Abstract
Background:
Although obstructive sleep apnea (OSA) is strongly linked with obesity, both conditions have been associated with increased cardiovascular risk including glucose intolerance, dyslipidemia, and hypertension independent of one another. Weight loss is known to improve both cardiovascular risk and OSA severity. The aim of this study was to evaluate cardiovascular and metabolic changes, including compartment-specific fat loss in obese OSA subjects undergoing a weight loss program.
Design:
Observational study.
Participants:
93 men with moderate-severe OSA.
Interventions:
6-month open-label weight loss trial combining sibutramine (a serotonin and noradrenaline reuptake inhibitor) with a 600-kcal deficit diet and exercise.
Measurements and Results:
At baseline and following 6 months of weight loss, OSA was assessed together with CT-quantified intra-abdominal and liver fat and markers of metabolic and cardiovascular function. At 6 months, weight loss and improvements in OSA were accompanied by improved insulin resistance (HOMA), increased HDL cholesterol, and reduced total cholesterol/HDL ratio. There were also reductions in measures of visceral and subcutaneous abdominal fat and liver fat. Reductions in liver fat and sleep time spent below 90% oxyhemoglobin saturation partly explained the improvement in HOMA (R2 = 0.18). In contrast, arterial stiffness (aortic augmentation index), heart rate, blood pressure, and total cholesterol did not change.
Conclusions:
Weight loss with sibutramine was associated with improvements in metabolic and body composition risk factors but not blood pressure or arterial stiffness. Improved insulin resistance was partly associated with reductions in liver fat and hypoxemia associated with sleep apnea.
Citation:
Phillips CL; Yee BJ; Trenell MI; Magnussen JS; Wang D; Banerjee D; Berend N; Grunstein RR. Changes in regional adiposity and cardio-metabolic function following a weight loss program with sibutramine in obese men with obstructive sleep apnea. J Clin Sleep Med 2009;5(5):416-421.
Keywords: Obstructive sleep apnea, obesity, weight loss, insulin resistance, sibutramine, visceral fat, liver fat
Obesity is a major risk factor for both obstructive sleep apnea (OSA) and cardiovascular disease. However there is increasing evidence that OSA may promote cardiovascular morbidity and mortality independent of obesity. This may be related to an obesity-independent increase in the prevalence of the metabolic syndrome in OSA subjects1 and in the risk factors that define the syndrome.2 In addition, there is limited data suggesting that treatment of OSA with nasal continuous positive airway pressure (CPAP) may improve several important cardiovascular risk factors.3,4 However, apart from hypertension3 there is still no definitive data from randomized controlled trials that CPAP treatment improves other cardiovascular risk factors.5,6 Moreover compliance with CPAP remains suboptimal7 and there is a recognized need for adjunctive therapeutic approaches to improve overall risk.
Apart from CPAP, weight loss is one potential approach that may be useful in reducing cardiovascular risk in OSA. Even moderate weight loss results in marked improvements to a range of cardiovascular risk factors including lipids, insulin resistance (IR), and blood pressure.8 Sibutramine is a serotonin and noradrenaline reuptake inhibitor (SNRI) that promotes weight loss through increased energy expenditure and satiety.9 Previous studies have shown that weight loss with sibutramine is accompanied by marked loss of abdominal fat10,11 and improvements in metabolic function.12,13 However the effectiveness of weight loss medications to reduce weight and improve cardiovascular and metabolic function has never been specifically evaluated in OSA subjects. Some evidence suggests that sympathetic overactivity in patients with OSA may alter regulation of body weight.14 Indeed, there is a suggestion that sympathetic overactivity may contribute to increased abdominal obesity.15 It follows that cardio-metabolic improvements associated with weight loss may be modulated by both the presence of OSA and the extent to which OSA resolves. Therefore establishing the effectiveness of weight loss in reducing central adiposity and other cardio-metabolic risk factors in patients with OSA would have clinical relevance to management of this condition.
We have recently shown a reduction in sleep apnea severity following 6 months of sibutramine-assisted weight loss in subjects with moderate-severe OSA.16 The aim of this study was to determine (in the same group) the extent of improvement (if any) in a range of important cardio-metabolic risk factors including fat loss from visceral, subcutaneous, and liver stores. A secondary aim was to explore whether there was an association between OSA alleviation with weight loss and cardio-metabolic improvements (specifically IR and arterial stiffness) independent of changes in body composition.
METHODS
Study Population and Weight Loss Program
Selection criteria were as previously described16 and consisted of male OSAS subjects (aged 30–70 years, BMI 30–38 kg/m2, RDI ≥ 15 events/hour, and Epworth Sleepiness Scale score > 10). Subjects with inadequately controlled blood pressure (BP > 145/95 mm Hg) or elevated heart rate (HR > 100 bpm) or type 2 diabetes were excluded from the study. Twenty-six subjects were taking antihypertensive medication. All but 9 subjects were newly diagnosed with OSA and were CPAP naïve. The remainder had previously failed to initiate CPAP therapy. All subjects underwent a 24-week period of sibutramine intake (10 or 15 mg per day) combined with a 2500 kJ (600 kcal) deficit diet and exercise advice (≥ 30 minutes brisk walking per day).16
Measurements
All measurements were performed at baseline and at 24 weeks. Sleep staging and respiratory events were scored from polysomnographic recordings (Compumedics, Melbourne, Australia) using standard techniques.16 Nasal flow was determined from nasal pressure recordings. Arterial oxygen saturation (SpO2) was measured by pulse oximetry. Apneas were defined as a complete cessation of airflow ≥ 10 sec associated with an EEG arousal and/or ≥ 3% oxygen desaturation. Hypopneas were defined as a reduction of airflow > 50% from baseline, lasting ≥ 10 sec, associated with an EEG arousal and/or ≥ 3% oxygen desaturation. The respiratory disturbance index (RDI) and oxygen desaturation index (ODI) were defined respectively by the number of apneas plus hypopneas or arterial oxygen desaturations ≥ 3% per hour of sleep. The total sleep time with SpO2 less than 90% (SpO2T90) was also quantified.
After 15 minutes rest, BP and HR were measured in triplicate from the nondominant arm while seated.16 Arterial stiffness and central aortic BP were subsequently measured in the supine position by pulse wave analysis (PWA).17 This technique provides measurements for the aortic augmentation index (AIx) and central aortic BP using a validated transfer algorithm. All augmentation index measurements were standardized to a heart rate of 75 beats per minute (ΔAIXHR75). Measurements were always made at the same time of day, when subjects had spent ≥ 5 h without food, caffeine, or smoking.18
Anthropometric measurements were taken16 together with fasting venous blood for analysis of metabolic variables using standard techniques available through the local hospital biochemistry laboratory. Insulin sensitivity was estimated by the HOMA (homeostasis model assessment) method. HOMA is determined from fasting plasma insulin and glucose where: HOMA = fasting insulin (μU/mL) × fasting glucose (mmol/L) / 22.5. The volume of subcutaneous and visceral adipose tissue (SCAT and VAT) was quantified using a validated software tool (Hippo Fat)19 from 5 contiguous 1-mm axial computed tomography (CT) slices starting at the level of the umbilicus between the L3 and L5 vertebra. Liver and spleen Hounsfield unit attenuations (E-FILM) were measured from unenhanced CT slices in several homogenous areas (≥ 10 cm2) that were free of vasculature adjacent to the T12/L1 intervertebral space. The liver to spleen attenuation ratio (L/S ratio) was then used to estimate liver fat. This method has been validated against the gold standard of liver biopsy.20 Values < 1.0 are consistent with fatty liver (steatosis).21
Statistical Analysis
All changes were assessed using paired-samples t tests or Wilcoxon signed ranks tests where appropriate. Univariate correlations (Pearson r or Spearman rho) were used to explore associations for the magnitude of change (Δ) between variables. Stepwise multiple linear regression (MLR) analysis was used to specifically explore changes in body compositional variables (ΔBMI and Δabdominal fat) and OSA variables (ΔRDI and ΔSpO2T90) with IR (ΔHOMA). A more simplified MLR was used to explore changes in BMI and OSA variables with arterial stiffness (Δaortic augmentation index). All independent variables were examined for co-linearity. Statistical analyses were performed with the SPSS software (Chicago, IL).
The study is registered with the Australian Clinical Trials Registry (ACTR #12605000330640) and was approved by the local hospital ethics committee.
RESULTS
Paired data (baseline and 6 months) for the 93 subjects are presented in Table 1. Incomplete data for some variables was due to processing errors in the biochemistry laboratory and to regional non-alignment of CT images.
Table 1.
Measurement of Variables at Baseline and at 6 Months
| Variable | Baseline | 6 Months | p-value |
|---|---|---|---|
| Sleep Disordered Breathing | |||
| RDI (per h)93 | 45.9 ± 23.0 | 30.2 ± 20.4 | 0.0001 |
| SpO2T90 (min)93 | 2.9 (0.2-7.8) | 1 (0.1-5.5) | 0.008 |
| ODI (per h)91 | 35.0 ± 24 | 25.8 ± 22 | 0.00002 |
| ESS (0-20)90 | 13.3 ± 3.7 | 8.7 ± 4.4 | 0.0001 |
| Anthropometry | |||
| Weight (kg)92 | 107.1 ± 12.4 | 99.1 ± 12.6 | 0.0001 |
| BMI (kg/m2)92 | 34.1 ± 2.7 | 31.6 ± 3.0 | 0.0001 |
| Neck circ (cm)91 | 44.7 ± 2.4 | 43.1 ± 2.4 | 0.0001 |
| Waist circ (cm)91 | 115.8 ± 8.3 | 107.6 ± 9.1 | 0.0001 |
| Sagittal ht (cm)90 | 28.2 ± 2.8 | 25.0 ± 3.1 | 0.0001 |
| Hemodynamics (sitting) | |||
| SBP (mm Hg)90 | 119.1 ± 12.1 | 117.7 ± 11.0 | 0.2 |
| DBP (mm Hg)92 | 79.5 ± 8.4 | 79.2 ± 8.5 | 0.73 |
| HR (beats/min)83 | 69 ± 10 | 72 ± 11 | 0.004 |
| Hemodynamics (supine)63 | |||
| PSBP (mm Hg) | 123.7 ± 11.0 | 122.7 ± 10.0 | 0.28 |
| PDBP (mm Hg) | 79 ± 6.6 | 79.4 ± 7.3 | 0.8 |
| CSBP (mm Hg) | 114.2 ± 11.7 | 113.2 ± 10.9 | 0.3 |
| CDBP (mm Hg) | 80.1 ± 6.7 | 80.4 ± 7.4 | 0.85 |
| HR (beats/min) | 64.1 ± 8.4 | 63.9 ± 8.9 | 0.77 |
| AIXHR75 (%) | 16.2 ± 8.0 | 16.0 ± 8.1 | 0.8 |
| APHR75 (mm Hg) | 7.8 ± 4.9 | 7.4 ± 4.0 | 0.3 |
| Metabolic | |||
| Insulin (μIU/mL)84 | 16.6 ± 8.4 | 13.5 ± 7.4 | 0.001 |
| Glucose (mmol/L)88 | 4.78 ± 0.84 | 4.94 ± 0.73 | 0.09 |
| HOMA81 | 3.60 ± 1.97 | 2.99 ± 1.77 | 0.01 |
| Leptin (ng/mL)79 | 14.1 ± 7.1 | 12.3 ± 6.9 | 0.05 |
| HDL (mmol/L)88 | 1.11 ± 0.23 | 1.18 ± 0.25 | 0.0001 |
| Total cholesterol/HDL-C ratio42 | 4.99 ± 1.18 | 4.72 ± 1.1 | 0.043 |
| Abdominal and Liver Fat | |||
| VAT (cm3)64 | 141.1 ± 38.0 | 113.5 ± 36.4 | 0.0001 |
| SCAT (cm3)64 | 212.7 ± 51.7 | 179.9 ± 51.1 | 0.0001 |
| VAT/SCAT ratio64 | 0.706 ± 0.275 | 0.673 ± 0.276 | 0.05 |
| Liver/Spleen ratio65 | 0.89 ± 0.33 | 1.06 ± 0.25 | 0.0001 |
Superscript denotes number of paired comparisons. Change denotes mean change ± SD (paired samples t test) or median (interquartile range) (Wilcoxon signed ranks test).
RDI = respiratory disturbance index per hour of sleep. ODI = oxygen desaturation index per hour of sleep. ESS = Epworth Sleepiness Scale. SpO2T90 = Sleep time below 90% oxyhemoglobin saturation. PSBP, CSBP, PDBP, CDBP = peripheral or central systolic and diastolic blood pressure. AIXHR75 and APHR75 = aortic augmentation index and augmentation pressure corrected to a heart rate of 75 bpm. HOMA = Homeostasis Model Assessment of insulin sensitivity. VAT = visceral adipose tissue volume. SCAT = subcutaneous adipose tissue volume. L/S ratio = Liver/Spleen Hounsfield unit attenuation ratio (a measure of liver fat content).
As previously reported,16 subjects were middle-aged (46.5 ± 9.3 years) and moderately obese (BMI 34.2 ± 2.7 kg/m2) with moderate-severe OSA (RDI 46.0 ± 23/h). There was a moderate amount of weight loss (7.9 ± 4.9 kg), and RDI was reduced by 30% (16.0 ± 19.1/h) together with hypoxemia (SpO2T90 and ODI) and sleepiness (ESS fell by 4.6).
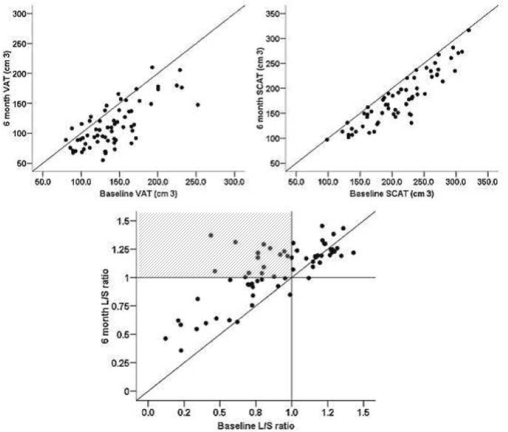
In parallel with weight (−7.5%) and anthropometric changes, there were marked reductions in visceral (VAT −19.6%) and subcutaneous (SCAT −15.4%) abdominal fat and liver fat (L/S ratio −19.1%). At baseline, ∼55% of subjects had L/S ratio scores (< 1) compatible with steatosis (fatty liver).21 At 6 months, this had decreased to ∼35% and included 14 subjects whose fatty liver had resolved (Figure 1). There was also an overall modest improvement in insulin sensitivity (HOMA −16.9%) and in plasma lipid and leptin levels. In contrast, apart from a small increase in sitting HR, there were no changes in any hemodynamic variables (Table 1).
Figure 1.
Visceral (VAT), Subcutaneous (SAT) and liver (L/S HU ratio) fat changes. An increase in L/S ratio indicates a reduction in liver fat. The shaded area (last panel) includes subjects whose fatty liver resolved.
In univariate analyses (Table 2), the change to IR (ΔHOMA) correlated with ΔBMI, ΔSCAT, and ΔL/S ratio, but not with ΔVAT. In addition, there was also a strong trend for an association with improved sleep related hypoxemia (ΔSpO2T90). Subsequent regression analysis (Table 3) revealed that ΔHOMA was best predicted by changes in liver fat (ΔL/S ratio) and hypoxemia during sleep (ΔSpO2T90). This model explained 18.4% of the variance in ΔHOMA, with hypoxemia accounting for half of that variance.
Table 2.
Correlation Coefficients and p Values for Change (Δ) in IR (ΔHOMA)
| Variable | Δ HOMA | p-value |
|---|---|---|
| Δ BMI81 | 0.310 | 0.005 |
| Δ VAT57 | 0.13 | 0.33 |
| Δ SCAT57 | 0.253 | 0.06 |
| Δ L/S ratio59 | −0.281 | 0.03 |
| Δ RDI81 | 0.16 | 0.16 |
| Δ SpO2T9081 | 0.21 | 0.057 |
BMI = Body Mass Index. VAT = Visceral Adipose Tissue volume. SCAT = Subcutaneous Adipose Tissue volume. L/S ratio = Liver/Spleen Hounsfield unit attenuation ratio (a measure of liver fat content). RDI = respiratory disturbance index per hour of sleep. SpO2T90 = Sleep time below 90% oxyhemoglobin saturation.
Table 3.
Multiple Regression Analyses for Change in IR (ΔHOMA)
| Variable | B | SE B | p-value |
|---|---|---|---|
| Δ HOMA | |||
| Model 1 R2 = 0.099, p = 0.019 | |||
| Δ Liver/Spleen ratio | −3.409 | 0.342 | 0.019 |
| Model 2 R2 = 0.184, p = 0.005 | |||
| Δ Liver/Spleen ratio | −3.854 | 1.371 | 0.007 |
| Δ SpO2T90 | 0.059 | 0.025 | 0.024 |
Variables excluded from the final model for Δ HOMA: Δ BMI, Δ VAT, Δ SCAT, Δ RDI (all p > 0.2)
B = Unstandardized coefficients
DISCUSSION
This study has shown that the previously documented loss of weight and reduction in sleep apnea severity following 6 months of a weight loss program with sibutramine16 were accompanied by significant metabolic changes and changes in body composition. Apart from there being only limited data on the concomitant cardiovascular and metabolic changes associated with weight loss in subjects with objectively defined OSA,22 this is one of the largest cohorts of men to be examined with sibutramine that has included comprehensive assessment of both abdominal and liver fat changes. Changes in body composition included significant loss of visceral and subcutaneous abdominal fat and liver fat; these were accompanied by improvements in insulin resistance, HDL cholesterol, and total cholesterol/HDL ratio. An important additional finding was that the improvement in insulin sensitivity (albeit only modest) was partially associated with a reduction in apnea-related hypoxemia. In contrast, however, there were no parallel improvements in relation to blood pressure or arterial stiffness.
Abdominal Fat Changes
A number of studies with sibutramine have demonstrated marked improvements in visceral and subcutaneous abdominal fat assessed by numerous methods including CT.11,23 The findings from these studies demonstrate greater percentage loss of visceral fat (VAT) than subcutaneous fat (SCAT) and are reflected by results from the present study. The greater loss of VAT may be a consequence of a greater capacity for catecholamine induced lipolysis in VAT than SCAT.24 The observed loss of visceral fat (which is a strong independent predictor of all cause mortality in men25) is a particularly important outcome for a population of OSA subjects who already have increased cardio-metabolic risk.26
However, despite the overall reduction of fat stores in the present study the relative amount of fat loss was markedly reduced when compared to a previous study that used a similar (3-dimensional) method of fat assessment.27 This diminished loss of fat (37% versus 20% and 24% versus 15% for VAT and SCAT respectively) may be related to the inadequate resolution of OSA which in the present cohort was reduced by a modest ∼30%. In support of this, a previous study has shown that visceral fat decreases with CPAP treatment but only in compliant users.28
Liver Fat Changes
Liver fat accumulation is an important factor (independent of obesity) in the development of IR and other features of the metabolic syndrome as well being increasingly recognized as a predictor of cardiovascular risk.29 In the current study weight loss was also accompanied by marked reductions in liver fat and in the proportion of subjects with L/S ratios indicative of steatosis. These changes were also strongly associated with improved IR, but there was no association with improvement in OSA.
Metabolic Changes
In the present study, there was a modest improvement in IR as determined by HOMA. From a mechanistic perspective, the changes in abdominal fat (VAT and SCAT) and liver fat (L/S ratio) were quantified to establish whether they influenced the improvement in IR observed with weight loss. The results are in agreement with a previous study10 showing that the change in VAT was not associated with the change in IR. This was an unexpected finding, since VAT is considered to be an important predictor of insulin resistance,30 and it may reflect the relative inaccuracy of HOMA as a measure of insulin sensitivity compared with the gold standard hyperinsulinemic euglycemic clamp. Alternatively, the current results may suggest that liver fat changes have a more important influence on IR following weight loss with sibutramine. Liver fat has been associated with hepatic IR independent of obesity and intra-abdominal fat,31 and a recent study has shown that increased liver fat is associated with both impaired insulin clearance and hepatic insulin resistance independent of BMI.32
In addition to liver fat changes, an important new finding in this study was the association between improvements in hypoxemia (associated with OSA) and IR. In a large epidemiological study, hypoxemia has been shown to predict impaired glucose tolerance.33 Furthermore, an improvement in OSA symptoms in patients losing weight has been associated with a lower 2-year incidence of diabetes and hypertriglyceridemia.34
Finally, there was also a modest reduction in the total cholesterol/HDL ratio which represents over half the improvement observed with compliant (≥ 4 h/night) CPAP therapy.35 This marker has been associated with ischemic heart disease risk.36 The magnitude of improvement in leptin also represents almost half of that observed with compliant CPAP therapy.28
Arterial Stiffness and Blood Pressure
We have previously documented a dose-dependent relationship between OSA severity and arterial stiffness18 and we hypothesized that a reduction in both weight and OSA would be associated with improvements in arterial stiffness and/or blood pressure. However no changes were detected in any variable. The lack of change in arterial stiffness contrasts with a previous study that found progression and regression of arterial stiffness (measured by pulse wave velocity) associated respectively with weight gain or loss over 2 years.37 The lack of change in blood pressure is also in contrast to numerous studies that have documented moderate falls accompanying weight loss in short term trials that did not involve sibutramine. 38 However favorable blood pressure changes have not been consistently demonstrated in weight loss studies with sibutramine,39 and no study has examined changes in arterial stiffness by any method.
Ultimately, the lack of change in arterial stiffness and blood pressure in the present study may be a consequence of the weight loss effects being negated by possible vasoconstrictive effects associated with noradrenaline reuptake inhibition by sibutramine and also possibly by persistent residual OSA. In this context, although supine heart rate did not change, there was a small rise in sitting heart rate. This is consistent with many studies documenting small increases in heart rate with sibutramine and highlights the need to closely monitor heart rate in patients taking this medication.39 This heart rate rise could potentially be ameliorated if patients received concomitant treatment for their OSA, since falls in heart rate have been observed with CPAP treatment.40
Study Limitations
It is important to recognize several limitations with the current study. Firstly, the experimental design did not include a placebo arm. However the original aim of the (previously published) efficacy study16 was to determine whether a weight loss program with sibutramine would improve sleep apnea without exacerbating blood pressure. There were ethical concerns at the time in using a blinded design with a drug that could potentially elevate BP in patients with OSA, a disorder strongly associated with hypertension. A second limitation is that the study cohort consisted exclusively of males, and the changes observed may be influenced by gender. However it is important to recognize that central obesity and its impact on metabolic health is more pertinent to the male population because of the preponderance for central obesity in males. Previous studies with sibutramine have mainly enrolled female subjects. In contrast, to the best of our knowledge the present study provides the most extensive dataset on males. Another limitation is that physical activity and diet (caloric intake) were not quantified and some of the observed improvements may be independently influenced by inter-individual differences in either. However, a previous placebo-controlled trial has already demonstrated that in the absence of sibutramine, weight loss is not sustained despite ongoing diet and exercise.41 Finally, as previously mentioned, there are limitations associated with the use of the HOMA method for assessing insulin sensitivity, and blood pressure changes would have been better assessed using 24-h ambulatory monitoring than office-based measurements.
Clinical Implications
The current data shows that weight loss targeted towards OSA subjects results in some clinically important reductions in cardio-metabolic risk factors, though it is possible that persisting OSA may hinder this effect. While CPAP remains the gold standard therapy for moderate to severe OSA, many patients refuse this therapy,7 and the effect of CPAP on long term cardiovascular risk remains controversial. In the very least, weight loss may prove beneficial in subjects who refuse CPAP therapy while at most it may enhance any positive effects that occur with compliant CPAP use. Ultimately consideration needs to be given what treatment approach improves overall cardio-metabolic risk in these patients. Combining weight loss with CPAP treatment may be more efficacious than either treatment alone. Finally, although sibutramine was used to assist weight loss in the current study, there was a lack of reduction in office blood pressure and arterial stiffness. Newer anti-obesity agents that do not alter vascular tone may have advantages in weight loss studies involving patients with OSA.
DISCLOSURE STATEMENT
This study was investigator driven but did receive assistance from Abbot Pharmaceuticals Australasia in the form of financial support and provision of Sibutramine medication for the participants. The authors indicated no other conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Yeow Lee for carrying out the abdominal and liver fat analyses in this study.
All work was performed at the Woolcock Institute of Medical Research and the Royal Prince Alfred Hospital, Sydney.
REFERENCES
- 1.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PMA, Wilding JPH. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–41. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 2.Newman AB, Nieto FJ, Guidry U, et al. Relation of Sleep-disordered breathing to cardiovascular disease risk factors: The Sleep Heart Health Study. Am J Epidemiol. 2001;154:50–9. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 3.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–23. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 4.Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–62. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JPH, Calverley PMA. Cardiovascular and metabolic effects of CPAP in obese men with OSA. Eur Respir J. 2007;29:720–7. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 6.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. The effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–74. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cistulli PA, Grunstein RR. Medical devices for the diagnosis and treatment of obstructive sleep apnea. Expert Rev Med Devices. 2005;2:749–63. doi: 10.1586/17434440.2.6.749. [DOI] [PubMed] [Google Scholar]
- 8.Klein S, Burke LE, Bray GA, et al. Clinical implications of obesity with specific focus on cardiovascular disease: A Statement for Professionals From the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: Endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–67. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 9.Stock MJ. Sibutramine: a review of the pharmacology of a novel anti-obesity agent. Int J Obes Relat Metab Disord. 1997;21:S25–9. [PubMed] [Google Scholar]
- 10.Faria AN, Ribeiro Filho FF, Kohlmann NE, Gouvea Ferreira SR, Zanella MT. Effects of sibutramine on abdominal fat mass, insulin resistance and blood pressure in obese hypertensive patients. Diabetes Obes Metab. 2005;7:246–53. doi: 10.1111/j.1463-1326.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Gaal LF, Wauters MA, Peiffer FW, De Leeuw IH. Sibutramine and fat distribution: is there a role for pharmacotherapy in abdominal/visceral fat reduction? Int J Obes Relat Metab Disord. 1998;22:S38–40. [PubMed] [Google Scholar]
- 12.Krejs GJ. Metabolic benefits associated with sibutramine therapy. Int J Obes Relat Metab Disord. 2002;26:S34–7. doi: 10.1038/sj.ijo.0802217. [DOI] [PubMed] [Google Scholar]
- 13.Tambascia MA, Geloneze B, Repetto EM, Geloneze SR, Picolo M, Magro DO. Sibutramine enhances insulin sensitivity ameliorating metabolic parameters in a double-blind, randomized, placebo-controlled trial. Diabetes Obes Metab. 2003;5:338–44. doi: 10.1046/j.1463-1326.2003.00285.x. [DOI] [PubMed] [Google Scholar]
- 14.Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279:H234–7. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 15.Greenfield J, Campbell L. Role of the autonomic nervous system and neuropeptides in the development of obesity in humans: targets for therapy? Curr Pharm Des. 2008;14:1815–20. doi: 10.2174/138161208784746716. [DOI] [PubMed] [Google Scholar]
- 16.Yee BJ, Phillips CL, Banerjee D, Caterson I, Hedner JA, Grunstein RR. The effect of sibutramine-assisted weight loss in men with obstructive sleep apnoea. Int J Obes. 2007;31:161–8. doi: 10.1038/sj.ijo.0803363. [DOI] [PubMed] [Google Scholar]
- 17.O'Rourke MF, Gallagher DE. Pulse wave analysis. J Hypertens Suppl. 1996;14:S147–57. [PubMed] [Google Scholar]
- 18.Phillips C, Hedner J, Berend N, Grunstein R. Diurnal and obstructive sleep apnea influences on arterial stiffness and central blood pressure in men. Sleep. 2005;28:604–9. doi: 10.1093/sleep/28.5.604. [DOI] [PubMed] [Google Scholar]
- 19.Demerath EW, Ritter KJ, Couch WA, et al. Validity of a new automated software program for visceral adipose tissue estimation. Int J Obes. 2006;31:285–91. doi: 10.1038/sj.ijo.0803409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson LE, Kuk JL, Church TS, Ross R. Protocol for measurement of liver fat by computed tomography. J Appl Physiol. 2006;100:864–8. doi: 10.1152/japplphysiol.00986.2005. [DOI] [PubMed] [Google Scholar]
- 21.Ricci C, Longo R, Gioulis E, et al. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol. 1997;27:108–13. doi: 10.1016/s0168-8278(97)80288-7. [DOI] [PubMed] [Google Scholar]
- 22.Dixon JB, Schachter LM, O'Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond) 2005;29:1048–54. doi: 10.1038/sj.ijo.0802960. [DOI] [PubMed] [Google Scholar]
- 23.Kim DM, Yoon SJ, Ahn CW, et al. Sibutramine improves fat distribution and insulin resistance, and increases serum adiponectin levels in Korean obese nondiabetic premenopausal women. Diabetes Res Clin Pract. 2004;66:S139–44. doi: 10.1016/j.diabres.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Ramis JM, Salinas R, Garcia-Sanz JM, Moreiro J, Proenza AM, Llado I. Depot- and gender-related differences in the lipolytic pathway of adipose tissue from severely obese patients. Cell Physiol Biochem. 2006;17:173–80. doi: 10.1159/000092079. [DOI] [PubMed] [Google Scholar]
- 25.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obes Res. 2006;14:336–41. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- 26.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 27.Kamel EG, McNeill G, Van Wijk MC. Change in intra-abdominal adipose tissue volume during weight loss in obese men and women: correlation between magnetic resonance imaging and anthropometric measurements. Int J Obes Relat Metab Disord. 2000;24:607–13. doi: 10.1038/sj.ijo.0801204. [DOI] [PubMed] [Google Scholar]
- 28.Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab. 2007;9:679–87. doi: 10.1111/j.1463-1326.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 29.Kotronen A, Yki-Jarvinen H. Fatty liver. A novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 30.Gan SK, Kriketos AD, Poynten AM, et al. Insulin action, regional fat, and myocyte lipid: altered relationships with increased adiposity. Obes Res. 2003;11:1295–305. doi: 10.1038/oby.2003.176. [DOI] [PubMed] [Google Scholar]
- 31.Seppala-Lindroos A, Vehkavaara S, Hakkinen A-M, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–28. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 32.Kotronen A, Vehkavaara S, Seppala-Lindroos A, Bergholm R, Yki-Jarvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab. 2007;293:1709–15. doi: 10.1152/ajpendo.00444.2007. [DOI] [PubMed] [Google Scholar]
- 33.Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep. 2006;29:777–83. doi: 10.1093/sleep/29.6.777. [DOI] [PubMed] [Google Scholar]
- 34.Grunstein RR, Stenlof K, Hedner JA, Peltonen M, Karason K, Sjostrom L. Two year reduction in sleep apnea symptoms and associated diabetes incidence after weight loss in severe obesity. Sleep. 2007;30:703–10. doi: 10.1093/sleep/30.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steiropoulos P, Tsara V, Nena E, et al. Effect of continuous positive airway pressure treatment on serum cardiovascular risk factors in patients with obstructive sleep apnea-hypopnea syndrome. Chest. 2007;132:843–51. doi: 10.1378/chest.07-0074. [DOI] [PubMed] [Google Scholar]
- 36.Lemieux I, Lamarche B, Couillard C, et al. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: The Quebec Cardiovascular Study. Arch Intern Med. 2001;161:2685–92. doi: 10.1001/archinte.161.22.2685. [DOI] [PubMed] [Google Scholar]
- 37.Wildman RP, Farhat GN, Patel AS, et al. Weight Change is associated with change in arterial stiffness among healthy young adults. Hypertension. 2005;45:187–92. doi: 10.1161/01.HYP.0000152200.10578.5d. [DOI] [PubMed] [Google Scholar]
- 38.Aucott L, Poobalan A, Smith WCS, Avenell A, Jung R, Broom J. Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: a systematic review. Hypertension. 2005;45:1035–41. doi: 10.1161/01.HYP.0000165680.59733.d4. [DOI] [PubMed] [Google Scholar]
- 39.Jordan J, Scholze J, Matiba B, Wirth A, Hauner H, Sharma AM. Influence of Sibutramine on blood pressure: evidence from placebo-controlled trials. Int J Obes Relat Metab Disord. 2005;29:509–16. doi: 10.1038/sj.ijo.0802887. [DOI] [PubMed] [Google Scholar]
- 40.Mills PJ, Kennedy BP, Loredo JS, Dimsdale JE, Ziegler MG. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol. 2006;100:343–8. doi: 10.1152/japplphysiol.00494.2005. [DOI] [PubMed] [Google Scholar]
- 41.James WPT, Astrup A, Finer N, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. Lancet. 2000;356:2119–25. doi: 10.1016/s0140-6736(00)03491-7. [DOI] [PubMed] [Google Scholar]