Abstract
Successful treatment of cancer patients with a combination of monoclonal antibodies (mAb) and chemotherapeutic drugs has spawned various other forms of additional combination therapies, including vaccines or adoptive lymphocyte transfer combined with chemotherapeutics. These therapies were effective against established tumors in animal models and showed promising results in initial clinical trials in cancer patients, awaiting testing in larger randomized controlled studies. Although combination between immunotherapy and chemotherapy has long been viewed as incompatible as chemotherapy, especially in high doses meant to increase anti-tumor efficacy, has induced immunosuppression, various mechanisms may explain the reported synergistic effects of the two types of therapies. Thus direct effects of chemotherapy on tumor or host environment, such as induction of tumor cell death, elimination of regulatory T cells, and/or enhancement of tumor cell sensitivity to lysis by CTL may account for enhancement of immunotherapy by chemotherapy. Furthermore, induction of lymphopenia by chemotherapy has increased the efficacy of adoptive lymphocyte transfer in cancer patients. On the other hand, immunotherapy may directly modulate the tumor’s sensitivity to chemotherapy. Thus, anti-tumor mAb can increase the sensitivity of tumor cells to chemotherapeutic drugs and patients treated first with immunotherapy followed by chemotherapy showed higher clinical response rates than patients that had received chemotherapy alone. In conclusion, combination of active specific immunotherapy or adoptive mAb or lymphocyte immunotherapy with chemotherapy has great potential for the treatment of cancer patients which needs to be confirmed in larger controlled and randomized Phase III trials.
Keywords: Cancer, Immunotherapy, Chemotherapy, Antibody, Vaccine, Lymphocyte
Introduction
Combination between immunotherapy and chemotherapy has long been viewed as incompatible as chemotherapy, especially at high doses meant to increase the anti-tumor efficacy, has induced immunosuppression. Possible mechanisms of immune suppression by chemotherapy are induction of lymphopenia, immunosuppressive cytokines, immune tolerance by high doses of antigens released by the dying tumor cells, and inhibition of immune effector cell function [3, 90, 94, 155]. However, in the 1960s, Mihich already demonstrated in murine leukemia model that the curative effects of chemotherapy are due to the induction of immune response directed against the tumor cells [91–93]. Immunoaugmentation has also been shown in later studies following chemotherapy with some drugs at low doses [3, 47, 90, 94, 155]. Treatment of cytotoxic T lymphocytes (CTL) with certain chemotherapeutic drugs enhanced their capacity to lyse Epstein Barr virus (EBV)-transformed lymphocytes, whereas other drugs showed inhibitory activities [86]. Experimental evidence has shown that direct effects of chemotherapy on tumor and host environment, which are discussed in detail below, may counteract its immunosuppressive effects, leading to enhancement of anti-tumor immune response.
We have reviewed here experimental and clinical approaches to combining active specific immunotherapy, or adoptive antibody or cellular immunotherapy with chemotherapy in the treatment of cancer. Most of the previous review articles did not cover combination of adoptive antibody or cellular immunotherapy with chemotherapy in pre-clinical and clinical studies and, in contrast to our article, none (including also articles on combined active specific immunotherapy and chemotherapy) describe experimental details, which are important to better understand differences in the results obtained with similar combination therapies by different investigators [3, 18, 21, 32, 45, 48, 58, 73–75, 77, 83, 90, 95, 96, 101, 117, 123, 132, 137, 143, 144]. The experimental approaches in this review include only studies which are carefully controlled to demonstrate that a combination of both therapies is superior to the use of either therapy alone. Clinical trials with combination therapies are also included in this review although they were not randomized and have not yet reached phase III. This review article does not include studies in which non-specific immune modulators such as cytokines were combined with chemotherapeutic agents. These studies have recently been reviewed by Zitvogel et al. [155].
Pre-clinical and clinical studies of combined mAb IT and CT
MAb therapy, which has long been viewed as unsuccessful, has been greatly rejuvenated by its combination with chemotherapeutics. Naked and radiolabelled mAb in combination with chemotherapeutics, or mAb linked to drugs have been used for the treatment of various malignancies in mice and cancer patients (Tables 1, 2). In mice, the anti-tumor effects of these combination therapies were significantly greater compared to either therapy alone. Of note, in each of the experimental studies (Table 1), significant effects were seen against established tumors. In cancer patients, impressive clinical responses were reported with combination therapies targeting specifically CD33 in leukemias, CD20 in B cell lymphomas, HER-2 in breast carcinomas, and epidermal growth factor receptor (EGF-R) in head and neck carcinomas (Table 2). The possible mechanisms underlying therapeutic effects of this combination therapy are discussed below.
Table 1.
Effect of combined mAb IT and CT on tumor growth and/or survival in mice
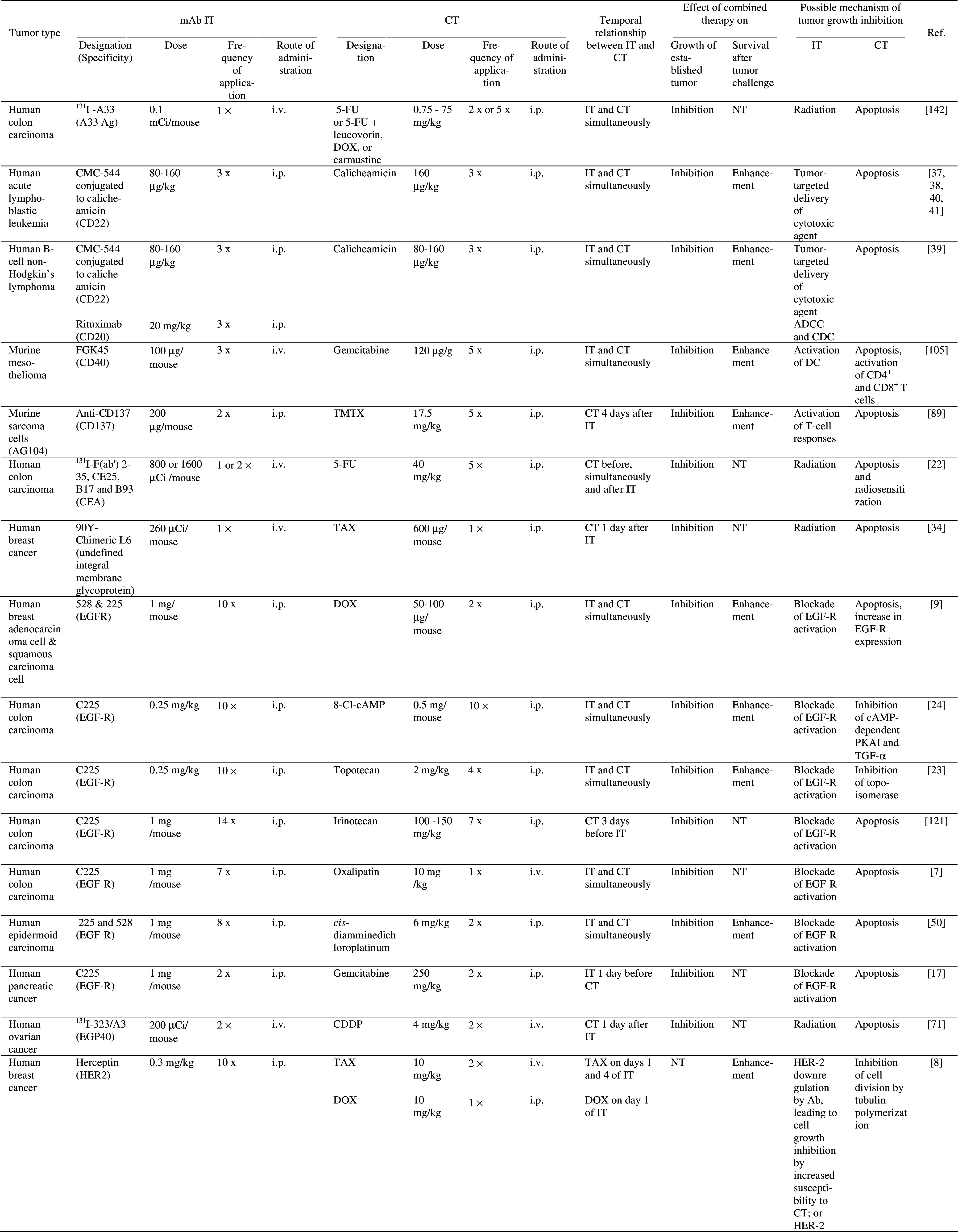

5-FU 5-fluorouracil, Ag antigen, CDDP cisplatin, CEA carcinoembryonic antigen, CT chemotherapy, CY cyclophosphamide, DC dendritic cells, DOX Doxorubicin, EGF-R epidermal growth factor receptor, EGP40 epithelial glycoprotein 40, i.p. intraperitoneally, IT immunotherapy, i.v. intravenously, mAb monoclonal antibody, MTX Methotrexate, NT not tested, PKA protein kinase, s.c. subcutaneously, TAX paclitaxel, TGF transforming growth factor, TMTX antifolate trimetrexate,VBL vinca alkaloid vinblastine, VP-16 Topoisomerase II inhibitor etoposide
Table 2.
Clinical trials of combined mAb IT and CT
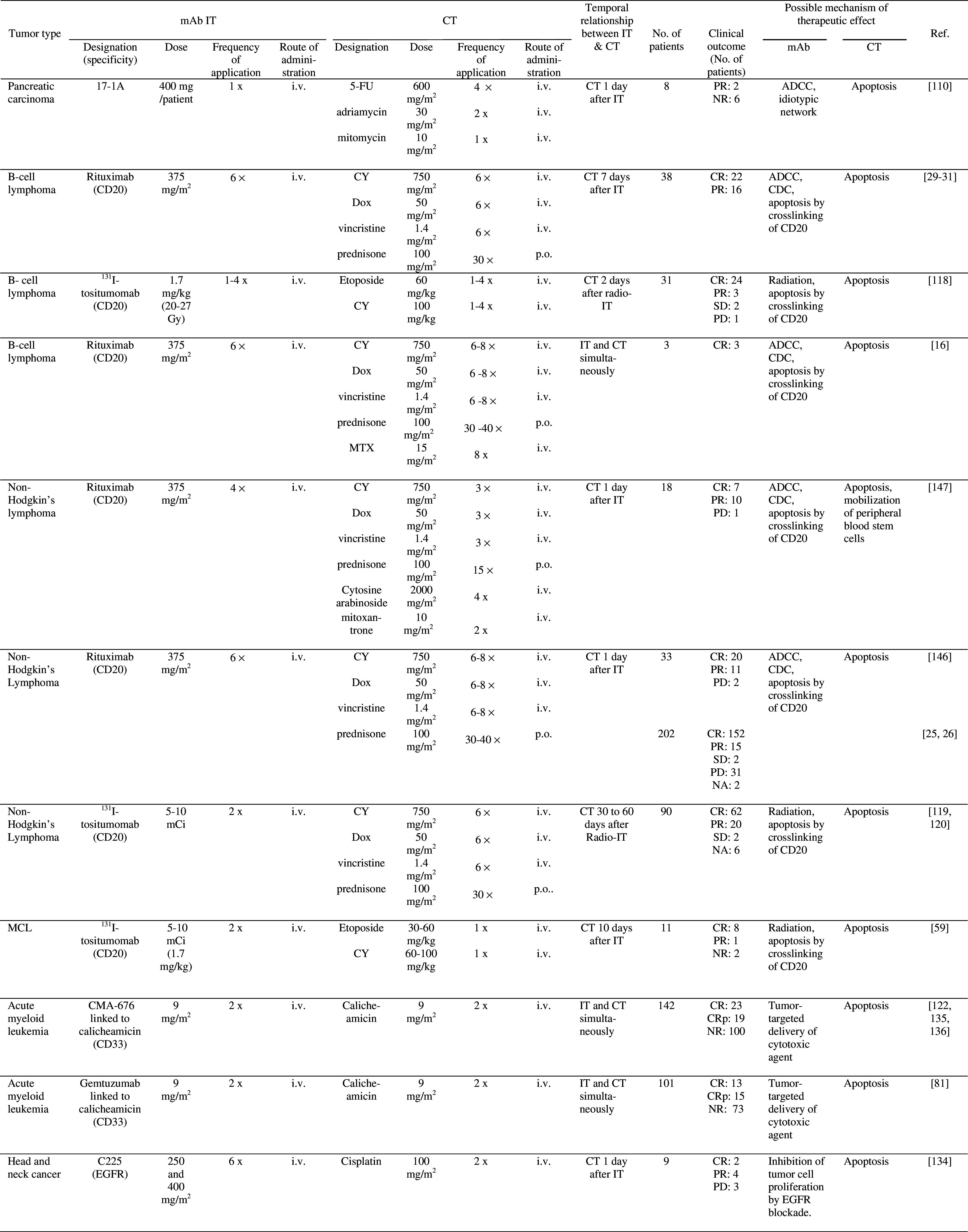
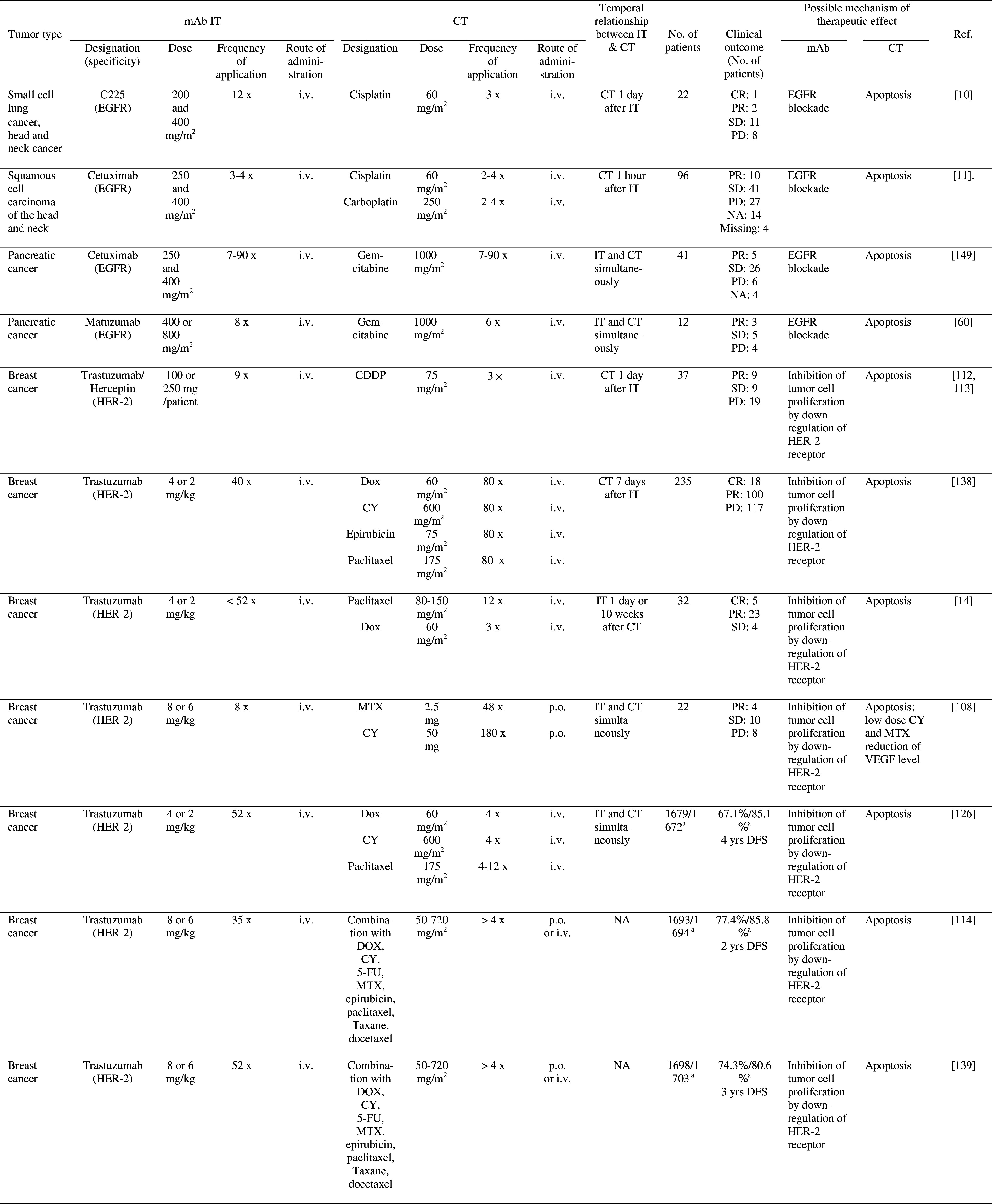
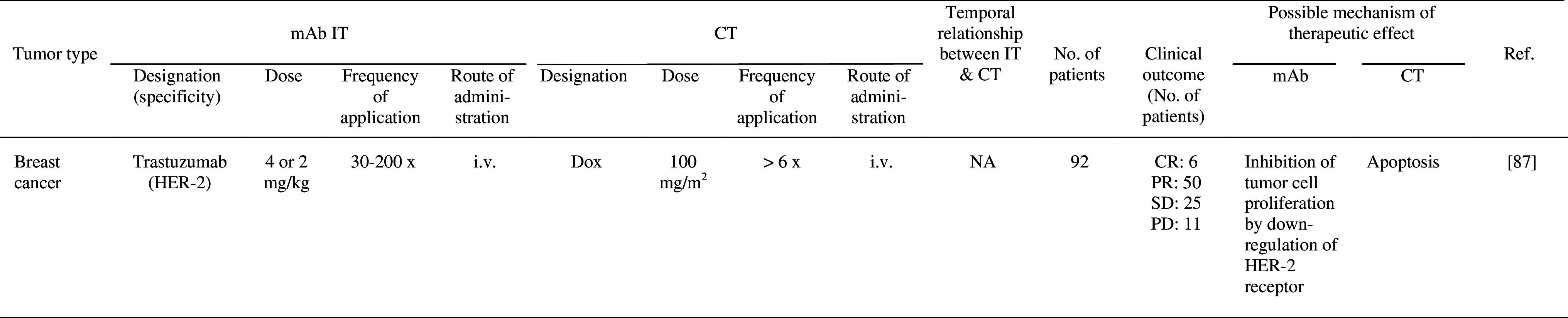
aCT/CT + IT
5-FU 5-fluorouracil, ADCC antibody-dependent cell-mediated cytotoxicity, CDC completment-dependent cytotoxicity, CDDP cisplatin, CR complete response, CRp remission with incomplete platelet recovery, CT chemotherapy, CY cyclophosphamide, DFS disease-free survival, Dox Doxorubicin, IT immunotherapy, i.v. intravenously, MCL Mantle cell lymphoma, MTX methotrexate, NA no assessment, NR no response, PD progressive disease, p.o. per os, PR partial response, SD stable disease
Pre-clinical and clinical studies of combined active specific IT and CT
The possible mechanisms underlying synergistic effects of active specific IT and CT are quite well understood, but selection of optimal dose of chemotherapy and timing of administration of the two therapies remain a challenge (see below). Various forms of vaccine delivery, such as irradiated tumor cells, tumor cell extract, tumor proteins or antigens expressed in naked plasmids or viral vectors have been used in combination with chemotherapeutics in several tumor models in mice (Table 3). In some of these studies, combination therapy was able to inhibit growth of established tumors [2, 19, 28, 46, 49, 61, 65, 67, 69, 70, 72, 76, 109, 130, 140, 141, 153, 154]. In clinical trials in which combined vaccine/chemotherapy was compared with either therapy or IT alone, promising clinical responses have been reported. Thus, the number of glioblastoma patients demonstrating 2 year disease-free survival was increased after treatment with dendritic cells (DC) loaded with tumor peptides or lysates, followed by chemotherapy with Temozolomide and BCNU as compared to treatment with either therapy alone [148] (Table 4). Clinical response rates of prostate cancer patients were increased following immunization with tumor peptides in combination with chemotherapy (Estramustine phosphate) as compared to IT alone [103] (Table 4). In another trial in prostate cancer patients, median time to tumor progression was increased after combination therapy (recombinant vaccinia virus expressing prostate specific antigen, followed by doxorubicin), compared to IT alone [6] (Table 4).
Table 3.
Effect of combined active specific IT and CT on tumor growth and/or survival in experimental animals
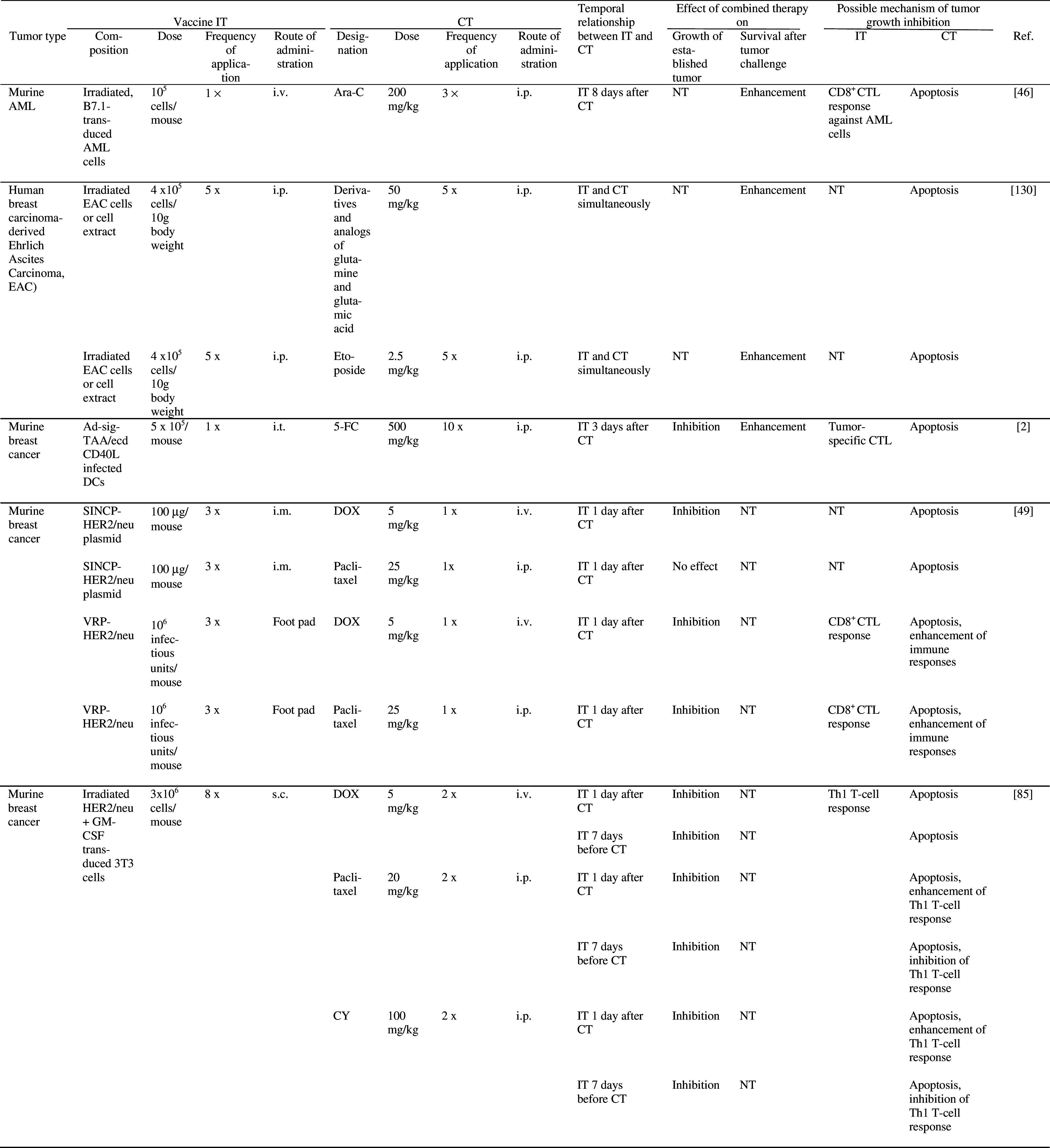
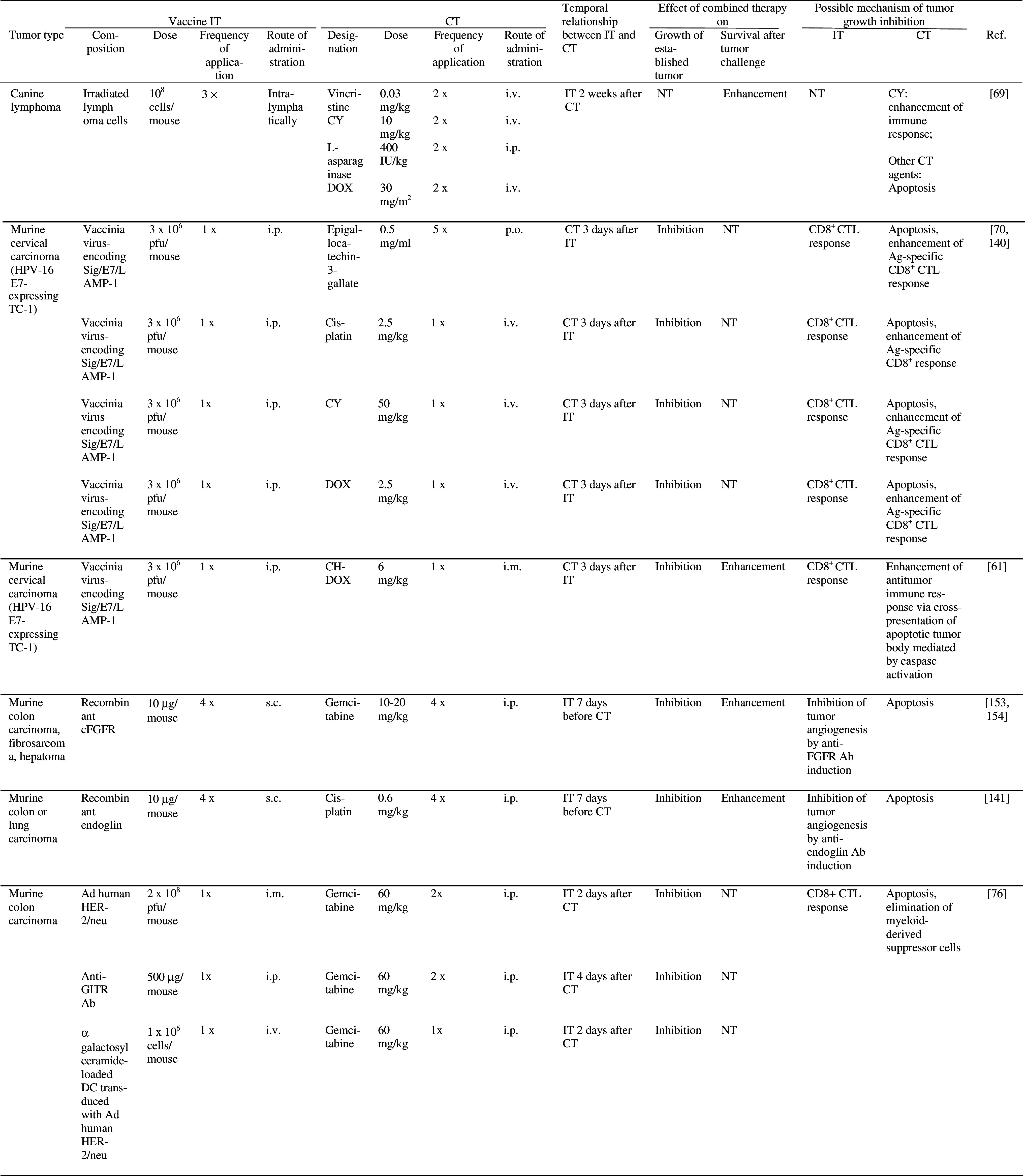
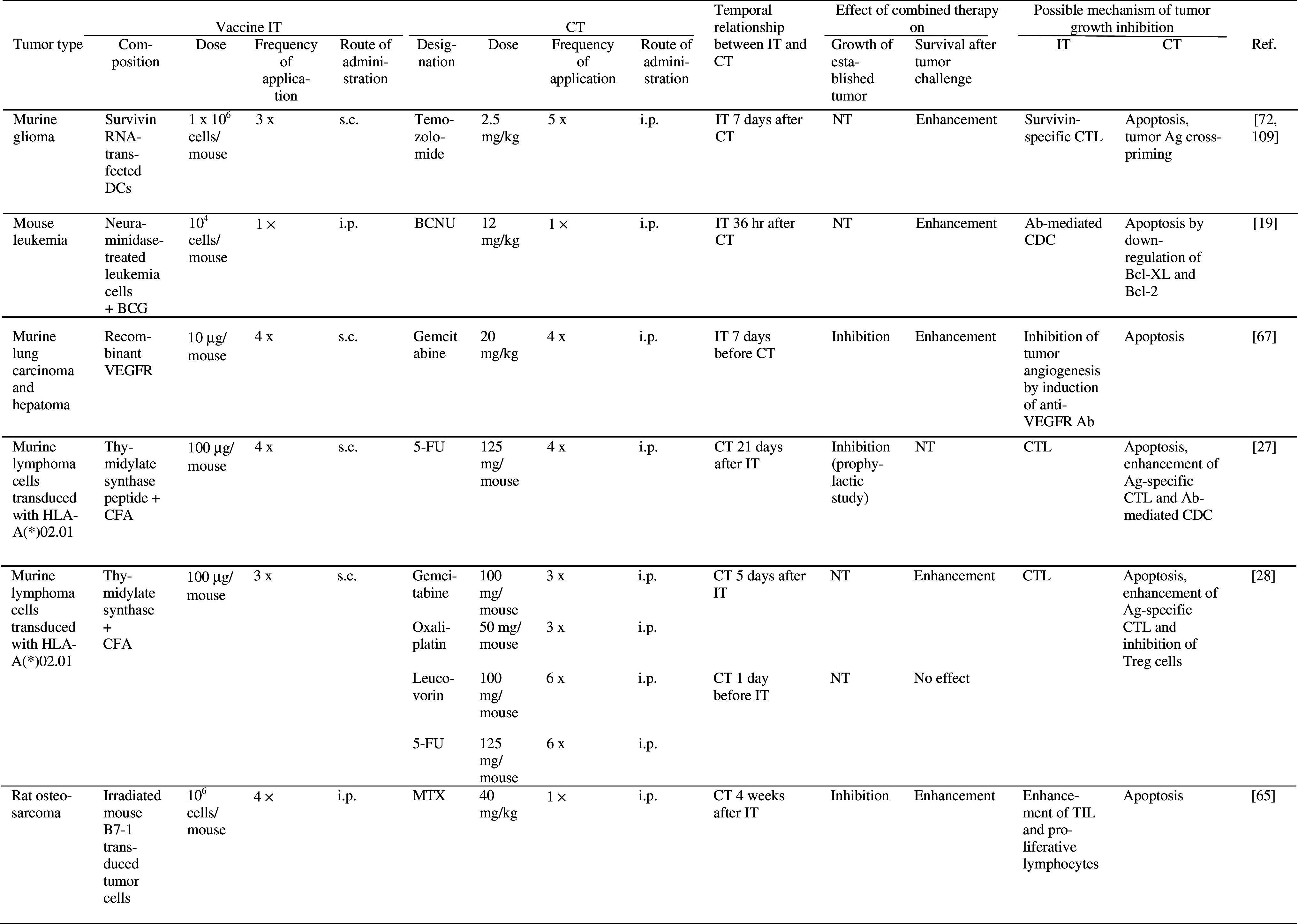
5-FC 5-fluorocytosine, 5-FU 5-fluorouracil, Ab antibody, Ad adenovirus, Ag antigen, AML acute myelogenous leukemia, BCG Bacillus Calmette Guerin, BCNU 1, 3-bis-(2-chloroethyl)-1-nitrosourea, CDC complement-dependent cytotoxicity, CFA complete Freund’s adjuvant, cFGFR chicken fibroblast growth factor receptor, CH-DOX chitosan hydrogel containing doxorubicin, CT chemotherapy, CTL cytotoxic T lymphocyte, CY cyclophosphamide, DC dendritic cells, DOX Doxorubicin, GITR glucocorticoid-induced TNFR family-related receptor, HPV human papilloma virus, i.m. intramuscularly, IT immunotherapy, LAMP lysosome-associated membrane protein, MTX methotrexate, NT not tested, pfu plaque forming units, s.c. subcutaneously; SINCP Sindbis virus, TIL tumor infiltrating lymphocytes, VEGFR vascular endothelial growth factor receptor, VRP Venezuelan equine encephalitis virus replicon particles
Table 4.
Clinical trials of combined active specific IT and CT
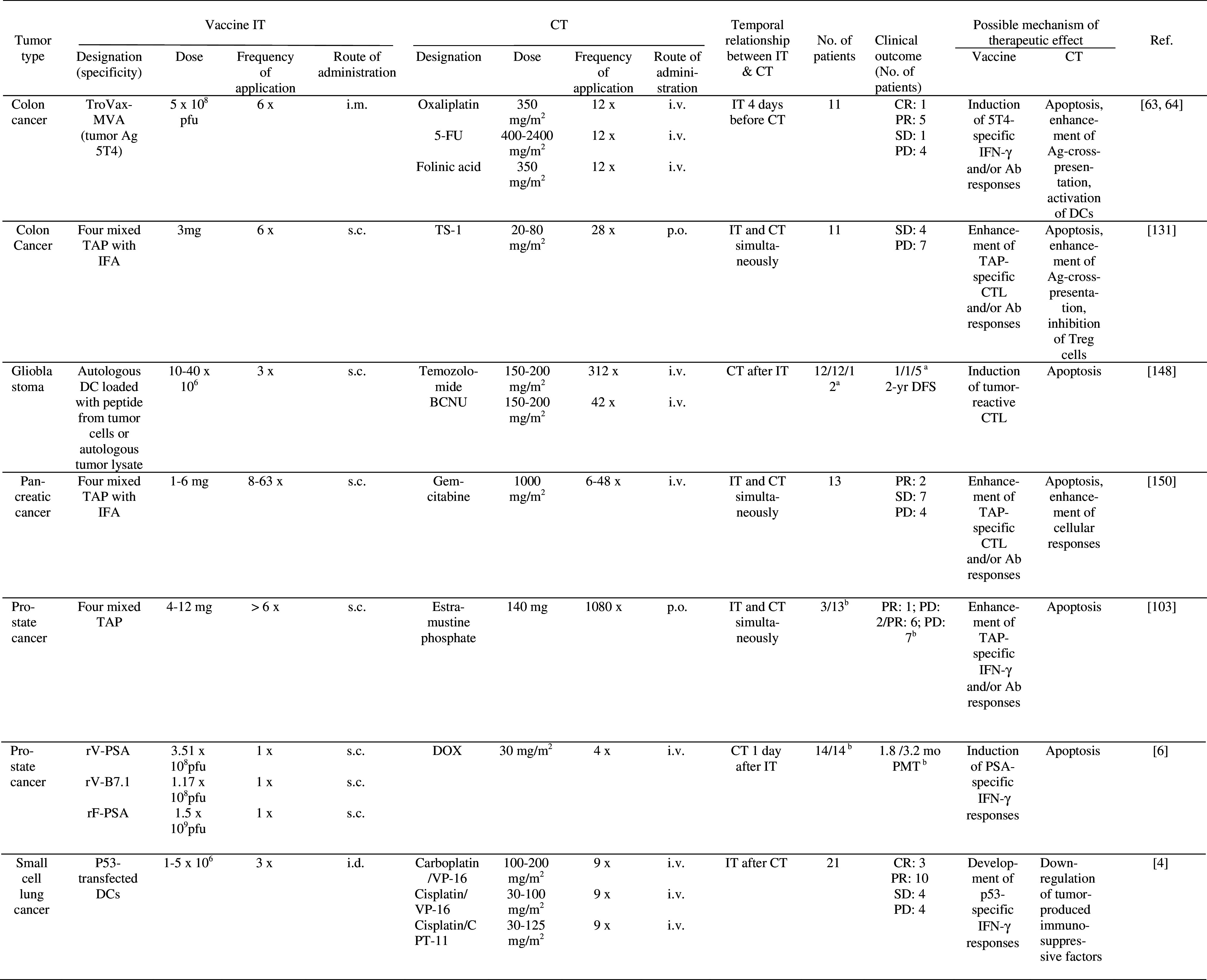
aCT/IT/CT + IT
bIT/IT + CT
Ab antibody, AML acute myelogenous leukemia, BCG Bacillus Calmette Guerin, BCNU 1, 3-bis-(2-chloroethyl)-1-nitrosourea, CPT-11 irinotecan, CR complete response, CT chemotherapy, CY cyclophosphamide, DC dendritic cells, DFS disease free survival, i.d. intradermally, IFA incomplete Freund’s adjuvant, IT immunotherapy, MR mixed response, MTX Methotrexate, MVA modified vaccinia Ankara, NR no response, NT not tested, OR objective (>50%) regression, PD progressive disease, p.o. per os, PMT progression median time, PR partial response, PSA prostate-specific antigen, rF recombinant fowlpox virus, rV recombinant vaccinia virus, s.c. subcutaneously, SD stable disease, TAP tumor associated peptides, TAX paclitaxel, TS-1 5-FU derivative, VP-16 etoposide
Pre-clinical and clinical studies of adoptive lymphocyte or active specific IT in combination with lymphodepletion by CT
The combination of adoptive lymphocyte IT with lymphodepletion by CT in patients with refractory metastatic (stage IV) melanoma has resulted in remarkable clinical response rates of approximately 50% [44] (Table 5), whereas clinical response rates with various CTs or adoptive lymphocyte transfer alone usually ranged between 10 and 34% in historical control patients [128, 129]. Various mechanisms may underly the synergistic effects of lymphodepletion on adoptive lymphocyte IT (see below). Lymphodepletion also has been combined with both active specific and adoptive lymphocyte IT in six metastatic melanoma patients. Thus, each patient received all three therapies [5] (Table 5). Only one of six patients showed a partial response to this combination therapy and it is unclear which form of therapy this response may be attributed to.
Table 5.
Pre-clinical and clinical studies of combined adoptive lymphocyte or active specific IT and CT
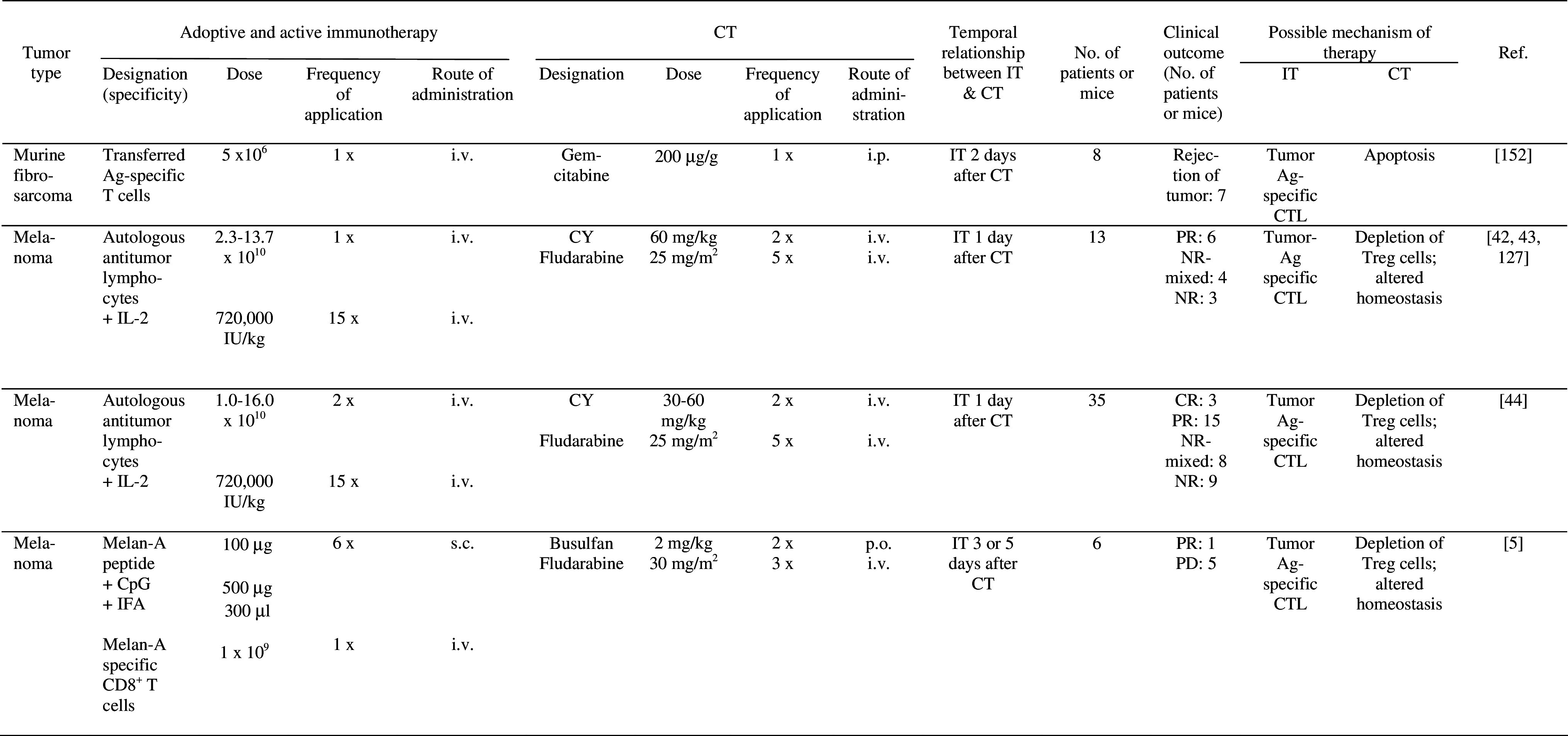
CR complete response, CT chemotherapy, CY cyclophosphamide, IFA incomplete Freund’s adjuvant; i.p. intraperitoneally, IT immunotherapy, i.v. intravenously, NR no response, NR-mixed mixed/no response, PD progressive disease, p.o.per os, PR partial response, s.c. subcutaneously
Treatment of well established tumors in mice with a chemotherapeutic drug, followed by adoptive lymphocyte IT resulted in tumor regression [152] (Table 5). Interestingly, synergism between the two therapies was dependent on the tumor microenvironment (see below).
Discussion and conclusions
The major possible direct effects of chemotherapy on tumor and/or host environment, which provide a rationale for combining CT with active and/or adoptive cellular IT, are:
Induction of tumor cell death
In the early studies by Bonmassar, it was shown that various types of immunogenic modification of tumor cells might occur in tumor-bearing hosts after treatment with drugs in vivo [15, 52, 68, 102, 125]. The molecular mechanism of drug-mediated immunogenic changes could be related to somatic mutations [51, 56]. Notably, chemotherapy of tumor-bearing mice and breast cancer patients was followed by induction of immune responses to the tumors [66, 97, 104, 109, 125]. Induction of necrosis and/or apoptosis in tumor cells in vitro has frequently been shown to increase their immunogenicity in vivo [3, 20, 54, 78, 90, 94, 107, 124]. Most likely, necrotic or apoptotic tumor cells induced by chemotherapy were phagocytosed by antigen-presenting cells (APC), presented to immune lymphocytes, followed by the stimulation of an anti-tumor responses in the lymphocytes [3, 55, 79, 90, 94]. Through induction of cell death by chemotherapeutics, a tumor could become its own cellular vaccine by crosspresentation of the apoptotic cells to APC, or induction of pro-inflammatory mediators such as heat shock proteins or interleukin (IL)-6, followed by crosspriming of immune effector cells [80, 145]. Although different chemotherapeutic agents may kill tumor cells through an apparently homogeneous apoptotic pathway, they may differ in the mechanism underlying the induction of immunogenic cell death. Thus, the chemotherapeutic agent anthracyclin induced an immune response to tumor cells only when apoptosis was preceded by translocation of calreticulin to the plasma membrane. Blockade or knock-down of calreticulin suppressed the phagocytosis of anthracyclin-treated tumor cells by dendritic cells and abolished their immunogenicity in mice [3, 90, 106, 107].
In principle, any therapy that delivers higher levels of cross-presented tumor antigens to the draining lymph nodes could synergize with immunotherapy. Thus, anti-tumor immunity induced by apoptotic tumor cells following chemotherapy can be boosted by active specific immunotherapy (see Tables 3, 4).
Elimination of regulatory T (Treg) cells
Cyclophosphamide (Cy) may down regulate the activity of Treg, especially when used in low doses [3, 82, 84, 90, 94, 99], whereas high doses may have direct tumor-cytotoxic effects [97–99]. Cy has been widely used in conjunction with active specific IT to enhance anti-tumor immune responses by down regulation of Treg, and this combination therapy has been pioneered by Berd et al. [12, 13] (Table 3).
Enhancement of tumor cell sensitivity to lysis by CTL
Active specific immunotherapy often induces low avidity CTL which do not effectively lyse tumors. However, when melanoma cells were treated with chemotherapeutic agents in vitro, they became highly sensitive to lysis by low avidity CTL. Cytotoxic drug-mediated sensitization primed both perforin/granzyme and Fas-mediated killing by the CTL [151]. In a related study, treatment of cancer cells with 5-aza-2′-deoxycytidine restored the expression of major histocompatibility complex (MHC) class I molecules and cancer testis antigens on tumor cells, rendering the tumor cells susceptible to CTL attack [133].
In a reverse manner, immunotherapy may directly modulate the tumor’s sensitivity to chemotherapy:
Monoclonal antibody Rituximab, used for passive immunotherapy of B cell lymphoma and non-Hodgkin’s lymphoma cancer patients, has reverted chemoresistance in B cell lymphoma cell lines to chemosensitivity [33]. Chemosensitization of tumor cells was due to downregulation of TNF-alpha secretion, but not to downmodulation of either the MDR-1 or bcl-2 gene products. Also, Her2-neu downregulation by mAb Herceptin increased tumor cell sensitivity to cisplatin by decreasing DNA repair activity following cisplatin-induced DNA damage [62, 115].
In several clinical trials, IT was followed by salvage CT [4, 6, 103, 148] (Table 4). Patients treated with this combination therapy showed higher clinical response rates as compared to historical controls treated with CT alone, although larger randomized and carefully controlled trials must be conducted to convincingly demonstrate beneficial effects of combination therapies. It is not known whether in the trials mentioned above IT “conditioned” the tumor to destruction by CT as shown for combinations of mAb and CT [33, 62, 115]. Gabrilovich [53] suggests that the anti-tumor effects of IT followed by CT are exerted independently by the two therapies and synergistic effects of this combination therapy may be dependent on optimal timing and scheduling of the two therapies. Specifically, CT may need to be started quickly after the administration of IT as anti-tumor immune responses generated by IT can not be sustained for a long period of time in cancer patients [53]. On the other hand, studies in tumor-bearing experimental animals have shown that delaying CT after IT increases the anti-tumor efficacy of this combined treatment, evidently through inhibition of vaccine-induced regulatory T cells by the chemotherapeutic drug [28] (Table 3).
The therapeutic induction of lymphopenia has raised considerable interest in the context of adoptive lymphocyte transfer therapies and vaccination of melanoma patients [100]. Transient lymphopenia is thought to enhance the efficiency of these therapies by activating homeostatic mechanisms that stimulate the tumor-reactive effector T cells and by counteracting tumor-induced suppression by mechanisms such as regulatory T cells or other mechanisms. Lymphodepletion also enhances T cell homing into tumor beds and intra-tumoral proliferation of effector cells [42, 44] (Table 5).
In an animal model, synergism between CT and adoptive lymphocyte IT was dependent on the involvement of the tumor microenvironment [152] (Table 5). Thus, treating well established tumors expressing low levels of antigen with a chemotherapeutic drug caused sufficient release of antigen to sensitize stromal cells for destruction by adoptively transferred cytotoxic T cells (CTL), resulting in tumor growth inhibition.
In summary, the demonstration of statistically significant survival enhancement in cancer patients treated in randomized phase III trials with mAb and CT vs patients treated with either therapy alone, raises great expectations for combination therapies consisting of active specific IT or adoptive lymphocyte IT with CT, as suggested by studies in experimental animals.
Acknowledgments
We thank Marion Sacks for editorial assistance. This work was supported by grants CA25874, CA93372, CA89480 and CA10815 from the National Institutes of Health.
Abbreviations
- 5-FC
5-Fluorocytosine
- 5-FU
5-Fluorouracil
- Ab
Antibody
- Ad
Adenovirus
- Ag
Antigen
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- AML
Acute myelogenous leukemia
- APC
Antigen-presenting cells
- BCG
Bacillus Calmette Guerin
- BCNU
1, 3-Bis-(2-chloroethyl)-1-nitrosourea
- CDC
Complement-dependent cytotoxicity
- CDDP
Cisplatin
- CEA
Carcinoembryonic antigen
- CFA
Complete Freund’s adjuvant
- cFGFR
Chicken fibroblast growth factor receptor
- CH-DOX
Chitosan hydrogel containing doxorubicin
- CPT
11, irinotecan
- CR
Complete response
- CRp
Remission with incomplete platelet recovery
- CT
Chemotherapy
- CTL
Cytotoxic T lymphocytes
- CY
Cyclophosphamide
- DC
Dendritic cells
- DFS
Disease-free survival
- DOX
Doxorubicin
- EBV
Epstein Barr virus
- EGF-R
Epidermal growth factor receptor
- EGP40
Epithelial glycoprotein 40
- GITR
Glucocorticoid-induced TNFR family-related receptor
- HPV
Human papilloma virus
- i.d.
Intradermally
- IFA
Incomplete Freund’s adjuvant
- IL
Interleukin
- i.m.
Intramuscularly
- i.p.
Intraperitoneally
- IT
Immunotherapy
- i.v.
Intravenously
- LAMP
Lysosome-associated membrane protein
- mAb
Monoclonal antibody
- MCL
Mantle cell lymphoma
- MHC
Major histocompatibility complex
- MR
Mixed response
- MTX
Methotrexate
- MVA
Modified vaccinia Ankara
- NA
No assessment
- NR
No response
- NT
Not tested
- OR
Objective (>50%) regression
- PD
Progressive disease
- pfu
Plaque forming units
- PKA
Protein kinase
- PMT
Progression median time
- p.o.
Per os
- PR
Partial response
- PSA
Prostate-specific antigen
- rF
Recombinant fowlpox virus
- rV
Recombinant vaccinia virus
- s.c.
Subcutaneously
- SD
Stable disease
- SINCP
Sindbis virus
- TAP
Tumor associated peptides
- TAX
Paclitaxel
- TGF
Transforming growth factor
- TIL
Tumor infiltrating lymphocytes
- TMTX
Antifolate trimetrexate
- Treg
Regulatory T cells
- TS-1
5-FU derivative
- VBL
Vinca alkaloid vinblastine
- VEGFR
Vascular endothelial growth factor receptor
- VP-16
Topoisomerase II inhibitor etoposide
- VRP
Venezuelan equine encephalitis virus replicon particles
References
- 1.Agus DB, Scher HI, Higgins B, Fox WD, Heller G, Fazzari M, Cordon-Cardo C, Golde DW. Response of prostate cancer to anti-Her-2/neu antibody in androgen-dependent and -independent human xenograft models. Cancer Res. 1999;59:4761–4764. [PubMed] [Google Scholar]
- 2.Akbulut H, Tang Y, Akbulut KG, Maynard J, Zhang L, Deisseroth A. Antitumor immune response induced by i.t. injection of vector-activated dendritic cells and chemotherapy suppresses metastatic breast cancer. Mol Cancer Ther. 2006;5:1975–1985. doi: 10.1158/1535-7163.MCT-06-0049. [DOI] [PubMed] [Google Scholar]
- 3.Andersen MH, Sorensen RB, Schrama D, Svane IM, Becker JC, Thor Straten P. Cancer treatment: the combination of vaccination with other therapies. Cancer Immunol Immunother. 2008;57:1735–1743. doi: 10.1007/s00262-008-0480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, Bepler G, Simon G, Janssen W, Lee JH, Menander K, Chada S, Gabrilovich DI. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 5.Appay V, Voelter V, Rufer N, Reynard S, Jandus C, Gasparini D, Lienard D, Speiser DE, Schneider P, Cerottini JC, Romero P, Leyvraz S. Combination of transient lymphodepletion with busulfan and fludarabine and peptide vaccination in a phase I clinical trial for patients with advanced melanoma. J Immunother. 2007;30:240–250. doi: 10.1097/01.cji.0000211332.68643.98. [DOI] [PubMed] [Google Scholar]
- 6.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosenbach DW, Feldman J, Steinberg SM, Jones E, Chen C, Marte J, Schlom J, Dahut W. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balin-Gauthier D, Delord JP, Rochaix P, Mallard V, Thomas F, Hennebelle I, Bugat R, Canal P, Allal C. In vivo and in vitro antitumor activity of oxaliplatin in combination with cetuximab in human colorectal tumor cell lines expressing different level of EGFR. Cancer Chemother Pharmacol. 2006;57:709–718. doi: 10.1007/s00280-005-0123-3. [DOI] [PubMed] [Google Scholar]
- 8.Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58:2825–2831. [PubMed] [Google Scholar]
- 9.Baselga J, Norton L, Masui H, Pandiella A, Coplan K, Miller WH, Jr, Mendelsohn J. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst. 1993;85:1327–1333. doi: 10.1093/jnci/85.16.1327. [DOI] [PubMed] [Google Scholar]
- 10.Baselga J, Pfister D, Cooper MR, Cohen R, Burtness B, Bos M, D’Andrea G, Seidman A, Norton L, Gunnett K, Falcey J, Anderson V, Waksal H, Mendelsohn J. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol. 2000;18:904–914. doi: 10.1200/JCO.2000.18.4.904. [DOI] [PubMed] [Google Scholar]
- 11.Baselga J, Trigo JM, Bourhis J, Tortochaux J, Cortes-Funes H, Hitt R, Gascon P, Amellal N, Harstrick A, Eckardt A. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23:5568–5577. doi: 10.1200/JCO.2005.07.119. [DOI] [PubMed] [Google Scholar]
- 12.Berd D. Low doses of chemotherapy to inhibit suppressor T cells. Prog Clin Biol Res. 1989;288:449–458. [PubMed] [Google Scholar]
- 13.Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: depletion of CD4+, 2H4+ suppressor-inducer T-cells. Cancer Res. 1988;48:1671–1675. [PubMed] [Google Scholar]
- 14.Bianchi G, Albanell J, Eiermann W, Vitali G, Borquez D, Vigano L, Molina R, Raab G, Locatelli A, Vanhauwere B, Gianni L, Baselga J. Pilot trial of trastuzumab starting with or after the doxorubicin component of a doxorubicin plus paclitaxel regimen for women with HER2-positive advanced breast cancer. Clin Cancer Res. 2003;9:5944–5951. [PubMed] [Google Scholar]
- 15.Bonmassar E, Testorelli C, Franco P, Goldin A, Cudkowicz G. Changes of the immunogenic properties of a radiation-induced mouse lymphoma following treatment with antitumor drugs. Cancer Res. 1975;35:1957–1962. [PubMed] [Google Scholar]
- 16.Bouzani M, Karmiris T, Rontogianni D, Delimpassi S, Apostolidis J, Mpakiri M, Nikiforakis E. Disseminated intravascular B-cell lymphoma: clinicopathological features and outcome of three cases treated with anthracycline-based immunochemotherapy. Oncologist. 2006;11:923–928. doi: 10.1634/theoncologist.11-8-923. [DOI] [PubMed] [Google Scholar]
- 17.Bruns CJ, Harbison MT, Davis DW, Portera CA, Tsan R, McConkey DJ, Evans DB, Abbruzzese JL, Hicklin DJ, Radinsky R. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin Cancer Res. 2000;6:1936–1948. [PubMed] [Google Scholar]
- 18.Bruserud O, Wendelboe O. Biological treatment in acute myelogenous leukaemia: how should T-cell targeting immunotherapy be combined with intensive chemotherapy? Expert Opin Biol Ther. 2001;1:1005–1016. doi: 10.1517/14712598.1.6.1005. [DOI] [PubMed] [Google Scholar]
- 19.Cantrell JL, Killion JJ, Kollmorgen GM. Correlations between humoral immunity and successful chemotherapy–immunotherapy. Cancer Res. 1976;36:3051–3057. [PubMed] [Google Scholar]
- 20.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Metivier D, Pichard E, Aucouturier P, Pierron G, Garrido C, Zitvogel L, Kroemer G. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalandon Y, Mach JP, Pelegrin A, Folli S, Buchegger F. Combined radioimmunotherapy and chemotherapy of human colon carcinoma grafted in nude mice, advantages and limitations. Anticancer Res. 1992;12:1131–1139. [PubMed] [Google Scholar]
- 23.Ciardiello F, Bianco R, Damiano V, De Lorenzo S, Pepe S, De Placido S, Fan Z, Mendelsohn J, Bianco AR, Tortora G. Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225. Clin Cancer Res. 1999;5:909–916. [PubMed] [Google Scholar]
- 24.Ciardiello F, Damiano V, Bianco R, Bianco C, Fontanini G, De Laurentiis M, De Placido S, Mendelsohn J, Bianco AR, Tortora G. Antitumor activity of combined blockade of epidermal growth factor receptor and protein kinase A. J Natl Cancer Inst. 1996;88:1770–1776. doi: 10.1093/jnci/88.23.1770. [DOI] [PubMed] [Google Scholar]
- 25.Coiffier B. Rituximab in combination with CHOP improves survival in elderly patients with aggressive non-Hodgkin’s lymphoma. Semin Oncol. 2002;29:18–22. doi: 10.1053/sonc.2002.32749. [DOI] [PubMed] [Google Scholar]
- 26.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 27.Correale P, Del Vecchio MT, Di Genova G, Savellini GG, La Placa M, Terrosi C, Vestri M, Urso R, Lemonnier F, Aquino A, Bonmassar E, Giorgi G, Francini G, Cusi MG. 5-fluorouracil-based chemotherapy enhances the antitumor activity of a thymidylate synthase-directed polyepitopic peptide vaccine. J Natl Cancer Inst. 2005;97:1437–1445. doi: 10.1093/jnci/dji188. [DOI] [PubMed] [Google Scholar]
- 28.Correale P, Del Vecchio MT, La Placa M, Montagnani F, Di Genova G, Savellini GG, Terrosi C, Mannucci S, Giorgi G, Francini G, Cusi MG. Chemotherapeutic drugs may be used to enhance the killing efficacy of human tumor antigen peptide-specific CTLs. J Immunother. 2008;31:132–147. doi: 10.1097/CJI.0b013e31815b69c8. [DOI] [PubMed] [Google Scholar]
- 29.Czuczman MS. CHOP plus rituximab chemoimmunotherapy of indolent B-cell lymphoma. Semin Oncol. 1999;26:88–96. [PubMed] [Google Scholar]
- 30.Czuczman MS. Immunochemotherapy in indolent non-Hodgkin’s lymphoma. Semin Oncol. 2002;29:11–17. doi: 10.1053/sonc.2002.32748. [DOI] [PubMed] [Google Scholar]
- 31.Czuczman MS, Grillo-Lopez AJ, White CA, Saleh M, Gordon L, LoBuglio AF, Jonas C, Klippenstein D, Dallaire B, Varns C. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol. 1999;17:268–276. doi: 10.1200/JCO.1999.17.1.268. [DOI] [PubMed] [Google Scholar]
- 32.Del Poeta G, Ilaria Del Principe M, Buccisano F, Maurillo L, Niscola P, Venditti A, Amadori S. Role of immunochemotherapy in the treatment of chronic lymphocytic leukemia. Expert Rev Anticancer Ther. 2006;6:1787–1800. doi: 10.1586/14737140.6.12.1787. [DOI] [PubMed] [Google Scholar]
- 33.Demidem A, Lam T, Alas S, Hariharan K, Hanna N, Bonavida B. Chimeric anti-CD20 (IDEC-C2B8) monoclonal antibody sensitizes a B cell lymphoma cell line to cell killing by cytotoxic drugs. Cancer Biother Radiopharm. 1997;12:177–186. doi: 10.1089/cbr.1997.12.177. [DOI] [PubMed] [Google Scholar]
- 34.DeNardo SJ, Kukis DL, Kroger LA, O’Donnell RT, Lamborn KR, Miers LA, DeNardo DG, Meares CF, DeNardo GL. Synergy of Taxol and radioimmunotherapy with yttrium-90-labeled chimeric L6 antibody: efficacy and toxicity in breast cancer xenografts. Proc Natl Acad Sci USA. 1997;94:4000–4004. doi: 10.1073/pnas.94.8.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desrues B, Brichory F, Lena H, Bourguet P, Delaval P, Toujas L, Dazord L. Treatment of human lung carcinoma xenografts with a combination of 131I-labelled monoclonal antibody Po66 and doxorubicin. Cancer Immunol Immunother. 1996;43:269–274. doi: 10.1007/s002620050333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desrues B, Lena H, Brichory F, Ramee MP, Toujas L, Delaval P, Dazord L. Monoclonal antibody Po66 uptake by human lung tumours implanted in nude mice: effect of co-administration with doxorubicin. Br J Cancer. 1995;72:1076–1082. doi: 10.1038/bjc.1995.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiJoseph JF, Armellino DC, Boghaert ER, Khandke K, Dougher MM, Sridharan L, Kunz A, Hamann PR, Gorovits B, Udata C, Moran JK, Popplewell AG, Stephens S, Frost P, Damle NK. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood. 2004;103:1807–1814. doi: 10.1182/blood-2003-07-2466. [DOI] [PubMed] [Google Scholar]
- 38.Dijoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia. 2007;21:2240–2245. doi: 10.1038/sj.leu.2404866. [DOI] [PubMed] [Google Scholar]
- 39.DiJoseph JF, Dougher MM, Kalyandrug LB, Armellino DC, Boghaert ER, Hamann PR, Moran JK, Damle NK. Antitumor efficacy of a combination of CMC-544 (inotuzumab ozogamicin), a CD22-targeted cytotoxic immunoconjugate of calicheamicin, and rituximab against non-Hodgkin’s B-cell lymphoma. Clin Cancer Res. 2006;12:242–249. doi: 10.1158/1078-0432.CCR-05-1905. [DOI] [PubMed] [Google Scholar]
- 40.DiJoseph JF, Goad ME, Dougher MM, Boghaert ER, Kunz A, Hamann PR, Damle NK. Potent and specific antitumor efficacy of CMC-544, a CD22-targeted immunoconjugate of calicheamicin, against systemically disseminated B-cell lymphoma. Clin Cancer Res. 2004;10:8620–8629. doi: 10.1158/1078-0432.CCR-04-1134. [DOI] [PubMed] [Google Scholar]
- 41.DiJoseph JF, Popplewell A, Tickle S, Ladyman H, Lawson A, Kunz A, Khandke K, Armellino DC, Boghaert ER, Hamann P, Zinkewich-Peotti K, Stephens S, Weir N, Damle NK. Antibody-targeted chemotherapy of B-cell lymphoma using calicheamicin conjugated to murine or humanized antibody against CD22. Cancer Immunol Immunother. 2005;54:11–24. doi: 10.1007/s00262-004-0572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry RM, Marincola FM, Leitman SF, Seipp CA, Rogers-Freezer L, Morton KE, Nahvi A, Mavroukakis SA, White DE, Rosenberg SA. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunussi-Joannopoulos K. The combination of chemotherapy and systemic immunotherapy and the concept of cure in murine leukemia and lymphoma. Leuk Lymphoma. 2002;43:2075–2082. doi: 10.1080/1042819021000032926. [DOI] [PubMed] [Google Scholar]
- 46.Dunussi-Joannopoulos K, Krenger W, Weinstein HJ, Ferrara JL, Croop JM. CD8+ T cells activated during the course of murine acute myelogenous leukemia elicit therapeutic responses to late B7 vaccines after cytoreductive treatment. Blood. 1997;89:2915–2924. [PubMed] [Google Scholar]
- 47.Ehrke MJ. Immunomodulation in cancer therapeutics. Int Immunopharmacol. 2003;3:1105–1119. doi: 10.1016/S1567-5769(03)00021-3. [DOI] [PubMed] [Google Scholar]
- 48.Emens LA, Reilly RT, Jaffee EM. Breast cancer vaccines: maximizing cancer treatment by tapping into host immunity. Endocr Relat Cancer. 2005;12:1–17. doi: 10.1677/erc.1.00671. [DOI] [PubMed] [Google Scholar]
- 49.Eralp Y, Wang X, Wang JP, Maughan MF, Polo JM, Lachman LB. Doxorubicin and paclitaxel enhance the antitumor efficacy of vaccines directed against HER 2/neu in a murine mammary carcinoma model. Breast Cancer Res. 2004;6:R275–R283. doi: 10.1186/bcr787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan Z, Baselga J, Masui H, Mendelsohn J. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res. 1993;53:4637–4642. [PubMed] [Google Scholar]
- 51.Fioretti MC, Bianchi R, Romani L, Bonmassar E. Drug-induced immunogenic changes of murine leukemia cells: dissociation of onset of resistance and emergence of novel immunogenicity. J Natl Cancer Inst. 1983;71:1247–1251. [PubMed] [Google Scholar]
- 52.Fioretti MC, Romani L, Bonmassar E. Antigenic changes related to drug action. Prog Clin Biol Res. 1983;132B:435–445. [PubMed] [Google Scholar]
- 53.Gabrilovich DI. Combination of chemotherapy and immunotherapy for cancer: a paradigm revisited. Lancet Oncol. 2007;8:2–3. doi: 10.1016/S1470-2045(06)70985-8. [DOI] [PubMed] [Google Scholar]
- 54.Gamrekelashvili J, Kruger C, von Wasielewski R, Hoffmann M, Huster KM, Busch DH, Manns MP, Korangy F, Greten TF. Necrotic tumor cell death in vivo impairs tumor-specific immune responses. J Immunol. 2007;178:1573–1580. doi: 10.4049/jimmunol.178.3.1573. [DOI] [PubMed] [Google Scholar]
- 55.Ghiringhelli F, Apetoh L, Housseau F, Kroemer G, Zitvogel L. Links between innate and cognate tumor immunity. Curr Opin Immunol. 2007;19:224–231. doi: 10.1016/j.coi.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Giampietri A, Fioretti MC, Goldin A, Bonmassar E. Drug-mediated antigenic changes in murine leukemia cells: antagonistic effects of quinacrine, an antimutagenic compound. J Natl Cancer Inst. 1980;64:297–301. doi: 10.1093/jnci/64.2.297. [DOI] [PubMed] [Google Scholar]
- 57.Gold DV, Schutsky K, Modrak D, Cardillo TM. Low-dose radioimmunotherapy ((90)Y-PAM4) combined with gemcitabine for the treatment of experimental pancreatic cancer. Clin Cancer Res. 2003;9:3929S–3937S. [PubMed] [Google Scholar]
- 58.Gomez GG, Hutchison RB, Kruse CA. Chemo-immunotherapy and chemo-adoptive immunotherapy of cancer. Cancer Treat Rev. 2001;27:375–402. doi: 10.1053/ctrv.2001.0222. [DOI] [PubMed] [Google Scholar]
- 59.Gopal AK, Rajendran JG, Petersdorf SH, Maloney DG, Eary JF, Wood BL, Gooley TA, Bush SA, Durack LD, Martin PJ, Matthews DC, Appelbaum FR, Bernstein ID, Press OW. High-dose chemo-radioimmunotherapy with autologous stem cell support for relapsed mantle cell lymphoma. Blood. 2002;99:3158–3162. doi: 10.1182/blood.v99.9.3158. [DOI] [PubMed] [Google Scholar]
- 60.Graeven U, Kremer B, Sudhoff T, Killing B, Rojo F, Weber D, Tillner J, Unal C, Schmiegel W. Phase I study of the humanised anti-EGFR monoclonal antibody matuzumab (EMD 72000) combined with gemcitabine in advanced pancreatic cancer. Br J Cancer. 2006;94:1293–1299. doi: 10.1038/sj.bjc.6603083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han HD, Song CK, Park YS, Noh KH, Kim JH, Hwang T, Kim TW, Shin BC. A chitosan hydrogel-based cancer drug delivery system exhibits synergistic antitumor effects by combining with a vaccinia viral vaccine. Int J Pharm. 2008;350:27–34. doi: 10.1016/j.ijpharm.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 62.Hancock MC, Langton BC, Chan T, Toy P, Monahan JJ, Mischak RP, Shawver LK. A monoclonal antibody against the c-erbB-2 protein enhances the cytotoxicity of cis-diamminedichloroplatinum against human breast and ovarian tumor cell lines. Cancer Res. 1991;51:4575–4580. [PubMed] [Google Scholar]
- 63.Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, Kingsman SM, Naylor S, Griffiths R, Steven N, Hawkins RE. Vaccination of colorectal cancer patients with TroVax given alongside chemotherapy (5-Xuorouracil, leukovorin and irinotecan) is safe and induces potent immune responses. Cancer Immunol Immunother. 2008;57:977–986. doi: 10.1007/s00262-007-0428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, Kingsman SM, Naylor S, Melcher A, Nicholls J, Wassan H, Habib N, Anthoney A. Vaccination of colorectal cancer patients with modified vaccinia ankara encoding the tumor antigen 5T4 (TroVax) given alongside chemotherapy induces potent immune responses. Clin Cancer Res. 2007;13:4487–4494. doi: 10.1158/1078-0432.CCR-07-0704. [DOI] [PubMed] [Google Scholar]
- 65.Hayakawa M, Kawaguchi S, Ishii S, Murakami M, Uede T. B7-1-transfected tumor vaccine counteracts chemotherapy-induced immunosuppression and prolongs the survival of rats bearing highly metastatic osteosarcoma cells. Int J Cancer. 1997;71:1091–1102. doi: 10.1002/(sici)1097-0215(19970611)71:6<1091::aid-ijc28>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 66.Head JF, Elliott RL, McCoy JL. Evaluation of lymphocyte immunity in breast cancer patients. Breast Cancer Res Treat. 1993;26:77–88. doi: 10.1007/BF00682702. [DOI] [PubMed] [Google Scholar]
- 67.Hou JM, Liu JY, Yang L, Zhao X, Tian L, Ding ZY, Wen YJ, Niu T, Xiao F, Lou YY, Tan GH, Deng HX, Li J, Yang JL, Mao YQ, Kan B, Wu Y, Li Q, Wei YQ. Combination of low-dose gemcitabine and recombinant quail vascular endothelial growth factor receptor-2 as a vaccine induces synergistic antitumor activities. Oncology. 2005;69:81–87. doi: 10.1159/000087303. [DOI] [PubMed] [Google Scholar]
- 68.Houchens DP, Bonmassar E, Gaston MR, Kende M, Goldin A. Drug-mediated immunogenic changes of virus-induced leukemia in vivo. Cancer Res. 1976;36:1347–1352. [PubMed] [Google Scholar]
- 69.Jeglum KA, Young KM, Barnsley K, Whereat A. Chemotherapy versus chemotherapy with intralymphatic tumor cell vaccine in canine lymphoma. Cancer. 1988;61:2042–2050. doi: 10.1002/1097-0142(19880515)61:10<2042::aid-cncr2820611019>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 70.Kang TH, Lee JH, Song CK, Han HD, Shin BC, Pai SI, Hung CF, Trimble C, Lim JS, Kim TW, Wu TC. Epigallocatechin-3-gallate enhances CD8 + T cell-mediated antitumor immunity induced by DNA vaccination. Cancer Res. 2007;67:802–811. doi: 10.1158/0008-5472.CAN-06-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kievit E, Pinedo HM, Schluper HM, Boven E. Addition of cisplatin improves efficacy of 131I-labeled monoclonal antibody 323/A3 in experimental human ovarian cancer. Int J Radiat Oncol Biol Phys. 1997;38:419–428. doi: 10.1016/s0360-3016(97)82501-1. [DOI] [PubMed] [Google Scholar]
- 72.Kim CH, Woo SJ, Park JS, Kim HS, Park MY, Park SD, Hong YK, Kim TG. Enhanced antitumour immunity by combined use of temozolomide and TAT-survivin pulsed dendritic cells in a murine glioma. Immunology. 2007;122:615–622. doi: 10.1111/j.1365-2567.2007.02680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kipp RT, McNeel DG. Immunotherapy for prostate cancer—recent progress in clinical trials. Clin Adv Hematol Oncol. 2007;5(465–74):77–79. [PubMed] [Google Scholar]
- 74.Ko AH. Future strategies for targeted therapies and tailored patient management in pancreatic cancer. Semin Oncol. 2007;34:354–364. doi: 10.1053/j.seminoncol.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Ko EC, Wang X, Ferrone S. Immunotherapy of malignant diseases. Challenges and strategies. Int Arch Allergy Immunol. 2003;132:294–309. doi: 10.1159/000074897. [DOI] [PubMed] [Google Scholar]
- 76.Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 77.Kofler DM, Mayr C, Wendtner CM. Current status of immunotherapy in B cell malignancies. Curr Drug Targets. 2006;7:1371–1374. doi: 10.2174/138945006778559120. [DOI] [PubMed] [Google Scholar]
- 78.Kotera Y, Shimizu K, Mule JJ. Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer Res. 2001;61:8105–8109. [PubMed] [Google Scholar]
- 79.Krysko DV, D’Herde K, Vandenabeele P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis. 2006;11:1709–1726. doi: 10.1007/s10495-006-9527-8. [DOI] [PubMed] [Google Scholar]
- 80.Lake RA, Robinson BW. Immunotherapy and chemotherapy—a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 81.Larson RA, Boogaerts M, Estey E, Karanes C, Stadtmauer EA, Sievers EL, Mineur P, Bennett JM, Berger MS, Eten CB, Munteanu M, Loken MR, Van Dongen JJ, Bernstein ID, Appelbaum FR. Antibody-targeted chemotherapy of older patients with acute myeloid leukemia in first relapse using Mylotarg (gemtuzumab ozogamicin) Leukemia. 2002;16:1627–1636. doi: 10.1038/sj.leu.2402677. [DOI] [PubMed] [Google Scholar]
- 82.Liu JY, Wu Y, Zhang XS, Yang JL, Li HL, Mao YQ, Wang Y, Cheng X, Li YQ, Xia JC, Masucci M, Zeng YX. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother. 2007;56:1597–1604. doi: 10.1007/s00262-007-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luftner D, Pollmann D, Schildhauer S, Sehouli J, Possinger K. Perspectives of immunotherapy in metastatic breast cancer. Anticancer Res. 2005;25:4599–4604. [PubMed] [Google Scholar]
- 84.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 85.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 86.Markasz L, Skribek H, Uhlin M, Otvos R, Flaberg E, Eksborg S, Olah E, Stuber G, Szekely L. Effect of frequently used chemotherapeutic drugs on cytotoxic activity of human cytotoxic T-lymphocytes. J Immunother. 2008;31:283–293. doi: 10.1097/CJI.0b013e3181628b76. [DOI] [PubMed] [Google Scholar]
- 87.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Anton A, Lluch A, Kennedy J, O’Byrne K, Conte P, Green M, Ward C, Mayne K, Extra JM. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 88.Masters GR, Berger MA, Albone EF. Synergistic effects of combined therapy using paclitaxel and [90Y-DOTA]776.1 on growth of OVCAR-3 ovarian carcinoma xenografts. Gynecol Oncol. 2006;102:462–467. doi: 10.1016/j.ygyno.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 89.McMillin DW, Hewes B, Gangadharan B, Archer DR, Mittler RS, Spencer HT. Complete regression of large solid tumors using engineered drug-resistant hematopoietic cells and anti-CD137 immunotherapy. Hum Gene Ther. 2006;17:798–806. doi: 10.1089/hum.2006.17.798. [DOI] [PubMed] [Google Scholar]
- 90.Menard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother. 2008;57:1579–1587. doi: 10.1007/s00262-008-0505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mihich E. Combined effects of chemotherapy and immunity against leukemia L1210 in DBA–2 mice. Cancer Res. 1969;29:848–854. [PubMed] [Google Scholar]
- 92.Mihich E. Modification of tumor regression by immunologic means. Cancer Res. 1969;29:2345–2350. [PubMed] [Google Scholar]
- 93.Mihich E. Preclinical evaluation of the interrelationships between cancer chemotherapy and immunity. Natl Cancer Inst Monogr. 1971;34:90–102. [PubMed] [Google Scholar]
- 94.Mihich E. Anticancer drug-induced immunomodulation and cancer therapeutics. Curr Cancer Ther Rev. 2007;3:174–193. [Google Scholar]
- 95.Mitchell MS. Combinations of anticancer drugs and immunotherapy. Cancer Immunol Immunother. 2003;52:686–692. doi: 10.1007/s00262-003-0427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mitchell MS. Immunotherapy as part of combinations for the treatment of cancer. Int Immunopharmacol. 2003;3:1051–1059. doi: 10.1016/S1567-5769(03)00019-5. [DOI] [PubMed] [Google Scholar]
- 97.Mokyr MB, Dray S. Some advantages of curing mice bearing a large subcutaneous MOPC-315 tumor with a low dose rather than a high dose of cyclophosphamide. Cancer Res. 1983;43:3112–3119. [PubMed] [Google Scholar]
- 98.Mokyr MB, Hengst JC, Dray S. Role of antitumor immunity in cyclophosphamide-induced rejection of subcutaneous nonpalpable MOPC-315 tumors. Cancer Res. 1982;42:974–979. [PubMed] [Google Scholar]
- 99.Motoyoshi Y, Kaminoda K, Saitoh O, Hamasaki K, Nakao K, Ishii N, Nagayama Y, Eguchi K. Different mechanisms for anti-tumor effects of low- and high-dose cyclophosphamide. Oncol Rep. 2006;16:141–146. [PubMed] [Google Scholar]
- 100.Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, Restifo NP. Increased intensity lymphodepletion and adoptive immunotherapy—how far can we go? Nat Clin Pract Oncol. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Neelapu SS, Lee ST, Qin H, Cha SC, Woo AF, Kwak LW. Therapeutic lymphoma vaccines: importance of T-cell immunity. Expert Rev Vaccines. 2006;5:381–394. doi: 10.1586/14760584.5.3.381. [DOI] [PubMed] [Google Scholar]
- 102.Nicolin A, Spreafico F, Bonmassar E, Goldin A. Antigenic changes of L5178Y lymphoma after treatment with 5-(3, 3-dimethyl-1-triazeno) imidazole-4-carboxamide in vivo. J Natl Cancer Inst. 1976;56:89–93. doi: 10.1093/jnci/56.1.89. [DOI] [PubMed] [Google Scholar]
- 103.Noguchi M, Itoh K, Yao A, Mine T, Yamada A, Obata Y, Furuta M, Harada M, Suekane S, Matsuoka K. Immunological evaluation of individualized peptide vaccination with a low dose of estramustine for HLA-A24 + HRPC patients. Prostate. 2005;63:1–12. doi: 10.1002/pros.20157. [DOI] [PubMed] [Google Scholar]
- 104.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JA, Robinson BW. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 105.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–4496. [PubMed] [Google Scholar]
- 106.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 107.Obeid M, Tesniere A, Panaretakis T, Tufi R, Joza N, van Endert P, Ghiringhelli F, Apetoh L, Chaput N, Flament C, Ullrich E, de Botton S, Zitvogel L, Kroemer G. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev. 2007;220:22–34. doi: 10.1111/j.1600-065X.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 108.Orlando L, Cardillo A, Ghisini R, Rocca A, Balduzzi A, Torrisi R, Peruzzotti G, Goldhirsch A, Pietri E, Colleoni M. Trastuzumab in combination with metronomic cyclophosphamide and methotrexate in patients with HER-2 positive metastatic breast cancer. BMC Cancer. 2006;6:225. doi: 10.1186/1471-2407-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park SD, Kim CH, Kim CK, Park JA, Sohn HJ, Hong YK, Kim TG. Cross-priming by temozolomide enhances antitumor immunity of dendritic cell vaccination in murine brain tumor model. Vaccine. 2007;25:3485–3491. doi: 10.1016/j.vaccine.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 110.Paul AR, Engstrom PF, Weiner LM, Steplewski Z, Koprowski H. Treatment of advanced measurable or evaluable pancreatic carcinoma with 17–1A murine monoclonal antibody alone or in combination with 5-fluorouracil, adriamycin and mitomycin (FAM) Hybridoma. 1986;5(Suppl 1):S171–S174. [PubMed] [Google Scholar]
- 111.Pegram M, Hsu S, Lewis G, Pietras R, Beryt M, Sliwkowski M, Coombs D, Baly D, Kabbinavar F, Slamon D. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene. 1999;18:2241–2251. doi: 10.1038/sj.onc.1202526. [DOI] [PubMed] [Google Scholar]
- 112.Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA, Slamon DJ. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 113.Pegram MD, Slamon DJ. Combination therapy with trastuzumab (Herceptin) and cisplatin for chemoresistant metastatic breast cancer: evidence for receptor-enhanced chemosensitivity. Semin Oncol. 1999;26:89–95. [PubMed] [Google Scholar]
- 114.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 115.Pietras RJ, Fendly BM, Chazin VR, Pegram MD, Howell SB, Slamon DJ. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene. 1994;9:1829–1838. [PubMed] [Google Scholar]
- 116.Pietras RJ, Pegram MD, Finn RS, Maneval DA, Slamon DJ. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene. 1998;17:2235–2249. doi: 10.1038/sj.onc.1202132. [DOI] [PubMed] [Google Scholar]
- 117.Pinkerton R. Continuing challenges in childhood non-Hodgkin’s lymphoma. Br J Haematol. 2005;130:480–488. doi: 10.1111/j.1365-2141.2005.05598.x. [DOI] [PubMed] [Google Scholar]
- 118.Press OW, Eary JF, Gooley T, Gopal AK, Liu S, Rajendran JG, Maloney DG, Petersdorf S, Bush SA, Durack LD, Martin PJ, Fisher DR, Wood B, Borrow JW, Porter B, Smith JP, Matthews DC, Appelbaum FR, Bernstein ID. A phase I/II trial of iodine-131-tositumomab (anti-CD20), etoposide, cyclophosphamide, and autologous stem cell transplantation for relapsed B-cell lymphomas. Blood. 2000;96:2934–2942. [PubMed] [Google Scholar]
- 119.Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, Leblanc M, Fisher RI. Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131 tositumomab for previously untreated follicular non-Hodgkin’s lymphoma: five-year follow-up of Southwest Oncology Group Protocol S9911. J Clin Oncol. 2006;24:4143–4149. doi: 10.1200/JCO.2006.05.8198. [DOI] [PubMed] [Google Scholar]
- 120.Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, LeBlanc M, Gaynor ER, Rivkin SE, Fisher RI. A phase 2 trial of CHOP chemotherapy followed by tositumomab/iodine I 131 tositumomab for previously untreated follicular non-Hodgkin lymphoma: Southwest Oncology Group Protocol S9911. Blood. 2003;102:1606–1612. doi: 10.1182/blood-2003-01-0287. [DOI] [PubMed] [Google Scholar]
- 121.Prewett MC, Hooper AT, Bassi R, Ellis LM, Waksal HW, Hicklin DJ. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clin Cancer Res. 2002;8:994–1003. [PubMed] [Google Scholar]
- 122.Rajvanshi P, Shulman HM, Sievers EL, McDonald GB. Hepatic sinusoidal obstruction after gemtuzumab ozogamicin (Mylotarg) therapy. Blood. 2002;99:2310–2314. doi: 10.1182/blood.v99.7.2310. [DOI] [PubMed] [Google Scholar]
- 123.Ravindranath MH, Muthugounder S, Presser N, Viswanathan S. Anticancer therapeutic potential of soy isoflavone, genistein. Adv Exp Med Biol. 2004;546:121–165. doi: 10.1007/978-1-4757-4820-8_11. [DOI] [PubMed] [Google Scholar]
- 124.Reiter I, Krammer B, Schwamberger G. Cutting edge: differential effect of apoptotic versus necrotic tumor cells on macrophage antitumor activities. J Immunol. 1999;163:1730–1732. [PubMed] [Google Scholar]
- 125.Romani L, Fioretti MC, Bonmassar E. In vitro generation of primary cytotoxic lymphocytes against L5178Y leukemia antigenically altered by 5-(3, 3′-dimethyl-1-triazeno)-imidazole-4-carboxamide in vivo. Transplantation. 1979;28:218–222. doi: 10.1097/00007890-197909000-00013. [DOI] [PubMed] [Google Scholar]
- 126.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 127.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14639–14645. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, White DE. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–484. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 130.Samanta S, Alam SM, Basu S, Maji T, Roy DK, Jha T. Chemoimmunotherapeutic approach to prolonged survival time in combination with immunization and glutamic Acid derivatives with antitumor activity in tumor-bearing mice. Biol Pharm Bull. 2007;30:2334–2339. doi: 10.1248/bpb.30.2334. [DOI] [PubMed] [Google Scholar]
- 131.Sato Y, Fujiwara T, Mine T, Shomura H, Homma S, Maeda Y, Tokunaga N, Ikeda Y, Ishihara Y, Yamada A, Tanaka N, Itoh K, Harada M, Todo S. Immunological evaluation of personalized peptide vaccination in combination with a 5-fluorouracil derivative (TS-1) for advanced gastric or colorectal carcinoma patients. Cancer Sci. 2007;98:1113–1119. doi: 10.1111/j.1349-7006.2007.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schuster M, Nechansky A, Kircheis R. Cancer immunotherapy. Biotechnol J. 2006;1:138–147. doi: 10.1002/biot.200500044. [DOI] [PubMed] [Google Scholar]
- 133.Serrano A, Tanzarella S, Lionello I, Mendez R, Traversari C, Ruiz-Cabello F, Garrido F. Rexpression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2′-deoxycytidine treatment. Int J Cancer. 2001;94:243–251. doi: 10.1002/ijc.1452. [DOI] [PubMed] [Google Scholar]
- 134.Shin DM, Donato NJ, Perez-Soler R, Shin HJ, Wu JY, Zhang P, Lawhorn K, Khuri FR, Glisson BS, Myers J, Clayman G, Pfister D, Falcey J, Waksal H, Mendelsohn J, Hong WK. Epidermal growth factor receptor-targeted therapy with C225 and cisplatin in patients with head and neck cancer. Clin Cancer Res. 2001;7:1204–1213. [PubMed] [Google Scholar]
- 135.Sievers EL, Appelbaum FR, Spielberger RT, Forman SJ, Flowers D, Smith FO, Shannon-Dorcy K, Berger MS, Bernstein ID. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood. 1999;93:3678–3684. [PubMed] [Google Scholar]
- 136.Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H, Karanes C, Theobald M, Bennett JM, Sherman ML, Berger MS, Eten CB, Loken MR, van Dongen JJ, Bernstein ID, Appelbaum FR. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 137.Sinkovics JG, Horvath JC. Evidence accumulating in support of cancer vaccines combined with chemotherapy: a pragmatic review of past and present efforts. Int J Oncol. 2006;29:765–777. [PubMed] [Google Scholar]
- 138.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 139.Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sanchez Rovira P, Piccart-Gebhart MJ. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 140.Song CK, Han HD, Noh KH, Kang TH, Park YS, Kim JH, Park ES, Shin BC, Kim TW. Chemotherapy enhances CD8(+) T cell-mediated antitumor immunity induced by vaccination with vaccinia virus. Mol Ther. 2007;15:1558–1563. doi: 10.1038/sj.mt.6300221. [DOI] [PubMed] [Google Scholar]
- 141.Tan GH, Tian L, Wei YQ, Zhao X, Li J, Wu Y, Wen YJ, Yi T, Ding ZY, Kan B, Mao YQ, Deng HX, Li HL, Zou CH, Fu CH. Combination of low-dose cisplatin and recombinant xenogeneic endoglin as a vaccine induces synergistic antitumor activities. Int J Cancer. 2004;112:701–706. doi: 10.1002/ijc.20449. [DOI] [PubMed] [Google Scholar]
- 142.Tschmelitsch J, Barendswaard E, Williams C, Jr, Yao TJ, Cohen AM, Old LJ, Welt S. Enhanced antitumor activity of combination radioimmunotherapy (131I-labeled monoclonal antibody A33) with chemotherapy (fluorouracil) Cancer Res. 1997;57:2181–2186. [PubMed] [Google Scholar]
- 143.Untch M, Ditsch N, Hermelink K. Immunotherapy: new options in breast cancer treatment. Expert Rev Anticancer Ther. 2003;3:403–408. doi: 10.1586/14737140.3.3.403. [DOI] [PubMed] [Google Scholar]
- 144.van den Eertwegh AJ. Active specific immunotherapy in colon cancer. Recent Results Cancer Res. 2005;165:260–267. doi: 10.1007/3-540-27449-9_29. [DOI] [PubMed] [Google Scholar]
- 145.van der Most RG, Currie A, Robinson BW, Lake RA. Cranking the immunologic engine with chemotherapy: using context to drive tumor antigen cross-presentation towards useful antitumor immunity. Cancer Res. 2006;66:601–604. doi: 10.1158/0008-5472.CAN-05-2967. [DOI] [PubMed] [Google Scholar]
- 146.Vose JM, Link BK, Grossbard ML, Czuczman M, Grillo-Lopez A, Gilman P, Lowe A, Kunkel LA, Fisher RI. Phase II study of rituximab in combination with chop chemotherapy in patients with previously untreated, aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2001;19:389–397. doi: 10.1200/JCO.2001.19.2.389. [DOI] [PubMed] [Google Scholar]
- 147.Voso MT, Pantel G, Weis M, Schmidt P, Martin S, Moos M, Ho AD, Haas R, Hohaus S. In vivo depletion of B cells using a combination of high-dose cytosine arabinoside/mitoxantrone and rituximab for autografting in patients with non-Hodgkin’s lymphoma. Br J Haematol. 2000;109:729–735. doi: 10.1046/j.1365-2141.2000.02084.x. [DOI] [PubMed] [Google Scholar]
- 148.Wheeler CJ, Das A, Liu G, Yu JS, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10:5316–5326. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 149.Xiong HQ, Rosenberg A, LoBuglio A, Schmidt W, Wolff RA, Deutsch J, Needle M, Abbruzzese JL. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II Trial. J Clin Oncol. 2004;22:2610–2616. doi: 10.1200/JCO.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 150.Yanagimoto H, Mine T, Yamamoto K, Satoi S, Terakawa N, Takahashi K, Nakahara K, Honma S, Tanaka M, Mizoguchi J, Yamada A, Oka M, Kamiyama Y, Itoh K, Takai S. Immunological evaluation of personalized peptide vaccination with gemcitabine for pancreatic cancer. Cancer Sci. 2007;98:605–611. doi: 10.1111/j.1349-7006.2007.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang S, Haluska FG. Treatment of melanoma with 5-fluorouracil or dacarbazine in vitro sensitizes cells to antigen-specific CTL lysis through perforin/granzyme- and Fas-mediated pathways. J Immunol. 2004;172:4599–4608. doi: 10.4049/jimmunol.172.7.4599. [DOI] [PubMed] [Google Scholar]
- 152.Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, Yu P, Fu YX, Weichselbaum RR, Rowley DA, Kranz DM, Schreiber H. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zheng SJ, Zheng SP, Huang FY, Jiao CL, Wu RL. Synergistic anti-tumor effect of recombinant chicken fibroblast growth factor receptor-1-mediated anti-angiogenesis and low-dose gemcitabine in a mouse colon adenocarcinoma model. World J Gastroenterol. 2007;13:2484–2489. doi: 10.3748/wjg.v13.i17.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zheng SP, Zheng SJ, Wu RL, Huang FY, Cao LM, Jiao CL. Enhanced efficacy in anti-tumour activity by combined therapy of recombinant FGFR-1 related angiogenesis and low-dose cytotoxic agent. Eur J Cancer. 2007;43:2134–2139. doi: 10.1016/j.ejca.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 155.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]


