Abstract
Study Objectives:
To assess the feasibility and efficacy of a novel 16-week exercise and diet program for important clinical outcomes in obstructive sleep apnea (OSA).
Methods:
Cohort study assessing sleep disordered breathing, cardiovascular risk factors, and neurobehavioral function prior to and following completion of the 16-week program. The program used a proprietary very low energy diet (Optifast, Novartis), and subjects participated in a supervised exercise schedule, which included both aerobic and resistance training. Follow-up contact was made at 12 months after program exit. Consecutive patients with newly diagnosed sleep apnea were approached who had an apnea-hypopnea index (AHI) of 10 to 50, a body mass index (BMI) of greater than 30 kg/m2, no significant comorbidities, and able to exercise.
Results:
All data are presented as mean [SD]. Of 21 patients with OSA who were approached, 12 middle-aged (42.3 [10.4] years old), obese (BMI 36.1 [4.3] kg/m2), and predominantly female (75%) subjects with mild to moderate OSA were enrolled (AHI 24.6 [12.0]). Weight loss was significant (12.3 [9.6] kg, p < 0.001), and 5 of the 10 who completed the program were able to independently maintain good weight loss at 12 months. At the 16-week assessment, there was a small nonsignificant fall in the AHI. Six of the 10 subjects had a reduction in sleep disordered breathing, and the AHI was less than 10 in 3 patients. There were significant improvements in neurobehavioral and cardiometabolic outcomes. Snoring improved in most subjects, but the improvement was clinically important (a score of < 2) in only 7.
Conclusions:
A supportive diet and exercise program may be of benefit to obese patients with mild to moderate sleep apnea. The results of this feasibility study showed significant weight loss and improvement in clinically important neurobehavioral and cardiometabolic outcomes but no significant change in sleep disordered breathing. These promising preliminary results need confirmation with a larger randomized trial.
Citation:
Barnes M; Goldsworthy UR; Cary BA; Hill CJ. A diet and exercise program to improve clinical outcomes in patients with obstructive sleep apnea – a feasibility study. J Clin Sleep Med 2009;5(5):409-415.
Keywords: Sleep apnea, obstructive; obesity; diet, reducing; exercise
Obstructive sleep apnea (OSA) is a common condition characterized by repetitive obstruction of the upper airway during sleep with resultant episodic hypoxia and arousal. The etiology of OSA is multifactorial and includes underlying abnormalities of bony cranial structure, relaxation of the upper airway musculature during sleep, and impaired responses of the central respiratory control center. In addition, there are modifiable precipitating factors,1 the most important of which is obesity. Patients demonstrate behavioral and neuropsychological consequences to varying degrees, including excessive daytime sleepiness, psychomotor deficits,2 an increased risk of having motor vehicle crashes,3 and lost work productivity.4 The most common presenting symptoms are snoring and daytime sleepiness. Long-term studies of sleep clinic patients with OSA have shown that they have significantly more clinical cardiovascular events than do their counterparts without OSA and that treatment with continuous positive airways pressure (CPAP) only partially ameliorates this risk.5,6
CPAP is effective in controlling upper airway collapse across a wide range of disease severity, but symptom control is limited.7,8 This is due in part to poor usage in those with mild to moderate disease7 and also relates to a failure to address underlying pathophysiologic factors, including obesity. Management of these patients needs to be widened to involve other approaches, including weight reduction.
In addition to the association with OSA,9,10 obesity is an important risk factor for insulin resistance, coronary heart disease, and stroke.11 A 1-standard-deviation increase in body mass index (BMI) has been shown to be associated with a 4-fold increase in the relative risk of having OSA.12 Data from both the Wisconsin cohort and the Sleep Heart Health Study have shown that a 10% decrease in body weight is associated with a 30% improvement in AHI.13,14 In our own study of 114 subjects with mild to moderate OSA, 90% were either overweight or obese.7 Although weight loss is an excellent therapeutic target in this group, we do not suggest that body weight reduction will reduce sleep disordered breathing (SDB) to the same extent as does CPAP (when used). However, it is likely that symptom responses may be equivalent for 2 reasons. First, daytime sleepiness is caused in part by obesity per se (mediated by elevated levels of inflammatory cytokines, including tumor necrosis factor-α15) and will thus respond to weight loss through 2 separate mechanisms. Second, fewer than 50% of patients with mild to moderate OSA will consistently use CPAP (defined as 4 hours per night for 70% of nights),2 thus response to this treatment modality is limited.
Recognizing that obesity is a common precipitant for OSA and a contributing cause to significant cardiometabolic morbidity in these patients, many physicians recommend weight loss and exercise. This is rarely effective—most likely due to the lack of clinical programs that meet the specific needs of these patients. In other patient populations, initial weight loss of 10% has been shown to be both feasible and beneficial,16,17 particularly using very-low-energy diets.18 A meta-analysis of randomized controlled trials comparing diet alone with diet and exercise revealed that the diet and exercise strategy yielded significant additional weight loss over that achieved with diet alone and furthermore, improved the likelihood of this weight loss being maintained in the long term.19 An exercise program alone is beneficial for OSA and has been suggested as adjunctive therapy.20
A reduction in OSA severity and some improvement in cardiovascular measures with a weight-loss program have been shown previously, but the evidence base is limited.21,22 A recent study23 provided the best evidence to date supporting the use of a lifestyle intervention as effective treatment of OSA. This randomized controlled trial of a very low energy–based diet in participants with mild OSA (apnea-hypopnea index [AHI] 5-15) showed significant weight loss and an improvement in SDB, snoring and plasma insulin and triglyceride levels, which were maintained 1 year after enrolment. The study program did not include exercise or record the amount of physical activity that subjects undertook.
In the current study, we investigated the feasibility and efficacy of a 16-week very low energy–based diet in association with a supervised exercise program in patients with mild to moderate OSA.
METHODS
The study was approved by the Austin Health Human Research Ethics Committee, registered with the Australian Clinical Trials Registry (# 12606000465550), and all subjects gave written informed consent.
Research staff approached 21 consecutive patients, seen at the sleep clinic, who had a BMI greater than 30 kg/m2, a diagnostic sleep study showing an AHI between 10 and 50 and who had no significant sleep hypoxemia (oxygen saturation did not fall below 70%); 9 subjects declined to participate, all because of time constraints. Polysomnography was done according to our usual clinical routine, based on a computerized system (Compumedics, Melbourne, Australia). This routine provides a comprehensive assessment of both respiratory and sleep-state variables. Respiratory disturbances were identified as events lasting longer than 10 seconds, with a 50% or greater decrease in peak-to-peak nasal pressure or a discernible drop in any 1 of 3 parameters (nasal pressure, thoracic respitrace, or abdominal respitrace) associated with either an oxygen desaturation of at least 3% or an arousal.24
Subjects were excluded if they had significant or unstable medical or psychological morbidities or were unable to exercise due to musculoskeletal conditions. Those with insulin-requiring diabetes, renal failure, or liver failure were also excluded.
Initial Evaluation
Initial evaluation of subjects comprised anthropometry; blood tests for electrolytes, liver function, C-reactive protein, insulin, triglyceride, cholesterol, and glucose levels; 24-hour ambulatory blood pressure; bioelectrical impedance analysis (an estimation of body-fat percentage); and a neurobehavioral evaluation using questionnaires for subjective daytime sleepiness (Epworth Sleepiness Scale), mood (Beck Depression Index and the Profile of Moods States), and quality of life (Functional Outcomes of Sleep Questionnaire and the SF-36). Symptoms were evaluated using the Sleep Apnea Symptom Questionnaire, a well-validated questionnaire that we have used extensively in our previous research.2,7,25 The Sleep Apnea Symptom Questionnaire (SASQ) asks subjects to rate 14 common symptoms of sleep apnea on a 10-point Likert scale from never (0) to always (10); the maximum score is 140.
A maximal cardiopulmonary exercise test was performed to exclude any cardiac ischemia or rhythm irregularities and also provided a baseline assessment of cardiovascular fitness.26 Muscular fitness is a term that describes the integrated status of muscular strength and muscular endurance; the best measure of this is the 1-repetition maximum test (1-RM).27 A 5-repetition maximum test was done weekly and from this, an estimated 1-RM was calculated. This was used in the final evaluation of improvement in muscle fitness with participation in the program.27
Weight-Reduction Diet
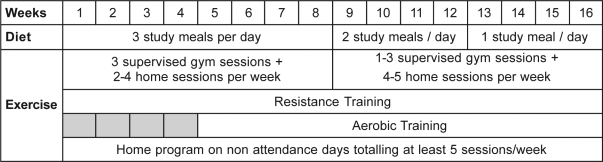
All subjects had an initial 1-hour interview with the study dietician to discuss their current diet and the dietary program of the study. A proprietary very low energy diet (Optifast®, Novartis Medical Nutrition, Basel, Switzerland) was used according to Figure 1 to initially replace, 3 meals per day, reducing to 2 meals per day, and then continuing on to replace 1 meal per day. The dietician provided detailed advice, menus, and suggestions for the low-calorie meals (introduced at weeks 9 and 13), based on each individual's energy requirement, as needed in addition to that provided by the Optifast. This calculation was based on the resting metabolic rate and the estimated energy expenditure from the exercise program, in addition to normal daily activity.27
Figure 1.
The diet consisted initially of 3 very low energy meals per day, reducing to 2 meals per day, and then provided as only breakfast as subjects were educated on the replacement of these meals with low-calorie home-prepared meals. The exercise program consisted of both resistance and aerobic training, a minimum of 5 sessions per week. Initially, 3 of these sessions were supervised in the hospital gymnasium; the number of supervised sessions was gradually reduced as subjects were educated about exercise in the community.
Exercise Program
Subjects participated in a hospital-based exercise program 3 evenings per week for the first 8 weeks and then a minimum of 1 hospital session per week for the next 8 weeks, with the aim of continuing a similar and sustainable exercise program at home (see Figure 1). All hospital-based sessions were supervised by an exercise physiologist and a physiotherapist.
Exercise consisted of resistance training 3 times per week for the duration of the study and aerobic training 5 times per week from weeks 5 to 16. Resistance training was employed during the early rapid-weight-loss phase of the very-low-energy diet to attenuate the loss of fat free mass.28 Training was prescribed at 80% of the estimated 1-RM for 3 sets of 8 to 12 repetitions and was performed for 7 major upper limb and lower limb muscle groups. An estimated 1-RM measure was calculated weekly and intensity progressed accordingly.
Aerobic training commenced in week 5 and was performed 5 times per week using a combination of cycling, walking with or without an incline, and jogging for up to 40 minutes. Initial intensity was prescribed at 80% of VO2peak, predicted from the baseline exercise test, and was progressed weekly to maintain the equivalent target heart rate.
Monitoring
The research assistant kept full records of exercise performance outcomes and weighed all subjects weekly. In addition, exercise and food diaries were checked to ensure that subjects understood the diet they were using and any queries or concerns were answered as they arose. The study dietician attended 1 gym session each week to answer any diet-related queries or concerns.
Outcome Evaluation
At the conclusion of the 16-week program, all subjects underwent repeat assessment of baseline parameters—polysomnography, blood pressure, blood tests, cardiopulmonary exercise test, and neurobehavioral assessment. One year after beginning the program, all subjects were contacted by mail and asked about their current weight, ongoing exercise and diet, and whether they felt that it would have been of benefit to have had a structured follow-up program.
Statistics
Analysis of variance for continuous variables using paired t-tests was used to compare baseline with posttreatment outcomes. All analyses were intention to treat.
RESULTS
All data are shown as mean (SD) unless otherwise indicated. Twenty-one patients from the sleep clinic were approached, and 12 subjects were enrolled during the 4-week recruitment period. The reason for not participating in all those who declined was not having enough spare time for the requirements of the program. There were no differences between those who declined participation and those who enrolled in terms of severity of SDB, obesity, or age. Participants had mild to moderate OSA (AHI 24.6 [12.0], range 11.3 - 48.4); they were middle-aged (42.3 [10.4] years old) and were predominantly women (9 of 12). Two subjects dropped out, 1 at 2 weeks and 1 at 3 weeks; 1 subject underwent minor ankle surgery in the 10th week, so was unable to exercise for 5 weeks. She continued on the diet while immobile and completed the remainder of the study to the best of her ability. All subjects were heavy snorers, 2 subjects had preexisting cardiovascular disease, 7 were current smokers for 8.8 (5.8) pack years, 6 had previously been diagnosed with hypertension, and 2 had stable type 2 diabetes (1 diagnosed 4 months before the study began and 1 diagnosed 3 years prior to study participation). They weighed 95.6 kg (12.1 kg) and had a BMI of 36.1 kg/m2 (4.3 kg/m2), a waist circumference of 117.3 cm (11.3 cm), and a neck circumference of 42.0 cm (4.5 cm).
Weight Loss and Exercise Response (Tables 1 and 2)
Table 1.
Outcomes: Weight and Exercise
| Baseline | 16 weeks | p value | |
|---|---|---|---|
| Weight, kg | 95.6 (12.1) | 82.9 (11.5) | 0.001 |
| BMI, kg/m2 | 36.1 (4.3) | 30.1 (4.2) | < 0.001 |
| Body fat, % | 42.9 (9.2) | 36.5 (9.7) | 0.009 |
| Waist, cm | 117.3 (11.3) | 97.7 (8.4) | < 0.001 |
| Neck, cm | 42.0 (4.5) | 37.2 (3.2) | 0.001 |
| Max work, watts | 117.8 (37.7) | 126.3 (39.4) | 0.003 |
| VO2peak, mL/min per kg | 17.1 (3.0) | 20.9 (5.0) | 0.003 |
| HRisowork, bpm | 158.4 (17.3) | 150.8 (17.3) | 0.02 |
| 1-RM (kg, calculated) | 40.9 (9.0) | 97.0 (33.3) | 0.03 |
Data are shown as mean (SD). BMI refers to body mass index; HRisowork = heart rate at maximum workload achieved on both testing occasions
Table 2.
Weight and AHI Outcomes
| Subject no. | Weight, kg |
AHI, events/hour |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 16 wks | ΔWeight | 12 mo | ΔWeight | Baseline | 16 wks | Δ AHI | |
| 1 | 88.0 | 70.0 | −18.0 | 76.0 | −12.0 | 11.3 | 20.7 | 9.4 |
| 2 | 89.7 | 71.9 | −17.8 | 90.0 | 0.30 | 48.4 | 39.8 | −8.6 |
| 3 | 82.3 | 71.2 | −11.1 | 71.0 | −11.3 | 17.2 | 6.4 | −10.8 |
| 4 | 85.0 | 76.9 | −8.1 | 83.0 | −2.0 | 19.0 | 21.4 | 2.4 |
| 5 | 89.0 | 73.3 | −15.7 | 80.0 | −9.0 | 17.3 | 6.0 | −11.3 |
| 6 | 86.0 | 74.9 | −11.1 | 80.0 | −6.0 | 31.1 | 15.2 | −15.9 |
| 7 | 102.0 | 88.2 | −13.8 | 95.0 | −7.0 | 28.1 | 34.7 | 6.6 |
| 8 | 108.2 | 97.5 | −10.7 | 103.0 | −5.2 | 12.1 | 21.2 | 9.1 |
| 9 | 100.0 | 100.4a | 0.4 | 16.7 | ||||
| 10 | 137.0 | 99.6 | −37.4 | 110.0 | −27.0 | 43.9 | 5.1 | −38.8 |
| 11 | 88.8 | 85.7a | −3.1 | 30.2 | ||||
| 12 | 91.3 | 85.0 | −6.3 | 88.0 | −3.3 | 20.3 | 12.5 | −7.8 |
| Mean | 95.6 | 82.5 | −12.7 | 87.6 | −8.3 | 24.6 | 18.3 | −6.6 |
| SD | 15.1 | 11.5 | 9.6 | 12.2 | 7.7 | 12.0 | 11.9 | 14.6 |
| P value | 0.001 | 0.008 | 0.19 | |||||
Weight at last attended visit. Δ Weight refers to the change in weight from baseline; Δ AHI, the change in the apnea-hypopnea index (AHI, the number of apneas and hypopneas per hour of sleep) from baseline. P value refers to the change from baseline
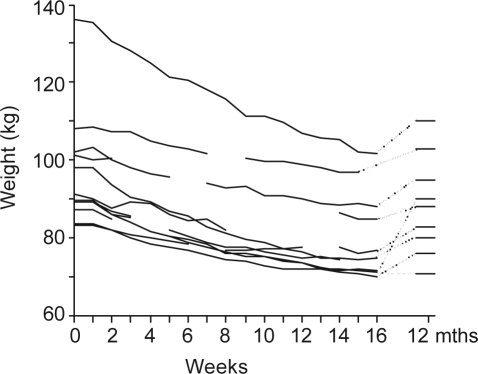
Subjects lost 12.3 kg (9.6) or 12.9% (7.7%) of their baseline total body weight. There was a significant reduction in BMI, body-fat percentage, abdominal girth and neck circumference. The last measurements for the 2 subjects who failed to complete the study were included as their final measurements. All subjects were contacted 12 months after program commencement. The 10 who had completed the program responded and of those, 9 had regained some weight. Weight gain was 8.5% (6.6%) of weight at program completion; however, the subjects were still significantly lighter than at study entry (95.6 kg [12.1] vs 87.6 kg [12.2]). Weight loss from study entry to follow-up at 12 months after program commencement was 8.2% (6.1%, with a range of weight loss from 0.3 to 27kg (Figure 2). Five subjects had a long-term weight loss of at least 7%. At 12 months, all subjects said that they had maintained an exercise program but that regular face-to-face contact would have assisted with ongoing adherence to a low-energy diet.
Figure 2.
Weight loss was steady throughout the active program. Subjects gained some weight after exiting from the study, but overall weight loss was still significant.
Subjects demonstrated a 7% increase in their maximal work load achieved and a 20% increase in peak oxygen consumption, which was independent of weight loss. At the equivalent maximum work capacity (VO2 isowork), heart rate was significantly reduced following the lifestyle program, also indicating improved cardiovascular fitness. Although the 1-RM was not measured directly, but estimated from the 5-RM training results, there was evidence of significantly improved strength outcomes.
Sleepiness, Mood, Quality of Life, and Symptoms of OSA (Table 3)
Table 3.
Symptoms, Mood, and Quality of Life
| Baseline | 16 weeks | p value | |
|---|---|---|---|
| ESSa | 8.7 (5.1) | 6.0 (4.3) | 0.04 |
| POMS-TMD | −23.1 (19.2) | −7.5 (24.3) | 0.11 |
| BDI | 13.5 (5.8) | 5.6 (4.3) | < 0.001 |
| FOSQ total | 3.0 (0.6) | 3.5 (0.5) | 0.07 |
| SF36 total | 56.2 (13.0) | 74.4 (15.6) | 0.03 |
| SASQ - total | 65.7 (17.8) | 44.9 (25.9) | 0.08 |
| SASQ-snoring | 7.8 (2.5) | 4.6 (3.9) | 0.02 |
Note: Data are presented as mean (SD). ESS refers to Epworth Sleepiness Scale; POMS-TMD, Profile of Mood State—Total Mood Disorder; BDI, Beck Depression Inventory; FOSQ, Functional Outcomes of Sleep Questionnaire; SASQ, Sleep Apnea Symptom Questionnaire.
There was a significant improvement in subjective daytime sleepiness (Epworth Sleepiness Scale) and a trend to improvement in sleep apnea symptoms (Sleep Apnea Symptom Questionnaire) (Table 3). Quality of life was shown to improve in the generic questionnaire, SF-36 but not in the sleep-specific quality of life questionnaire (Functional Outcomes of Sleep Questionnaire), although there was a significant improvement in the activity domain of the Functional Outcomes of Sleep Questionnaire. The overall Profile of Mood States score showed no significant treatment response, but there was a significant improvement in the domains of confusion-bewilderment and vigor. There was also a significant improvement in depression, as measured by the Beck Depression Inventory.
All participants described themselves as heavy snorers when they enrolled in the study. After 4 months, 9 of the 10 subjects who completed this portion of the study felt that their snoring had improved noticeably. In the 1 subject whose snoring did not improve, this was despite significant weight loss and a fall in the AHI from 17.2 to 6.4. The first question of the symptom questionnaire asks “How often do you snore?” The mean score for this response fell from 7.8 (2.5) at baseline to 4.6 (3.9) after the program, with 8 subjects having an improvement of at least 50% and 7 subjects having a score less than 2 out of 10, which is considered to be clinically significant.
Cardiometabolic Outcomes (Table 4)
Table 4.
Cardiometabolic Outcomes
| Parameter | Baseline | 16 weeks | p value |
|---|---|---|---|
| Blood pressure (mmHg) | |||
| Mean arterial pressure | |||
| 24-h | 92.1 (12.0) | 89.3 (7.1) | 0.04 |
| Day | 94.8 (11.6) | 92.0 (6.7) | 0.03 |
| Night | 85.7 (14.0) | 81.3 (8.8) | 0.06 |
| Systolic, 24-h | 125.8 (14.0) | 120.5 (9.8) | 0.02 |
| Diastolic, 24-h | 77.3 (10.6) | 73.3 (6.3) | 0.09 |
| Cholesterol, mmol/L | |||
| Total | 5.3 (1.1) | 4.4 (1.0) | 0.006 |
| LDL | 3.5 (1.0) | 2.9 (0.8) | 0.04 |
| Triglycerides, mmol/L | 1.5 (0.8) | 1.0 (0.6) | 0.003 |
| C-reactive protein, mg/L | 7.1 (3.1) | 4.2 (2.2) | 0.01 |
| Glucose, mg/L | 6.1 (2.2) | 5.8 (1.5) | 0.37 |
| Insulin, mIU/L | 15.5 (6.0) | 10.7 (4.7) | < 0.001 |
| GammaGT, IU/L | 26.9 (14.7) | 20.8 (9.2) | 0.004 |
Note: Data are presented as mean (SD). LDL refers to low-density lipoprotein; GammaGT, Glutamyl Transpeptidase.
Six of the 12 subjects had previously diagnosed hypertension; there was a statistically significant reduction in 24-hour mean arterial blood pressure, 24-hour systolic blood pressure, and day and night systemic blood pressure. There was a trend for a fall in day diastolic blood pressure, night diastolic blood pressure and night mean arterial blood pressure. Fasting total cholesterol and low-density lipoprotein cholesterol both fell significantly, as did triglyceride level. There was no significant change in glucose level, but the fasting insulin level fell by 30%. C-reactive protein level fell significantly, as did the liver enzyme, glutamyl transpeptidase (gammaGT).
Polysomnogram Outcomes (Table 2)
The AHI fell from 24.6 (12.0) to 18.3 (11.9), a reduction of 25%, but this was not statistically significant. Of the 10 subjects for whom a follow-up polysomnogram was done, 6 had a reduction in AHI and of those, 3 had an AHI less than 10. There was a significant correlation between weight loss and change in AHI (R = 0.66, p = 0.04). There were no significant changes in sleep architecture. Sleep efficiency improved significantly (p = 0.02) from 74.7% (10.7%) to 84.1% (8.6%) and minimum oxygen saturation showed a trend to improvement from 88.1% (6.6%) to 89.9% (4.7%). There was no significant correlation between weight loss and change in any other polysomnographic outcome.
DISCUSSION
The management of mild to moderate OSA is difficult and alternative treatment strategies are needed. Current treatment options are often poorly tolerated, have limited effectiveness, and do not directly address the major underlying risk factor of obesity or the associated cardiovascular risk.
In this small cohort study, 10 of our 12 middle-aged participants with mild to moderate OSA (AHI 11-48) completed the 16-week diet and exercise program. On an intention to treat basis, weight loss was significant, with a mean (SD) weight loss of 12.9% (7.7%) of total body weight from baseline to posttreatment.
These results compare favorably with weight loss programs in other patient groups and are significant in terms of expected improvement in cardiovascular and diabetic outcomes.29 Twelve months after exit from the active program and with no contact during that time, all but 1 subject regained some weight, but 5 of the 10 subjects who completed the program had maintained a weight loss of at least 7% from their entry weight. In addition, our subjects showed an improvement in physical fitness (VO2peak and heart rate at isowork level) that has been shown to be independently associated with reduction in mortality.30 However, there was only a small and nonsignificant improvement in the AHI, with only 3 subjects achieving an AHI below 10. Clinical outcome measures (subjective sleepiness, quality of life, mood, and symptoms, including snoring) improved significantly; there was a significant improvement or a trend to improvement in blood pressure measurements and levels of lipids, C-reactive protein (an inflammatory cytokine that is closely linked with cardiovascular risk31), insulin, and gammaGT. In these patients, fasting serum insulin levels indicated a prediabetic state and gammaGT, a marker of liver inflammation, most likely reflected fatty infiltration of the liver.
The program we have devised was quite onerous for participants, particularly in the first 4 weeks, which required attendance at a supervised exercise session 3 times each week. Despite this, the agree-to-participate rate was high (12 of the 21 patients approached agreed to do so) and our dropout rate was low (17%). Those who declined participation cited time constraints as the predominant reason; this may have led to the subjects being mostly women and also contributed to the low dropout rate. There were 2 reasons for incorporating exercise into this program—the first was the known beneficial effect of exercise on fitness, cardiovascular health, and weight. Exercise alone has been shown to improve SDB in the absence of weight loss.20 The second reason for the exercise component was the anticipated morale-building benefit of the group dynamics, which we hoped would improve adherence to the entire program. Previous studies in other patient populations have shown that this group-therapy effect is a more important addition to a weight-reduction program than are weight-loss drugs,32 that exercise improves long-term maintenance of weight loss33 and that a combined exercise and diet program results in more weight loss than does diet alone.19
The limitations of this study are that it has small numbers of participants, predominantly women, no control group; and only 1 mail-out inquiry at 12 months for long-term follow-up. Despite these limitations, we have shown that this diet and exercise program is feasible in this patient group, that the subjects achieved significant weight loss for the duration of their participation in the program, that ancillary benefits in terms of cardiometabolic and neurobehavioral outcomes can be expected and that half of those who complete the program will have significant long-term weight loss. These data support the recently published Finnish study, which also showed significant weight loss maintained to 12 months and improvement in metabolic outcomes. However, our data suggest the efficacy of this treatment approach in those subjects with more severe SDB and that a benefit in terms of symptoms and quality of life, as well as cardiometablic markers, might be anticipated in a study with more subjects.
The mechanism by which obesity increases the risk for OSA is likely multifactorial. The upper airway is more collapsible in the obese; with weight loss, the critical closing pressure (Pcrit) falls and SDB resolves.34 However, it is likely that both an elevated mechanical load and impaired neuromuscular response are required for the development of OSA.35,36 The mechanical load may be due to restricted lung expansion caused by both abdominal visceral fat accumulation impairing diaphragmatic excursion and chest wall obesity impairing rib cage expansion. Functional residual capacity and residual volume are therefore reduced in obese individuals,37 which reduces caudal traction on the trachea and thus increases the collapsibility of the upper airway.38 Imaging of the upper airway has consistently shown a reduced airway size, predominantly in the lateral dimension.39 This has variously been attributed to increased size of the lateral pharyngeal fat pads40 or to increased muscle thickness in the lateral pharyngeal wall.41
OSA may also contribute to obesity. Short sleep duration has been shown to be associated with obesity42,43 and high levels of the obesity-related hormones ghrelin and leptin are seen in patients with OSA.44–46 The concept of leptin resistance in patients with OSA is further supported by the significant correlation that has been shown between leptin receptor gene polymorphism and OSA.47
Acknowledging that these are uncontrolled data, we have shown a significant improvement in sleepiness in the absence of a significant improvement in AHI, supporting the concept that obesity itself contributes to daytime somnolence independent of OSA. Multivariate analysis in a larger randomized and controlled study of patients undergoing weight loss would enable a measurement of the separate contribution that weight loss and improved SDB make to the improvement in symptoms, blood pressure, and other important clinical outcomes. We hypothesize that the diet and exercise program may be as effective as CPAP for SDB, but due to the ancillary benefits of weight loss, the program would be of superior efficacy for decreasing daytime sleepiness, improving quality of life, and reducing cardiovascular risk. There is little benefit in investing time, energy, and money in a weight-reduction program that is effective only for the duration of the intervention; such a program must be subjected to long-term follow-up and a comprehensive health-economic evaluation.
Obese patients with mild to moderate OSA comprise the most numerous group of patients who are seen in a sleep clinic. This diet and exercise program has the potential to deliver significant health benefits in terms of treating SDB and reducing cardiovascular risk, however, no conclusions can be drawn from this feasibility study. It is important that large, randomized, controlled studies are now conducted that follow participants for at least 2 years to explore the efficacy of medical weight loss and clinical benefits in terms of SDB, neurobehavioral, and cardiometabolic outcomes.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Erin McKenzie-McHarg (Nutrition Department, Austin Health) for her assistance with the dietary protocol, A/Prof. Steve Selig (Department of Exercise Physiology, Victoria University) for his assistance with the exercise protocol, and Professor Rob Pierce for his review of the manuscript and support.
Institution at which the work was performed: Austin Hospital, Heidelberg, Victoria, Australia
Financial Support: This study was supported by the Institute for Breathing and Sleep (Austin Health) and the Physiotherapy Research Foundation, Australia. Novartis Medical Nutrition, Basel, Switzerland, provided the Optifast at cost price but provided no actual funding for the study. Novartis Medical Nutrition was not involved in study design, data collection, analysis or interpretation, manuscript preparation, or the decision to submit the paper for publication.
Trial Registration Number: Australian Clinical Trials Registry No. 12606000465550
REFERENCES
- 1.White DP. The pathogenesis of obstructive sleep apnea: Advances in the past 100 years. Am J Respir Cell Mol Biol. 2006;34:1–6. doi: 10.1165/rcmb.2005-0317OE. [DOI] [PubMed] [Google Scholar]
- 2.Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:656–664. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 3.George CFP. Sleepiness, sleep apnea, and driving: still miles to go before we safely sleep. Am J Respir Crit Care Med. 2004;170:927–928. doi: 10.1164/rccm.2408009. [DOI] [PubMed] [Google Scholar]
- 4.Lavie P. Incidence of sleep apnoea in a presumably healthy working population: a significant relationship with excessive daytime sleepiness. Sleep. 1983;64:312–8. [PubMed] [Google Scholar]
- 5.Peker Y, Hedner J, Norum J, et al. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: A 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 6.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 7.Barnes M, Houston D, Worsnop CJ, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:773–780. doi: 10.1164/ajrccm.165.6.2003166. [DOI] [PubMed] [Google Scholar]
- 8.Engleman HM, McDonald JP, Graham D, et al. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med. 2002;166:855–859. doi: 10.1164/rccm.2109023. [DOI] [PubMed] [Google Scholar]
- 9.Bearpark H, Elliott L, Grunstein R, et al. Snoring and sleep apnea. A population study in Australian men. Am J Respir Crit Care Med. 1995;151:1459–1465. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- 10.Grunstein RR, Stenlof K, Hedner JA, et al. Impact of self-reported sleep-breathing disturbances on psychosocial performance in the Swedish Obese Subjects (SOS) Study. Sleep. 1995;18:635–43. doi: 10.1093/sleep/18.8.635. [DOI] [PubMed] [Google Scholar]
- 11.York DA, Rossner S, Caterson I, et al. Prevention conference VII: obesity, a worldwide epidemic related to heart Disease and stroke: group I: worldwide demographics of obesity. Circulation. 2004;110:e463–470. doi: 10.1161/01.CIR.0000140125.26161.49. [DOI] [PubMed] [Google Scholar]
- 12.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 13.Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 14.Newman AB, Foster G, Givelber R, et al. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165:2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 15.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 16.Wing R. Behavioural approaches to the treatment of obesity. In: Bray GA, Bouchard C, editors. Handbook of Obesity: clinical applications. New York: Marcel Dekker; 2004. pp. 147–67. [Google Scholar]
- 17.Eckel RH. Nonsurgical management of obesity in adults. N Engl J Med. 2008;358:1941–1950. doi: 10.1056/NEJMcp0801652. [DOI] [PubMed] [Google Scholar]
- 18.Mustajoki P, Pekkarinen T. Very low energy diets in the treatment of obesity. Obes Rev. 2001;2:61–72. doi: 10.1046/j.1467-789x.2001.00026.x. [DOI] [PubMed] [Google Scholar]
- 19.Curioni CC, Lourenco PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes (Lond) 2005;29:1168–74. doi: 10.1038/sj.ijo.0803015. [DOI] [PubMed] [Google Scholar]
- 20.Netzer N, Lormes W, Giebelhaus V, et al. Physical training of patients with sleep apnea. Pneumologie. 1997;(Suppl 3):779–82. [PubMed] [Google Scholar]
- 21.Suratt PM, McTier RF, Findley LJ, et al. Changes in breathing and the pharynx after weight loss in obstructive sleep apnea. Chest. 1987;92:631–7. doi: 10.1378/chest.92.4.631. [DOI] [PubMed] [Google Scholar]
- 22.Kansanen M, Vanninen E, Tuunainen A, et al. The effect of a very low-calorie diet-induced weight loss on the severity of obstructive sleep apnoea and autonomic nervous function in obese patients with obstructive sleep apnoea syndrome. Clin Physiol. 1998;18:377–385. doi: 10.1046/j.1365-2281.1998.00114.x. [DOI] [PubMed] [Google Scholar]
- 23.Tuomilehto HP, Seppa JM, Partinen MM, et al. Lifestyle intervention with weight reduction-first line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2008:200805–669OC. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 24.AASM Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 25.Goudge R, Goh N, Barnes M, et al. Validation of a sleep apnoea symptom questionnaire (SASQ) Am J Resp Crit Care Med. 2001;163:A933. [Google Scholar]
- 26.Pretto J, Braun G, Guy P. Using baseline respiratory function data to optimize cycle exercise test duration. Respirology. 2001;6:287–291. doi: 10.1046/j.1440-1843.2001.00356.x. [DOI] [PubMed] [Google Scholar]
- 27.American College of Sports Medicine . ACSM guidelines for exercise testing and prescription. 7th edition. Baltimore, USA: Lippincott Williams – Wilkins Senior Editor Whaley MH; 2006. [Google Scholar]
- 28.Kraemer WJ, Volek JS, Clark KL, et al. Influence of exercise training on physiological and performance changes with weight loss in men. Med Sci Sports Exercise. 1999;31:1320–9. doi: 10.1097/00005768-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Horvath K, Jeitler K, Siering U, et al. Long-term effects of weight-reducing interventions in hypertensive patients: systematic review and meta-analysis Arch Intern Med. 2008;168:571–580. doi: 10.1001/archinte.168.6.571. [DOI] [PubMed] [Google Scholar]
- 30.LaMonte MJ, Blair SN. Physical activity, cardiorespiratory fitness, and adiposity: contributions to disease risk. Curr Opin Clin Nutr Metab Care. 2006;9:540–6. doi: 10.1097/01.mco.0000241662.92642.08. [DOI] [PubMed] [Google Scholar]
- 31.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more Am J Respir Crit Care Med. 2008;177:369–375. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 33.Wing RR, Tate DF, Gorin AA, et al. A self-regulation program for maintenance of weight loss. New Engl J Med. 2006;355:1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz A, Gold A, Schubert N, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144:494–8. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 35.Patil SP, Schneider H, Marx JJ, et al. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102:547–556. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 36.McGinley BM, Schwartz AR, Schneider H, et al. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol. 2008;105:197–205. doi: 10.1152/japplphysiol.01214.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutherland TJT, Cowan JO, Young S, et al. The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med. 2008;178:469–475. doi: 10.1164/rccm.200802-301OC. [DOI] [PubMed] [Google Scholar]
- 38.Heinzer RC, Stanchina ML, Malhotra A, et al. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61:435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haponik EF SP, Bohlman ME, Allen RP, et al. Computerized tomography in obstructive sleep apnea. Correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis. 1983;127:221–6. doi: 10.1164/arrd.1983.127.2.221. [DOI] [PubMed] [Google Scholar]
- 40.Horner RL MR, Lowell DG, Shea SA, et al. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur Respir J. 1989;2:613–22. [PubMed] [Google Scholar]
- 41.Schwab RJ, Gupta KB, Gefter WB, et al. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152:1673–89. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- 42.Vorona RD, Winn MP, Babineau TW, et al. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165:25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 43.Singh M, Drake CL, Roehrs T, et al. The association between obesity and short sleep duration: a population-based study. J Clin Sleep Med. 2005;1:357–63. [PubMed] [Google Scholar]
- 44.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia J Clin Endocrinol Metab. 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 45.Ozturk L, Unal M, Tamer L, et al. The association of the severity of obstructive sleep apnea with plasma leptin levels. Arch Otolaryngol Head Neck Surg. 2003;129:538–540. doi: 10.1001/archotol.129.5.538. [DOI] [PubMed] [Google Scholar]
- 46.Phillips BG, Kato M, Narkiewicz K, et al. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279:H234–237. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 47.Popko K, Gorska E, Wasik M, et al. Frequency of distribution of leptin receptor gene polymorphism in obstructive sleep apnea patients. J Physiol Pharmacol. 2007;(Suppl 5):551–61. [PubMed] [Google Scholar]