Abstract
Hydrogen peroxide and other reactive oxygen species are intimately involved in endothelial cell signaling. In many cell types, the AMP-activated protein kinase (AMPK) has been implicated in the control of metabolic responses, but the role of endothelial cell redox signaling in the modulation of AMPK remains to be completely defined. We used RNA interference and pharmacological methods to establish that H2O2 is a critical activator of AMPK in cultured bovine aortic endothelial cells (BAECs). H2O2 treatment of BAECs rapidly and significantly increases the phosphorylation of AMPK. The EC50 for H2O2-promoted phosphorylation of AMPK is 65 ± 15 μM, within the physiological range of cellular H2O2 concentrations. The Ca2+/calmodulin-dependent protein kinase kinase-β (CaMKKβ) inhibitor STO-609 abolishes H2O2-dependent AMPK activation, whereas eNOS inhibitors enhance AMPK activation. Similarly, siRNA-mediated knockdown of CaMKKβ abrogates AMPK activation, whereas siRNA-mediated knockdown of eNOS leads to a striking increase in AMPK phosphorylation. Cellular imaging studies using the H2O2 biosensor HyPer show that siRNA-mediated eNOS knockdown leads to a marked increase in intracellular H2O2 generation, which is blocked by PEG-catalase. eNOS−/− mice show a marked increase in AMPK phosphorylation in liver and lung compared to wild-type mice. Lung endothelial cells from eNOS−/− mice also show a significant increase in AMPK phosphorylation. Taken together, these results establish that CaMKKβ is critically involved in mediating the phosphorylation of AMPK promoted by H2O2 in endothelial cells, and document that eNOS is an important negative regulator of AMPK phosphorylation and intracellular H2O2 generation in endothelial cells.
Keywords: eNOS, signal transduction
The term “reactive oxygen species” (ROS) is used to describe a class of molecules capable of oxidizing molecular targets in cells and tissues. ROS modulate both physiological and pathophysiological responses, and increased production of ROS is implicated in cardiovascular disease states (1, 2). Hydrogen peroxide (H2O2) is a stable cell-permeant ROS that modulates diverse endothelial cell processes, including vascular remodeling and endothelium-regulated vasorelaxation (1, 3, 4). The molecular mechanisms of H2O2-dependent modulation of these endothelial cell responses are incompletely understood. Basal intracellular levels of H2O2, typically in the range of 25–75 μM (5), are involved in normal processes of endothelial cell function (6). However, under pathological conditions, excessive ROS production has detrimental consequences in the vascular wall (7).
In many cells, H2O2-induced cellular responses involve the modulation of protein kinase signaling pathways (8, 9). AMPK is a heterotrimeric serine/threonine kinase (10) that can be activated by a rise in the AMP/ATP ratio; activated AMPK may phosphorylate metabolic enzymes that switch on ATP-generating catabolic pathways and switch off anabolic pathways. However, AMP-dependent activation of AMPK is only part of the regulatory pathway: full AMPK activation requires the phosphorylation of the enzyme at threonine-172 in the α subunit (11). At least two AMPK-activating kinases have been identified: the tumor suppressor kinase LKB1 and the Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) (12, 13).
The regulation of AMPK has been most thoroughly studied in “metabolic” tissues such as adipose tissue, muscle, and liver. More recently, AMPK has been characterized in vascular endothelial cells. AMPK in endothelial cells plays a role in cell energy flux (14), apoptosis (15), and regulation of inflammation, angiogenesis, and tissue perfusion (16). Endothelial AMPK is activated by a broad range of stimuli, including hypoxia (17); metformin, peroxynitrite (18), and adiponectin (19); and vasoactive mediators such as S1P, VEGF (20, 21), bradykinin (29), or thrombin (22). The link between AMPK and eNOS activation has been extensively studied. Kemp and colleagues first showed that AMPK phosphorylates eNOS at serine-1177, leading to enzyme activation (23). NO formation has been implicated in the angiogenic effects of AMPK (24). Several studies reported that H2O2 promotes eNOS activation and phosphorylation in endothelial cells—but without implicating AMPK—and yet other studies reported that H2O2 treatment promotes AMPK phosphorylation, but without implicating eNOS (13, 25, 26). Thus, multiple reports have suggested intriguing correlations and complex interactions between H2O2, AMPK, and eNOS activation pathways, yet the relationship between AMPK, H2O2, and eNOS remains incompletely understood.
In the present studies, we investigate the signaling mechanisms involved in AMPK activation in endothelial cells stimulated with H2O2. We demonstrate that H2O2 activates AMPK in endothelial cells, and establish that CaMKKβ is the key upstream kinase responsible for H2O2-dependent AMPK phosphorylation and endothelial tube formation. Importantly, we identify a role of eNOS in regulating AMPK phosphorylation and activation: eNOS negatively modulates AMPK phosphorylation both in cultured endothelial cells and in tissues from eNOS−/− mice. These studies establish stimulatory and inhibitory links between H2O2, CaMKKβ, AMPK, and eNOS signaling pathways in the vascular endothelium.
H2O2-Mediated AMPK Activation.
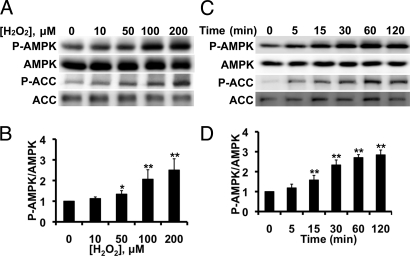
The effects of H2O2 on phosphorylation of AMPK and its substrate acetyl CoA carboxylase (ACC) in BAECs are shown in Fig. 1A. BAECs were treated for 30 min with varying concentrations of H2O2, and cell lysates were analyzed in immunoblots probed with antibodies against phospho-AMPK, total AMPK, phospho-ACC, or total ACC. H2O2 treatment increases AMPK and ACC phosphorylation in a dose-dependent manner; total AMPK or ACC abundance does not change, and there is no change in cell viability (Fig. S1). Fig. 1B and Fig. S2A shows pooled data from five similar dose-response experiments quantitating AMPK and ACC phosphorylation, respectively; the EC50 for H2O2-promoted AMPK phosphorylation is 65 ± 15 μM, a value close to the physiological H2O2 concentration in these cells (6). Fig. 1C shows a time course of H2O2-induced phosphorylation of AMPK and ACC, and Fig. 1D and Fig. S2B shows the quantitative analysis of pooled data from five similar experiments. Within 5 min after addition of H2O2 (200 μM) to BAECs, AMPK and ACC phosphorylation increase significantly, and this signal is sustained for at least 120 min. Furthermore, as previously reported (25), we found that H2O2 promoted phosphorylation of eNOS at serine-1179 (Fig. S2 C–F).
Fig. 1.
H2O2−mediated AMPK phosphorylation in endothelial cells. Shown in this figure are the results of immunoblots analyzed in endothelial cells treated with H2O2. (A) Representative immunoblot from a dose-response experiment analyzed in cells stimulated with the indicated concentrations of H2O2 for 30 min and probed with antibodies as shown; (B) pooled data from five independent experiments, analyzing the intensities corresponding to phospho-AMPK and total AMPK by quantitative chemiluminescence. (C) Representative time course experiment in BAECs treated with 200 μM H2O2 for the indicated times and analyzed in immunoblots probed with antibodies as shown; (D) pooled data from five independent experiments. *, P < 0.05, and **, P < 0.01 by ANOVA.
Effects of Protein Kinase or NOS Inhibitors on H2O2-Mediated Phosphorylation Responses.
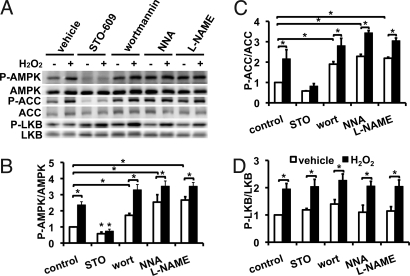
Fig. 2 shows experiments analyzing H2O2-induced AMPK phosphorylation in BAECs treated with the CaMKKβ inhibitor STO-609, with the phosphoinositide 3-kinase (PI3K) inhibitor wortmannin, or with the NOS inhibitors N-nitro-L-arginine (NNA) or L-arginine methyl ester (L-NAME). STO-609 suppresses basal phosphorylation of AMPK and ACC and effectively abrogates H2O2-stimulated phosphorylation of AMPK and ACC (n = 5, P < 0.05); however, STO-609 does not block H2O2-promoted Akt phosphorylation (Fig. S3). The PI3K inhibitor wortmannin induces a small but statistically significant increase in basal and H2O2-mediated AMPK and ACC phosphorylation (n = 3, P < 0.05). While wortmannin does not inhibit H2O2-promoted AMPK phosphorylation, wortmannin blocks H2O2-promoted Akt phosphorylation, as previously reported (25; Fig. S3). As shown in Fig. 2, NOS inhibitors significantly increase basal AMPK and ACC phosphorylation (2.1 ± 0.2-fold increase, n = 6, P < 0.05), to the point that there was only nominal additional phosphorylation when H2O2 was added (Fig. 2). We explored the effects of STO-609, wortmannin, NNA, and L-NAME on phosphorylation of another AMPK kinase LKB1. As shown in Fig. 2A, STO-609 treatment had no effect on LKB1 phosphorylation, while suppressing AMPK phosphorylation; LKB1 phosphorylation was unaffected by the NOS inhibitors and by wortmannin.
Fig. 2.
Effects of protein kinase inhibitors and NOS inhibitors on H2O2-induced AMPK phosphorylation. (A) Representative immunoblot analyzed in endothelial cells treated with H2O2 (200 μM, 30 min) after first being incubated for 30 min with inhibitors as shown: STO-609 (CaMKKβ inhibitor, 10 μg/mL); wortmannin (PI3-kinase inhibitor, 10 μM); N-nitro-L-arginine (NNA, NOS inhibitor 10 μM); or L-arginine methyl ester (L-NAME, NOS inhibitor, 100 μM). Cell lysates were subjected to immunoblotting using antibodies as shown. (B–D) Quantitative analyses of pooled data from three to five independent experiments, analyzing the phosphorylation responses for AMPK, ACC, and LKB1, respectively.
Effects of siRNA-Mediated Knockdown of CaMKKβ on H2O2-Mediated Phosphorylation Responses.
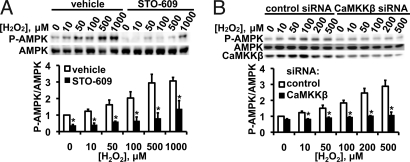
Fig. 3A shows the H2O2 dose response for AMPK phosphorylation in the presence and absence of the CaMKKβ inhibitor STO-609. At H2O2 concentrations up to 500 μM, AMPK phosphorylation is abrogated by STO-609. At a H2O2 concentration of 1 mM, some AMPK phosphorylation was seen despite the presence of STO-609, suggesting that other mechanisms for AMPK phosphorylation may come into play at high H2O2 concentrations. We next performed experiments using CaMKKβ siRNA, and found that siRNA-mediated knockdown of CaMKKβ blocked AMPK phosphorylation in response to H2O2 (Fig. 3B).
Fig. 3.
CaMMKβ inhibitor STO-609 and siRNA-mediated CaMMKβ knockdown: Effects on H2O2-stimulated AMPK phosphorylation. This figure shows the results of dose-response experiments exploring AMPK phosphorylation in BAECs treated with the CaMKKβ inhibitor STO-609 (A), or transfected with CaMKKβ or control siRNA (B). The cells were then incubated for 30 min with the indicated concentrations of H2O2. For each panel, an immunoblot from a representative experiment is shown above; the results from pooled data analyzed by quantitative chemiluminescence are shown below; *, P < 0.05.
Roles of eNOS in AMPK Activation.
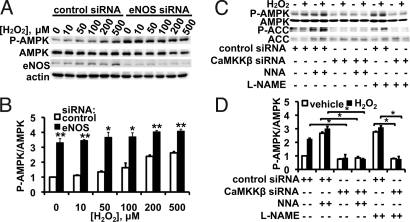
siRNA-mediated knockdown of eNOS led to a marked increase in AMPK phosphorylation (Fig. 4): there was a 3.3 ± 0.3-fold increase in basal AMPK phosphorylation (n = 4, P < 0.01), to the point that only a nominal (albeit statistically significant) increase in AMPK phosphorylation was seen with the addition of H2O2. Cells treated with eNOS inhibitors show robust AMPK phosphorylation, to the point that there is only a small increase in phosphorylation following addition of H2O2 (Fig. 4B). However, siRNA-mediated CaMKKβ knockdown attenuates the NOS inhibitor-mediated increase in AMPK phosphorylation (Fig. 4B), suggesting that eNOS-dependent AMPK activation requires CaMKKβ.
Fig. 4.
siRNA-mediated eNOS knockdown and H2O2-mediated AMPK phosphorylation. In the experiment shown in (A) endothelial cells were transfected with control or eNOS siRNA; 48 h after transfection, cells were treated with indicated concentrations of H2O2 for 30 min. The blot shown is a representative of five similarly designed experiments that yielded equivalent results. (B) Endothelial cells were transfected with control siRNA or with siRNA targeting CaMKKβ; 48 h after transfection, cells were first treated with vehicle or with the NOS inhibitors NNA (10 μM) or L-NAME (100 μM) for 30 min, and incubated with H2O2 (200 μM for 30 min) or vehicle as indicated. The blot shown is a representative of five similar experiments.
Intracellular H2O2 in AMPK Activation.
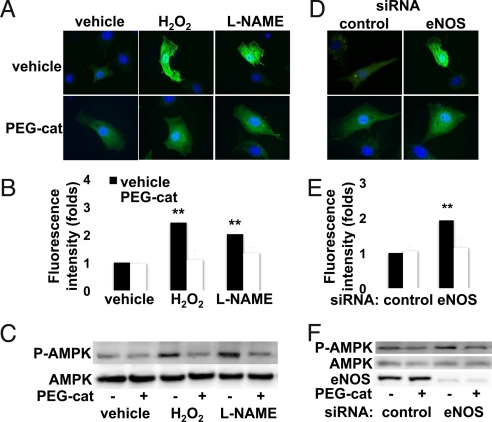
Cellular imaging approaches to detect intracellular H2O2 used the H2O2 biosensor, HyPer (28), transfected into BAECs (Fig. 5). Exogenous H2O2 causes an approximate 2.5-fold increase in fluorescence in HyPer-transfected endothelial cells relative to vehicle-treated HyPer-transfected cells (P < 0.01). We next used HyPer to detect endogenous production of H2O2. Treatment of BAECs with the NOS inhibitor L-NAME leads to a striking 2.0-fold increase in intracellular H2O2 production (Fig. 5). Importantly, siRNA-mediated eNOS knockdown increases the production of H2O2 to a similar magnitude (Fig. 5). These same interventions (L-NAME treatment and siRNA-mediated eNOS knockdown) also similarly increase AMPK phosphorylation (Fig. 4). Conversely, treatment of endothelial cells with PEG-catalase to degrade intracellular H2O2 (27) suppresses both the increase in H2O2 generation (Fig. 5 A, B, D, and E) as well as the increase in AMPK phosphorylation (Fig. 5 C and F) that are seen after eNOS inhibition with L-NAME (Fig. 5 A–C) or following siRNA-mediated eNOS knockdown (Fig. 5 D–F). Cells cultured in high glucose media are known to show an increase in AMPK phosphorylation as well an increase in reactive oxygen species, including H2O2, relative to cells cultured in physiological levels of glucose (1, 2). Using the HyPer biosensor to detect intracellular H2O2, we found that BAECs cultured in high glucose (30 mM) had elevated levels of H2O2 compared to cells cultured in 5 mM glucose, associated with an increase in AMPK phosphorylation (Fig. S4).
Fig. 5.
Intracellular H2O2 detection by HyPer: Effects of eNOS inhibition and reversal by PEG-catalase. Endothelial cells were transfected with the HyPer plasmid (28), and single cell images were obtained 48 h later; cells were incubated with PEG-catalase or vehicle, as noted, and processed either for imaging or immunoblot analyses. (A–C) Results of treatments with H2O2 (200 μM) or L-NAME (100 μM) following incubation of cells with PEG-catalase or vehicle. The results in (D–F) are from endothelial cells transfected with control or eNOS siRNA constructs and then treated with PEG-catalase or vehicle, as shown. (A and D) Representative images of HyPer-transfected cells that were treated as shown, then fixed and stained with Hoechst 33342, and analyzed for HyPer fluorescence. (B and E) Pooled data from 15 individual HyPer transfected cells from three experiments, quantitated for the determination of fluorescence intensity using MetaMorph software. (C and F) Representative immunoblot experiments in which endothelial cells were treated with PEG-catalase or vehicle, and either treated with L-NAME or H2O2 (C) or transfected with control or eNOS siRNA (F) as described in the text, and then analyzed in immunoblots probed with antibodies as shown. **,P < 0.01.
AMPK Activation in Tissues and Cells from eNOS−/− Mice.
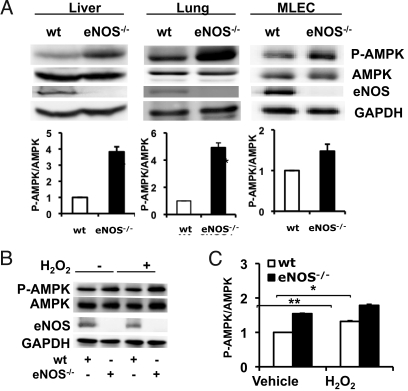
We next studied AMPK phosphorylation and expression in tissues and cells isolated from wild type and eNOS−/− mice. In the liver and lung of eNOS−/− mice, AMPK phosphorylation is strikingly increased compared to the level of AMPK phosphorylation seen in corresponding tissues of wild-type mice, with no change in overall levels of AMPK expression (Fig. 6). In lung tissue of eNOS−/− mice, AMPK phosphorylation is increased by 4.9 ± 0.3-fold compared to wild-type littermates (n = 3, P < 0.001). There is also a 3.8 ± 0.3-fold increase in AMPK phosphorylation in the liver of eNOS−/− mice compared to wild-type mice (n = 3, P < 0.001). In contrast to the increase in AMPK phosphorylation in liver and lung, in several other tissues we examined, including arterial preparations from aorta and carotid artery, heart, brown fat, and white fat, we found no difference in AMPK phosphorylation or expression in eNOS−/− mice compared to wild-type animals. As shown in Fig. 6, AMPK phosphorylation in lung endothelial cells cultured from eNOS−/− mice is significantly increased compared to wild-type mice (1.5 ± 0.1-fold increase, n = 3, P < 0.01). H2O2-induced AMPK phosphorylation also is enhanced in lung endothelial cells isolated from eNOS−/− mice compared to cells from wild-type mice (n = 3, P < 0.05; Fig. 6 B and C).
Fig. 6.
AMPK phosphorylation in tissues and cells from eNOS−/− mice. This figure shows immunoblot analyses of liver, lung, or endothelial cells from wild-type (wt) or eNOS−/− mice. (A) Liver, lung tissues and isolated lung endothelial cells (MLECs) from wild-type and eNOS−/− mice were analyzed in immunoblots probed with antibodies as indicated. Shown below are pooled data from at least three experiments quantitating AMPK phosphorylation; ***, P < 0.001. (B) MLECs from wild-type or eNOS−/− mice were treated with H2O2 (100 μM for 30 min). The experiment shown is a representative of five similar experiments showing that MLECs from eNOS−/− mice have increased basal as well as H2O2-stimulated AMPK phosphorylation relative to MLECs from wild-type mice; pooled data are also shown,*, P < 0.05.
The Role of AMPK and CaMKKβ in Endothelial Cell Tube Formation.
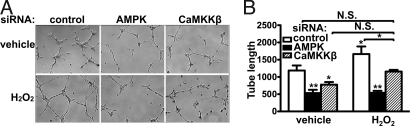
H2O2 has been reported to play a role in angiogenesis (29). We investigated the effects of siRNA-mediated knockdown of AMPK and CaMKKβ on H2O2 responses in the Matrigel tube formation assay, which is commonly used as an index of angiogenesis (30).H2O2 treatment enhances endothelial tube formation. siRNA-mediated knockdown of AMPK or CaMKKβ suppresses basal endothelial tube formation and blocks the response to H2O2; control siRNA is without effect (Fig. 7A). Quantitative analyses of tube formation (20) confirm that siRNA-mediated knockdown of AMPK or CaMKKβ reduces both basal and H2O2-stimulated endothelial tube formation (Fig. 7B).
Fig. 7.
siRNA-mediated knockdown of AMPK and CaMKKβ impairs H2O2-induced endothelial cell tube formation. Endothelial cells were transfected with siRNA constructs targeting AMPK or CaMKKβ, and analyzed in the Matrigel tube formation assay. (A) Representative images of endothelial Matrigel tube formation in H2O2-treated endothelial cells transfected with siRNA constructs as shown. (B) Pooled data from three independent tube formation experiments, plotting the total normalized tube length relative to the total tube length as measured for untreated endothelial cells transfected with control siRNA. *, P < 0.05, **, P < 0.01.
Discussion
These studies have explored the endothelial cell signaling pathways modulated by H2O2 in the context of AMPK regulation. Reactive oxygen species are produced by diverse vascular cells (31–33). The production of superoxide anion (O2·) in the vasculature has been extensively analyzed, yet the short half-life and small radius of diffusion of O2· limit its role as an important paracrine agent in vascular biology (1, 3). The O2· metabolite H2O2 is an important cellular signaling agent that modulates diverse aspects of endothelial cell physiology and pathophysiology (1, 3, 34). H2O2 has been reported to activate AMPK by inducing oxidative stress (13, 27), and H2O2 is involved in some receptor-mediated pathways of AMPK activation (35). We assessed AMPK activation in endothelial cells by measuring AMPK phosphorylation and by quantitating phosphorylation of the AMPK substrate ACC (Fig. 1). Under all conditions, the level of AMPK phosphorylation paralleled the phosphorylation of its substrate ACC, indicating that phosphorylation of AMPK can serve as an effective marker for enzyme activation. We found that H2O2 induces AMPK phosphorylation in a time-dependent and dose-dependent manner, with an EC50 value of 65 ± 15 μM, a concentration in the range of physiological concentrations of H2O2 (6). Following siRNA-mediated knockdown of AMPK or CaMKKβ, H2O2 treatment no longer increases endothelial tube formation (Fig. 7), suggesting that H2O2-modulated angiogenic responses can be modulated by the CaMKKβ/AMPK pathway.
Two separate lines of evidence establish a key role for CaMKKβ in AMPK activation by H2O2. Pharmacological inhibition of CaMKKβ by STO-609 completely abolishes H2O2-induced AMPK activation (Fig. 2). In addition, siRNA-mediated downregulation of CaMKKβ suppresses H2O2-induced AMPK activation (Fig. 3). These findings suggest that CaMKKβ is critically involved in modulating H2O2-induced AMPK activation. We also found that phosphorylation of the AMPK kinase LKB1 is increased by H2O2 treatment (Fig. 2). However, the H2O2-promoted increase in LKB1 phosphorylation is not blocked by the CaMKKβ inhibitor STO-609 under conditions where H2O2-promoted AMPK phosphorylation is completely blocked. Taken together, these findings argue against a central role for LKB1 in H2O2-induced AMPK phosphorylation.
The mechanisms whereby H2O2 modulates CaMKKβ remain to be completely defined. H2O2-induced oxidative stress may lead to an increased level of intracellular AMP, leading to AMPK phosphorylation. However, AMP has no effect on the activity of CaMKKβ (13), so our finding that CaMKKβ inhibition abolishes H2O2-stimulated AMPK activation suggests that H2O2-induced AMPK phosphorylation does not importantly involve changes in AMP levels, at least at physiological levels of H2O2 (Fig. 3). The signaling pathways leading to activation of CaMKKβ are incompletely understood, although a role for calcium-calmodulin has been established. A growing literature on protein kinase regulation has identified redox-active cysteine thiols as critical determinants of the activity of some kinases (4, 8). For example, the cGMP-dependent protein kinase undergoes oxidation at key thiol residues, leading to kinase activation independent of cGMP (4, 8). An intriguing if speculative hypothesis in the context of these studies is that redox-active cysteine thiols in CaMKKβ might undergo reversible oxidation as well as S-nitrosylation, each modification with opposing effects on CaMKKβ activity.
eNOS is a Ca2+/calmodulin-dependent enzyme that is regulated by phosphorylation at multiple residues (23, 36). AMPK is one of several kinases that stimulate eNOS phosphorylation (17, 20, 37). In contrast to the inhibitory effects of the CaMKKβ inhibitor STO-609 on AMPK activation, the PI3K inhibitor wortmannin failed to suppress AMPK phosphorylation (Fig. 2). Treatment of endothelial cells with NOS inhibitors leads to a significant increase in basal AMPK and ACC phosphorylation (Fig. 2), suggesting that blockade of NO synthesis leads to an increase in AMPK phosphorylation. Indeed, following eNOS inhibition with NNA or L-NAME, AMPK appears to become fully activated, with only a nominal additional response after subsequent treatment with H2O2 (Figs. 2 and 4). siRNA-mediated knockdown of CaMKKβ abolishes the effect of eNOS inhibitors on AMPK activation (Fig. 4), suggesting that eNOS-dependent activation of AMPK involves CaMKKβ. Further evidence for an inhibitory role for eNOS in modulating AMPK signaling pathways comes from our studies using eNOS siRNA (Fig. 4), in which we found that siRNA-mediated eNOS knockdown enhances AMPK phosphorylation. Finally, our observations in eNOS−/− mice are consistent with our findings in cultured endothelial cells: AMPK phosphorylation is increased in liver and lung tissues and in lung endothelial cells in eNOS−/− mice compared to wild-type mice (Fig. 6).
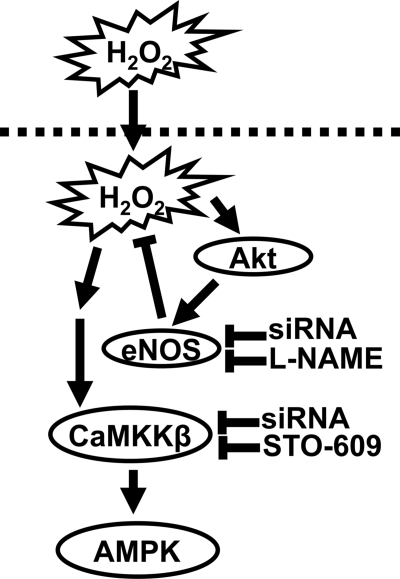
Our studies document an important relationship between H2O2 and eNOS in the reciprocal regulation of the CaMKKβ/AMPK pathway. Our experiments using the HyPer H2O2 biosensor have been particularly informative in the analysis of intracellular H2O2 generation in endothelial cells. Exogenous H2O2 promotes phosphorylation of AMPK (Fig. 1) and eNOS (Fig. S2), associated with a strong intracellular HyPer signal (Fig. 5). Importantly, the suppression of the eNOS pathway in endothelial cells—either by enzyme inhibition with L-NAME or by siRNA-mediated eNOS knockdown—leads both to AMPK phosphorylation (Fig. 4) as well as marked increases in intracellular H2O2 (Fig. 5). The effects of siRNA-mediated eNOS knockdown or eNOS enzyme inhibition on enhanced H2O2 generation or AMPK phosphorylation are fully reversed by treatment of endothelial cells with PEG-catalase (Fig. 5). Catalase catalyzes the decomposition of H2O2 into H2O and O2, and the striking inhibitory effects of PEG-catalase clearly identify eNOS as a critical determinant of endogenous H2O2 synthesis. In addition, stronger HyPer signals were observed in transfected BAEC treated with high glucose (30 mM) compared to cells in lower glucose (5 mM), in parallel with an increase in AMPK phosphorylation (Fig. S5). Thus, conditions leading to increased generation of intracellular H2O2 are associated with enhanced AMPK activation. Importantly, these studies implicate eNOS as a key negative regulator of intracellular H2O2 generation and also as an inhibitor of CaMKKβ-dependent AMPK phosphorylation. The role (if any) of NADPH oxidases in the control of CaMKKβ-regulated responses, as well as the mechanisms whereby eNOS modulates H2O2 production and CaMKKβ-dependent AMPK phosphorylation, remain to be definitively explicated. Our evidence points away from cGMP/PKG as a key modulator, in that siRNA-mediated PKG knockdown does not attenuate H2O2-promoted AMPK phosphorylation (Fig. S6). We speculate that suppression of eNOS activity may lead to enhanced mitochondrial respiration (1–3, 45) and thereby increase H2O2 production, which then promotes CaMKKβ activation by oxidation of key thiols (5, 8) either in CaMKKβ or in an upstream kinase or phosphatase, leading then to AMPK phosphorylation (13, 20) and activation (see model in Fig. 8).
Fig. 8.
Model for eNOS regulation of CaMKKβ/AMPK pathway via H2O2. This figure presents a plausible if speculative model for eNOS modulation of CaMKKβ/AMPK via H2O2 in endothelial cells. The present studies have shown that H2O2 promotes the phosphorylation of AMPK in endothelial cells (Fig. 1); the response is blocked by the CaMKKβ inhibitor STO-609 (Fig. 2) or by siRNA-mediated CaMKKβ knockdown (Fig. 3). Conversely, the NOS inhibitor L-NAME potentiates AMPK phosphorylation (Fig. 2), as does siRNA-mediated eNOS knockdown (Fig. 4); both of these effects are inhibited by CaMKKβ inhibition (Fig. 4). siRNA-mediated eNOS knockdown or enzyme inhibition with L-NAME leads to a marked increase in intracellular H2O2 generation (Fig. 5); the increase in intracellular H2O2 generation is blocked by PEG-catalase, which also suppresses the increase in AMPK phosphorylation. Since previous work have shown that eNOS modulates mitochondrial function, we propose that suppression of the eNOS-NO pathway (by siRNA or enzyme inhibition) enhances H2O2 production and thereby leads to CaMKKβ -dependent phosphorylation of AMPK. We propose that H2O2 directly or indirectly activates CaMKKβ. The phosphorylated AMPK in turn phosphorylates and activates eNOS, representing a feedback mechanism controlling this pathway.
These findings contrast with the conclusions of a recent publication, which reported that nitric oxide donors promote an increase in AMPK phosphorylation (38). The conclusions of this previous paper were largely based on experiments in which treatment of cultured cells with pharmacological NO donors, such as SNP, increased AMPK phosphorylation in HeLa cells and in human umbilical vein endothelial cells. However, we did not observe AMPK activation in BAECs treated with SNP under the same conditions (Fig. S5). These discrepant findings may indicate that subtle differences in culture conditions, possibly associated with variations in nitrosative or oxidative stress, may lead to differential effects of eNOS on the AMPK pathway. Our observations consistently reveal an inhibitory role for endogenous eNOS in AMPK activation, an effect seen both in cultured native endothelial cells and in tissues from eNOS−/− mice. It is plausible that under some conditions, prolonged exposure to high doses of pharmacological NO donors might lead to AMPK activation because of an inhibition of cellular energy flux. The previous observations on the activation of AMPK by pharmacological NO donors probably reflect a pathway distinct from the inhibition of AMPK by endogenous eNOS. Our finding that eNOS enzyme inhibition as well as siRNA-mediated eNOS knockdown enhances AMPK phosphorylation leads us to conclude that eNOS negatively regulates AMPK activation in endothelial cells.
There may be some interesting implications for organismal energy metabolism that reflect this interplay between eNOS and AMPK pathways. For example, AMPKα2 transgenic mice show reduced maximal exercise capacity, suggesting a role for AMPK in physical activity (39). Likewise, eNOS-dependent responses in both mice and rats appear to affect exercise capacity, oxygen consumption, and aerobic work (40). Interestingly, eNOS−/− mice show marked reductions in physical work capacity (41). Our finding that eNOS negatively regulates AMPK activation might suggest that the decreased physical activity in eNOS−/− mice could lead to the activation of AMPK-dependent metabolic pathways, which in turn would suppress the accumulation of energy stores and thereby lead to a decrease in exercise capacity.
In summary, our studies have demonstrated that H2O2 activates AMPK in endothelial cells through CaMKKβ, and establish a key role for the CaMKKβ/AMPK pathway in endothelial cell signaling. These studies also identify a previously unrecognized role of eNOS in the inhibition of H2O2 generation and AMPK activation in the vascular endothelium.
Materials and Methods
Reagents and siRNA Constructs.
Fetal bovine serum (FBS) was from HyClone; Dulbecco's modified Eagle medium (DMEM), Lipofectamine 2000 transfection reagent and other cell culture reagents were from Invitrogen. The CaMKKβ inhibitor STO-609, was from Calbiochem. Polyclonal antibodies against phospho-AMPK (Thr172), AMPK, phospho-ACC (Ser79), ACC, phospho-eNOS (Ser1179), LKB1, and phospho-LKB1 (Ser428) were from Cell Signaling Technologies. eNOS monoclonal antibody was from BD Transduction Laboratories. CaMKKβ monoclonal antibody was from Novus Biologicals. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody and PEG-catalase were from Sigma. Super Signal substrate and secondary antibodies conjugated with horseradish peroxidase were from Pierce. Gelatin, H2O2 and other reagents were from Sigma. Custom duplex siRNA constructs targeting eNOS and CaMKKβ were from Ambion, as well as control siRNA from Dharmacon and have been characterized in detail previously (20). The HyPer plasmid construct was from Evrogen.
Cell Culture and Immunoblotting.
BAECs were from Cell Systems and cultured in DMEM supplemented with 10% (vol/vol) fetal bovine serum as described (42). Cells were maintained in 0.2% gelatin-coated culture dishes and starved in serum-free media overnight before treatment. BAECs were transfected with specific siRNA-targeting constructs as described previously (43), and analyzed 48 h after transfection. For PEG-catalase treatment (27), BAECs were incubated overnight with PEG-catalase (100 U/mL) or vehicle. Cell lysates were prepared and immunoblotted with specific antibodies using protocols provided by the manufacturers. Immunoblots were analyzed by quantitative chemiluminescence using a ChemiImager 4000 (Alpha-Innotech) and reported in arbitrary units.
Intracellular H2O2 Detection by HyPer.
BAECs were transfected with the cytosol-targeted Hyper plasmid and imaged 48 h after transfection following the protocol described (28). After cell treatments, single-cell imaging was performed using a Nikon TE2000 microscope with a Perkin-Elmer spinning disk confocal system. Image intensities were quantified using MetaMorph software.
Tube Formation Assay.
One hundred microliters of growth-factor reduced Matrigel (BD Biosciences) were added to wells in a 48-well plate, and 104 cells were added to each Matrigel-coated well. Quantitative assays of tube formation were performed as reported (20).
Analyses of Tissues and Cells from Wild-Type and eNOSnull Mice.
C57BL/6J wild type and eNOS−/− mice from Jackson Laboratory were euthanized, and liver and lung were harvested and homogenized using a Polytron homogenizer in a 50 mM Tris-HCl pH7.4; 5 mM EGTA; 2 mM EDTA; 100 mM NaF; 2 mM Na3VO3; and Sigma protease inhibitor mixture. Following determination of protein concentrations, equal quantities of liver or lung lysates were analyzed in immunoblots. Mouse lung endothelial cells were isolated (44) and maintained in DMEM supplemented with endothelial cell growth factor and 20% (vol/vol) FBS. Cells between passages 3 and 5 were studied.
Statistical Analysis.
All experiments were performed at least three times. Mean values for individual experiments were expressed as means ± SE. Statistical differences were assessed by ANOVA. A P value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments.
We thank Drs. Alison Lin and David Golan for helpful discussions, and the Nikon Imaging Center at Harvard Medical School for support of cellular imaging studies. This work was supported in part by National Institutes of Health Grants GM36259, HL46457, and HL48743 (to T.M.) and a Postdoctoral Fellowship from the American Diabetes Association (J.L.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907409106/DCSupplemental.
References
- 1.Cai H. Hydrogen peroxide regulation of endothelial function: Origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 3.Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: Regulation and signaling leading to dysfunction. Exp Biol Med. 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- 4.Matsushita K, et al. Hydrogen peroxide regulation of endothelial exocytosis by inhibition of N-ethylmaleimide sensitive factor. J Cell Biol. 2005;170:73–79. doi: 10.1083/jcb.200502031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroder E, Eaton P. Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: Issues and considerations. Curr Opin Pharmacol. 2008;8:153–159. doi: 10.1016/j.coph.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Stone JR, Collins T. The role of hydrogen peroxide in endothelial proliferative responses. Endothelium. 2002;9:231–238. doi: 10.1080/10623320214733. [DOI] [PubMed] [Google Scholar]
- 7.Zafari AM, et al. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 8.Burgoyne JR, et al. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu S, et al. Hydrogen peroxide stimulates tetrahydrobiopterin synthesis through activation of the Jak2 tyrosine kinase pathway in vascular endothelial cells. Int J Biochem Cell Biol. 2008;40:755–765. doi: 10.1016/j.biocel.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Hardie DG. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 11.Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J. 2000;345:437–443. [PMC free article] [PubMed] [Google Scholar]
- 12.Woods A, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Woods A, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Dagher Z, Ruderman N, Tornheim K, Ido Y. The effect of AMP-activated protein kinase and its activator AICAR on the metabolism of human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1999;265:112–115. doi: 10.1006/bbrc.1999.1635. [DOI] [PubMed] [Google Scholar]
- 15.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: Inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 16.Gaskin FS, Kamada K, Yusof M, Korthuis RJ. 5′-AMP-activated protein kinase activation prevents postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol. 2007;292:H326–332. doi: 10.1152/ajpheart.00744.2006. [DOI] [PubMed] [Google Scholar]
- 17.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278:31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 18.Xie Z, et al. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281:6366–6375. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 19.Ouchi N, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine YC, Li GK, Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK -> Rac1 -> Akt -> endothelial nitric-oxide synthase pathway. J Biol Chem. 2007;282:20351–20364. doi: 10.1074/jbc.M702182200. [DOI] [PubMed] [Google Scholar]
- 21.Mount PF, et al. Bradykinin stimulates endothelial cell fatty acid oxidation by CaMKK-dependent activation of AMPK. Atherosclerosis. 2008;200:28–36. doi: 10.1016/j.atherosclerosis.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol. 2006;26:5933–5945. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen ZP, et al. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 24.Reihill JA, Ewart MA, Hardie DG, Salt IP. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem Biophys Res Commun. 2007;354:1084–1088. doi: 10.1016/j.bbrc.2007.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas SR, Chen K, Keaney JF., Jr Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem. 2002;277:6017–6024. doi: 10.1074/jbc.M109107200. [DOI] [PubMed] [Google Scholar]
- 26.Hu Z, Chen J, Wei Q, Xia Y. Bidirectional actions of hydrogen peroxide on endothelial nitric-oxide synthase phosphorylation and function: Co-commitment and interplay of Akt and AMPK. J Biol Chem. 2008;283:25256–25263. doi: 10.1074/jbc.M802455200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckman JS, et al. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988;263:6884–6892. [PubMed] [Google Scholar]
- 28.Belousov VV, et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda M, et al. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with ETS-1 in endothelial cells. Life Sci. 1999;64:249–258. doi: 10.1016/s0024-3205(98)00560-8. [DOI] [PubMed] [Google Scholar]
- 30.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: A critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 31.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 32.Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol. 1994;266:H2568–2572. doi: 10.1152/ajpheart.1994.266.6.H2568. [DOI] [PubMed] [Google Scholar]
- 33.Tang EH, et al. Calcium and reactive oxygen species increase in endothelial cells in response to releasers of endothelium-derived contracting factor. Br J Pharmacol. 2007;151:15–23. doi: 10.1038/sj.bjp.0707190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matoba T, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang M, et al. Thromboxane receptor activates the AMP-activated protein kinase in vascular smooth muscle cells via hydrogen peroxide. Circ Res. 2008;102:328–337. doi: 10.1161/CIRCRESAHA.107.163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 37.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, et al. Identification of nitric oxide as an endogenous activator of the AMP-activated protein kinase in vascular endothelial cells. J Biol Chem. 2008;283:27452–27461. doi: 10.1074/jbc.M802578200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Viollet B, et al. Physiological role of AMP-activated protein kinase (AMPK): Insights from knockout mouse models. Biochem Soc Trans. 2003;31:216–219. doi: 10.1042/bst0310216. [DOI] [PubMed] [Google Scholar]
- 40.Wang MX, et al. Nitric oxide in skeletal muscle: Inhibition of nitric oxide synthase inhibits walking speed in rats. Nitric Oxide. 2001;5:219–232. doi: 10.1006/niox.2001.0348. [DOI] [PubMed] [Google Scholar]
- 41.Momken I, Lechene P, Ventura-Clapier R, Veksler V. Voluntary physical activity alterations in endothelial nitric oxide synthase knockout mice. Am J Physiol Heart Circ Physiol. 2004;287:H914–920. doi: 10.1152/ajpheart.00651.2003. [DOI] [PubMed] [Google Scholar]
- 42.Michel T, Li GK, Busconi L. Phosphorylation and subcellular translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1993;90:6252–6256. doi: 10.1073/pnas.90.13.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez E, Nagiel A, Lin AJ, Golan DE, Michel T. Small interfering RNA-mediated downregulation of caveolin-1 differentially modulates signaling pathways in endothelial cells. J Biol Chem. 2004;279:40659–40669. doi: 10.1074/jbc.M407051200. [DOI] [PubMed] [Google Scholar]
- 44.Dong QG, et al. A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler Thromb Vasc Biol. 1997;17:1599–1604. doi: 10.1161/01.atv.17.8.1599. [DOI] [PubMed] [Google Scholar]
- 45.Nisoli E, et al. Mitochondrial biogenesis in mammals: The role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.