Abstract
Multifunctional adaptor protein APPL1 [adaptor protein containing PH (pleckstrin homology) domain, PTB (phosphotyrosine binding) domain and leucine zipper motif] belongs to a growing group of endocytic proteins which actively participate in various stages of signalling pathways. Owing to its interaction with the small GTPase Rab5, APPL1 localizes predominantly to a subpopulation of early endosomes but is also capable of nucleocytoplasmic shuttling. Among its various binding partners, APPL1 was reported to associate with the nuclear co-repressor complex NuRD (nucleosome remodelling and deacetylase), containing both nucleosome remodelling and HDAC (histone deacetylase) activities, but the biochemical basis or functional relevance of this interaction remained unknown. Here we characterized the binding between APPL1 and NuRD in more detail, identifying HDAC2 as the key NuRD subunit responsible for this association. APPL1 interacts with the NuRD complex containing enzymatically active HDAC2 but not HDAC1 as the only deacetylase. However, the cellular levels of HDAC1 can regulate the extent of APPL1–NuRD interactions, which in turn modulates the nucleocytoplasmic distribution of APPL1. Increased binding of APPL1 to NuRD upon silencing of HDAC1 promotes the nuclear localization of APPL1, whereas HDAC1 overexpression exerts an opposite effect. Moreover, we also uncovered a NuRD-independent interaction of APPL1 with HDAC1. APPL1 overexpression affects the composition of the HDAC1-containing NuRD complex and the expression of HDAC1 target p21WAF1/CIP1. Cumulatively, these data reveal a surprising complexity of APPL1 interactions with HDACs, with functional consequences for the modulation of gene expression. In a broader sense, these results contribute to an emerging theme of endocytic proteins playing alternative roles in the cell nucleus.
Keywords: APPL1 adaptor protein, histone deacetylase (HDAC), multiprotein complex, nucleocytoplasmic shuttling, nucleosome remodelling and deacetylase (NuRD) complex
Abbreviations: APPL, adaptor protein containing pleckstrin homology (PH) domain, phosphotyrosine binding (PTB) domain and leucine zipper motif; BAR domain, Bin1/amphiphysin/Rvs167 domain; EEA1, early endosome antigen 1; esiRNA, endoribonuclease-prepared siRNA; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GST, glutathione transferase; HDAC, histone deacetylase; HEK-293 cell, human embryonic kidney 293 cell; HIP1, Huntingtin-interacting protein 1; MBD, methyl CpG-binding domain; MTA, metastasis tumour antigen; NLS, nuclear localization signal; NuRD, nucleosome remodelling and deacetylase; PH domain, pleckstrin homology domain; PTB domain, phosphotyrosine binding domain; RbAp, retinoblastoma-associated protein; RNAi, RNA interference; siRNA, small interfering RNA; TCF, T-cell factor; TSA, trichostatin A
INTRODUCTION
During the last years many reports highlighted the impact of endocytosis on cellular signalling (reviewed in [1–4]). On one hand, endocytosis negatively regulates signal transduction by downregulation and degradation of ligand–receptor complexes from the plasma membrane, thus leading to signal attenuation. On the other hand, endocytic trafficking can also positively contribute to signal propagation, as in several cases signalling from internalized receptors continues intracellularly in endosomal compartments and certain signalling events require endocytosis to occur [5,6]. Consistent with this idea, there is a growing list of endocytic proteins actively participating in signal transduction. Most of them are localized to the membranes of endosomal compartments where they regulate the function of bona fide signalling proteins such as kinases, GTPases or transcription factors [6]. Interestingly, several endocytic proteins are capable of nuclear translocation themselves [7,8]. Although the exact mechanisms and significance of this phenomenon are largely unknown, it seems that these endocytic proteins may interact with nuclear molecules involved in transcription or chromatin remodelling, changing their localization and/or activity and may directly modulate the levels or specificity of gene transcription [9–13]. Certain endocytic proteins translocate to the nucleus in response to extracellular signals in order to exert a specific biological effect, as reported for β-arrestin 1 [14] or for HIP1 (Huntingtin-interacting protein 1) [15]. However, in most cases it is unclear to what extent the endocytic and nuclear functions are related or exclusive for each compartment.
APPL1 [adaptor protein containing PH (pleckstrin homology) domain, PTB (phosphotyrosine binding) domain and leucine zipper motif] represents an interesting example of an endocytic protein implicated in signal transduction and capable of nuclear translocation. The structure of APPL1 (and of its less well-characterized homologue APPL2) comprises the N-terminal BAR (Bin1/amphiphysin/Rvs167) domain, followed by the PH and the C-terminal PTB domain [16,17]. APPL1 is localized to the membranes of a particular subpopulation of early endosomes, where it is recruited via interaction with an active form of Rab5 GTPase, a key regulator of early steps of endocytosis [16]. In addition, APPL1 is present in the cytosol and to a lower degree in the cell nucleus, despite the lack of a canonical NLS (nuclear localization signal). APPL1 has been shown to interact with many partners involved in various signalling pathways mediating apoptosis [18], cell survival [19], cell proliferation and chromatin remodelling [16]. In particular, APPL1 interacts with a diverse set of receptors, including netrin-1 receptor DCC (deleted in colorectal cancer) [18], nerve growth factor receptor TrkA (tropomyosin receptor kinase A) [20,21], follicle-stimulating hormone receptor [22,23] and the adiponectin receptors AdipoR1 and AdipoR2 [24,25], as well as with signalling proteins: Akt [17,23,26]; the p85 and p110 subunits of phosphatidylinositol-3 kinase [17,27]; OCRL (oculocerebrorenal syndrome of Lowe); and inositol polyphosphate-5-phosphatase [28]. We have recently shown that APPL1 protein binds Reptin, a transcriptional repressor of the Wnt pathway and thus acts as a positive regulator of β-catenin/TCF (T-cell factor)-dependent transcription [12]. In general, APPL1 appears to play a role of an adaptor or a scaffold protein for distinct signalling pathways.
Interestingly, it was previously documented that APPL1 interacts with the NuRD (nucleosome remodelling and deacetylase) complex [16]. This multiprotein co-repressor complex is unique with respect to combining the two usually separate activities of chromatin remodelling and histone deacetylation in one macromolecular assembly [29–32]. These activities are provided by the nucleosome remodelling ATPase Mi-2 and two related class I HDACs (histone deacetylases) HDAC1 and HDAC2. The other complex components include histone-binding proteins RbAp46 (retinoblastoma-associated protein 46; Rbbp7) and RbAp48 (Rbbp4), one member of the MTA (metastasis tumour antigen) family (alternatively MTA1, MTA2 or MTA3) [33], a member of the MBD (methyl CpG-binding domain) family of proteins (alternatively MBD2 or MBD3) [34] and transcriptional repressors p66α (Gatad2A) and p66β (Gatad2B) interacting with histones and MBD2/MBD3 [35,36]. Based on the number of alternative subunits of this complex and their partially tissue-specific expression, it was suggested that the NuRD complex is, in fact, not a single molecular species but a set of distinct although similar complexes, possibly exhibiting partly specialized functions [37–39]. The main function of all NuRD complexes is transcriptional repression, mediated by HDAC1 and HDAC2, which are involved in deacetylation of histone H3 and H4 tails [40]. Overall, NuRD regulates fundamental cellular processes such as proliferation and differentiation and thus plays important roles in development or carcinogenesis [33,41,42].
In the present study, we investigated the relationship between the endocytic protein APPL1 and the NuRD complex in more detail. First, we characterized the biochemical basis of this interaction and identified HDAC2 as a key NuRD subunit mediating the association with APPL1. Moreover, we demonstrated that binding to HDAC2-containing NuRD complex contributes to the nuclear localization of APPL1. Interestingly, APPL1 is also capable of forming another complex with HDAC1 which seems independent of HDAC2-mediated association with NuRD. Finally, our results suggest that APPL1 may regulate HDAC functions, as APPL1 levels influence the expression of the HDAC1 target p21WAF1/CIP1.
EXPERIMENTAL
Cell lines
HEK-293 (human embryonic kidney 293) and A431 cells were maintained in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin. HeLa cells were grown in MEM (modified Eagle's medium) supplemented as above.
Plasmids
APPL1-encoding plasmids (pcDNA3/APPL1, pcDNA3/APPL1–MYC, pGEX-6P-3/APPL1 amino acids 1–428, pGEX-6P-3/APPL1 amino acids 429–709) were previously described in [16]. C-terminally FLAG-tagged HDAC1 was recloned into pcDNA3 (Invitrogen) from pBJ5-HDAC1–FLAG plasmid which was a gift from Dr Stuart Schreiber (Broad Institute of Harvard and MIT, Cambridge, MA, U.S.A.).
siRNA (small interfering RNA)
The following siRNA oligonucleotides were purchased from Ambion: against HDAC1: a (ID # 120420), b (ID # 5436); HDAC2: a (ID # 120209), b (ID # 120210); MTA2: a (ID # 5314), b (107516); RbAp46: a (ID # 121182), b (ID # 12277), c (ID # 142450); RbAp48: a (ID # 16640), b (ID # 142862), c (ID # 142863); and non-specific siRNA (negative control ID # 4611).
Production and purification of short double-stranded RNA duplexes
Optimal esiRNA (endoribonuclease-prepared siRNA) target regions with a length of 400–600 bp were selected using the DEQOR web server (http://cluster-1.mpi-cbg.de/Deqor/deqor.html). In brief, T7 promoter sequence was added to the selected regions of APPL1 cDNAs by two PCRs: the first PCR was carried out by using gene-specific primer pairs that were tagged at 5′ ends with a part of the T7 promoter (underlined). During the second PCR, primers specific to T7 promoter were used to amplify the whole T7 sequence. The sequences for these primers are as follows: for APPL1, 5′-TCACTATAGGGAGAGGATTCTCTTGTTGCCCCAGA-3′ (forward primer) and 5′-TCACTATAGGGAGACTCCCCCTCATTGTTTGACTC-3′ (reverse primer); for T7 promoter, 5′-GCTAATACGACTCACTATAGGGAGAG-3′ (forward primer) and 5′-GCTAATACGACTCACTATAGGGAGAC-3′ (reverse primer). For control luciferase esiRNA, the PCRs were performed in one step using the following primers (T7 sequence underlined): luciferase FLuc, 5′-GCTAATACGACTCACTATAGGGAGAGGAGCAACTGCATAAGG-3′ (forward primer) and 5′-GCTAATACGACTCACTATAGGGAGACAATCTGACGCAGGCAGT3′ (reverse primer) or RLuc, 5′-GCTAATACGACTCACTATAGGGAGAGGATAACTGGTCCGCAGTGGT-3′ (forward primer) and 5′-GCTAATACGACTCACTATAGGGAGACCCATTCATCCCATGATTCAA-3′ (reverse primer). Further esiRNA synthesis was carried out as described previously [43]. The concentration of esiRNA was determined by measuring the absorbance at 260 nm.
Antibodies
The following antibodies were used: polyclonal against HDAC1 (Abcam), MTA2 (Oncogene), RbAp46 (Affinity BioReagents), EEA1 (early endosome antigen 1; BD Biosciences), GAPDH (glyceraldehyde-3-phosphate dehydrogenase; Santa Cruz Biotechnology), histone H3 (Sigma), histone H3 acetylated on Lys9 (Upstate), p21 (Santa Cruz Biotechnology) and APPL1 [16]; mouse monoclonal against HDAC1 (Abcam), HDAC2 (Upstate), RbAp48 (Upstate), FLAG-M2 (Sigma) and Myc (Sigma); secondary horseradish peroxidase-conjugated goat anti-mouse and anti-rabbit antibodies (Jackson ImmunoResearch), Alexa Fluor® 488-conjugated anti-mouse and Alexa Fluor® 568-conjugated anti-rabbit antibodies (Invitrogen).
Plasmid, siRNA and esiRNA transfection
For microscopy analysis, HeLa human cervical carcinoma cells were transfected with 0.2 μg of plasmid DNA in 24-well plates using FuGENE™ reagent (Roche) and fixed 48 h after transfection. HEK-293 cells used for immunoprecipitation or preparation of nuclear/cytoplasmic fractions were transfected in 10 cm plates with 18–24 μg of plasmid DNA using Lipofectamine™ 2000 (Invitrogen) and harvested 48 h after transfection. For siRNA and esiRNA transfection, cells were transfected for 72 h with 10 nM siRNA or 33 nM esiRNA using HiPerFect transfection reagent (Qiagen). All transfections were performed according to the manufacturers' instructions.
Western blot analysis
Cells were extracted in lysis buffer containing 1% Triton X-100, 0.1% SDS, 5 μg/ml DNase and protease inhibitor cocktail (6 μg/ml chymostatin, 0.5 μg/ml leupeptin, 10 μg/ml antipain, 2 μg/ml aprotinin, 0.7 μg/ml pepstatin A and 10 μg/ml 4-amidinophenylmethanesulfonyl fluoride hydrochloride; Sigma) in PBS. Samples of 10–20 μg total protein were subjected to SDS/PAGE on 8, 10 or 12% polyacrylamide gels. Resolved proteins were transferred to nitrocellulose membrane (Whatman) for immunoblot analysis, probed with specific antibodies diluted 1:1000, and detected with enhanced chemiluminescence.
Preparation of nuclear/cytoplasmic fractions
HeLa cells were washed in ice-cold PBS, scraped from the plate and centrifuged (800 g, 3 min, 4 °C). The cell pellet was resuspended in a lysis buffer (10 mM Tris/HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.5% Nonidet P40 and protease inhibitors) by passing 10 times through a pipette tip and then applied on the top of 6 ml of sucrose buffer (0.7 M sucrose, 60 mM KCl, 15 mM NaCl, 15 mM Tris/HCl, pH 7.5, 2 mM EDTA, 0.5 mM EGTA, 14 mM 2-mercaptoethanol and 0.1% Triton X-100). After 10 min of centrifugation (1300 g, 4 °C), the cytoplasmic fraction was harvested from the top of the sucrose buffer, and the nuclei forming a pellet at the bottom of the tube were lysed in RIPA buffer (50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 0.5 mM EDTA, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, 5 μg/ml DNase and protease inhibitors) for 20 min on ice. Both fractions were centrifuged for 15 min at 20000 g to remove insoluble complexes. For HEK-293 cells, the above protocol was modified to obtain clean fractions. HEK-293 cells were trypsinized, centrifuged and resuspended in a buffer consisting of 20 mM Hepes, pH 7.9, 20 mM NaF, 1 mM Na3VO4, 1 mM Na3P2O7, 1 mM EDTA, 1 mM EGTA, 1 mM DTT (dithiothreitol), DNase and protease inhibitor cocktail. After 15 min of lysis on ice, Nonidet P40 was added to the cell extracts to a final concentration of 0.2% for a further 15 min on ice. Afterwards cell lysates were processed as above with centrifugation in the sucrose buffer. The purity of fractions was tested by immunoblotting for EEA1 and GAPDH as cytoplasmic markers and histone H3 as a nuclear marker.
Immunoprecipitation and GST (glutathione transferase) pull-down assay
APPL1, MTA2 or HDAC1 were immunoprecipitated from HeLa or HEK-293 cells. First, cells were lysed in ice-cold PBS containing 1% Triton X-100, 0.1% SDS, 5 μg/ml DNase and protease inhibitor cocktail. Between 100 and 250 μg of protein was used per reaction. Proteins of interest were immunoprecipitated by overnight incubation with an appropriate antibody at 4 °C with constant rotation. Immune complexes were recovered by 2 h incubation with Protein G–agarose beads (Roche) at 4 °C with rotation, followed by centrifugation and five washes in a wash buffer for immunoprecipitation (50 mM Hepes, pH 7.5, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100, 10% glycerol, 5 μg/ml DNase and protease inhibitor cocktail). Next, samples were incubated at 95 °C for 5 min with Laemmli buffer and subjected to electrophoresis on 8% polyacrylamide gels. In some experiments, antibodies were first cross-linked with dimethyl pimelimidate (Pierce) to Protein G agarose, incubated with extracts or fractions at 4 °C overnight and washed extensively with the wash buffer as described above. In such cases, the final elution was performed with 100 mM glycine, pH 2.5, instead of Laemmli buffer.
GST, GST–APPL1-N (comprising 428 amino acids from the N-terminus) and GST–APPL1-C (comprising amino acids 429–709) fusion proteins used in pull-down assays as bait were expressed and purified according to the manufacturer's instructions (GE Healthcare). Isopropyl β-D-thiogalactoside (Sigma) at a concentration of 0.5 mM was used to induce the expression. In vitro translated HDAC1–FLAG, HDAC2, RbAp46 and RbAp48 proteins (synthesized using TNT T7 Coupled Reticulocyte Lysate System from Promega according to the manufacturer's protocol) were incubated overnight at 4 °C with constant rotation with equal amounts of glutathione–Sepharose beads (GE Healthcare) complexed with GST, GST–APPL1-N or GST–APPL1-C fusion proteins. Beads were washed 5 times with the wash buffer used for immunoprecipitation. GST-fusion proteins together with bound proteins were eluted with 10 mM glutathione in 50 mM Tris/HCl, pH 8.0, for 15 min at room temperature (22 °C) with shaking. Eluates were resuspended in Laemmli buffer, subjected to SDS/PAGE (10% gels) and immunoblotted for the proteins of interest.
HDAC activity assay
HDAC activity was measured using the HDAC Fluorimetric Cellular Activity Assay kit according to the instructions from the manufacturer (kit AK503 from BIOMOL). Briefly, immunoprecipitates bound to Protein G beads were washed three times and resuspended in the assay buffer containing 100 μM substrate, with or without the presence of 1 μM TSA (trichostatin A; Sigma) or 1 mM nicotinamide (BIOMOL). The reaction mixtures were incubated for 2 h at room temperature and stopped by adding developer solution. The fluorescence of the modified substrate was measured after 30 min at 360 nm excitation/450 nm emission using a spectrofluorophotometer (Shimadzu RF-530IPC).
Microscopy
HeLa cells grown on coverslips were washed twice in PBS and fixed with 3% paraformaldehyde in PBS for 15 min at room temperature. Cells were then washed and permeabilized in 0.1% Triton X-100 in PBS for 2 min at room temperature. After washing, free aldehyde groups were quenched by 15 min incubation with 50 mM NH4Cl in PBS. Washed coverslips were blocked in 10% fetal bovine serum in PBS for 1 h, and incubated with primary antibodies diluted in 5% fetal bovine serum. To ensure a complete staining of nuclear proteins (HDAC2 and APPL1), the incubation with primary antibodies was performed overnight in a humid chamber. The coverslips were washed twice for 5 min in PBS, and Alexa Fluor®-tagged secondary antibodies were added for 2 h. Nuclei were visualized by adding Hoechst reagent (Invitrogen) at a concentration of 1 μM to the secondary antibody mix. The coverslips were washed three times in PBS, rinsed in water, and mounted onto glass slides using Moviol (Fluka). Images were acquired on a laser-scanning confocal microscope Leica TCS SP2 with AOBS (acousto-optical beam splitter). Cells were scanned along the z-axis in 1 μm steps. Z-stacks were built and converted into maximal projections using the Metamorph 4.6 r10 program (Universal Imaging). Fluorescence intensity of APPL1 in the cell nuclei (manually outlined based on the Hoechst staining) was calculated using the Metamorph 4.6 r10 program. Nuclear levels of APPL1 presented as average pixel intensity reflect integrated pixel intensity divided by the area of an outlined cell nucleus region. Results were calculated and statistical significance between groups was evaluated by GraphPad Prism 4.02 (GraphPad Software, Inc.). The presented microscopy images were assembled using Adobe Photoshop 7.0.
RESULTS
Binding between APPL1 and the NuRD complex depends on HDAC2
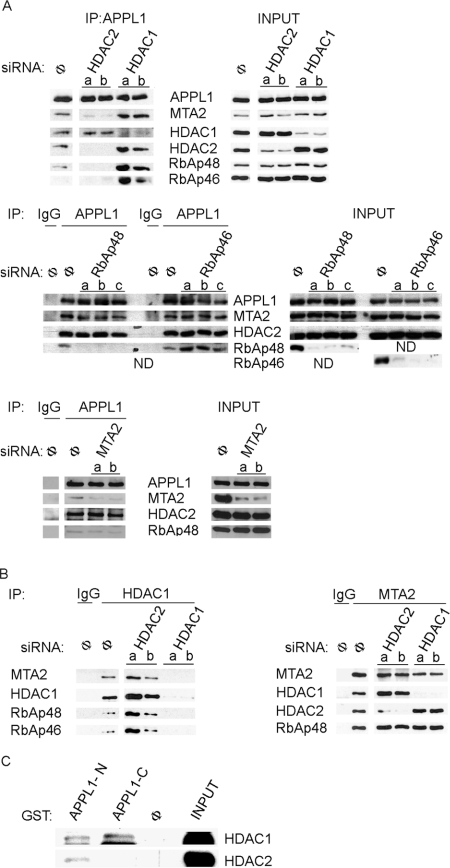
In order to understand the biological significance of interactions between APPL1 protein and the multisubunit NuRD complex, we started with the biochemical characterization of this association. In particular, we set out to identify a subunit of NuRD responsible for creating contact between APPL1 and the rest of the multiprotein complex. To this end, we tested the ability of APPL1 to interact with the NuRD complex in lysates of cells depleted of individual components of the complex (silenced one at a time). We reduced the levels of four core NuRD subunits: HDAC1, HDAC2, RbAp48 or RbAp46, or additionally MTA2 protein (which modulates the enzymatic activity of the HDAC core complex) [44] by employing the RNAi (RNA interference) technique with at least two different siRNA oligos per gene in HeLa cells. Extracts from such cells were subjected to co-immunoprecipitation assay using anti-APPL1 antibody. As shown in Figure 1(A), in cells transfected with non-specific siRNA, APPL1 co-immunoprecipitated with MTA2, HDAC1, HDAC2 and RbAp48 proteins, as previously reported [16]. In contrast, depletion of HDAC2 precluded binding of APPL1 to the NuRD complex, as shown by the lack of MTA2 and RbAp48 in the APPL1 immunoprecipitate compared with control cells. The depletion of HDAC1, RbAp48, RbAp46 or MTA2 did not disrupt the APPL1–NuRD interactions, since other NuRD subunits were still present in APPL1 immunoprecipitate under such conditions. Thus these results pointed out that HDAC2 is a crucial component, mediating the APPL1–NuRD interaction. Interestingly, the ability of APPL1 to bind HDAC1 remained unaffected under conditions when HDAC2 was depleted, and interactions with MTA2 and RbAp48 were prohibited (Figure 1A). This result indicated that, in addition to HDAC2-mediated association with NuRD, APPL1 exhibits an independent interaction with HDAC1 or an HDAC1-containing complex which does not involve other core subunits of NuRD. Strikingly, in cells silenced for HDAC1, the interactions between APPL1 and the NuRD subunits were clearly enhanced, as demonstrated by increased amounts of MTA2, HDAC2, RbAp48 and RbAp46 in APPL1 immunoprecipitates in comparison with control cells (Figure 1A). This fact implies that lack of HDAC1 promotes binding of APPL1 to NuRD via HDAC2, further pointing to the equilibrium between the amounts of complexes containing APPL1–HDAC1 and APPL1–HDAC2–NuRD present in cells under normal conditions.
Figure 1. HDAC2 is critical for binding of APPL1 to the NuRD complex.
(A and B) Extracts from HeLa cells transfected for 72 h with two (a, b) or three (a, b, c) different siRNA oligonucleotides per gene against: HDAC1, HDAC2, MTA2, RbAp48 and RbAp46 or non-specific siRNA (Φ) were subjected to immunoprecipitation (IP) using: (A) anti-APPL1 antibody; (B) anti-HDAC1 antibody (left panel) or anti-MTA2 antibody (right panel). Non-specific antibodies (IgG) were used as controls. Input indicates 10% of total cell extracts used for immunoprecipitation. Immunoprecipitates and input extracts were analysed by Western blotting using different antibodies as indicated. (C) To verify the direct interactions between APPL1 and HDAC1 or HDAC2, in vitro translated HDAC1–FLAG and untagged HDAC2 were subjected to GST pull-down assay using GST alone (Φ) or GST fused to the N- or C-terminal parts of APPL1 (APPL1-N or APPL1-C, respectively). Input indicates 10% of in vitro translated material used for the pull-down assay. Bound proteins were analysed by Western blotting using anti-HDAC1 and anti-HDAC2 antibodies. ND, not determined.
In order to verify further that HDAC2 mediates the association between APPL1 and NuRD, we tested whether the inability of APPL1 to associate with NuRD upon knockdown of HDAC2 is not due to the disruption of interactions between other NuRD subunits under such conditions. To this end, we used extracts depleted for HDAC1 or HDAC2 and performed co-immunoprecipitation experiments with anti-MTA2 or anti-HDAC1 antibodies. As shown in Figure 1(B), lack of HDAC2 did not preclude the interactions between the remaining NuRD subunits (HDAC1, MTA2, RbAp46 and RbAp48). These results demonstrate that lack of HDAC2 does not affect the overall integrity of the NuRD complex, but specifically prevents the binding between NuRD and APPL1, further confirming that HDAC2 mediates the APPL1–NuRD interactions. We next wished to test whether APPL1 was able to bind directly to HDAC2. To this end, we conducted GST pull-down experiments with in vitro translated HDAC2 using as bait two non-overlapping fragments of APPL1 fused to GST: the N-terminal part (amino acids 1–428; comprising the BAR and PH domains) and the C-terminal part (amino acids 429–709; containing the PTB domain). We could detect weak direct binding of HDAC2 to the N-terminal part of APPL1 (Figure 1C). These results could potentially explain the role of HDAC2 in bridging the association between APPL1 and NuRD; however, most likely, the binding of HDAC2 to APPL1 is more efficient when HDAC2 is present in the context of the whole NuRD complex. In addition, we performed similar experiments with in vitro translated HDAC1, RbAp46 and RbAp48 proteins. Although no binding to APPL1 was detected for RbAp46 and RbAp48 (which were demonstrated not to be critical for APPL1–NuRD interactions; results not shown), HDAC1 appeared to weakly interact with both N- and C-terminal parts of APPL1 (Figure 1C). These data suggest that HDAC1 and HDAC2 may share a binding site in the N-terminal part of APPL1, whereas HDAC1 can additionally interact with the C-terminus of APPL1. The ability of APPL1 to interact directly with HDAC1 can explain the binding between these proteins, which is independent of HDAC2. Cumulatively, these findings demonstrated that APPL1 interacts with the NuRD complex via HDAC2 and forms another complex with HDAC1.
HDAC2-dependent interactions between APPL1 and NuRD occur in both the cytoplasmic and nuclear fractions, also in the presence of HDAC inhibitors
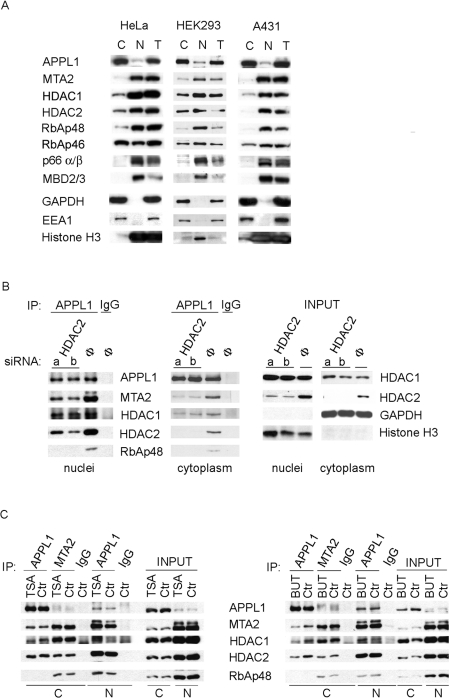
The initial data demonstrating the binding between APPL1 and NuRD came from experiments using HeLa nuclear extracts [16]. Although the NuRD components are considered to be largely nuclear proteins, no comprehensive analysis of their intracellular distribution has been performed, even though some of them were reported to also be present, to different degrees, in the cytoplasm [45,46]. In order to address the question of the intracellular localization of the APPL1–NuRD interaction, first we systematically investigated the distribution of the individual NuRD subunits in the cell. Fractionation experiments from different cell lines presented in Figure 2(A) revealed that most NuRD subunits are predominantly nuclear but their cytoplasmic pools are also significant in several cases, particularly for RbAp46 (but not related RbAp48), HDAC1 and HDAC2. Different proportions of various NuRD subunits found in the nucleus and in the cytoplasm argue that these proteins are not exclusively complexed to each other but are also present free or as components of complexes other than NuRD. Interestingly, the cytoplasmic pools of the NuRD components appear generally larger in HeLa and HEK-293 than in A431 cells, which may imply some kind of physiological relevance depending on the cell type. Our observation of cytoplasmic pools of HDAC1 and HDAC2 is in agreement with other studies demonstrating that the NuRD components can form complexes with cytoplasmic proteins [e.g. HDAC2 with IRS-1 (insulin receptor substrate 1)] [46]. These two facts prompted us to investigate the intracellular site of APPL1–NuRD interaction. As shown in Figure 2(B), APPL1 co-immunoprecipitates the NuRD components from both cytoplasmic and nuclear fractions. In both compartments, these interactions are HDAC2-dependent, as they were clearly reduced upon HDAC2 knockdown. Cumulatively, these results clearly demonstrate that APPL1 can interact with the NuRD components both in the nucleus and in the cytoplasm.
Figure 2. APPL1 interacts with the NuRD subunits in both cytoplasmic and nuclear fractions independently of HDAC enzymatic activity.
(A) Cytoplasmic (C) and nuclear (N) fractions along with total extracts (T) of three different cell lines, HeLa, HEK-293 and A431, were analysed for the presence of several NuRD subunits by Western blotting with different antibodies as indicated. For detection with a given antibody, equal amounts of proteins from all fractions and three cell lines were loaded (20 μg of protein for blotting with anti-p66α/β, -MBD2/3 and -EEA1 antibodies; 15 μg of protein for anti-APPL1, -HDAC2, -RbAp46 and -GAPDH; 10 μg of protein for anti-MTA2, -HDAC1, -RbAp48 and -Histone H3; the different amounts of protein loaded were chosen to match different sensitivities of the antibodies used). Cytoplasmic (GAPDH and EEA1) and nuclear (histone H3) markers were used to demonstrate the purity of fractions. (B) HeLa cells were transfected for 72 h with two oligonucleotides (a, b) against HDAC2 or with non-specific control oligonucleotide (Φ). Cytoplasmic and nuclear fractions were prepared and subjected to immunoprecipitation (IP) using anti-APPL1 antibody or non-specific immunoglobulins (IgG). Immunoprecipitates were tested for the presence of several NuRD subunits by immunoblotting with various antibodies as indicated. Right panel: 10% of the input material (cytoplasmic and nuclear fractions) were analysed for the knockdown efficiency using anti-HDAC1 and anti-HDAC2 antibodies, as well as for the fraction purity with anti-GAPDH and anti-histone H3 antibodies. (C) Immunoprecipitation from cytoplasmic (C) or nuclear (N) fractions of HeLa cells treated for 20 h with 100 ng/ml of TSA (left panel) or 25 mM sodium butyrate (BUT; right panel) was performed using anti-APPL1, anti-MTA2 or non-specific rabbit IgG. Precipitates along with 10% of the input material were analysed by Western blotting using different antibodies, as indicated. Ctr, control.
Since APPL1 binds the NuRD complex in a strictly HDAC2-dependent manner, we could consider two, non-mutually exclusive, explanations for this dependence. In one case, the enzymatic activity of HDAC2, and in the other case its physical presence could be crucial for APPL1–NuRD binding. To address this issue, we conducted co-immunoprecipitation experiments using anti-APPL1 antibody in nuclear and cytoplasmic fractions of HeLa cells pretreated with the HDAC class I and II inhibitors, TSA or sodium butyrate. Both treatments caused a large increase in the levels of acetylated histone H3, as determined by Western blotting (results not shown). The formation of the NuRD complex under such conditions was controlled by immunoprecipitation with anti-MTA2 antibody. As depicted in Figure 2(C), the APPL1–NuRD associations in inhibitor-treated cells are generally preserved, with only minor effects. Sodium butyrate treatment leads to a slight reduction in APPL1 interactions with the HDAC2-containing complex, as well as with HDAC1 in the cytoplasm. In TSA-treated cells, only the interactions of APPL1 with HDAC1 in the cytoplasmic fraction were slightly reduced. The observed effects were specific for APPL1 interactions, since MTA2 association with the NuRD subunits remained unchanged upon treatment with HDAC inhibitors (Figure 2C). Moreover, in nuclear fractions, the reduction of HDAC enzymatic activity by either TSA or sodium butyrate enhanced the binding of APPL1 to HDAC1, without affecting the association with NuRD. In conclusion, the enzymatic activity of HDAC1 or HDAC2 seems not to have a critical role for APPL1–NuRD interactions, even though it may slightly modulate them depending on the intracellular compartment. As there are many examples of non-histone proteins being targets for HDAC enzymatic activities (reviewed in [47]), we also tested if APPL1 was acetylated. By probing APPL1 immunoprecipitates with antibodies against acetylated lysine residues, we were not able to detect modification of APPL1, even upon treatment with HDAC inhibitors (results not shown), further indicating that the interactions between APPL1 and HDAC1 or HDAC2 are unlikely to have a substrate–enzyme character.
Interactions with the NuRD complex promote the nuclear localization of APPL
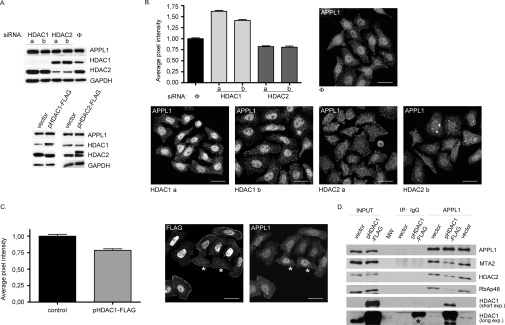
Knowing that APPL1 binds the NuRD components in both cytoplasmic and nuclear fractions in an HDAC2-dependent manner, we attempted to investigate whether these interactions played a role in regulating the nucleocytoplasmic distribution of APPL1. APPL1 lacks a classical NLS, and the mechanism of its nuclear targeting is unknown. There are reported examples of endocytic proteins which are imported to the nucleus via interactions with an NLS-harbouring partner (reviewed in [7]). Despite the fact that we found some NuRD components in the cytoplasm, this complex still remains mainly nuclear. We therefore hypothesized that an increased interaction of APPL1 with NuRD (observed upon HDAC1 silencing, Figure 1A) could result in an enhancement of the nuclear localization of APPL1, whereas lack of such interactions upon knockdown of HDAC2 could reduce the nuclear presence of APPL1. We first verified that the total intracellular levels of APPL1 protein were not affected by silencing of HDAC1 or HDAC2 (Figure 3A) or both together (results not shown), or by overexpression of HDAC1 or HDAC2 (Figure 3A). We then downregulated the levels of HDAC1 or HDAC2 by RNAi in HeLa cells and measured the amounts of APPL1 present in the cell nucleus by quantitative microscopy. Consistent with the biochemical experiments demonstrating the opposite effects of HDAC1 and HDAC2 depletion on the extent of APPL1–NuRD interaction (Figure 1A), we observed that knockdown of HDAC2 reduces the nuclear localization of APPL1 by approx. 20%, whereas knockdown of HDAC1 enhances it by at least 30% (Figure 3B). These data indicate that binding of APPL1 to HDAC2-containing NuRD complex favours the nuclear localization of APPL1. Moreover, the overexpression of HDAC1 exerts an effect opposite to the knockdown of HDAC1, leading to the approx. 20% reduction in the nuclear localization of APPL1 (Figure 3C). To explain this phenomenon, we investigated the extent of interactions between APPL1 and the NuRD complex subunits upon HDAC1 overexpression and observed a decrease in APPL1 interactions with HDAC2, RbAp48 and MTA2 (Figure 3D). The observed destabilization of association between APPL1 and HDAC2-containing NuRD complex upon HDAC1 overexpression could explain the reduced nuclear localization of APPL1. These results, in combination with the results demonstrating the increase in APPL1–NuRD interactions upon HDAC1 silencing (Figure 1A), clearly show that the intracellular level of HDAC1 influences the extent of binding between APPL1 and the HDAC2-containing NuRD complex. Interestingly, HDAC2 overexpression did not enhance the interactions between APPL1 and the HDAC2-containing NuRD complex, as tested by immunoprecipitation and, consequently, no changes in the nuclear localization of APPL1 were observed (results not shown). Overall, the extent of interactions with the NuRD complex appears to be modulated by HDAC1 and to regulate the nucleocytoplasmic distribution of APPL1.
Figure 3. The interactions with NuRD affect cellular distribution of APPL1.
(A) APPL1 protein levels do not depend on HDAC1 or HDAC2. Extracts of HeLa cells with reduced HDAC1 or HDAC2 levels by siRNA [two different oligonucleotides (a, b) per gene or non-specific oligo (Φ), transfected for 72 h; top panel] and HEK-293 cells transfected for 48 h with plasmids encoding FLAG-tagged HDAC1 (pHDAC1–FLAG), HDAC2 (pHDAC2–FLAG) or with a control vector (bottom panel), were analysed by Western blotting using anti-APPL1 antibodies. To demonstrate the efficiency of silencing or overexpression, extracts were probed using anti-HDAC1 and anti-HDAC2 antibodies. GAPDH was included as a loading control. (B) Microscopy-based analysis of APPL1 nuclear localization upon silencing of HDAC1 or HDAC2. HeLa cells were transfected with siRNA oligonucleotides: one non-specific (Φ) and two specific per gene (a and b) against HDAC1 and HDAC2 for 72 h, followed by fixation and immunostaining for APPL1. Acquired microscopic images were analysed by Metamorph software and the average pixel intensities corresponding to APPL1 in the nuclei (as visualized by Hoechst staining, not shown) were calculated. The results of a representative experiment are shown in the graph. The values are normalized with respect to the average pixel intensity of nuclear APPL1 in cells transfected with non-specific siRNA, assigned one arbitrary unit. Error bars indicate standard error (minimum 100 cells from each transfection were used for the analysis). The results were statistically analysed by GraphPad Prism4 software, and the values obtained for each knockdown experiment were significantly different from the control at P<0.0001. The images demonstrate the cellular localization of APPL1 upon silencing of HDAC1 or HDAC2 (as indicated) and represent a maximal projection of z-stacks. Scale bar: 24 μm. (C) HeLa cells transfected with the plasmid encoding FLAG-tagged HDAC1 (pHDAC1–FLAG) were analysed with respect to the nuclear localization of APPL1 as described above. Untransfected cells (marked with asterisks) were used as a control (the average pixel intensity of nuclear APPL1 set to one unit). The statistical analysis was performed on 50 transfected and 50 untransfected cells, and the difference between them was statistically significant at P<0.0001. The images represent a maximal projection of z-stacks. FLAG staining (left image) is shown to discriminate between transfected and untransfected cells with respect to APPL1 staining (right image). Scale bar: 24 μm. (D) HDAC1 overexpression leads to the destabilization of binding between APPL1 and HDAC2-containing NuRD complex. Extracts of HEK-293 cells transfected with HDAC1-FLAG or with a control vector were subjected to immunoprecipitation (IP) with anti-APPL1 or non-specific (IgG) antibodies. Immunoprecipitates, along with 10% of the extracts used (input), were analysed by Western blotting using the indicated antibodies. MW indicates a lane loaded with a molecular mass (weight) marker. Two exposures (short and long) of the HDAC1 blot are shown. Overexpressed HDAC1 exhibits some non-specific binding to IgG-covered protein G beads (visible at the long exposure of the blot and marked with an asterisk); however, its binding to beads containing anti-APPL1 antibodies is higher.
APPL1-bound HDAC2 exhibits enzymatic activity
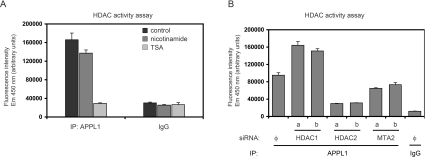
Having established the importance of HDAC2-mediated interactions with NuRD for the nuclear localization of APPL1, we next investigated whether APPL1 can influence any properties of the NuRD complex or its components. First, we studied whether APPL1 associates with enzymatically active HDAC. To this end, we employed enzymatic assays with a fluorogenic substrate and demonstrated that the immunoprecipitates of APPL1 exhibit deacetylase activity sensitive to the class I/II HDAC inhibitor TSA (Figure 4A). This enzymatic activity is not affected by the class III HDAC inhibitor nicotinamide, confirming that APPL1 binds to active class I enzymes, such as HDAC1 or HDAC2. We further wished to determine whether both HDAC1 and HDAC2 contribute to APPL1-associated deacetylase activity. We observed that this activity was significantly inhibited upon knockdown of HDAC2 and only slightly reduced upon silencing of another NuRD subunit, MTA2 (Figure 4B). Interestingly, in HDAC1-depleted HeLa cells, deacetylase activity bound to APPL1 was considerably increased, which could be explained by an enhanced binding of HDAC2 and NuRD to APPL1 under such conditions (Figure 1A). This fact strongly implies that most of the deacetylase activity found in the APPL1 complex is derived from HDAC2, whereas HDAC1 associated to APPL1 seems to be less active or less abundant than HDAC2. These results confirm that APPL1 interacts with the NuRD complex containing enzymatically active HDAC2.
Figure 4. Deacetylase activity detected in APPL1 complexes derives mainly from HDAC2.
HDAC enzymatic activity was measured using a fluorimetric method (as described in the Experimental section) in immunoprecipitates from HeLa cells. (A) APPL1 binds active HDACs from class I or II. The APPL1 immunoprecipitate (IP) was divided into three equal parts: one left untreated and the other two treated with HDAC inhibitors: 1 mM nicotinamide and 1 μM TSA. Non-specific rabbit IgG was used as a control. (B) HeLa cells were silenced for HDAC1, HDAC2 and MTA2 [using two (a, b) different siRNA oligonucleotides per gene or non-specific siRNA Φ] prior to immunoprecipitation using APPL1 antibodies or non-specific rabbit IgG. The same extracts as presented in Figure 1(A) were used (one third of the immunoprecipitates was measured in the HDAC activity assay, two thirds blotted as shown in Figure 1A). The intensity of fluorescence emitted by the deacetylated substrate is expressed in arbitrary units in (A) and (B).
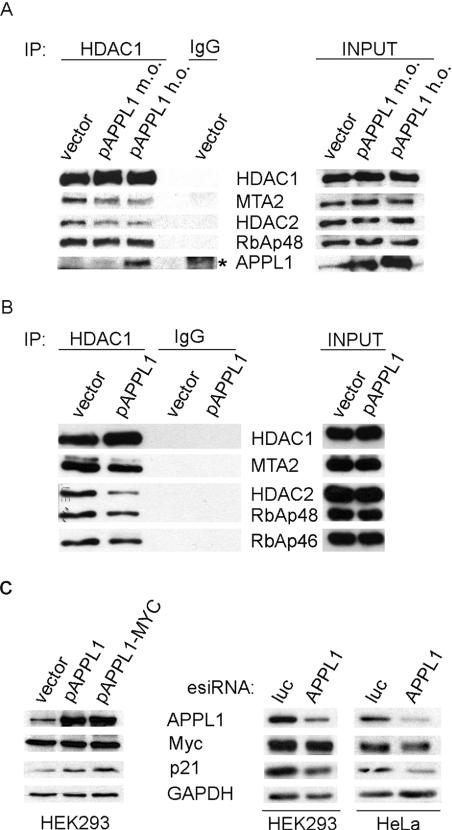
APPL1 overexpression affects the composition of HDAC1-containing NuRD complex and the expression of HDAC1 target p21WAF1/CIP
We tested further whether overexpression of APPL1 could affect the overall activity of HDAC1 or HDAC2, or their ability to bind with other NuRD subunits. No significant changes were observed in the enzymatic activities of HDAC1 or HDAC2 immunoprecipitated from cells silenced for or overproducing APPL1, as measured in vitro using a fluorogenic substrate (results not shown). Instead, overexpression of APPL1 appeared to modulate the amounts of NuRD components interacting with HDAC1, without affecting their overall intracellular levels (Figure 5A). Immunoprecipitates of HDAC1 from cells overproducing APPL1 contained reduced amounts of MTA2 and HDAC2, and occasionally also of RbAp48. This effect correlated with the levels of APPL1 and was stronger upon its higher overexpression (Figure 5A). Similar reduction of the NuRD components associated with HDAC1 was observed when HDAC1 immunoprecipitates were isolated from the nuclear fractions of cells overexpressing APPL1 (Figure 5B). In contrast, the composition and amounts of proteins present in HDAC2 immunoprecipitates were not altered upon APPL1 overexpression (results not shown). These data indicate that an excess of APPL1 specifically affects the assembly of HDAC1-containing NuRD complex but not of the NuRD complex containing HDAC2, possibly by competing out some of the HDAC1-binding proteins from their complex with HDAC1.
Figure 5. APPL1 overexpression affects the composition of HDAC1-containing NuRD complex and the expression of HDAC1 target p21WAF1/CIP.
(A) APPL1 overexpression impairs the interactions of HDAC1 with other NuRD subunits. HEK-293 cells overexpressing untagged APPL1 at moderate or high levels (m.o., moderate overexpression of pAPPL1; h.o., high overexpression of pAPPL1) were subjected to immunoprecipitation (IP) with anti-HDAC1 or non-specific rabbit (IgG) antibodies. Immunoprecipitates along with 10% of the extracts used (input, right panel) were tested by immunoblotting for the presence of several NuRD subunits, as indicated. Some non-specific binding of APPL1 to IgG-covered Protein G beads is marked with an asterisk. (B) APPL1 overexpression reduces the association of HDAC1 with other NuRD components in the nuclear fraction. HDAC1 was immunoprecipitated from the nuclear extracts of HEK-293 cells with endogenous (vector) or overexpressed APPL1. Immunoprecipitates and 5% of the starting material (input, right panel) were blotted for the presence of the indicated NuRD components. (C) APPL1 influences the level of HDAC1 target gene product p21WAF1/CIP1. The level of p21WAF1/CIP1 expression was analysed by Western blotting using anti-p21 antibody in extracts of cells with overexpression or silenced expression of APPL1. Left panel: Extracts of HEK-293 cells overexpressing APPL1 (either untagged, pAPPL1, or MYC-tagged, pAPPL1–MYC) or transfected with a control vector for 48 h were immunoblotted as indicated. No efficient overexpression of APPL1 could be achieved in HeLa cells. Right panel: APPL1 expression was reduced by esiRNA against APPL1 in HEK-293 or HeLa cells, using esiRNA against luciferase (luc) as a specificity control. Transfections with esiRNA were performed for 72 h (HEK-293) or 48 h (HeLa). The resulting extracts were immunoblotted against APPL1, Myc and p21. GAPDH was included as a loading control.
In order to verify whether the observed changes in the interaction status of HDAC1 may have any relevance for the expression of HDAC1 targets in vivo, we investigated the levels of the cyclin-dependent kinase inhibitor p21WAF1/CIP1. In proliferating cells, HDAC1 is bound directly to the promoter of p21WAF1/CIP1 repressing its transcription, whereas in HDAC1 knockout cells, the p21WAF1/CIP1 expression is upregulated and these changes cannot be compensated by the increased levels of HDAC2 present in these cells [40,48]. Strikingly, the cellular amounts of p21WAF1/CIP1 were increased upon overexpression of two different constructs of APPL1 in HEK-293 cells (Figure 5C). This effect was specific for p21WAF1/CIP1, as the levels of Myc, another proliferation-related protein reported to exhibit increased expression upon HDAC1 silencing [49], were not changed. We could further confirm that the downregulation of APPL1 by esiRNA caused an opposite effect, namely a decrease in p21WAF1/CIP1 protein levels, in both HEK-293 and HeLa cells (Figure 5C). These data demonstrate that APPL1 can selectively modulate the expression of HDAC1 targets.
DISCUSSION
In the present study, we characterized the interactions between an endocytic adaptor APPL1 and the NuRD co-repressor complex. We provide evidence that binding between NuRD and APPL1 involves HDAC2, although APPL1 can also associate with HDAC1 in a NuRD-independent manner. Overall, our results point to a complexity of interactions between APPL1 and HDAC-containing complexes, which appear to regulate APPL1 nuclear localization and HDAC function. APPL1 belongs to a growing group of endocytic proteins which undergo nucleocytoplasmic shuttling, interact with nuclear partners and affect their functions, thus modulating gene expression.
APPL1 interaction with the NuRD complex depends on HDAC2
Here we have extended our previous studies which demonstrated an interaction of APPL1 protein with the core subunits of the NuRD complex such as HDAC1, HDAC2, RbAp46, RbAp48 and MTA2 [16]. In the present work, we identify HDAC2 as a key subunit which bridges the binding of APPL1 to NuRD and is indispensable for this association. Interestingly, HDAC1 cannot compensate for lack of HDAC2 in supporting the interaction of APPL1 with NuRD, which strongly argues for their non-redundant roles within the NuRD complex. HDAC1 and HDAC2 are highly homologous proteins found, apart from NuRD [29,31,32], also in other multiprotein complexes such as Sin3 [50–52] or CoREST [45]. Importantly, it has been reported that NuRD as well as CoREST complexes may exist as entities containing either HDAC1 or HDAC2 or both [45]. Our results from the present study support this conclusion, as we show that APPL1 interacts with the NuRD complex containing HDAC2 (and possibly with the complex including both HDAC1 and HDAC2), but not with the NuRD complex comprising HDAC1 as the only deacetylase. The exact basis for this binding selectivity or for the functional differences between various NuRD variants is unknown. Despite high homology and common appearance in several complexes, the two deacetylase enzymes are not functionally redundant, as shown by an embryonic lethality of HDAC1 knockout mice in which an increased expression of HDAC2 and HDAC3 cannot compensate for lack of HDAC1 [40]. One surprising aspect of our present study is the observation that the extent of HDAC2-mediated association between APPL1 and NuRD is regulated by the intracellular levels of HDAC1, pointing to a tightly controlled equilibrium between the interactions exhibited by both HDACs. This picture is further complicated by the fact that APPL1 is also found in complex with HDAC1, which occurs independently of HDAC2 or other NuRD components. This association probably reflects an interaction of APPL1 with Reptin, a transcriptional repressor acting in the Wnt/β-catenin pathway and capable of binding HDAC1 [12]. We have recently shown that the presence of APPL1 in a complex containing β-catenin, Reptin and HDAC1 is important for the modulation of β-catenin/TCF-dependent transcription [12]. Here we demonstrate that, in agreement with the immunoprecipitation data obtained from cell extracts, in vitro translated HDAC1 and HDAC2 deacetylases both exhibit a weak direct binding to recombinant APPL1 via its N-terminus, and in case of HDAC1 also via the C-terminus of APPL1, indicating that interactions of APPL1 with both deacetylases may be mechanistically different. In general, recombinant HDAC1 and HDAC2 are difficult to express and have little enzymatic activity, which can be restored upon co-expression of RbAp46, RbAp48 and MTA2 that appear to stabilize proper folding of HDAC1/2 [44,45,53]. In this light, the weak direct interactions between APPL1 and HDAC1/2 in vitro may not be surprising, although the associations in the context of other cellular proteins seem to be reasonably efficient.
The significance of APPL1 interactions with HDACs
Another important finding of our study are the differences in enzymatic activity of HDACs associated with APPL1. Our analysis revealed that deacetylase activity in APPL1 immunoprecipitates was largely derived from HDAC2, indicating that APPL1 associates with the active NuRD complex, whereas HDAC1 bound to APPL1 is less active or inactive. The experiments using HDAC inhibitors did not reveal significant changes in the amounts of HDAC1 or HDAC2 bound to APPL1, thus it is unlikely that APPL1 preferentially associates with inhibited or activated enzymes. Furthermore, we could not detect any changes in the global deacetylase activity of HDAC1- or HDAC2-immunoprecipitates from extracts of cells overexpressing or silenced for APPL1, arguing that APPL1 itself does not change the enzymatic properties of HDACs, as measured using artificial substrates. This is not surprising, considering that APPL1 is not a stoichiometric component of the NuRD complex, and APPL1-bound pools of HDAC2 or HDAC1 constitute only a minor fraction of their total cellular content, arguing for their fine-tuning regulatory character. Although we were unable to detect any measurable changes in the properties of HDAC2-containing NuRD complex upon alterations of APPL1 levels in cells, we could nevertheless observe the impact of APPL1 on the interactions exhibited by HDAC1. Overexpression of APPL1 reduced the binding of HDAC1 with the other core NuRD subunits and further correlated in vivo with the increased expression of p21WAF1/CIP1, a gene specifically repressed by HDAC1 under normal conditions. Silencing of APPL1 evoked an opposite effect and resulted in reduced expression of p21WAF1/CIP1. These results suggest that increased APPL1 levels negatively modulate the repressor potential of HDAC1, indicating that APPL1 might act as a sequestering factor for HDAC1 to restrict its function in vivo. We have recently shown that overexpression of APPL1 reduces the amounts of HDAC1, HDAC2 and β-catenin associated with Reptin and decreases the levels of Reptin and HDAC1 on the promoters of β-catenin target genes, thereby stimulating β-catenin/TCF-mediated transcription by relieving Reptin-dependent repression [12]. Thus, APPL1 could indeed modulate the composition and activity of various complexes containing HDACs. HDACs themselves do not bind DNA and are usually recruited to their histone substrates by sequence-specific DNA-binding proteins [41], therefore their action is regulated by various protein–protein interactions. By reducing the binding of HDAC1 to other NuRD components, overexpressed APPL1 may reduce the enzymatic activity of HDAC1 and/or affect its potential to be recruited to DNA. Indeed, our results showing low enzymatic activity of HDAC1 bound to APPL1 suggests that this pool of HDAC1 is sequestered and lacks other interacting partners, which ensures its proper conformation and thus activity (see above) [44,45,53].
The role of NuRD in mediating the nuclear localization of APPL1
One of the main questions regarding the endocytic proteins acting in the cell nucleus is the mechanism of their nuclear translocation. Some of such endocytic adaptors possess classical NLSs, whereas others enter the nucleus via interactions with NLS-harbouring partners [7]. APPL1 lacks a canonical NLS, and its nuclear translocation must occur via the latter piggy-back mechanism. An analogous phenomenon was reported for endosomal adaptors such as Epsin1, which binds PLZF (promyelocytic leukemia zinc finger) transcription factor [54], or for CALM (clathrin assembly lymphoid myeloid leukemia) which associates with a transcriptional regulator CATS (CALM interactor expressed in thymus and spleen) in order to translocate to the nucleus [55]. In the present study, we identified a similar mechanism operating for APPL1 and uncovered the role of HDAC2-containing NuRD complexes in mediating APPL1 nuclear localization. The components of NuRD are predominantly nuclear, although according to our systematic analysis of their cellular localization, some components are also present in the cytoplasm, and the APPL1–NuRD interactions are detected in both the nuclear and cytoplasmic fractions. However, we observed that the increased association between APPL1 and NuRD occurring upon silencing of HDAC1 results in a higher accumulation of APPL1 in the nucleus. In contrast, when the APPL1–NuRD interactions are destabilized upon HDAC2 knockdown or HDAC1 overexpression, the nuclear levels of APPL1 are diminished. These results argue that the interactions with the NuRD complex favour the nuclear localization of APPL1. However, it is important to mention that, despite these effects, the association with NuRD represents only one factor contributing to the nuclear import of APPL1, because under conditions of HDAC2 silencing when the APPL1–NuRD binding is not detectable, a certain pool of APPL1 is still nuclear. It is therefore likely that binding of APPL1 to other proteins accounts for this localization. Although to various degrees in different cells, APPL1 appears to be constitutively present in the nucleus. Its nuclear localization can be enhanced further via treatment with epidermal growth factor [16], but we could not detect any changes in the extent of APPL1–NuRD binding upon such stimulation (results not shown).
Nuclear functions of endocytic proteins – a case of signalling or moonlighting?
A growing body of evidence indicates that endocytic proteins can actively participate in the regulation of gene expression, either by a nuclear translocation in response to a specific stimulus or being constitutively present in the nucleus [7,8]. Although for the majority of endocytic proteins their nuclear functions remain poorly understood, there are some studies describing the molecular mechanisms of such phenomena. β-arrestin 1, an endocytic and signalling adaptor for G-protein coupled receptors, enters the nucleus upon stimulation of the δ-opioid receptor and recruits the histone acetyltransferase p300 to the promoter regions of p27 and c-fos genes [14]. This event enhances local histone H4 acetylation and transcription, which is further regulated by an interaction of β-arrestin 1 and the transcription factor CREB (cAMP-response element-binding protein). HIP1, which acts as an adaptor in clathrin-mediated endocytosis, can also bind the androgen receptor. Upon androgen stimulation, HIP1 is recruited to appropriate DNA-response elements and acts as a positive regulator of transcription [15]. Also, the heavy chain of clathrin (in its monomeric form) stimulates p53-mediated transcription through the recruitment of histone acetyltransferase p300 [11]. In most described cases, it is not clear whether the endocytic and nuclear pools of such proteins are largely independent or interchangeable, thus potentially serving as signalling molecules between the endocytic organelles and the nucleus. The latter possibility is appealing, considering that histone modifications and chromatin remodelling occur in response to extracellular cues which activate plasma membrane receptors, followed by endocytosis of ligand–receptor complexes [41,56]. On the other hand, even if the endocytic and nuclear pools are not linked via a direct signalling event, the mechanisms determining a particular nucleocytoplasmic distribution are of importance to secure an appropriate balance between the two functions. Future studies will determine to what extent the interactions of APPL1 with NuRD and HDAC1 are related to its function on the early endosomes.
AUTHOR CONTRIBUTION
Magdalena Banach-Orlowska, Iwona Pilecka, Anna Torun and Beata Pyrzynska designed and performed experiments, and analysed the data with the help of Marta Miaczynska. Marta Miaczynska conceived and directed the project; Magdalena Banach-Orlowska and Marta Miaczynska wrote the manuscript.
ACKNOWLEDGEMENTS
We are grateful to Dr Andrzej Dziembowski (Warsaw University) for help and advice on biochemistry of protein complexes and to Dr Stuart Schreiber for the gift of pBJ5-HDAC1FLAG plasmid. Dr Sajid Rashid, Anna Hupalowska, Marta Olchowik and Lukasz Sadowski are acknowledged for critical reading of the manuscript prior to submission.
FUNDING
This work was supported by an International Research Scholar grant from the Howard Hughes Medical Institute, a Senior Research Fellowship from the Wellcome Trust [grant number 076469/Z/05/Z], by the European Union [grant number LSHG-CT-2006-019050] (EndoTrack), Polish Ministry of Science and Higher Education [grant number 2P04A03828] and Mobilitas.pl network and Max Planck Society (Partner Group programme) to M. M. M. B.-O. was supported by the START fellowship of the Foundation for Polish Science. I. P. was supported by a grant from Iceland, Liechtenstein and Norway through the EEA Financial Mechanism via Homing Programme from the Foundation for Polish Science.
References
- 1.Hoeller D., Volarevic S., Dikic I. Compartmentalization of growth factor receptor signalling. Curr. Opin. Cell Biol. 2005;17:107–111. doi: 10.1016/j.ceb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Le Roy C., Wrana J. L. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell. Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 3.Miaczynska M., Pelkmans L., Zerial M. Not just a sink: endosomes in control of signal transduction. Curr. Opin. Cell Biol. 2004;16:400–406. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Polo S., Di Fiore P. P. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 5.von Zastrow M., Sorkin A. Signaling on the endocytic pathway. Curr. Opin. Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadowski L., Pilecka I., Miaczynska M. Signaling from endosomes: location makes a difference. Exp. Cell Res. 2008;315:1601–1609. doi: 10.1016/j.yexcr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Pilecka I., Banach-Orlowska M., Miaczynska M. Nuclear functions of endocytic proteins. Eur. J. Cell Biol. 2007;86:533–547. doi: 10.1016/j.ejcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Pyrzynska B., Pilecka I., Miaczynska M. Endocytic proteins in the regulation of nuclear signaling, transcription and tumorigenesis. Mol. Oncol. 2009;3:321–338. doi: 10.1016/j.molonc.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colaluca I. N., Tosoni D., Nuciforo P., Senic-Matuglia F., Galimberti V., Viale G., Pece S., Di Fiore P. P. NUMB controls p53 tumour suppressor activity. Nature. 2008;451:76–80. doi: 10.1038/nature06412. [DOI] [PubMed] [Google Scholar]
- 10.Ma L., Pei G. β-Arrestin signaling and regulation of transcription. J. Cell Sci. 2007;120:213–218. doi: 10.1242/jcs.03338. [DOI] [PubMed] [Google Scholar]
- 11.Ohmori K., Endo Y., Yoshida Y., Ohata H., Taya Y., Enari M. Monomeric but not trimeric clathrin heavy chain regulates p53-mediated transcription. Oncogene. 2008;27:2215–2227. doi: 10.1038/sj.onc.1210854. [DOI] [PubMed] [Google Scholar]
- 12.Rashid S., Pilecka I., Torun A., Olchowik M., Bielinska B., Miaczynska M. Endosomal adaptor proteins APPL1 and APPL2 are novel activators of β-catenin/TCF-mediated transcription. J. Biol. Chem. 2009;284:18115–18128. doi: 10.1074/jbc.M109.007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slagsvold T., Pattni K., Malerod L., Stenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–326. doi: 10.1016/j.tcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Kang J., Shi Y., Xiang B., Qu B., Su W., Zhu M., Zhang M., Bao G., Wang F., Zhang X., et al. A nuclear function of β-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell. 2005;123:833–847. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Mills I. G., Gaughan L., Robson C., Ross T., McCracken S., Kelly J., Neal D. E. Huntingtin interacting protein 1 modulates the transcriptional activity of nuclear hormone receptors. J. Cell Biol. 2005;170:191–200. doi: 10.1083/jcb.200503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miaczynska M., Christoforidis S., Giner A., Shevchenko A., Uttenweiler-Joseph S., Habermann B., Wilm M., Parton R. G., Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 17.Mitsuuchi Y., Johnson S. W., Sonoda G., Tanno S., Golemis E. A., Testa J. R. Identification of a chromosome 3p14.3-21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene. 1999;18:4891–4898. doi: 10.1038/sj.onc.1203080. [DOI] [PubMed] [Google Scholar]
- 18.Liu J., Yao F., Wu R., Morgan M., Thorburn A., Finley R. L., Jr, Chen Y. Q. Mediation of the DCC apoptotic signal by DIP13α. J. Biol. Chem. 2002;277:26281–26285. doi: 10.1074/jbc.M204679200. [DOI] [PubMed] [Google Scholar]
- 19.Schenck A., Goto-Silva L., Collinet C., Rhinn M., Giner A., Habermann B., Brand M., Zerial M. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 20.Lin D. C., Quevedo C., Brewer N. E., Bell A., Testa J. R., Grimes M. L., Miller F. D., Kaplan D. R. APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction. Mol. Cell. Biol. 2006;26:8928–8941. doi: 10.1128/MCB.00228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varsano T., Dong M. Q., Niesman I., Gacula H., Lou X., Ma T., Testa J. R., Yates J. R., 3rd, Farquhar M. G. GIPC is recruited by APPL to peripheral TrkA endosomes and regulates TrkA trafficking and signaling. Mol. Cell. Biol. 2006;26:8942–8952. doi: 10.1128/MCB.00305-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nechamen C. A., Thomas R. M., Cohen B. D., Acevedo G., Poulikakos P. I., Testa J. R., Dias J. A. Human follicle-stimulating hormone (FSH) receptor interacts with the adaptor protein APPL1 in HEK 293 cells: potential involvement of the PI3K pathway in FSH signaling. Biol. Reprod. 2004;71:629–636. doi: 10.1095/biolreprod.103.025833. [DOI] [PubMed] [Google Scholar]
- 23.Nechamen C. A., Thomas R. M., Dias J. A. APPL1, APPL2, Akt2 and FOXO1a interact with FSHR in a potential signaling complex. Mol. Cell. Endocrinol. 2007;260–262:93–99. doi: 10.1016/j.mce.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao X., Kikani C. K., Riojas R. A., Langlais P., Wang L., Ramos F. J., Fang Q., Christ-Roberts C. Y., Hong J. Y., Kim R. Y., et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 25.Cheng K. K., Lam K. S., Wang Y., Huang Y., Carling D., Wu D., Wong C., Xu A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–1394. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- 26.Saito T., Jones C. C., Huang S., Czech M. P., Pilch P. F. The interaction of Akt with APPL1 is required for insulin-stimulated Glut4 translocation. J. Biol. Chem. 2007;282:32280–32287. doi: 10.1074/jbc.M704150200. [DOI] [PubMed] [Google Scholar]
- 27.Yang L., Lin H. K., Altuwaijri S., Xie S., Wang L., Chang C. APPL suppresses androgen receptor transactivation via potentiating Akt activity. J. Biol. Chem. 2003;278:16820–16827. doi: 10.1074/jbc.M213163200. [DOI] [PubMed] [Google Scholar]
- 28.Erdmann K. S., Mao Y., McCrea H. J., Zoncu R., Lee S., Paradise S., Modregger J., Biemesderfer D., Toomre D., De Camilli P. A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev. Cell. 2007;13:377–390. doi: 10.1016/j.devcel.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong J. K., Hassig C. A., Schnitzler G. R., Kingston R. E., Schreiber S. L. Chromatin deacetylation by an ATP-dependent nucleosome remodelling complex. Nature. 1998;395:917–921. doi: 10.1038/27699. [DOI] [PubMed] [Google Scholar]
- 30.Wade P. A., Jones P. L., Vermaak D., Wolffe A. P. A multiple subunit Mi-2 histone deacetylase from Xenopus laevis cofractionates with an associated Snf2 superfamily ATPase. Curr. Biol. 1998;8:843–846. doi: 10.1016/s0960-9822(98)70328-8. [DOI] [PubMed] [Google Scholar]
- 31.Xue Y., Wong J., Moreno G. T., Young M. K., Cote J., Wang W. NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol. Cell. 1998;2:851–861. doi: 10.1016/s1097-2765(00)80299-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y., LeRoy G., Seelig H. P., Lane W. S., Reinberg D. The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell. 1998;95:279–289. doi: 10.1016/s0092-8674(00)81758-4. [DOI] [PubMed] [Google Scholar]
- 33.Manavathi B., Kumar R. Metastasis tumor antigens, an emerging family of multifaceted master coregulators. J. Biol. Chem. 2007;282:1529–1533. doi: 10.1074/jbc.R600029200. [DOI] [PubMed] [Google Scholar]
- 34.Le Guezennec X., Vermeulen M., Brinkman A. B., Hoeijmakers W. A., Cohen A., Lasonder E., Stunnenberg H. G. MBD2/NuRD and MBD3/NuRD, two distinct complexes with different biochemical and functional properties. Mol. Cell. Biol. 2006;26:843–851. doi: 10.1128/MCB.26.3.843-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brackertz M., Boeke J., Zhang R., Renkawitz R. Two highly related p66 proteins comprise a new family of potent transcriptional repressors interacting with MBD2 and MBD3. J. Biol. Chem. 2002;277:40958–40966. doi: 10.1074/jbc.M207467200. [DOI] [PubMed] [Google Scholar]
- 36.Brackertz M., Gong Z., Leers J., Renkawitz R. p66α and p66β of the Mi-2/NuRD complex mediate MBD2 and histone interaction. Nucleic Acids Res. 2006;34:397–406. doi: 10.1093/nar/gkj437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowen N. J., Fujita N., Kajita M., Wade P. A. Mi-2/NuRD: multiple complexes for many purposes. Biochim. Biophys. Acta. 2004;1677:52–57. doi: 10.1016/j.bbaexp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Denslow S. A., Wade P. A. The human Mi-2/NuRD complex and gene regulation. Oncogene. 2007;26:5433–5438. doi: 10.1038/sj.onc.1210611. [DOI] [PubMed] [Google Scholar]
- 39.Feng Q., Zhang Y. The NuRD complex: linking histone modification to nucleosome remodeling. Curr. Top. Microbiol. Immunol. 2003;274:269–290. doi: 10.1007/978-3-642-55747-7_10. [DOI] [PubMed] [Google Scholar]
- 40.Lagger G., O'Carroll D., Rembold M., Khier H., Tischler J., Weitzer G., Schuettengruber B., Hauser C., Brunmeir R., Jenuwein T., Seiser C. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J. 2002;21:2672–2681. doi: 10.1093/emboj/21.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunliffe V. T. Eloquent silence: developmental functions of class I histone deacetylases. Curr. Opin. Genet. Dev. 2008;18:404–410. doi: 10.1016/j.gde.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonel P., Costello I., Hendrich B. Keeping things quiet: Roles of NuRD and Sin3 co-repressor complexes during mammalian development. Int. J. Biochem. Cell Biol. 2009;41:108–116. doi: 10.1016/j.biocel.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kittler R., Heninger A. K., Franke K., Habermann B., Buchholz F. Production of endoribonuclease-prepared short interfering RNAs for gene silencing in mammalian cells. Nat. Meth. 2005;2:779–784. doi: 10.1038/nmeth1005-779. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y., Ng H. H., Erdjument-Bromage H., Tempst P., Bird A., Reinberg D. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999;13:1924–1935. doi: 10.1101/gad.13.15.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Humphrey G. W., Wang Y., Russanova V. R., Hirai T., Qin J., Nakatani Y., Howard B. H. Stable histone deacetylase complexes distinguished by the presence of SANT domain proteins CoREST/kiaa0071 and Mta-L1. J. Biol. Chem. 2001;276:6817–6824. doi: 10.1074/jbc.M007372200. [DOI] [PubMed] [Google Scholar]
- 46.Kaiser C., James S. R. Acetylation of insulin receptor substrate-1 is permissive for tyrosine phosphorylation. BMC Biol. 2004;2:23. doi: 10.1186/1741-7007-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glozak M. A., Sengupta N., Zhang X., Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Lagger G., Doetzlhofer A., Schuettengruber B., Haidweger E., Simboeck E., Tischler J., Chiocca S., Suske G., Rotheneder H., Wintersberger E., Seiser C. The tumor suppressor p53 and histone deacetylase 1 are antagonistic regulators of the cyclin-dependent kinase inhibitor p21/WAF1/CIP1 gene. Mol. Cell. Biol. 2003;23:2669–2679. doi: 10.1128/MCB.23.8.2669-2679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Senese S., Zaragoza K., Minardi S., Muradore I., Ronzoni S., Passafaro A., Bernard L., Draetta G. F., Alcalay M., Seiser C., Chiocca S. Role for histone deacetylase 1 in human tumor cell proliferation. Mol. Cell. Biol. 2007;27:4784–4795. doi: 10.1128/MCB.00494-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hassig C. A., Fleischer T. C., Billin A. N., Schreiber S. L., Ayer D. E. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell. 1997;89:341–347. doi: 10.1016/s0092-8674(00)80214-7. [DOI] [PubMed] [Google Scholar]
- 51.Laherty C. D., Yang W. M., Sun J. M., Davie J. R., Seto E., Eisenman R. N. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y., Iratni R., Erdjument-Bromage H., Tempst P., Reinberg D. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell. 1997;89:357–364. doi: 10.1016/s0092-8674(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 53.Li J., Staver M. J., Curtin M. L., Holms J. H., Frey R. R., Edalji R., Smith R., Michaelides M. R., Davidsen S. K., Glaser K. B. Expression and functional characterization of recombinant human HDAC1 and HDAC3. Life Sci. 2004;74:2693–2705. doi: 10.1016/j.lfs.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 54.Hyman J., Chen H., Di Fiore P. P., De Camilli P., Brunger A. T. Epsin 1 undergoes nucleocytosolic shuttling and its eps15 interactor NH(2)-terminal homology (ENTH) domain, structurally similar to Armadillo and HEAT repeats, interacts with the transcription factor promyelocytic leukemia Zn2+ finger protein (PLZF) J. Cell Biol. 2000;149:537–546. doi: 10.1083/jcb.149.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Archangelo L. F., Glasner J., Krause A., Bohlander S. K. The novel CALM interactor CATS influences the subcellular localization of the leukemogenic fusion protein CALM/AF10. Oncogene. 2006;25:4099–4109. doi: 10.1038/sj.onc.1209438. [DOI] [PubMed] [Google Scholar]
- 56.Hamon M. A., Cossart P. Histone modifications and chromatin remodeling during bacterial infections. Cell Host Microbe. 2008;4:100–109. doi: 10.1016/j.chom.2008.07.009. [DOI] [PubMed] [Google Scholar]