabbit active complement. Results were expressed as the mean log10 reduction in GBS colony-forming units before and after 60 min of incubation at 37 °C.
Generation of BM-DCs.
Mouse BM-DCs were generated culturing femoral bone marrow with recombinant murine GM-CSF (PeproTech) as described in ref. 40. At day 6, BM-DCs were cultured for 20 h in complete medium with β-mercaptoethanol 50 μM (Sigma) and 100 U/mL mGM-CSF, by using pro-bind U-bottom 96-well plates (Becton-Dickinson). Where indicated, cells were treated with Pam3CSK4 (Alexis Biochemicals). Cells were stained with PE-conjugated anti-CD86, FITC-conjugated anti-MHC class II, and allophycocyanin-conjugated anti-CD11c. Rabbit serum was used as a blocking agent. The acquisition was made on a LSR-II and data analyzed by using DIVA software (Becton-Dickinson).
Supplementary Material
Acknowledgments.
We thank Chiara Sammicheli and Sandra Nuti for support on FACS analysis; Francesca Necchi for challenge experiments; Giuseppe Teti for making TLR2 ko mice available and for stimulating discussions and experiments; Paolo Costantino, Stefania Crotta, and Ennio De Gregorio for critically reading the manuscript and valuable comments; and Giorgio Corsi for artwork.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.L.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903313106/DCSupplemental.
References
- 1.Weintraub A. Immunology of bacterial polysaccharide antigens. Carbohydr Res. 2003;338:2539–2547. doi: 10.1016/j.carres.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Lesinski GB, Westerink MA. Novel vaccine strategies to T-independent antigens. J Microbiol Methods. 2001;47:135–149. doi: 10.1016/s0167-7012(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 3.Paoletti LC, Kasper DL. Glycoconjugate vaccines to prevent group B streptococcal infections. Expert Opin Biol Ther. 2003;3:975–984. doi: 10.1517/14712598.3.6.975. [DOI] [PubMed] [Google Scholar]
- 4.Paoletti LC, et al. Effects of alum adjuvant or a booster dose on immunogenicity during clinical trials of group B streptococcal type III conjugate vaccines. Infect Immun. 2001;69:6696–6701. doi: 10.1128/IAI.69.11.6696-6701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guttormsen HK, Wetzler LM, Finberg RW, Kasper DL. Immunologic memory induced by a glycoconjugate vaccine in a murine adoptive lymphocyte transfer model. Infect Immun. 1998;66:2026–2032. doi: 10.1128/iai.66.5.2026-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paoletti LC, Kennedy RC, Chanh TC, Kasper DL. Immunogenicity of group B Streptococcus type III polysaccharide-tetanus toxoid vaccine in baboons. Infect Immun. 1996;64:677–679. doi: 10.1128/iai.64.2.677-679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalka-Moll WM, et al. Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J Immunol. 2002;169:6149–6153. doi: 10.4049/jimmunol.169.11.6149. [DOI] [PubMed] [Google Scholar]
- 8.Stephen TL, et al. Effect of B7–2 and CD40 signals from activated antigen-presenting cells on the ability of zwitterionic polysaccharides to induce T-cell stimulation. Infect Immun. 2005;73:2184–2189. doi: 10.1128/IAI.73.4.2184-2189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzianabos AO, et al. The capsular polysaccharide of Bacteroides fragilis comprises two ionically linked polysaccharides. J Biol Chem. 1992;267:18230–18235. [PubMed] [Google Scholar]
- 10.Tzianabos AO, Kasper DL, Onderdonk AB. Structure and function of Bacteroides fragilis capsular polysaccharides: Relationship to induction and prevention of abscesses. Clin Infect Dis 20 Suppl. 1995;2:S132–140. doi: 10.1093/clinids/20.supplement_2.s132. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, et al. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med. 2006;203:2853–2863. doi: 10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stingele F, et al. Zwitterionic polysaccharides stimulate T cells with no preferential V beta usage and promote anergy, resulting in protection against experimental abscess formation. J Immunol. 2004;172:1483–1490. doi: 10.4049/jimmunol.172.3.1483. [DOI] [PubMed] [Google Scholar]
- 13.Wang B, et al. A Toll-like receptor-2-directed fusion protein vaccine against tuberculosis. Clin Vaccine Immunol. 2007;14:902–906. doi: 10.1128/CVI.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson DC, et al. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Acad Sci USA. 2004;101:15440–15445. doi: 10.1073/pnas.0406740101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chua BY, Zeng W, Jackson DC. Synthesis of toll-like receptor-2 targeting lipopeptides as self-adjuvanting vaccines. Methods Mol Biol. 2008;494:247–261. doi: 10.1007/978-1-59745-419-3_14. [DOI] [PubMed] [Google Scholar]
- 16.Gallorini S, et al. Introduction of zwitterionic motifs into bacterial polysaccharides generates TLR2 agonists able to activate APCs. J Immunol. 2007;179:8208–8215. doi: 10.4049/jimmunol.179.12.8208. [DOI] [PubMed] [Google Scholar]
- 17.Wessels MR, et al. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J Clin Invest. 1990;86:1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latz E, Franko J, Golenbock DT, Schreiber JR. Haemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J Immunol. 2004;172:2431–2438. doi: 10.4049/jimmunol.172.4.2431. [DOI] [PubMed] [Google Scholar]
- 19.Rodewald AK, Onderdonk AB, Warren HB, Kasper DL. Neonatal mouse model of group B streptococcal infection. J Infect Dis. 1992;166:635–639. doi: 10.1093/infdis/166.3.635. [DOI] [PubMed] [Google Scholar]
- 20.Tzianabos AO, Onderdonk AB, Rosner B, Cisneros RL, Kasper DL. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 21.Kalka-Moll WM, et al. Effect of molecular size on the ability of zwitterionic polysaccharides to stimulate cellular immunity. J Immunol. 2000;164:719–724. doi: 10.4049/jimmunol.164.2.719. [DOI] [PubMed] [Google Scholar]
- 22.Tzianabos AO, Onderdonk AB, Smith RS, Kasper DL. Structure-function relationships for polysaccharide-induced intra-abdominal abscesses. Infect Immun. 1994;62:3590–3593. doi: 10.1128/iai.62.8.3590-3593.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellman I, Steinman RM. Dendritic cells: Specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 24.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 25.Medzhitov R, Janeway CA., Jr Innate immune recognition and control of adaptive immune responses. Semin Immunol. 1998;10:351–353. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 26.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 27.Querec T, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sen G, Khan AQ, Chen Q, Snapper CM. In vivo humoral immune responses to isolated pneumococcal polysaccharides are dependent on the presence of associated TLR ligands. J Immunol. 2005;175:3084–3091. doi: 10.4049/jimmunol.175.5.3084. [DOI] [PubMed] [Google Scholar]
- 29.Khan S, et al. Distinct uptake mechanisms but similar intracellular processing of two different toll-like receptor ligand-peptide conjugates in dendritic cells. J Biol Chem. 2007;282:21145–21159. doi: 10.1074/jbc.M701705200. [DOI] [PubMed] [Google Scholar]
- 30.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukhopadhyay S, Herre J, Brown GD, Gordon S. The potential for Toll-like receptors to collaborate with other innate immune receptors. Immunology. 2004;112:521–530. doi: 10.1111/j.1365-2567.2004.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dennehy KM, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinman RM, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J Clin Invest. 2002;109:1519–1526. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boscardin SB, et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J Exp Med. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertozzi CR, Kiessling LL. Chemical glycobiology. Science. 2001;291:2357–2364. doi: 10.1126/science.1059820. [DOI] [PubMed] [Google Scholar]
- 36.Jennings HJ, Roy R, Gamian A. Induction of meningococcal group B polysaccharide-specific IgG antibodies in mice by using an N-propionylated B polysaccharide-tetanus toxoid conjugate vaccine. J Immunol. 1986;137:1708–1713. [PubMed] [Google Scholar]
- 37.Ashton FE, Ryan JA, Michon F, Jennings HJ. Protective efficacy of mouse serum to the N-propionyl derivative of meningococcal group B polysaccharide. Microb Pathog. 1989;6:455–458. doi: 10.1016/0882-4010(89)90087-9. [DOI] [PubMed] [Google Scholar]
- 38.Guttormsen HK, et al. Rational chemical design of the carbohydrate in a glycoconjugate vaccine enhances IgM-to-IgG switching. Proc Natl Acad Sci USA. 2008;105:5903–5908. doi: 10.1073/pnas.0710799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeuchi O, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 40.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.