Abstract
AMPK (AMP-activated protein kinase) is a heterotrimetric enzyme that is expressed in many tissues, including the heart and vasculature, and plays a central role in the regulation of energy homoeostasis. It is activated in response to stresses that lead to an increase in the cellular AMP/ATP ratio caused either by inhibition of ATP production (i.e. anoxia or ischaemia) or by accelerating ATP consumption (i.e. muscle contraction or fasting). In the heart, AMPK activity increases during ischaemia and functions to sustain ATP, cardiac function and myocardial viability. There is increasing evidence that AMPK is implicated in the pathophysiology of cardiovascular and metabolic diseases. A principle mode of AMPK activation is phosphorylation by upstream kinases [e.g. LKB1 and CaMK (Ca2+/calmodulin-dependent protein kinase], which leads to direct effects on tissues and phosphorylation of various downstream kinases [e.g. eEF2 (eukaryotic elongation factor 2) kinase and p70 S6 kinase]. These upstream and downstream kinases of AMPK have fundamental roles in glucose metabolism, fatty acid oxidation, protein synthesis and tumour suppression; consequently, they have been implicated in cardiac ischaemia, arrhythmias and hypertrophy. Recent mechanistic studies have shown that AMPK has an important role in the mechanism of action of MF (metformin), TDZs (thiazolinediones) and statins. Increased understanding of the beneficial effects of AMPK activation provides the rationale for targeting AMPK in the development of new therapeutic strategies for cardiometabolic disease.
Keywords: 5-amino-4-imidazolecarboxamide riboside-1-β-D-ribofuranoside (AICAR), AMP-activated protein kinase (AMPK), cardiovascular disease, insulin resistance, metformin, obesity
Abbreviations: ACC, acetyl-CoA carboxylase; AICAR, 5-amino-4-imidazolecarboxamide riboside-1-β-D-ribofuranoside; AMPK, AMP-activated protein kinase; CaMK, Ca2+/calmodulin-dependent protein kinase; CPT-1, carnitine palmitoyltransferase-1; CVD, cardiovascular disease; eEF2, eukaryotic elongation factor 2; eNOS, endothelial NO synthase; GLUT-4, glucose transporter-4; HF, heart failure; CHF, chronic HF; HMG-CoA, 3-hydroxy-3-methyl-CoA; IL-6, interleukin-6; LV, left ventricular; MF, metformin; MI, myocardial infarction; MO25, mouse protein 25; mTOR, mammalian target of rapamycin; NEFA, non-esterified fatty acid (‘free fatty acid’); p70RSK, p70 ribosomal protein S6 kinase; PDH, pyruvate dehydrogenase; PFK-2, phosphofructokinase-2; PPAR-γ, peroxisome-proliferator-activated receptor-γ; PROactive, PROspective pioglitAzone Clinical Trial In macroVascular Events; STRAD, Ste20-related adaptor; TNF-α, tumour necrosis factor-α; TZD, thiazolinedione
INTRODUCTION
The prevalence of cardiometabolic diseases is reaching epidemic proportions in industrialized nations and in developing countries [1–3]. Despite aggressive treatment of the individual cardiometabolic risk factors, death from cardiometabolic conditions remains unacceptably high. Therefore there is an urgent need to identify new strategies for treating and preventing cardiometabolic diseases. In this respect, the AMPK (AMP-activated protein kinase) pathway has become the focus of a great deal of attention as a novel therapeutic target in cardiometabolic disease because it has been demonstrated to mediate, at least in part, the effects of a number of physiological and pharmacological factors that exert beneficial effects on the vasculature and the heart. AMPK has several important metabolic effects, including increasing muscle glucose uptake [4,5] and ameliorating insulin resistance [6]. It regulates cardiac muscle glucose and lipid metabolism both directly and indirectly in order to provide ATP in response to energy depletion (specifically a rise in the AMP/ATP ratio). AMPK activity can also be modulated by hormones and adipocytokines which may have protective effects against cardiovascular disease. AMPK has also been shown to regulate transcription of genes involved in lipid and glucose metabolism [7,8]. Dysregulation of this process (for example in obesity) can lead to the development of insulin resistance and dyslipidaemia, both of which are major risks factors for CVD (cardiovascular disease). Thus the identification of a compound that specifically and safely activates the AMPK pathway might contribute significantly to the treatment, management and even prevention of CVD. The aim of the present review is to discuss the direct and indirect role of AMPK in normal cardiac physiology and in cardiometabolic disease, and therapeutic strategies in modulating APMK activity.
STRUCTURE AND REGULATION OF AMPK
Understanding of the role of AMPK in key physiological pathways has increased several fold in recent years. Its discovery can be traced back to two independent findings reported in 1973 that observed that crude preparations of ACC (acetyl-CoA carboxylase) [9] and HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) reductase [9a] became inactivated when incubated with ATP. Both groups predicted that the effects were due to phosphorylation of the enzymes by an endogenous protein kinase that contaminated their preparations. It was subsequently shown that this protein kinase was itself activated by phosphorylation by an upstream kinase [10]. In 1987, Carling et al. [11] made the discovery that the inactivation of ACC and HMG-CoA reductase were both catalysed by a single protein kinase. As it became clear that it was a true multi-substrate kinase, they renamed it AMP-activated protein kinase after its allosteric activator 5΄-AMP [12]. Hardie [13] has described AMPK as a ‘fuel gauge’ and ‘guardian of energy status’, implying the fundamental role of AMPK in energy metabolism and maintaining body energy balance. AMPK is a heterotrimeric enzyme complex which consists of α, β and γ subunits, each of which has two or more isoforms that are encoded by distinct genes and are differentially expressed in various tissues. The α subunit contains the catalytic domain, including the important regulatory Thr172 residue, which is phosphorylated by upstream kinases. The β subunit has glycogen-binding C-terminal domains that are sufficient on their own to form a complex with the α and γ subunits. High cellular glycogen content exerts an inhibitory effect on AMPK through an interaction with the β subunit in skeletal muscle, although the exact mechanism is unknown [14]. The γ subunit of AMPK was first recognized by Bateman [15] and contains four repeats forming two domains. Each of these domains binds one molecule of AMP or ATP ion in a mutually exclusive manner [16], consistent with the findings that high concentrations of ATP antagonize activation of AMPK by AMP.
For many years, the upstream kinase(s) that phosphorylates Thr172 on the α subunit of AMPK remained unidentified. In recent years, it has been established that the major upstream kinase in mammalian cells is a complex of the protein kinase LKB1 and two accessory subunits STRAD (Ste20-related adaptor) and MO25 (mouse protein 25) [17–19]. LKB1 also acts as an upstream kinase of at least 12 other AMPK-related kinases [20,21]. It has also been found to be a tumour suppressor and was identified in humans as a gene carrying an autosomal-dominant mutation in Peutz–Jeghers syndrome [19,22]. The STRAD subunit is essential for the ability of the LKB1 complex to phosphorylate Thr172 on AMPK [18]. Besides LKB1, STRAD and MO25, AMPK can also be activated by an LKB1-independent mechanism involving CaMKs (Ca2+/calmodulin-dependent protein kinases).
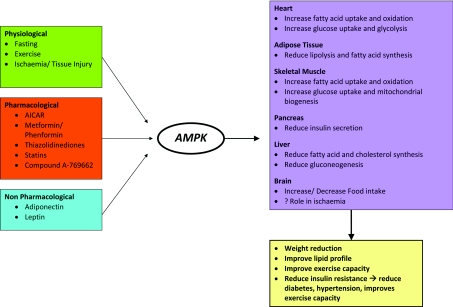
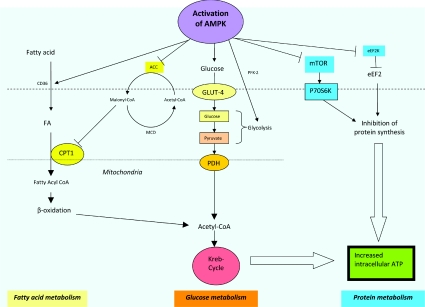
AMPK exerts its metabolic effects through its interactions with various metabolic pathways. Activation of these metabolic pathways via AMPK activation leads to remodelling of various components of the metabolic syndrome [23] (Figure 1). AMPK plays a major role in providing ATP in the midst of energy depletion via its interactions with various metabolic pathways (Figure 2). Furthermore, AMPK also has direct and indirect effects on the cardiovascular system, and the understanding of such effects provides the rationale of targeting AMPK as a new therapeutic modality for the treatment and prevention of CVD.
Figure 1. Physiological and pharmacological activation of AMPK results in remodelling of various components of the metabolic syndrome.
Figure 2. AMPK activation leads to activation of different metabolic pathways.
AMPK plays an important role in whole-body energy homoeostasis as it regulates and interacts with different key metabolic pathways. Activation of AMPK, secondary to a change in the AMP/ATP ratio or activation by upstream kinases, such as CAMKK (CaMK kinase) and LKB1 leads to a switching on of energy-production pathways, such as glucose and lipid metabolism, and a turning off of energy-metabolic processes, such as protein synthesis, which is not required for immediate cell survival. (i) Fatty acid metabolism. AMPK activation leads to increased translocation of CD36, a fatty-acid-transport protein, which increases fatty acid (FA) flux into cells and subsequent uptake into mitochondria for β-oxidation. CPT-1 inhibits fatty acid influx and acts as a gatekeeper for the mitochondrial uptake of fatty acids. Activation of AMPK leads to the inhibition of ACC2, which normally converts acetyl-CoA into malonyl-CoA. The inhibitory effect of malonyl-CoA on CPT-1 is hence removed, leading to unopposed intake of fatty acids into mitochondria. Furthermore, phosphorylating and inactivation of ACC1 by AMPK activation reduces fatty acid synthesis and turns off the expression of lipogenic genes, such as fatty acid synthase. MCD, malonyl-CoA decarboxylase. (ii) Glucose metabolism. Activation of AMPK increases translocation and retention of GLUT-4 in the plasma membrane, as well as increased transcription of the GLUT-4 gene, leading to increased glucose uptake. It also enhances glycolysis via activation and phosphorylation of PFK-2. (iii) Protein metabolism. p70RSK (p70S6K) is a one of the key kinases involved in protein synthesis. mTOR activates p70RSK and leads to increased protein synthesis. When AMPK is activated, the activation of p70RSK is blocked as a result of the inhibition of mTOR. Activation of AMPK also results in phosphorylation and inactivation of eEF2, and subsequent inhibition of protein synthesis. eEF2K, eEF2 kinase.
AMPK: DIRECT EFFECTS ON THE CARDIOVASCULAR SYSTEM
Congestive cardiac failure, LV (left ventricular) hypertrophy, myocardial ischaemia and diabetic cardiomyopathy are all associated with disturbances of cardiac energy homoeostasis. In these pathological states, AMPK activity is up-regulated in response to an increased AMP/ATP ratio (energy-depleted state). AMPK switches on energy-generating pathways to increase cardiac myocyte fatty acid uptake [24] and increase glucose uptake by increasing the translocation of GLUT-4 (glucose transporter-4) in a PI3K (phosphoinositide 3-kinase)-independent manner [5], while also enhancing glycolysis via PFK-2 (phosphofructokinase-2) activation [25]. At the same time, AMPK turns off protein synthesis pathways by activating eEF2 (eukaryotic elongation factor 2) kinase, resulting in the phosphorylation and inactivation of eEF2 and by decreasing Thr389 phosphorylation of p70RSK (p70 ribosomal protein S6 kinase), another important kinase which is involved in protein synthesis [26] via mTOR (mammalian target of rapamycin) inhibition [26,27] (see Figure 2).
AMPK and cardiac ischaemia
During cardiac ischaemia, the AMP/ATP ratio is increased as a result of decreased oxidative metabolism of both NEFAs [non-esterified fatty acids (‘free fatty acids’)] and glucose due to diminished oxygen supply in the face of increased glycolytic ATP production and glucose transport [28]. Russell et al. [5] have shown that AMPK activation using AICAR (5-amino-4-imidazolecarboxamide riboside-1-β-D-ribofuranoside) in an in vitro rat model increased translocation of glucose transporters (i.e. GLUT-4) into the sarcolemma and, hence, increased glucose uptake. Furthermore, AMPK also phosphorylates and activates PFK-2, leading to the production of fructose 2,6-bisphosphate, a potent stimulator of glycolysis. AMPK may be necessary for adiponectin to exert its cardioprotective effect against ischaemia/perfusion injury [29]. Both the α1 and α2 subunits of AMPK are activated during myocardial ischaemia, with the α2 subunit being activated to a greater extent [30,31]. Previous studies in transgenic mice have shown that decreased α2 activity resulted in reduced cardiac glucose uptake following ischaemia [32] and impaired recovery of LV systolic function [31]. Additionally, in transgenic mice expressing a kinase-dead form of the enzyme, phosphocreatinine was also lower after reperfusion [31]. These observations suggest that activation of AMPK following ischaemia has a cardioprotective effect and results in lesser cardiac injury and faster recovery. Calvert et al. [33] have also shown that activation of AMPK with MF (metformin) resulted in decreased myocardial injury in both diabetic and non-diabetic mice. This may be a result of deriving ATP from more energy-efficient glucose metabolism from increased AMPK-mediated glucose uptake and glycolytic flux in the face of oxygen deprivation [34,35].
However, ischaemic-induced activation of AMPK may be detrimental to the ischaemic heart, as suggested by Dyck and Lopaschuk [36]. During ischaemia, circulating fatty acid levels are elevated [37], which may be detrimental to the ischaemic heart [38,39]. Activation of AMPK leads to increased fatty acid uptake and inhibition of malonyl-CoA, a potent endogenous inhibitor of mitochondrial fatty acid uptake. This results in accelerated mitochondrial fatty acid uptake and, hence, increased mitochondrial acetyl-CoA production from β-oxidation. High levels of acetyl-CoA have an inhibitory effect on PDH (pyruvate dehydrogenase), reducing the amount of pyruvate being converted into acetyl-CoA and, hence, reduced glucose oxidation (the exact mechanisms remain undefined). The proposed mechanisms for these detrimental effects of high circulating fatty acids include: (i) accumulation of toxic intermediates of fatty acid oxidation, such as long-chain acyl-CoA thioesters and long-chain acylcarnitines [38], (ii) inhibition of glucose oxidation via inhibition of the PDH complex by fatty-acid-derived acetyl-CoA, and (iii) accumulation of glycolytic by-products such as protons and lactate. These valuable observations have affirmed the role of AMPK in cardiac ischaemia and suggested a potential role for therapeutic targeting in the treatment of myocardial ischaemia and MI (myocardial infarction).
AMPK and cardiac arrhythmias
Mutations of the γ2 subunit of AMPK have also been shown to contribute to glycogen storage disease and Wolff–Parkinson–White syndrome [40]. Gollob et al. [40] identified in 2001 a mutation (Arg531Gly) in the AMPK γ2 subunit (PRKAG2 gene) to be responsible for Wolff–Parkinson–White Syndrome and early onset of atrial fibrillation and conduction disease. Using a transgenic model targeting the murine gene, Davies et al. [41] demonstrated striking cardiac manifestations, such as hypertrophy, impaired contractile function, electrical conduction abnormalities and marked glycogen accumulation. Furthermore, Sidhu et al. [42] have identified a distinct atrial ventricular accessory pathway and a prolonged QRS in this transgenic mice model. However, the effects of the mutations described in this gene on the overall activity of AMPK varies in the different experimental models [43,44]. It is still uncertain whether these cardiac manifestations are the result of disease-causing mutations or secondary to glycogen deposition. Murphy et al. [45] postulated that the manifestations of AMPK disease may be due to defects in energy utilization or in specific cellular substrates, rather than mere passive deposition of glycogen. Nonetheless, these findings illustrate an important role for AMPK in cardiac hypertrophy and arrhythmias.
AMPK and cardiac hypertrophy, cell growth and gene transcription
AMPK may play a further role in the regulation of normal cardiac cell growth [31,32] and energy regulation in the hypertrophied heart [46], via its effects on protein synthesis [47,48]. Mutations in γ2 subunits not only cause glycogen overload in the heart and Wolff–Parkinson–White syndrome, but also hypertrophy and HF (heart failure) [40,49–51]. The severity of the defect also correlates with the severity of the disease. eEF2 is the main mediator of the translocation step in protein synthesis and is inhibited through phosphorylation of eEF2 kinase. p70RSK regulates protein synthesis through the same pathway or via phosphorylation of ribosomal protein S6. Chan et al. [48] have shown that AMPK not only regulates eEF2 kinase, but also exerts effects on protein synthesis via the mTOR pathway, ultimately leading to inhibition of p70RSK. Furthermore, Chan et al. [48] have shown that activation of AMPK using MF and AICAR results in inhibition of protein synthesis, and is associated with prevention and regression of cardiac hypertrophy. However, studies in transgenic mice have shown that elevated AMPK activity is associated with the accumulation of large amounts of glycogen, leading to dramatic LV hypertrophy and arrhythmias [46,52]. It remains uncertain, therefore, whether AMPK activation in the hypertrophied heart is beneficial [48,53] or deleterious, and further studies are required.
AMPK and vascular and endothelial function
AMPK also plays an important role in the regulation of vascular function and structure. It activates eNOS (endothelial NO synthase) in endothelial cells and cardiac myocytes by phosphorylation at Ser1177 (human sequence) [54,55]. eNOS activation leads to augmentation of vascular tone, platelet aggregation, leucocyte adherence and vascular smooth muscle proliferation [56].
Using a diabetic rat model, Suzuki et al. [57] have shown that activation of AMPK using a cAMP phosphodiesterase inhibitor, cilostazol, restores endothelial function independently of cAMP. Administration of cilostazol leads to phosphorylation of AMPK and subsequent phosphorylation of eNOS and increased NO production. Other AMPK activators, AICAR [58], MF [59] and rosiglitazone [60], have all been shown to increase NO production in human aortic endothelial cells via the AMPK pathway. Additionally, AMPK also appears to have a role in angiogenesis, promoting the action of the HIF-1α (hypoxia-inducible factor-1α)/VEGF (vascular endothelial growth factor) pathway [61,62], and inhibiting AngII (angiotensin II)-induced smooth muscle cell proliferation [63]. Furthermore, activation of AMPK using AICAR has been shown to inhibit palmitate-induced endothelial cell apoptosis through suppression of ROS (reactive oxygen species) [64]. It is clear that AMPK plays a central role in vascular biology.
AMPK: INDIRECT EFFECTS ON THE CARDIOVASCULAR SYSTEM
Recent findings have shown that levels of adipocytokines such as adiponectin and leptin correlate with the development of different components of the metabolic syndrome [65]. AMPK has been suggested to play a role in mediating the metabolic and vascular effects of the key adipocytokines [66,67].
AMPK and leptin
Leptin is an adipocyte-secreted hormone that plays a pivotal role in the regulation of food intake, energy expenditure, body weight and neuroendocrine function [68]. Leptin stimulates fatty acid oxidation [69] and glucose uptake [70], and prevents lipid accumulation outwith adipose tissue, preventing lipotoxicity [71]. Deposition of ectopic fat in pancreatic β-cells, myocardium and skeletal muscle contributes to the pathogenesis of Type 2 diabetes mellitus, cardiomyopathy and insulin resistance respectively. Leptin is known to exert its effects via the AMPK pathway, stimulating phosphorylation and activation of the α2 catalytic subunit of AMPK selectively in skeletal muscle [69]. Leptin also suppresses ACC2 activity, thereby stimulating fatty acid oxidation in muscle. AMPK also inhibits lipogenesis and ectopic fat deposition in the liver [72]. AMPK is also a key regulator of leptin action in the hypothalamus and a ‘master regulator’ of food intake. Minokoshi et al. [73] have shown that inhibition of AMPK activity by leptin specifically in the arcuate and paraventricular nuclei is essential for its anorexigenic and weight-loss effects.
AMPK and adiponectin
Adiponectin, an adipose-specific protein present in high concentrations in the circulation, was first identified in 1996. It possesses anti-atherogenic, insulin-sensitizing and anti-inflammatory properties. Yamauchi et al. [66] have shown that adiponectin stimulates glucose utilization and fatty acid oxidation via the AMPK pathway. Furthermore, adiponectin has been shown to reduce infarct size, improve LV function and remodelling, and increase coronary flow during reperfusion in animal models. The underlying mechanisms are thought to be phosphorylation of eNOS, AMPK Thr172 and Akt Ser473 [74]. Adiponectin-deficient mice have been shown to have progressive cardiac remodelling in a pressure-overloaded condition due to reduced AMPK signalling and worsening insulin resistance [75]. Therefore the AMPK pathway is not only critical for the metabolic and insulin-sensitizing actions of adiponectin, but also its cardioprotective effects in myocardial ischaemia and reperfusion.
AMPK ACTIVATORS: PHARMACOLOGICAL TOOLS AND THERAPEUTIC POTENTIAL
As we have seen above, AMPK is a pivotal enzyme that regulates diverse signals in metabolic pathways and has direct and indirect effects on the heart and vasculature. AMPK activation has not only been shown to alleviate various components of the metabolic syndrome, but may also improve LV hypertrophy and reduce cardiac injury in ischaemia. AMPK is also a key mediator of the anti-atherosclerotic and insulin-sensitizing effects of adiponectin. Therefore it is clearly an attractive therapeutic target in cardiometabolic disease. A number of AMPK activators are available as pharmacological tools and some are in clinical use (Table 1).
Table 1. Different AMPK ‘activators’ and their limitations in clinical use.
For further details of AICAR and MF studies, see Tables 2 and 3 respectively. PKC, protein kinase C.
| AMPK activator | Possible mechanism(s) of AMPK activation | Activation of other pathways | Limitation(s) |
|---|---|---|---|
| AICAR | (i) Direct activation followed by allosteric modification | (i) Stimulates adiponectin release; (ii) inhibits cytokines such as TNF-α and IL-6 | (i) Short half-life; (ii) variable effectiveness; (iii) only intravenous forms available; (iv) may cause bradycardia and significant hypoglycaemia |
| MF | (i) Indirect activation; (ii) via alteration of the AMP/ATP ratio as a result of inhibition of Complex I in the respiratory chain; (iii) other unknown mechanisms | (i) Anticancer effects via its effects on p53; (ii) up-regulates eNOS and increases NO bioactivity; (iii) enhances fatty acid oxidation, which leads to alleviation of endothelial lipotoxicity | (i) Indirect AMPK activation; (ii) doses and duration of MF required for AMPK activation are not determined; (iii) higher doses of MF result in intolerable gastrointestinal side effects |
| TZDs | (i) Indirect activation; (ii) via alteration of the AMP/ATP ratio, possibly similar to MF; (iii) via adiponectin | (i) Anti-atherosclerotic and anti-inflammatory effects via adiponectin; (ii) effects on mitochondrial biogenesis; (iii) exerts antioxidative effects by inhibiting PKC via AMPK activation | (i) Indirect inhibition; (ii) risk of developing fluid retention; (iii) risk of developing cardiovascular events is yet to be determined |
| Statins | (i) Indirect activation; (ii) does not alter the AMP/ATP ratio; (iii) other unknown mechanisms | (i) HMG-CoA reductase inhibition; (ii) activation of AMPK/eNOS/ACC | (i) Doses required for AMPK activation in humans are still to be determined |
| Compound A-769662 | (i) Direct activation | (i) Increased fatty acid oxidation; (ii) decreased plasma and liver triacylglycerol levels; (iii) inhibits fatty acid synthesis | (i) Poor oral bioavailability; (ii) data on long-term AMPK activation are awaited |
AICAR
AICAR is an adenosine analogue which activates AMPK through direct binding, followed by allosteric modification. It is initially taken up by adenosine transporters and subsequently phosphorylated to ZMP (5-aminoimidazole-4-carboxamide-1-β-D-furanosyl 5′-monophosphate) within the cell, which mimics AMP in AMPK signalling [76]. AICAR was first developed to block adenosine reuptake in the ischaemic heart, promoting stimulation of adenosine membrane receptors. In 1997, treatment with acadesine (AICAR) before and during surgery was shown to reduce early cardiac death, MI and combined adverse cardiovascular outcomes [77], although the mode of action via AMPK was not fully appreciated at that time.
AICAR is now widely used in the laboratory setting, particularly in experiments relating to glucose metabolism, insulin signalling pathways and lipid metabolism. In recent years, AICAR has been shown to reverse various aspects of the metabolic syndrome in animal models [23,78–80] and healthy human subjects [81] (Table 2). AICAR has also been shown to stimulate adiponectin and inhibit cytokines, such as TNF-α (tumour necrosis factor-α) and IL-6 (interleukin-6), which have been implicated in the development of obesity-induced insulin resistance [82–85]. Unfortunately, AICAR is far from an ideal activator of the AMPK pathway in the clinical settings because of its short half-life, requirement for intravenous infusion and variable effectiveness. It also causes bradycardia and can lead to hypoglycaemia when administered intravenously. Therefore there is great interest in developing a more potent, safer and more specific activator.
Table 2. Various studies of AMPK activation using AICAR and their major findings.
HDL, high-density lipoprotein; IR, insulin-resistant; OGTT, oral glucose tolerance test; SBP, systolic blood pressure.
| Study | Type of subjects | Dosage | Duration | Major finding(s) |
|---|---|---|---|---|
| Iglesias et al. [80] | IR high-fat-fed rats | Subcutaneous injection of 250 mg/kg of body weight | 24 h | (i) Enhanced whole-body, muscle and liver insulin action; (ii) reduced hepatic glucose output |
| Buhl et al. [23] | Obese Zucker rats exhibiting IR, hyperlipidaemia and hypertension | Subcutaneous injection of 0.5 mg/g of body weight | 7 weeks | (i) Decreased plasma triacylglycerol and NEFAs, and increased HDL; (ii) lower SBP; (iii) normalized OGTT and decreased fasting glucose and insulin; (iv) tendency towards decreased intra-abdominal fat content |
| Bergeron et al. [79] | Obese Zucker rats | Bolus at 100 mg/kg of body weight and constant infusion at 10 mg· kg−1 of body weight·min−1 | 60 min | (i) Increased glucose transport in red gastrocnemius muscle, whereas insulin had no effects; (ii) suppression of endogenous glucose production and lipolysis |
| Song et al. [78] | ob/ob mice | Subcutaneous at 1 mg/g of body weight | 7 days | (i) Corrected hyperglycaemia, improved glucose tolerance, and increased GLUT-4 and hexokinase II protein expression in skeletal muscle |
| Cuthbertson et al. [81] | Healthy men | Intravenous infusion at 10 mg·kg−1 of body weight·h−1 | 9 h | (i) Increased human skeletal muscle 2-deoxyglucose uptake and whole-body glucose disposal |
Metformin
MF has been used to treat diabetes for more than 50 years and is associated in observational studies with reduced mortality and improved outcomes in patients with CHF (chronic HF) [86,87]. It is the preferred antidiabetic medication for obese patients with Type 2 diabetes mellitus because of its property to stabilize weight and reduce cardiovascular events when used as monotherapy [88]. Recent clinical studies have shown that the effects of MF may go beyond improving HbA1c (glycated haemoglobin) and may include reductions in cardiovascular end points in Type 2 diabetes mellitus and HF. This wide spectrum of cardiovascular-protective effects may be attributable to its activation of AMPK and its downstream pathways.
MF has been shown to activate AMPK in myocytes [89–91], hepatocytes [92] and skeletal muscle cells [92]. MF decreases hepatic glucose production and increases skeletal muscle glucose disposal. Therapeutic doses of MF have been shown to increase AMPK α2 subunit activity in human skeletal muscle with an associated increase in phosphorylation of AMPK on Thr172 and decreased ACC2 activity [93]. MF can also up-regulate eNOS and increases NO bioactivity via AMPK activation [94]. Furthermore, AMPK activation by MF enhances fatty acid oxidation, which leads to alleviation of endothelial lipotoxicity and improved endothelial function [95]. Moreover, MF has also been shown to have anticancer effects in a recent study via its indirect AMPK activation [96]. However, the precise mechanisms of how MF activates AMPK are still poorly understood.
Even though MF is regarded as an AMPK activator, it has not been shown to bind directly to AMPK; neither does it regulates its own phosphorylation and dephosphorylation in cell-free assays [97]. One hypothesis is that it activates AMPK by inhibiting Complex I of the respiratory chain, which subsequently causes an increase in the AMP/ATP ratio [98,99]. In fact, inhibition of the respiratory chain in the intestinal mucosa may account for the gastrointestinal side effects of the drug, and this property may account for the propensity of its predecessor biguanides phenformin to cause lactic acidosis. MF is transported into intestinal cells mainly by OCT-1 (organic cation transporter-1), but phenformin penetrates cell membranes without active transport. Identification of polymorphisms in genes encoding cation transporter proteins may ultimately explain differences in tolerance and response to MF [100]. Interestingly, there are also studies suggesting that AMPK can be activated by MF without changes in the AMP/ATP ratio [97,101], and MF can also exert its beneficial metabolic effects on cardiac myocytes in an AMPK-independent manner [102].
However, we should be mindful that extra caution is required if we are to use these results to extrapolate to the effects of MF on AMPK. First, variable doses of MF have been used in these studies. The plasma MF concentration in clinical use is usually approx. 10 μmol/l [103], whereas the doses used in in vivo and in vitro experiments are consistently higher, in the range of 1–10 mmol/l (Table 3). Saeedi et al. [102] have shown that lower doses of MF (i.e. 2 mmol/l) failed to activate AMPK and caused no changes in energetic state. On the contrary, Hawley et al. [97] have shown that lower doses of MF can actually produce AMPK activation without a significant change in the cellular AMP/ATP ratio. Other research groups have reported that AMPK activation required higher doses of MF (i.e. 5–10 mmol/l) [89,91] (Table 3) and suggested that higher doses of MF are required to cause changes in the energetic state and, hence, subsequent AMPK activation. However, these divergent results may be the result of different exposure time of MF. For instance, Yang and Holman [90] have shown that a lower dose of MF (1 mmol/l) activated AMPK and increased cardiac myocyte glucose uptake after a prolonged exposure of 18 h. On the other hand, Bertrand et al. [104] have shown that short exposure (4 h) of MF can result in AMPK activation if much higher doses of MF were used (5–10 mmol/l). Therefore AMPK can be activated by MF in a time- and concentration-dependent manner. Clearly further studies are required to determine the time and concentration of MF which will result in the maximal beneficial effects of AMPK activation without intolerable side effects.
Table 3. Studies of AMPK activation using MF and their major findings.
IRI, ischaemia/reperfusion injury; MAPK, mitogen-activated protein kinase; PKC, protein kinase C.
| Study | Aim(s) | Subjects | Dosage | Key finding(s) | Clinical application |
|---|---|---|---|---|---|
| Calvert et al. [33] | To examine the cardioprotective effects of MF | Murine models | 125 μg/kg of body weight compared with saline (286-fold lower than maximum antihyperglycaemic dose) | (i) Reduction in myocardial injury in both diabetic and non-diabetic mice; (ii) increased AMPK activity and eNOS phosphorylation | Cardioprotective effects of MF might be secondary to eNOS activation via AMPK pathway |
| Solskov et al. [134] | To determine the effects of a single dose of MF on cardiac protection against IRI | Wistar rats | Single dose of MF (250 mg/kg of body weight) compared with saline | (i) Reduction in MI size; (ii) 2-fold increase in AMPK α1 subunit activity | MF might reduce MI size in pre-treated subjects via AMPK activation |
| Saeedi et al. [102] | To determine whether MF has effects on the metabolism of heart muscle, independent of the AMPK pathway | Sprague–Dawley rats | 2 mmol/l (this dose has greatest cellular metabolic effects without an impact on cellular energy status) | (i) Increased rate of glycolysis, glucose uptake and fatty acid oxidation; (ii) AMPK was not activated by 2 mmol/l MF | MF has AMPK-independent metabolic effects, possibly via PKC and p38 MAPK pathways |
| Kovacic et al. [91] | To determine whether Akt activation induced by insulin negatively regulates AMPK activities | Akt transgenic mice and adenovirus-infected neonatal rat cardiac myocytes with mutant forms of Akt1 and Akt2 | 5 mmol/l MF | (i) Insulin increased Akt phosphorylation and reduced AMPK phosphorylation; (ii) administration of MF overcame Akt-dependent AMPK suppression; (iii) suggests a cross-talk between Akt and AMPK pathway | AMPK can be activated by MF via insulin- independent pathways, but higher doses of MF are required |
| Zhang et al. [89] | To examine whether MF activates AMPK in the heart via increasing cytosolic AMP | Sprague–Dawley rats | 10 mmol/l MF | (i) MF increases AMPK activity preceded by and correlated with increased cytosolic AMP, but the overall AMP/ATP ratio remained unchanged | MF activates AMPK without altering the total AMP/ATP ratio; a high dosage of MF is required for AMPK activation. |
TZDs (thiazolinediones)
TZDs (i.e. rosiglitazone and pioglitazone) are ligands for the nuclear hormone receptor family member PPAR-γ (peroxisome-proliferator-activated receptor-γ) [105]. Both rosiglitazone and pioglitazone have been shown to activate AMPK in intact cells [106,107]. TZDs can also activate AMPK by stimulating the release and expression of circulating adiponectin from adipose tissue [66,67] or indirectly by increasing the cellular AMP/ATP ratio, possibly by a similar mechanism to biguanides [108]. Both rosiglitazone and pioglitazone have been suggested to have additional and protective beneficial anti-atherosclerotic and anti-inflammatory effects [109]. Furthermore, TZDs have also been shown to have diverse beneficial effects on endothelial function, TNF-α, NO and endothelial cell proliferation via AMPK-dependent and PPAR-γ-independent mechanisms [110–114]. These effects may translate into an improvement in clinical outcomes in patients with cardiometabolic disease. Previous studies have raised the intriguing possibilities that these effects may be mediated via AMPK activation [107,115,116]. However, as with MF, we are not certain whether AMPK activation is the key to these beneficial clinical effects on cardiovascular system. Furthermore, we also need to be very cautious when we try to translate these observations in animal studies to the clinical setting. The doses and type of TZDs that have been shown to activate AMPK vary among different research groups and the doses used in these animal studies may not be applicable to human subjects. Furthermore, the majority of these in vivo studies are short-term studies examining the effects of acute AMPK activation and its metabolic effects; however, the effects of long-term AMP activation by TZDs have yet to be determined. Nonetheless, the cardiovascular-protective effects of TZDs are evidenced in the recently published post-hoc analysis from the PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events) study [116]. This showed that patients who have chronic kidney disease and received pioglitazone are less likely to reach composite end points of all-cause death, MI and stroke, independent of the severity of renal impairment.
However, it should be noted that TZD use is associated with the risk of fluid retention which may exacerbate HF [117]. In a recent meta-analysis, Lago et al. [118] reported that TZDs increased the risk of developing CHF, probably as a result of fluid retention, across a wide background of cardiac risk [relative risk, 1.72 (95% confidence interval, 1.21–2.42); P=0.002]. There is also a concern that these agents may be associated with additional cardiovascular (MI and stroke) risk in patients with Type 2 diabetes mellitus with rosiglitazone. However, it should be noted that these meta-analyses included many small trials [119], whereas large clinical trial data have shown no sign of these cardiovascular events [RECORD (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of glycemia in Diabetes) and PROactive] [117,120]. The EMEA (European Medicines Evaluation Agency) for Medicinal Products for Human Use has adopted a scientific opinion in January 2008 recommending the inclusion of a new warning stating that the use of rosiglitazone in patients with ischaemic heart disease and/or peripheral arterial disease is not recommended. A recent FDA (Federal Drug Administration) review suggested that more large randomized studies with active comparators should be conducted by the manufacturers.
Statins
Statins are widely prescribed in patients with the metabolic syndrome owing to the high incidence of hypercholesterolaemia in this group of patients. There is mounting evidence to suggest that the clinical benefits of statins are beyond its lipid-lowering effects. The clinical efficacy of statin treatment in reducing cardiovascular mortality and morbidity in patients without and with diabetes are well-proven in clinical trials, such as the HPS (Heart Protection Study) and the CARDS (Collaborative Atorvastatin Diabetes Study) [121–124]. Besides its cholesterol-lowering effect via HMG-CoA reductase inhibition, statins have also been shown to activate AMPK in human and bovine endothelial cells [125]. Xenos et al. [126] have shown that AMPK protein levels in human endothelial cells are increased after being treated with fluvastatin for 2 days. Sun et al. [125] have also shown that atorvastatin and lovastatin cause a rapid activation of AMPK/eNOS/ACC in mouse myocardium and endothelial cells. The atorvastatin dose used in that study was 50 mg/kg of body weight in mice which is equivalent to 80 mg/day in human. That study has shown that atorvastatin did not alter the cellular AMP/ATP ratio, suggesting a different mechanism of AMPK activation. The beneficial effects of statins on endothelial function have been suggested to be the result of its ability to up-regulate eNOS [121,122,127–129] and its anti-inflammatory and anti-artherogenic effects [124,130]. These observations have all suggested that AMPK activation might be the key to the pleiotropic effects of statins on cardiovascular protection; however, further mechanistic and translational studies are required to show that AMPK activation is indeed the key to the effects of statin treatment, as well as examining the doses of different statins required to activate AMPK.
Compound A-769662
Cool et al. [131] have identified a thienopyridone family of AMPK activators, compound A-769662, which stimulates AMPK directly in partially purified rat liver and inhibits fatty acid synthesis in primary rat hepatocytes. Short-term treatment of normal Sprague–Dawley rats with A-769662 decreases liver malonyl-CoA levels and the respiratory exchange ratio, V̇CO2/V̇O2 (carbon dioxide production/oxygen consumption), indicating an increased rate of whole-body fatty acid oxidation. In ob/ob mice, treatment with compound A-769662 has been shown to decrease plasma glucose, reduce weight gain and significantly decrease both plasma and liver triacylglycerol (triglyceride) levels. These results demonstrate that small-molecule-mediated activation of AMPK in vivo is feasible and, therefore, represents a promising approach for the treatment of Type 2 diabetes mellitus and the metabolic syndrome. However, the compound has poor oral bioavailability, limiting its use in clinical settings.
An alternative small-molecule compound that is safe, potent, acts directly on AMPK and has good oral bioavailability would be an attractive candidate to progress towards clinical development.
CONCLUSIONS
Activation of the AMPK pathway may be the key in treating and preventing various cardiometabolic diseases; however, it is still uncertain whether direct activation of the AMPK pathway in the absence of a physiological stress will be beneficial or deleterious overall in humans. It is hoped that chronic activation of AMPK will not result in ‘over-compensatory’ activation of other systems such as the RAAS (renin–angiotensin–aldosterone system) activation in HF. Alterations in cardiac AMPK activity are associated with a number of cardiovascular-related diseases, such as pathological cardiac hypertrophy [50], myocardial ischaemia [36], glycogen storage cardiomyopathy [52] and Wolff–Parkinson–White syndrome [51], suggesting a possible maladaptive role in such conditions. Andersson et al. [132] described antisatiety effects of AMPK, which may lead to weight gain. Furthermore, McCullough et al. [133] also demonstrated that activated AMPK may be harmful in stroke. All of these uncertainties will need to be clarified by further translational studies, and much effort is still required to define the roles of AMPK activation in various conditions that have been discussed above. Furthermore, it is also a great challenge for pharmaceutical companies to produce a specific AMPK activator that has predictable effects owing to its heterotrimeric structure and its complex interactions with various upstream and downstream kinases. The other approach in which many researchers have adopted is to develop a compound that targets the downstream kinases of AMPK [i.e. a malonyl-CoA activator or CPT-1 (carnitine palmitoyltransferase-1) activator]. The AMPK/malonyl-CoA/CPT-1 axis might represent an interesting pathway for further research in cardiac substrate utilization and fatty acid metabolism. The AMPK–adipocytokine interaction has also formed the rationale for the development of new treatment modalities for the treatment of obesity. Lastly, AMPK/mTOR/eEF2/p70RSK axis modulation may be the key to understanding the pathogenesis of cardiac myocyte hypertrophy and mitochondrial biogenesis. A greater understanding of the biochemistry and physiology of AMPK and a better understanding of the mechanisms of action of existing agents have nonetheless opened up a new horizon for the treatment and prevention of cardiovascular and metabolic disease.
FUNDING
This work was supported by the British Heart Foundation [grant number PG/06/143/21897].
References
- 1.Ford E. S. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 2.Ford E. S. Prevalence of the metabolic syndrome in US populations. Endocrinol. Metab. Clinics North Am. 2004;33:333–350. doi: 10.1016/j.ecl.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Ford E. S. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome, a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron R., Russell R. R., III, Young L. H., Ren J. M., Marcucci M., Lee A., Shulman G. I. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am. J. Physiol. 1999;276:E938–E944. doi: 10.1152/ajpendo.1999.276.5.E938. [DOI] [PubMed] [Google Scholar]
- 5.Russell R. R., III, Bergeron R., Shulman G. I., Young L. H. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am. J. Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 6.Ruderman N. B., Cacicedo J. M., Itani S., Yagihashi N., Saha A. K., Ye J. M., Chen K., Zou M., Carling D., Boden G., et al. Malonyl-CoA and AMP-activated protein kinase (AMPK), possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochem. Soc. Trans. 2003;31:202–206. doi: 10.1042/bst0310202. [DOI] [PubMed] [Google Scholar]
- 7.Long Y. C., Barnes B. R., Mahlapuu M., Steiler T. L., Martinsson S., Leng Y., Wallberg-Henriksson H., Andersson L., Zierath J. R. Role of AMP-activated protein kinase in the coordinated expression of genes controlling glucose and lipid metabolism in mouse white skeletal muscle. Diabetologia. 2005;48:2354–2364. doi: 10.1007/s00125-005-1962-5. [DOI] [PubMed] [Google Scholar]
- 8.Berasi S. P., Huard C., Li D., Shih H. H., Sun Y., Zhong W., Paulsen J. E., Brown E. L., Gimeno R. E., Martinez R. V. Inhibition of gluconeogenesis through transcriptional activation of EGR1 and DUSP4 by AMP-activated kinase. J. Biol. Chem. 2006;281:27167–27177. doi: 10.1074/jbc.M602416200. [DOI] [PubMed] [Google Scholar]
- 9.Carlson C. A., Kim K. H. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J. Biol. Chem. 1973;248:378–380. [PubMed] [Google Scholar]
- 9a.Beg Z. H., Allmann D. W., Gibson D. M. Modulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity with cAMP and with protein fractions of rat liver cytosol. Biochem. Biophys. Res. Commun. 1973;54:1362–1369. doi: 10.1016/0006-291x(73)91137-6. [DOI] [PubMed] [Google Scholar]
- 10.Ingebritsen T. S., Lee H. S., Parker R. A., Gibson D. M. Reversible modulation of the activities of both liver microsomal hydroxymethylglutaryl coenzyme A reductase and its inactivating enzyme. Evidence for regulation by phosphorylation-dephosphorylation. Biochem. Biophys. Res. Commun. 1978;81:1268–1277. doi: 10.1016/0006-291x(78)91273-1. [DOI] [PubMed] [Google Scholar]
- 11.Carling D., Zammit V. A., Hardie D. G. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–222. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- 12.Munday M. R., Campbell D. G., Carling D., Hardie D. G. Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase. Eur. J. Biochem. 1988;175:331–338. doi: 10.1111/j.1432-1033.1988.tb14201.x. [DOI] [PubMed] [Google Scholar]
- 13.Hardie D. G. The AMP-activated protein kinase cascade, the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 14.Polekhina G., Gupta A., Michell B. J., van Denderen B., Murthy S., Feil S. C., Jennings I. G., Campbell D. J., Witters L. A., Parker M. W., et al. AMPK β subunit targets metabolic stress sensing to glycogen. Curr. Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 15.Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem. Sci. 1997;22:12–13. doi: 10.1016/s0968-0004(96)30046-7. [DOI] [PubMed] [Google Scholar]
- 16.Scott J. W., Hawley S. A., Green K. A., Anis M., Stewart G., Scullion G. A., Norman D. G., Hardie D. G. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J. Clin. Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods A., Johnstone S. R., Dickerson K., Leiper F. C., Fryer L. G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 18.Hawley S. A., Boudeau J., Reid J. L., Mustard K. J., Udd L., Makela T. P., Alessi D. R., Hardie D. G. Complexes between the LKB1 tumor suppressor, STRAD α/β and MO25 α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw R. J., Kosmatka M., Bardeesy N., Hurley R. L., Witters L. A., DePinho R. A., Cantley L. C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lizcano J. M., Goransson O., Toth R., Deak M., Morrice N. A., Boudeau J., Hawley S. A., Udd L., Makela T. P., Hardie D. G., Alessi D. R. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaleel M., McBride A., Lizcano J. M., Deak M., Toth R., Morrice N. A., Alessi D. R. Identification of the sucrose non-fermenting related kinase SNRK, as a novel LKB1 substrate. FEBS Lett. 2005;579:1417–1423. doi: 10.1016/j.febslet.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 22.Hemminki A., Markie D., Tomlinson I., Avizienyte E., Roth S., Loukola A., Bignell G., Warren W., Aminoff M., Hoglund P., et al. A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 23.Buhl E. S., Jessen N., Pold R., Ledet T., Flyvbjerg A., Pedersen S. B., Pedersen O., Schmitz O., Lund S. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002;51:2199–2206. doi: 10.2337/diabetes.51.7.2199. [DOI] [PubMed] [Google Scholar]
- 24.Luiken J. J., Coort S. L., Willems J., Coumans W. A., Bonen A., van der Vusse G. J., Glatz J. F. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes. 2003;52:1627–1634. doi: 10.2337/diabetes.52.7.1627. [DOI] [PubMed] [Google Scholar]
- 25.Marsin A. S., Bertrand L., Rider M. H., Deprez J., Beauloye C., Vincent M. F., Van den Berghe G., Carling D., Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 26.Krause U., Bertrand L., Hue L. Control of p70 ribosomal protein S6 kinase and acetyl-CoA carboxylase by AMP-activated protein kinase and protein phosphatases in isolated hepatocytes. Eur. J. Biochem. 2002;269:3751–3759. doi: 10.1046/j.1432-1033.2002.03074.x. [DOI] [PubMed] [Google Scholar]
- 27.Horman S., Browne G., Krause U., Patel J., Vertommen D., Bertrand L., Lavoinne A., Hue L., Proud C., Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr. Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- 28.Young L. H., Renfu Y., Russell R., Hu X., Caplan M., Ren J., Shulman G. I., Sinusas A. J. Low-flow ischemia leads to translocation of canine heart GLUT-4 and GLUT-1 glucose transporters to the sarcolemma in vivo. Circulation. 1997;95:415–422. doi: 10.1161/01.cir.95.2.415. [DOI] [PubMed] [Google Scholar]
- 29.Shibata R., Sato K., Pimentel D. R., Takemura Y., Kihara S., Ohashi K., Funahashi T., Ouchi N., Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat. Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J., Coven D. L., Miller E. J., Hu X., Young M. E., Carling D., Sinusas A. J., Young L. H. Activation of AMPK α- and γ-isoform complexes in the intact ischemic rat heart. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1927–H1934. doi: 10.1152/ajpheart.00251.2006. [DOI] [PubMed] [Google Scholar]
- 31.Russell R. R., III, Li J., Coven D. L., Pypaert M., Zechner C., Palmeri M., Giordano F. J., Mu J., Birnbaum M. J., Young L. H. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J. Clin. Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xing Y., Musi N., Fujii N., Zou L., Luptak I., Hirshman M. F., Goodyear L. J., Tian R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative α2 subunit of AMP-activated protein kinase. J. Biol. Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- 33.Calvert J. W., Gundewar S., Jha S., Greer J. J., Bestermann W. H., Tian R., Lefer D. J. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 34.Sakamoto K., Zarrinpashneh E., Budas G. R., Pouleur A. C., Dutta A., Prescott A. R., Vanoverschelde J. L., Ashworth A., Jovanovic A., Alessi D. R., Bertrand L. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKα2 but not AMPKα1. Am. J. Physiol. Endocrinol. Metab. 2006;290:E780–E788. doi: 10.1152/ajpendo.00443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baron S. J., Li J., Russell R. R., III, Neumann D., Miller E. J., Tuerk R., Wallimann T., Hurley R. L., Witters L. A., Young L. H. Dual mechanisms regulating AMPK kinase action in the ischemic heart. Circ. Res. 2005;96:337–345. doi: 10.1161/01.RES.0000155723.53868.d2. [DOI] [PubMed] [Google Scholar]
- 36.Dyck J. R., Lopaschuk G. D. AMPK alterations in cardiac physiology and pathology, enemy or ally? J. Physiol. 2006;574:95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopaschuk G. D., Collins-Nakai R., Olley P. M., Montague T. J., McNeil G., Gayle M., Penkoske P., Finegan B. A. Plasma fatty acid levels in infants and adults after myocardial ischemia. Am. Heart J. 1994;128:61–67. doi: 10.1016/0002-8703(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 38.Hendrickson S. C., St Louis J. D., Lowe J. E., Abdel-aleem S. Free fatty acid metabolism during myocardial ischemia and reperfusion. Mol. Cell. Biochem. 1997;166:85–94. doi: 10.1023/a:1006886601825. [DOI] [PubMed] [Google Scholar]
- 39.Lopaschuk G. D., Spafford M. A. Energy substrate utilization by isolated working hearts from newborn rabbits. Am. J. Physiol. 1990;258:H1274–H1280. doi: 10.1152/ajpheart.1990.258.5.H1274. [DOI] [PubMed] [Google Scholar]
- 40.Gollob M. H., Seger J. J., Gollob T. N., Tapscott T., Gonzales O., Bachinski L., Roberts R. Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Circulation. 2001;104:3030–3033. doi: 10.1161/hc5001.102111. [DOI] [PubMed] [Google Scholar]
- 41.Davies J. K., Wells D. J., Liu K., Whitrow H. R., Daniel T. D., Grignani R., Lygate C. A., Schneider J. E., Noel G., Watkins H., Carling D. Characterization of the role of γ2 R531G mutation in AMP-activated protein kinase in cardiac hypertrophy and Wolff-Parkinson-White syndrome. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1942–H1951. doi: 10.1152/ajpheart.01020.2005. [DOI] [PubMed] [Google Scholar]
- 42.Sidhu J. S., Rajawat Y. S., Rami T. G., Gollob M. H., Wang Z., Yuan R., Marian A. J., DeMayo F. J., Weilbacher D., Taffet G. E., et al. Transgenic mouse model of ventricular preexcitation and atrioventricular reentrant tachycardia induced by an AMP-activated protein kinase loss-of-function mutation responsible for Wolff-Parkinson-White syndrome. Circulation. 2005;111:21–29. doi: 10.1161/01.CIR.0000151291.32974.D5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carling D., Woods A., Thornton C., Cheung P. C., Smith F. C., Ponticos M., Stein S. C. Molecular characterization of the AMP-activated protein kinase and its role in cellular metabolism. Biochem. Soc. Trans. 1997;25:1224–1228. doi: 10.1042/bst0251224. [DOI] [PubMed] [Google Scholar]
- 44.Daniel T., Carling D. Functional analysis of mutations in the γ 2 subunit of AMP-activated protein kinase associated with cardiac hypertrophy and Wolff-Parkinson-White syndrome. J. Biol. Chem. 2002;277:51017–51024. doi: 10.1074/jbc.M207093200. [DOI] [PubMed] [Google Scholar]
- 45.Murphy R. T., Mogensen J., McGarry K., Bahl A., Evans A., Osman E., Syrris P., Gorman G., Farrell M., Holton J. L., et al. Adenosine monophosphate-activated protein kinase disease mimicks hypertrophic cardiomyopathy and Wolff-Parkinson-White syndrome, natural history. J. Am. Coll. Cardiol. 2005;45:922–930. doi: 10.1016/j.jacc.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 46.Tian R., Musi N., D'Agostino J., Hirshman M. F., Goodyear L. J. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 47.Bolster D. R., Crozier S. J., Kimball S. R., Jefferson L. S. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J. Biol. Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 48.Chan A. Y., Soltys C. L., Young M. E., Proud C. G., Dyck J. R. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J. Biol. Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 49.Arad M., Benson D. W., Perez-Atayde A. R., McKenna W. J., Sparks E. A., Kanter R. J., McGarry K., Seidman J. G., Seidman C. E. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J. Clin. Invest. 2002;109:357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blair E., Redwood C., Ashrafian H., Oliveira M., Broxholme J., Kerr B., Salmon A., Ostman-Smith I., Watkins H. Mutations in the γ subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy, evidence for the central role of energy compromise in disease pathogenesis. Hum. Mol. Genet. 2001;10:1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- 51.Gollob M. H., Green M. S., Tang A. S., Gollob T., Karibe A., Ali Hassan A. S., Ahmad F., Lozado R., Shah G., Fananapazir L. et al. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N. Engl. J. Med. 2001;344:1823–1831. doi: 10.1056/NEJM200106143442403. [DOI] [PubMed] [Google Scholar]
- 52.Arad M., Moskowitz I. P., Patel V. V., Ahmad F., Perez-Atayde A. R., Sawyer D. B., Walter M., Li G. H., Burgon P. G., Maguire C. T., et al. Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy. Circulation. 2003;107:2850–2856. doi: 10.1161/01.CIR.0000075270.13497.2B. [DOI] [PubMed] [Google Scholar]
- 53.Shibata R., Ouchi N., Ito M., Kihara S., Shiojima I., Pimentel D. R., Kumada M., Sato K., Schiekofer S., Ohashi K., et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen H., Montagnani M., Funahashi T., Shimomura I., Quon M. J. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 55.Chen Z. P., Mitchelhill K. I., Michell B. J., Stapleton D., Rodriguez-Crespo I., Witters L. A., Power D. A., Ortiz de Montellano P. R., Kemp B. E. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 56.Moncada S., Palmer R. M., Higgs E. A. Nitric oxide, physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 57.Suzuki K., Uchida K., Nakanishi N., Hattori Y. Cilostazol activates AMP-activated protein kinase and restores endothelial function in diabetes. Am. J. Hypertens. 2008;21:451–457. doi: 10.1038/ajh.2008.6. [DOI] [PubMed] [Google Scholar]
- 58.Morrow V. A., Foufelle F., Connell J. M., Petrie J. R., Gould G. W., Salt I. P. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J. Biol. Chem. 2003;278:31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 59.Xie Z., Dong Y., Scholz R., Neumann D., Zou M. H. Phosphorylation of LKB1 at serine 428 by protein kinase C-ζ is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117:952–962. doi: 10.1161/CIRCULATIONAHA.107.744490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyle J. G., Logan P. J., Ewart M. A., Reihill J. A., Ritchie S. A., Connell J. M., Cleland S. J., Salt I. P. Rosiglitazone stimulates nitric oxide synthesis in human aortic endothelial cells via AMP-activated protein kinase. J. Biol. Chem. 2008;283:11210–11217. doi: 10.1074/jbc.M710048200. [DOI] [PubMed] [Google Scholar]
- 61.Lee M., Hwang J. T., Lee H. J., Jung S. N., Kang I., Chi S. G., Kim S. S., Ha J. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J. Biol. Chem. 2003;278:39653–39661. doi: 10.1074/jbc.M306104200. [DOI] [PubMed] [Google Scholar]
- 62.Reihill J. A., Ewart M. A., Hardie D. G., Salt I. P. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem. Biophys. Res. Commun. 2007;354:1084–1088. doi: 10.1016/j.bbrc.2007.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagata D., Takeda R., Sata M., Satonaka H., Suzuki E., Nagano T., Hirata Y. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004;110:444–451. doi: 10.1161/01.CIR.0000136025.96811.76. [DOI] [PubMed] [Google Scholar]
- 64.Kim J. E., Kim Y. W., Lee I. K., Kim J. Y., Kang Y. J., Park S. Y. AMP-activated protein kinase activation by 5-aminoimidazole-4-carboxamide- 1-β-D-ribofuranoside (AICAR) inhibits palmitate-induced endothelial cell apoptosis through reactive oxygen species suppression. J. Pharmacol. Sci. 2008;106:394–403. doi: 10.1254/jphs.fp0071857. [DOI] [PubMed] [Google Scholar]
- 65.Ingelsson E., Larson M. G., Yin X., Wang T. J., Meigs J. B., Lipinska I., Benjamin E. J., Keaney J. F., Jr, Vasan R. S. Circulating ghrelin, leptin, and soluble leptin receptor concentrations and cardiometabolic risk factors in a community-based sample. J. Clin. Endocrinol. Metab. 2008;93:3149–3157. doi: 10.1210/jc.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S., Yamashita S., Noda M., Kita S., Ueki K., et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 67.Tomas E., Tsao T. S., Saha A. K., Murrey H. E., Zhang C. c., Itani S. I., Lodish H. F., Ruderman N. B. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain, acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Friedman J. M., Halaas J. L. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 69.Minokoshi Y., Kim Y. B., Peroni O. D., Fryer L. G., Muller C., Carling D., Kahn B. B. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 70.Minokoshi Y., Haque M. S., Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- 71.Unger R. H. Lipotoxic diseases. Annu. Rev. Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 72.Viollet B., Foretz M., Guigas B., Horman S., Dentin R., Bertrand L., Hue L., Andreelli F. Activation of AMP-activated protein kinase in the liver, a new strategy for the management of metabolic hepatic disorders. J. Physiol. 2006;574:41–53. doi: 10.1113/jphysiol.2006.108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Minokoshi Y., Alquier T., Furukawa N., Kim Y. B., Lee A., Xue B., Mu J., Foufelle F., Ferre P., Birnbaum M. J., et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 74.Gonon A. T., Widegren U., Bulhak A., Salehzadeh F., Persson J., Sjoquist P. O., Pernow J. Adiponectin protects against myocardial ischaemia-reperfusion injury via AMP-activated protein kinase, Akt, and nitric oxide. Cardiovasc. Res. 2008;78:116–122. doi: 10.1093/cvr/cvn017. [DOI] [PubMed] [Google Scholar]
- 75.Liao Y., Takashima S., Maeda N., Ouchi N., Komamura K., Shimomura I., Hori M., Matsuzawa Y., Funahashi T., Kitakaze M. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc. Res. 2005;67:705–713. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 76.Merrill G. F., Kurth E. J., Hardie D. G., Winder W. W. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 77.Mangano D. T. Effects of acadesine on myocardial infarction, stroke, and death following surgery. A meta-analysis of the 5 international randomized trials. The Multicenter Study of Perioperative Ischemia (McSPI) Research Group. JAMA, J. Am. Med. Assoc. 1997;277:325–332. doi: 10.1001/jama.277.4.325. [DOI] [PubMed] [Google Scholar]
- 78.Song X. M., Fiedler M., Galuska D., Ryder J. W., Fernstrom M., Chibalin A. V., Wallberg-Henriksson H., Zierath J. R. 5-Aminoimidazole-4-carboxamide ribonucleoside treatment improves glucose homeostasis in insulin- resistant diabetic (ob/ob) mice. Diabetologia. 2002;45:56–65. doi: 10.1007/s125-002-8245-8. [DOI] [PubMed] [Google Scholar]
- 79.Bergeron R., Previs S. F., Cline G. W., Perret P., Russell R. R., III, Young L. H., Shulman G. I. Effect of 5-aminoimidazole-4-carboxamide-1-β- D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes. 2001;50:1076–1082. doi: 10.2337/diabetes.50.5.1076. [DOI] [PubMed] [Google Scholar]
- 80.Iglesias M. A., Ye J. M., Frangioudakis G., Saha A. K., Tomas E., Ruderman N. B., Cooney G. J., Kraegen E. W. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51:2886–2894. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- 81.Cuthbertson D. J., Babraj J. A., Mustard K. J., Towler M. C., Green K. A., Wackerhage H., Leese G. P., Baar K., Thomason-Hughes M., Sutherland C., Hardie D. G., Rennie M. J. 5-Aminoimidazole-4-carboxamide 1-β-D-ribofuranoside acutely stimulates skeletal muscle 2-deoxyglucose uptake in healthy men. Diabetes. 2007;56:2078–2084. doi: 10.2337/db06-1716. [DOI] [PubMed] [Google Scholar]
- 82.Lihn A. S., Jessen N., Pedersen S. B., Lund S., Richelsen B. AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochem. Biophys. Res. Commun. 2004;316:853–858. doi: 10.1016/j.bbrc.2004.02.139. [DOI] [PubMed] [Google Scholar]
- 83.Kern P. A., Ranganathan S., Li C., Wood L., Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 84.Senn J. J., Klover P. J., Nowak I. A., Mooney R. A. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–3399. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- 85.Hotamisligil G. S. The role of TNFα and TNF receptors in obesity and insulin resistance. J. Intern. Med. 1999;245:621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 86.Masoudi F. A., Inzucchi S. E., Wang Y., Havranek E. P., Foody J. M., Krumholz H. M. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure, an observational study. Circulation. 2005;111:583–590. doi: 10.1161/01.CIR.0000154542.13412.B1. [DOI] [PubMed] [Google Scholar]
- 87.Eurich D. T., Majumdar S. R., McAlister F. A., Tsuyuki R. T., Johnson J. A. Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care. 2005;28:2345–2351. doi: 10.2337/diacare.28.10.2345. [DOI] [PubMed] [Google Scholar]
- 88.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 89.Zhang L., He H., Balschi J. A. Metformin and phenformin activate AMP-activated protein kinase in the heart by increasing cytosolic AMP concentration. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H457–H466. doi: 10.1152/ajpheart.00002.2007. [DOI] [PubMed] [Google Scholar]
- 90.Yang J., Holman G. D. Long-term metformin treatment stimulates cardiomyocyte glucose transport through an AMP-activated protein kinase-dependent reduction in GLUT4 endocytosis. Endocrinology. 2006;147:2728–2736. doi: 10.1210/en.2005-1433. [DOI] [PubMed] [Google Scholar]
- 91.Kovacic S., Soltys C. L., Barr A. J., Shiojima I., Walsh K., Dyck J. R. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J. Biol. Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 92.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Musi N., Hirshman M. F., Nygren J., Svanfeldt M., Bavenholm P., Rooyackers O., Zhou G., Williamson J. M., Ljunqvist O., Efendic S., et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 94.Davis B. J., Xie Z., Viollet B., Zou M. H. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 95.McCarty M. F. AMPK activation as a strategy for reversing the endothelial lipotoxicity underlying the increased vascular risk associated with insulin resistance syndrome. Med. Hypoth. 2005;64:1211–1215. doi: 10.1016/j.mehy.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 96.Buzzai M., Jones R. G., Amaravadi R. K., Lum J. J., DeBerardinis R. J., Zhao F., Viollet B., Thompson C. B. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 97.Hawley S. A., Gadalla A. E., Olsen G. S., Hardie D. G. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 98.El-Mir M. Y., Nogueira V., Fontaine E., Averet N., Rigoulet M., Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 99.Owen M. R., Doran E., Halestrap A. P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000;348:607–614. [PMC free article] [PubMed] [Google Scholar]
- 100.Jonker J. W., Schinkel A. H. Pharmacological and physiological functions of the polyspecific organic cation transporters, OCT1, 2, and 3 (SLC22A1-SLC22A3) J. Pharmacol. Exp. Therap. 2004;308:2–9. doi: 10.1124/jpet.103.053298. [DOI] [PubMed] [Google Scholar]
- 101.Bergheim I., Guo L., Davis M. A., Lambert J. C., Beier J. I., Duveau I., Luyendyk J. P., Roth R. A., Arteel G. E. Metformin prevents alcohol-induced liver injury in the mouse, Critical role of plasminogen activator inhibitor-1. Gastroenterology. 2006;130:2099–2112. doi: 10.1053/j.gastro.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saeedi R., Parsons H. L., Wambolt R. B., Paulson K., Sharma V., Dyck J. R., Brownsey R. W., Allard M. F. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2497–H2506. doi: 10.1152/ajpheart.00873.2007. [DOI] [PubMed] [Google Scholar]
- 103.Bailey C. J., Turner R. C. Metformin. N. Engl. J. Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 104.Bertrand L., Ginion A., Beauloye C., Hebert A. D., Guigas B., Hue L., Vanoverschelde J. L. AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H239–H250. doi: 10.1152/ajpheart.01269.2005. [DOI] [PubMed] [Google Scholar]
- 105.Lehmann J. M., Moore L. B., Smith-Oliver T. A., Wilkison W. O., Willson T. M., Kliewer S. A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPAR γ) J. Biol. Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 106.Saha A. K., Avilucea P. R., Ye J. M., Assifi M. M., Kraegen E. W., Ruderman N. B. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem. Biophys. Res. Commun. 2004;314:580–585. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- 107.Fryer L. G., Parbu-Patel A., Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 108.Brunmair B., Staniek K., Gras F., Scharf N., Althaym A., Clara R., Roden M., Gnaiger E., Nohl H., Waldhausl W., Furnsinn C. Thiazolidinediones, like metformin, inhibit respiratory complex I, a common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53:1052–1059. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- 109.Stocker D. J., Taylor A. J., Langley R. W., Jezior M. R., Vigersky R. A. A randomized trial of the effects of rosiglitazone and metformin on inflammation and subclinical atherosclerosis in patients with type 2 diabetes. Am. Heart J. 2007;153:441–446. doi: 10.1016/j.ahj.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 110.Artwohl M., Furnsinn C., Waldhausl W., Holzenbein T., Rainer G., Freudenthaler A., Roden M., Baumgartner-Parzer S. M. Thiazolidinediones inhibit proliferation of microvascular and macrovascular cells by a PPARγ-independent mechanism. Diabetologia. 2005;48:586–594. doi: 10.1007/s00125-005-1672-z. [DOI] [PubMed] [Google Scholar]
- 111.Liu H. B., Hu Y. S., Medcalf R. L., Simpson R. W., Dear A. E. Thiazolidinediones inhibit TNFα induction of PAI-1 independent of PPARγ activation. Biochem. Biophys. Res. Commun. 2005;334:30–37. doi: 10.1016/j.bbrc.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 112.Polikandriotis J. A., Mazzella L. J., Rupnow H. L., Hart C. M. Peroxisome proliferator-activated receptor γ ligands stimulate endothelial nitric oxide production through distinct peroxisome proliferator-activated receptor γ-dependent mechanisms. Arterioscler. Thromb. Vasc. Biol. 2005;25:1810–1816. doi: 10.1161/01.ATV.0000177805.65864.d4. [DOI] [PubMed] [Google Scholar]
- 113.Sasaki M., Jordan P., Welbourne T., Minagar A., Joh T., Itoh M., Elrod J. W., Alexander J. S. Troglitazone, a PPAR-γ activator prevents endothelial cell adhesion molecule expression and lymphocyte adhesion mediated by TNF-α. BMC Physiol. 2005;5:3. doi: 10.1186/1472-6793-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ceolotto G., Gallo A., Papparella I., Franco L., Murphy E., Iori E., Pagnin E., Fadini G. P., Albiero M., Semplicini A., Avogaro A. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 2007;27:2627–2633. doi: 10.1161/ATVBAHA.107.155762. [DOI] [PubMed] [Google Scholar]
- 115.Forst T., Karagiannis E., Lubben G., Hohberg C., Schondorf T., Dikta G., Drexler M., Morcos M., Danschel W., Borchert M., Pfutzner A. Pleiotrophic and anti-inflammatory effects of pioglitazone precede the metabolic activity in type 2 diabetic patients with coronary artery disease. Atherosclerosis. 2008;197:311–317. doi: 10.1016/j.atherosclerosis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 116.Schneider C. A., Ferrannini E., Defronzo R., Schernthaner G., Yates J., Erdmann E. Effect of pioglitazone on cardiovascular outcome in diabetes and chronic kidney disease. J. Am. Soc. Nephrol. 2008;19:182–187. doi: 10.1681/ASN.2007060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dormandy J. A., Charbonnel B., Eckland D. J., Erdmann E., Massi-Benedetti M., Moules I. K., Skene A. M., Tan M. H., Lefebvre P. J., Murray G. D., et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events), a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 118.Lago R. M., Singh P. P., Nesto R. W. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones, a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 119.Nissen S. E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 120.Home P. D., Pocock S. J., Beck-Nielsen H., Gomis R., Hanefeld M., Jones N. P., Komajda M., McMurray J. J. Rosiglitazone evaluated for cardiovascular outcomes: an interim analysis. N. Engl. J. Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 121.Laufs U., Gertz K., Dirnagl U., Bohm M., Nickenig G., Endres M. Rosuvastatin, a new HMG-CoA reductase inhibitor, upregulates endothelial nitric oxide synthase and protects from ischemic stroke in mice. Brain Res. 2002;942:23–30. doi: 10.1016/s0006-8993(02)02649-5. [DOI] [PubMed] [Google Scholar]
- 122.Laufs U., Gertz K., Huang P., Nickenig G., Bohm M., Dirnagl U., Endres M. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000;31:2442–2449. doi: 10.1161/01.str.31.10.2442. [DOI] [PubMed] [Google Scholar]
- 123.Colhoun H. M., Betteridge D. J., Durrington P. N., Hitman G. A., Neil H. A., Livingstone S. J., Thomason M. J., Mackness M. I., Charlton-Menys V., Fuller J. H. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS), multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 124.Cahoon W. D., Jr, Crouch M. A. Preprocedural statin therapy in percutaneous coronary intervention. Ann. Pharmacother. 2007;41:1687–1693. doi: 10.1345/aph.1K248. [DOI] [PubMed] [Google Scholar]
- 125.Sun W., Lee T. S., Zhu M., Gu C., Wang Y., Zhu Y., Shyy J. Y. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;114:2655–2662. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- 126.Xenos E. S., Stevens S. L., Freeman M. B., Cassada D. C., Goldman M. H. Nitric oxide mediates the effect of fluvastatin on intercellular adhesion molecule-1 and platelet endothelial cell adhesion molecule-1 expression on human endothelial cells. Ann. Vasc. Surg. 2005;19:386–392. doi: 10.1007/s10016-005-0011-7. [DOI] [PubMed] [Google Scholar]
- 127.Laufs U., Endres M., Custodis F., Gertz K., Nickenig G., Liao J. K., Bohm M. Suppression of endothelial nitric oxide production after withdrawal of statin treatment is mediated by negative feedback regulation of rho GTPase gene transcription. Circulation. 2000;102:3104–3110. doi: 10.1161/01.cir.102.25.3104. [DOI] [PubMed] [Google Scholar]
- 128.Laufs U., Endres M., Stagliano N., Amin-Hanjani S., Chui D. S., Yang S. X., Simoncini T., Yamada M., Rabkin E., Allen P. G., et al. Neuroprotection mediated by changes in the endothelial actin cytoskeleton. J. Clin. Invest. 2000;106:15–24. doi: 10.1172/JCI9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wenzel P., Daiber A., Oelze M., Brandt M., Closs E., Xu J., Thum T., Bauersachs J., Ertl G., Zou M. H. et al. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis. 2008;198:65–76. doi: 10.1016/j.atherosclerosis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nissen S. E., Tuzcu E. M., Schoenhagen P., Crowe T., Sasiela W. J., Tsai J., Orazem J., Magorien R. D., O'Shaughnessy C., Ganz P. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 131.Cool B., Zinker B., Chiou W., Kifle L., Cao N., Perham M., Dickinson R., Adler A., Gagne G., Iyengar R., et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 132.Andersson U., Filipsson K., Abbott C. R., Woods A., Smith K., Bloom S. R., Carling D., Small C. J. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 133.McCullough L. D., Zeng Z., Li H., Landree L. E., McFadden J., Ronnett G. V. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J. Biol. Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- 134.Solskov L., Løfgren B., Kristiansen S. B., Jessen N., Pold R., Nielsen T. T., Bøtker H. E., Schmitz O., Lund S. Metformin induces cardioprotection against ischaemia/reperfusion injury in the rat heart 24 hours after administration. Basic Clin. Pharmacol. Toxicol. 2008;103:82–81. doi: 10.1111/j.1742-7843.2008.00234.x. [DOI] [PubMed] [Google Scholar]