DURING THE LAST DECADE, only three major immunosuppressants have been used extensively for whole organ transplantation, namely azathioprine, prednisone, and heterologous antilymphocyte globulin. None of these agents is sufficiently potent to permit consistent success when used alone in human beings. Consequently, the drugs have been administered in the double combination of azathioprine and prednisone or more recently in a triple combination of all three agents.
This article was undertaken to describe our experience with cyclophosphamide, a fourth major immunosuppressive drug which was given from the outset instead of azathioprine to 40 whole organ recipients and which was used to replace azathioprine at varying times after transplantation in another 54 patients. The results have shown cyclophosphamide to be equivalent to azathioprine in its therapeutic role and effectiveness.
METHODS
Cyclophosphamide from Outset
Material
There were 33, six, and one recipients, respectively, of 37 renal, six hepatic, and one cardiac homografts. The liver and heart transplants were used to replace the diseased organs in the orthotopic location, whereas most of the kidneys were placed heterotopically in the inferior portion of either the right or left extraperitoneal space. When the kidneys were obtained from related donors, bilateral nephrectomy and splenectomy were performed through a separate incision in the upper part of the abdomen on the same day as the transplantation. With cadaveric renal transplantation, the host kidneys or spleen usually was not disturbed.
Transplantation was not restricted to prime candidates in that 22 of the 40 patients fell into one, or more, putative high risk categories including age greater than 45 or less than 17 years, gastrointestinal ulceration, pancreatitis, lupus erythematosus, and a recent history of malignant neoplasia. Moreover, six of the 33 kidney recipients were entered into the cyclophosphamide series at the time of retransplantation after having rejected a first kidney at some past time.
Good histocompatibility matching was not required. For example, the consanguineous kidney donors had an A or B match with the recipients, as defined by Mickey and his associates (8), in only four of 18 instances. C, D, and E matches were present in another 13, and in one, the presence of antigraft antibodies caused the designation of F match. The 18 familial kidney donors included 11 siblings, six parents, and one aunt.
Within the 26 donor-recipient pairs of the 24 cadaveric renal, hepatic, and cardiac transplants, there were no good human leukocyte antigen (HL-A) matches. One human leukocyte antigen was mismatched in six instances. In the other 20, either two, three, or all four of the antigens were incompatible.
Immunosuppression
Treatment was adapted from the triple drug regimen which has been used at our institution since 1966 (16) except that cyclophosphamide was administered in place of azathioprine (15). The patients receiving consanguineous kidneys were given cyclophosphamide orally for three to nine days before operation, starting with 0.3 to 2.3 (mean 1.1) milligrams per kilogram per day and concluding with larger doses of 2.5 to 5.8 (mean 3.6) milligrams per kilogram per day on the day before and the day of transplantation. During the same pretreatment interval, daily intramuscular injections were given of horse antilymphocyte globulin that had leuko-agglutinin and lymphocytotoxin titers of 1 :4,000 to 1 :8,000 and 1 :4,000 to 1 :16,000, respectively, and a protein concentration of 5.0 to 7.2 grams per 100 milliliters. Finally, prednisone was given orally in preoperative doses of 0.4 to 1.2 (mean 0.71) milligrams per kilogram per day.
For the cadaveric renal, hepatic, and cardiac transplantations, pretreatment was not feasible, and immunosuppression was usually begun on the day of operation. Under these conditions, the initial dose of cyclophosphamide was 1.0 to 4.4 (mean 2.9) milligrams per kilogram by intravenous infusion over three hours, usually during the operation. Prednisone and antilymphocyte globulin were also started during or just after operation.
After transplantation, the protocol called for a decreasing number of antilymphocyte globulin injections after two weeks and for adjustments in prednisone dosage, especially after the first few weeks, according to the difficulty encountered with rejection. The average daily doses of cyclophosphamide used postoperatively started at 2 or 3 milligrams per kilogram, but eventually, these usually had to be reduced to considerably less than 1 milligram per kilogram to avoid leukopenia. The drug was given orally when possible, but in the absence of alimentation, it was administered intravenously each day in 5 per cent glucose in water over approximately three hours.
Azathioprine Control Studies
The results in the cyclophosphamide series were compared with those obtained in the three months from December 1970 to mid-March 1971 during which period a triple drug program of azathioprine, prednisone, and horse antilymphocyte globulin, of comparable potency to that used in the subsequent cyclophosphamide series, was used as primary treatment. The control patients included nine and two recipients of cadaveric kidneys and livers, respectively. Two of these patients had a single human leukocyte antigen mismatch with the donor, but the other nine had incompatibilities of two, three, or four antigens. The donors in the 20 consanguineous renal transplantations were ten siblings and ten parents. Within the sibling subgroup, there were four A matches connoting the favorable condition of double haplotype identity of the human leukocyte antigen system.
Histopathologic Studies
Organs recovered at autopsy or transplant nephrectomy from both the cyclophosphamide and azathioprine series were fixed in formalin and stained with hematoxylin and eosin, periodic acid-Schiff, Weigert's for elastic counterstained with hematoxylin and van Gieson, methyl greenpyronine, and Sudan III. The liver sections were also stained for reticulin fibers by Gordon and Sweet's silver impregnation method and for iron by Perls' Prussian blue method.
Delayed Cyclophosphamide
A total of 54 patients were taken off maintenance immunosuppressive therapy with azathioprine and were given cyclophosphamide instead. In four recipients of orthotopic liver transplants, the change was made from nine to 35 months after transplantation. A patient given a cardiac homograft 20 months earlier also had the substitution.
In addition, 49 renal recipients were given cyclophosphamide in place of azathioprine from one and one-half to 94 months postoperatively. In 11 patients, hepatotoxicity or other side effects of azathioprine, including drug fever, were suspected. Nine more patients were switched to cyclophosphamide because of the serologic diagnosis of chronic Australia antigenemia even though abnormalities in liver function were either minimal or absent. The switch in therapy in the other 29 patients was made in the hope of eventually maintaining graft function with smaller dosages of prednisone. Consequently, reductions in the daily quantities of steroids were usually made shortly after the institution of the cyclophosphamide.
In replacing azathioprine with cyclophosphamide, the same precautions were taken as when cyclophosphamide was used from the beginning. Frequent white blood cell counts were obtained, and appropriate dosage adjustments were made with any indication of impending leukopenia. Eventually, the typical patient was found to tolerate two-thirds the dosage of cyclophosphamide per kilogram as had previously been possible with azathioprine.
RESULTS
Cyclophosphamide from Outset
Intrafamilial renal transplantation
All 18 of the recipients primarily treated with cyclophosphamide are still alive. However, two of the 18 consanguineous kidneys failed because of hyperacute rejection and were removed within minutes, or hours, after transplantation. The serum of one of these patients had preformed cytotoxic antibodies with antigraft specificity, but this unfavorable condition was not demonstrable in the other patient. Both patients were subsequently given cadaveric kidneys and will be considered in the next section.
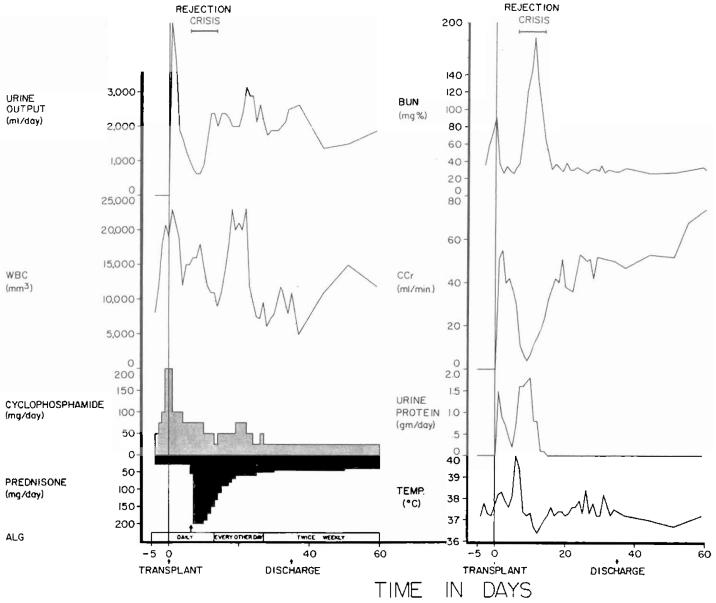
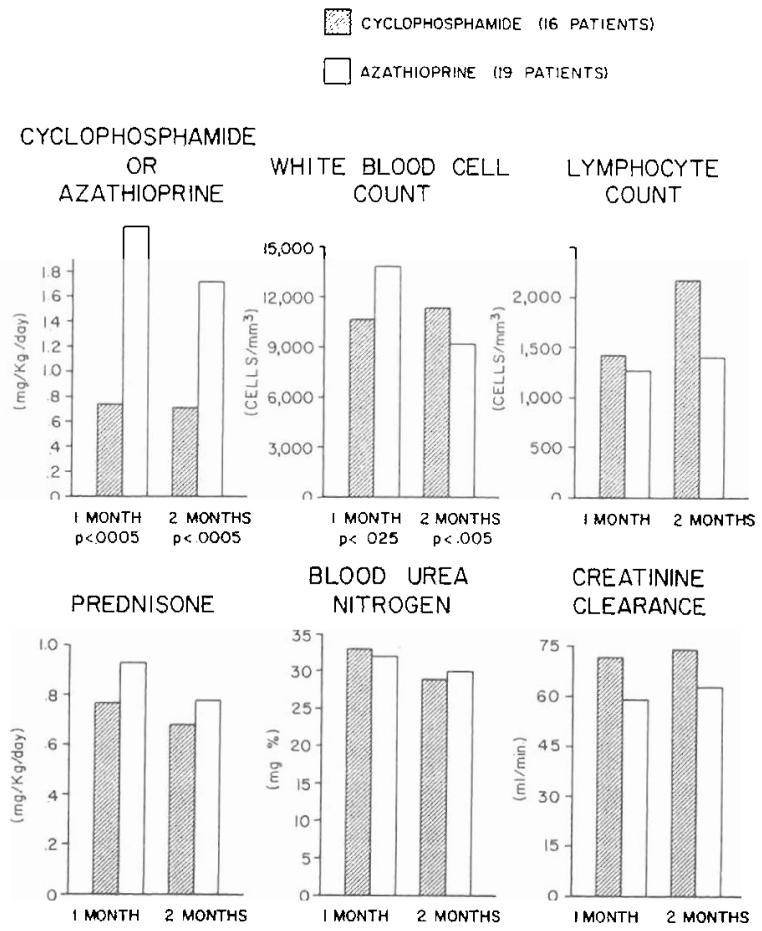
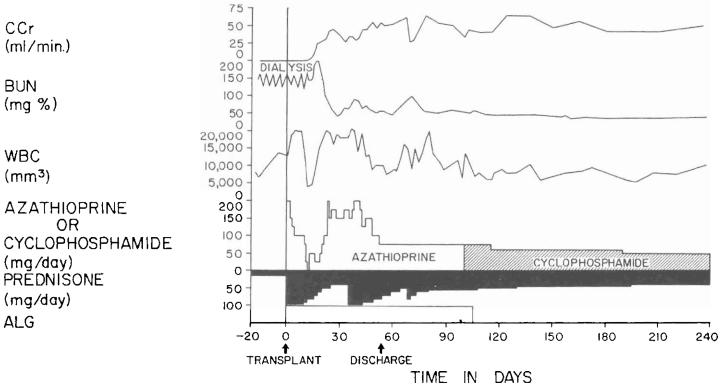
It was possible to evaluate the cyclophosphamide regimen in the other 16 recipients of related kidneys. All have had a satisfactory result with followup periods that are now two to six months. Rejection has never been diagnosed in seven patients, it occurred in a mild form in seven, and it was severe but eventually reversed in the other two (Fig. 1). The relations between the quantities of cyclophosphamide and prednisone versus graft function and white blood cell count after one and two postoperative months are summarized for the 16 patients in Figure 2.
Fig. 1.
The first 60 days after the transplantation of a kidney from a mother to her 14 year old daughter. The rejection crisis after a week was the most severe observed in the intrafamilial cyclophosphamide series, with the exception of two hyperacute rejections. However, it was easily and completely reversed. Note that leukopenia was never produced by the daily doses of cyclophosphamide that were usually between 0.5 to 1 milligram per kilogram per day. ALG, Horse antilymphocyte globulin; BUN, blood urea nitrogen level; CCr, creatinine clearance; WBC, white blood cell count; and arrow, 625 milligrams of methyl prednisolone intravenously.
Fig. 2.
Drug doses and laboratory measurements at the end of one and two months in patients given either cyclophosphamide or azathioprine. All these recipients of kidneys donated by blood relatives were also treated with prednisone and horse antilymphocyte globulin. The p values are noted only for differences that were statistically significant.
In the control series of 20 intrafamilial renal recipients treated with triple drug immunosuppression including azathioprine, there was one hyperacute rejection by a patient who did not have preformed antibodies. A subsequent attempt at cadaveric transplantation using the triple drug cyclophosphamide regimen resulted in the same complication, necessitating a probably permanent return to chronic dialysis.
Three of the remaining 19 patients under azathioprine therapy died of widespread infections for an over-all mortality rate of 15 per cent. Nine of the 19 recipients escaped rejection altogether. The other eight and two underwent mild and severe rejection episodes, respectively. The average pattern of recovery, as reflected in steroid requirement and graft function, was essentially the same as in the cyclophosphamide series, the principal difference being that the dosage of azathioprine per kilogram of body weight needed to achieve a comparable effect on the white blood cell count was approximately 2.5 times that of cyclophosphamide (Fig. 2).
Cadaveric renal transplantation
Among the 17 patients in the cyclophosphamide series, there were 12 who were undergoing transplantation for the first time. Eight or 67 per cent of the 12 still have good function of their original homografts after two to 4.5 months (average 3.7 months). One of the remaining four died 68 days postoperatively of an Aspergillus brain abscess following a protracted and incompletely controlled rejection. The other three recipients rejected their grafts which either became nonfunctional or were removed after one to nine weeks. Retransplantation was performed in two of these three patients with a good result four and two months later. The third patient is still on hemodialysis awaiting the retransplantation procedure.
The other five cadaveric renal recipients treated with cyclophosphamide were at high risk from immunologic sensitization in that two, two, and one of these patients had destroyed their first grafts by hyperacute, acute, and chronic rejection, respectively. After retransplantation under the cyclophosphamide regimen, three of the five secondary transplants failed, one because of hyperacute rejection and two more because the recipients died of cecal ulceration and gram-negative septicemia or Pneumocystis carinii pneumonitis, respectively. The other two grafts are functioning satisfactorily after four and six months.
There were nine control patients treated with a triple drug regimen containing azathioprine instead of cyclophosphamide. Only two of the group were undergoing primary transplantation. One has had an excellent result after nine months. The other rejected his kidney within a few days but then had successful retransplantation under cyclophosphamide treatment as previously has been described.
The other seven recipients were in the high risk retransplantation category. One died of diffuse interstitial pneumonitis and a second destroyed her kidney by hyperacute rejection. The other five still have variable life-sustaining function of their grafts after six to nine months.
The recipients of cadaveric renal transplants were such a heterogenous group and underwent so many retransplantations that comparisons of steroid dosages, graft function, and other parameters were not meaningful in the cyclophosphamide versus azathioprine-treated patients.
Cadaveric liver transplantation
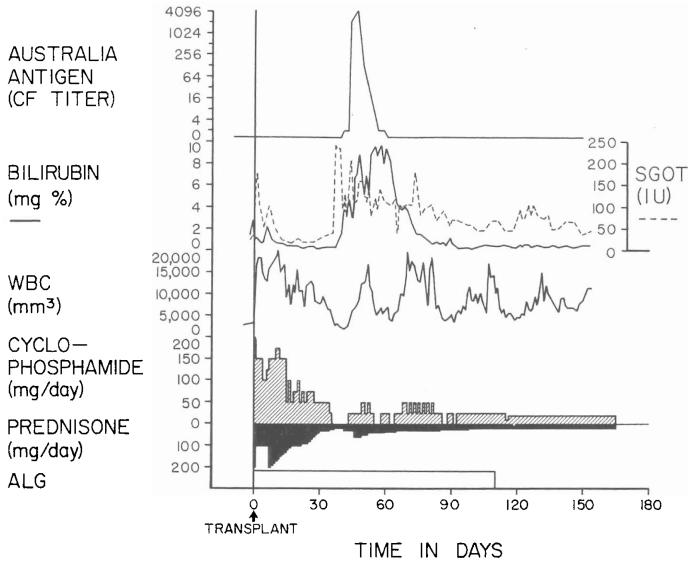
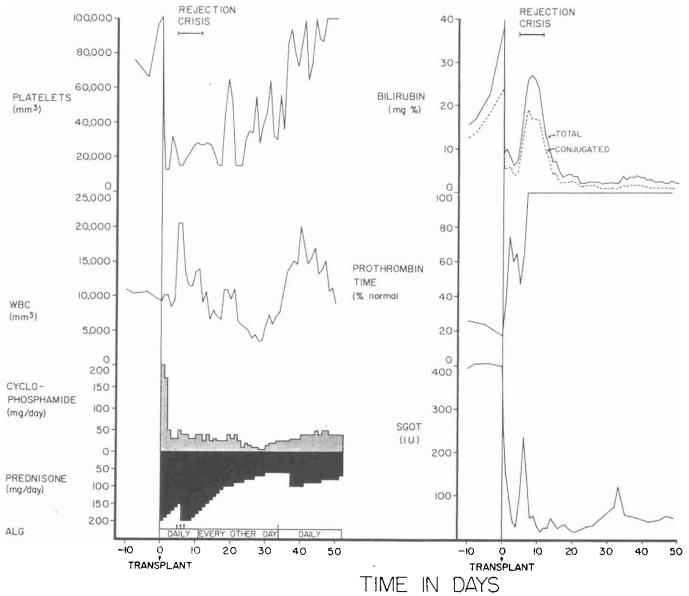
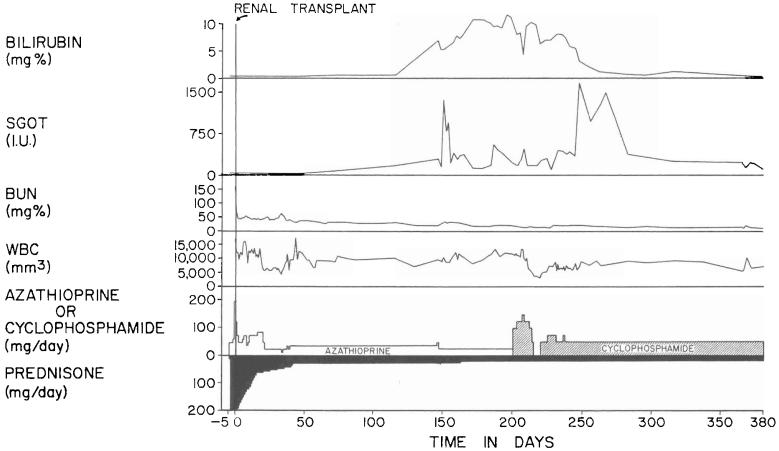
Two of the six patients died of cholangitis, liver abscesses, and bacteremia which were secondary to biliary obstruction. In both patients, cholecystoduodenostomy had been performed after ligation of the distal part of the common bile duct. The obstruction was at, or near, the junction of the cystic and common bile ducts. The other four patients, aged three, four, 15, and 52 years, are alive from one and one-half to six months postoperatively. The liver replacement was for biliary atresia in two recipients and for Wilson's disease and a hepatic hemangioendothelial sarcoma in the third and fourth. Two of the surviving patients have not had a rejection, although one of them had transient deterioration of hepatic function coincident with the development of Australia antigenemia (Fig. 3). The other two recipients had moderately severe rejections which were eventually controlled (Fig. 4).
Fig. 3.
Triple drug therapy with cyclophosphamide, prednisone, and horse antilymphocyte globulin in a 15 year old male who had an orthotopic liver transplantation for Wilson's disease. Rejection has never been diagnosed postoperatively. The temporary deterioration in hepatic function in the second postoperative month was accompanied by Australia antigenemia and consequently was thought to be a manifestation of serum hepatitis. ALG, Horse antilymphocyte globulin; CF, complement fixation; SGOT, serum glutamic oxalacetic transaminase in international units; and WBC, white blood cell count.
Fig. 4.
The early course of a 52 year old male who had total liver replacement as treatment for a hemangioendothelial sarcoma. This rare liver tumor usually causes death from fulminating hepatic failure in a few weeks or months, but it does not commonly metastasize. After transplantation, there was a severe rejection crisis. The protracted thrombocytopenia postoperatively is often noted after liver transplantation and is probably due to thrombocyte sequestration in the homograft. ALG, Horse antilymphocyte globulin; SGOT, serum glutamic oxalacetic transaminase in international units; and arrow, 625 milligrams of methyl prednisolone intravenously.
The two control patients received orthotopic livers under triple drug therapy including azathioprine. A 12 year old girl with chronic aggressive hepatitis had the same type of cystic duct obstruction described in the preceding paragraph which led to death nine weeks after transplantation despite secondary operative correction. The other recipient who was aged 44 years died of a brain stem hemorrhage four and one-half weeks after liver replacement for postnecrotic cirrhosis.
Cadaveric heart transplantation
A 39 year old recipient became remittently hypotensive beginning 27 days after transplantation for atherosclerotic coronary artery disease, coincident with multiple other signs of rejection. He had a cardiac arrest and died 32 days after operation.
Drug Toxicity
The incidence of several complications was compared in the renal recipients treated primarily with cyclophosphamide and in those given azathioprine from the outset. The results were converted to percentages and expressed in graphic form (Fig. 5), including only patients for whom full information was available for at least two postoperative months.
Fig. 5.
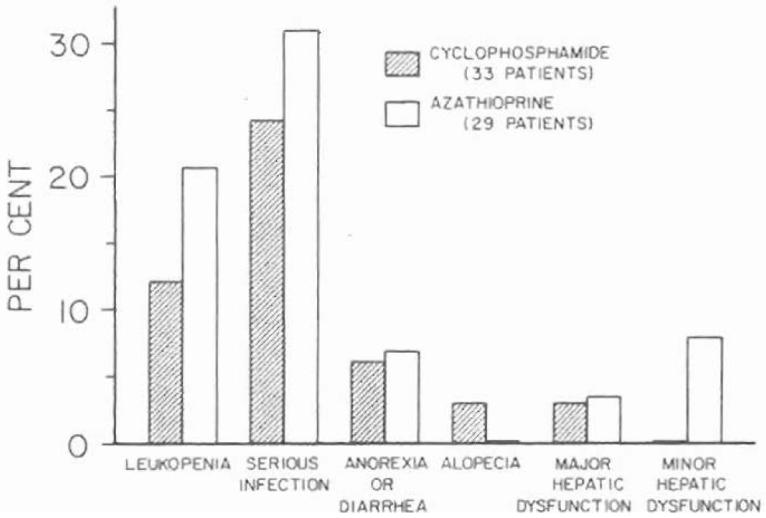
Incidence of toxicity in the first two months of treatment of renal homograft recipients with cyclophosphamide versus azathioprine. At the dosages used, there was little obvious difference between the two agents. The one patient under cyclophosphamide therapy in whom major hepatic dysfunction developed had a proved attack of acute serum hepatitis, Australia antigen positive.
Leukopenia severe enough to require the omission of the radiomimetic drugs for at least three consecutive days occurred somewhat more frequently in the azathioprine group (Fig. 5). This usually connoted white blood cell counts of less than 5,000 per cubic millimeter. Thrombocytopenia was also sporadically observed in both groups, but the finding frequently seemed more attributable to treatment with antilymphocyte globulin than to the cytotoxic drugs.
The incidence of serious infections was similar in the cyclophosphamide and azathioprine series (Fig. 5). Moreover, the nature of the responsible microorganisms tended to be comparable with a heavy representation of fungi, viruses, and protozoa of normally low pathogenicity.
Anorexia and diarrhea are well known complications of high dosage treatment with cyclophosphamide. In the low quantities given in this study, such complaints were uncommon. There was only one patient with intractable diarrhea, and at autopsy, its cause was found to be a large cecal ulcer. The incidence of gastrointestinal complaints was almost exactly the same in the azathioprine series (Fig. 5).
Abnormalities of liver function were considered major if the transaminase level exceeded 250 international units on more than two occasions, if jaundice developed, and if there was depression of protein synthesis, as measured by a serum albumin concentration of less than 3 grams per 100 milliliters, or persistent depressions of the prothrombin time. There was one example of liver malfunction in a cyclophosphamide-treated patient in whom acute serum hepatitis, Australia antigen positive, developed. The azathioprine series contained one recipient with major hepatic dysfunction and three with minor abnormalities (Fig. 5). None of these latter recipients was Australia antigen positive.
One patient became bald shortly after a cyclophosphamide-induced bout of leukopenia. Her hair returned completely after discontinuance of cyclophosphamide and the substitution of azathioprine.
Histopathologic Studies
Compared with those in the azathioprine studies, the renal and hepatic transplants from the cyclophosphamide-treated patients did not have any particularly distinguishing gross or microscopic features.
The kidneys of two of the cyclophosphamide-treated patients and three of the azathioprine-treated patients were normal except for a little tubular epithelial damage and slight interstitial mononuclear cell infiltration. Features compatible with hyperacute rejection having occurred were present in three of the cyclophosphamide group and two of the azathioprine group. There was a moderate amount of intimal thickening of the interlobular and arcuate arteries in the kidneys of one of the cyclophosphamide-treated patients and in one of the azathioprine-treated patients. The only differences between the two groups were that one kidney in the azathioprine-treated group showed acute cellular rejection and had ruptured and that the kidneys of two of the cyclophosphamide-treated patients showed areas of hemorrhagic necrosis in the cortex and venous thrombosis as well as arterial intimal thickening and interstitial mononuclear cell infiltration.
Three hepatic homografts, two from the cyclophosphamide and one from the azathioprine series, were examined microscopically. Specimens were not available from the other azathioprine-treated recipient who died. The three livers showed no evidence of rejection but had all the features of partial obstruction of the large biliary ducts. Cholangitis was present in two of the organs, one from each series.
Focal mononuclear cell infiltration was present in the myocardium of the cyclophosphamide-treated patient who received a cardiac transplant, but the coronary arteries and their branches were normal.
Delayed Cyclophosphamide
After renal transplantation
The 49 patients were one and one-half to 94 months after a primary or in some cases a repeat transplantation. The substitution of cyclophosphamide and azathioprine was almost never the only alteration in management. For example, upward or downward adjustments in steroids were made freely as it was thought indicated (Fig. 6). Moreover, a number of other less well defined variables could not be controlled at all or even measured. Consequently, the events following cyclophosphamide substitution, whether in the direction of improvement or deterioration, could not necessarily be attributed to the change in treatment.
Fig. 6.
The consecutive use of azathioprine and cyclophosphamide after cadaveric renal homotransplantation in a 20 year old male is charted. The change to cyclophosphamide was made because of dissatisfaction with the early postoperative course. There was slow improvement in the subsequent convalescence, but, retrospectively, there is no reason to believe that this might not have occurred even without making the drug switch. The globulin injections were daily for ten days, every other day for 20 days, and twice a week until they were discontinued because of anaphylactic reactions. ALG, Horse antilymphocyte globulin; BUN, blood urea nitrogen level; CCr, creatinine clearance; and WBC, white blood cell count.
Ten recipients had the cyclophosphamide substitution in the first three postoperative months during the time when therapy with horse antilymphocyte globulin was still in effect. Two of these ten patients had diffuse interstitial pneumonitis due to the protozoan Pneumocystis carinii, and a third had a through and through staphylococcal infection of the transplant wound. All three died. The fatal course of the pre-existing infections was not affected by the substitution of cyclophosphamide for azathioprine or by the later discontinuance of all immunosuppression.
The reasons for the cyclophosphamide substitution in the first three months were subnormal homograft function, a persistent fever, and difficulty with azathioprine dosage control in five, one, and one additional recipients, respectively. Homograft function has remained essentially the same in all seven patients for three to five months. The child with high fever became normothermic within a few days after the administration of azathioprine was stopped and has remained so.
Among the 39 patients with cyclophosphamide substitution after three months, all were either already off treatment with antilymphocyte globulin or else the globulin was stopped not long after the drug change. The only one who died was a 40 year old man with severe hepatic failure due to chronic aggressive hepatitis. Renal transplantation had been performed three and one-half years previously. His serum had contained antibodies against Australia antigen for the two preceding years. He died 45 days after changing to the cyclophosphamide. The alteration in treatment had no obvious effect on his course.
Three of the 39 renal recipients changed to cyclophosphamide later than three months after transplantation had subsequent deterioration of homograft function. However, in one, the kidney already was gradually failing after two years, a deterioration that has slowly continued during two and one-half months of cyclophosphamide treatment. The other two patients were of greater interest since excellent renal function preceded the drug substitution. The first recipient had been given five homografts, the last one from a cadaveric donor four and one-half months previously. Shortly after the conversion to cyclophosphamide, horse antilymphocyte globulin was stopped, and in addition, the prednisone dosage was significantly reduced. Renal failure recurred within three months which was not reversed by a switch back to azathioprine and by a large increase in steroids. The other patient was similar except that the substitution of cyclophosphamide was 16 months after transplantation of an uncle's kidney, long after discontinuance of antilymphocyte globulin therapy. Within three months, the blood urea nitrogen level rose from 11 to 125 milligrams per cent. Resumption of azathioprine and increases in prednisone have not restored good function.
The other 35 kidney recipients switched from azathioprine to cyclophosphamide have maintained their pre-existing renal homograft function during the one and one-half to nine months of follow-up study (Figs. 6 and 7). During this period, 18 of the 35 patients have had reductions of 10 to 35 (mean 20) per cent in their daily steroid dosage. Since the same type of late relaxation of prednisone therapy had been observed with other immunosuppressive regimens which contain azathioprine, the steroid reductions could not be attributed with assurance to the advent of cyclophosphamide.
Fig. 7.
The course of a 17 year old female in whom azathioprine was stopped because of the suspicion of hepatotoxicity. Multiple Australia antigen tests for serum hepatitis were negative. Note the recession of jaundice after the substitution of cyclophosphamide for azathioprine. BUN, blood urea nitrogen level; SGOT, serum glutamic oxalacetic transaminase in international units; and WBC, white blood cell count.
The commonest specific indication to replace azathioprine with cyclophosphamide was suspected hepatotoxicity. Prior to the drug change, there were five kidney recipients who had major liver malfunction as defined earlier by jaundice, persistent transaminasemia, or evidence of impaired protein synthesis. Excluding the patient who died of hepatic failure within a few weeks, liver function improved in all the others (Fig. 7).
After hepatic and cardiac transplantation
One of the four liver recipients had a dramatic, immediate fall in the serum transaminase level after the switch from azathioprine to cyclophosphamide, but within two months, these returned to the pre-existing level. The courses of the other three liver and one cardiac recipients have remained stable and satisfactory.
DISCUSSION
Although the principal use of cyclophosphamide in clinical medicine has been for cancer chemotherapy, its immunosuppressive qualities have been increasingly exploited. It has been used by Moncrieff (9), White (19), Fosdick (3), Novack (10), Greenspan (6), Buckley (1), Wong (20), Seah (14), and Laros (7) and their associates for the treatment of autoimmune disorders as diverse as the nephrotic syndrome, proliferative glomerulonephritis, rheumatoid arthritis, Wegener's granulomatosis, lethal midline granuloma, peripheral uveitis, ocular inflammatory disease, systemic lupus erythematosus, and thrombocytopenic purpura.
Even in the field of whole organ transplantation, cyclophosphamide had an early trial but under circumstances which did not permit its value to be fully appreciated. Almost a decade ago, Goodwin (5), Parsons (11), and Fox (4) and their associates reported the use of cyclophosphamide, alone or in combination with prednisone, in a total of nine kidney recipients. Although rejection was controlled in several patients, a high incidence of bone marrow depression and lethal infections discouraged further efforts. When cyclophosphamide was proved by Reams (12) as well as by subsequent investigators to be worthless for the prevention of renal homograft rejection in the dog, the seal of disapproval was nearly complete even though the poor results in dogs were probably due to a species peculiarity not relevant to human beings (15). Eventually, Santos and his associates (13) and a few others who gave large dosages of cyclophosphamide in an attempt to induce tolerance to bone marrow grafts remained the only spokesmen for the potential usefulness of this agent after any type of human transplantation procedure.
In retrospect, the reputation for toxicity acquired by cyclophosphamide in the early years of its use in cancer chemotherapy as well as for transplantation was the consequence of heavy dosage. In addition to bone marrow depression and infection, common complications included anorexia and other gastrointestinal disturbances, hemorrhagic cystitis, and alopecia. By giving smaller maintenance dosages which seldom exceeded 0.5 milligram per kilogram of body weight per day, these side reactions were reduced to an acceptable minimum in this study. To permit precise management, the standard 50 milligram tablets which cannot be accurately divided even in halves had to be repackaged in our local pharmacy into capsules containing 5, 10, and 25 milligrams.
Using the small dosages of cyclophosphamide, the effectiveness of the drug in preventing rejection of whole organ homografts was evaluated under two circumstances. To one group of recipients, cyclophosphamide was given from the outset in place of azathioprine as part of a triple drug regimen that also included prednisone and horse antilymphocyte globulin. Patient and organ survival; graft function and histopathologic condition; steroid requirement; and toxic and infectious complications were not essentially different from a companion series of recipients who were given azathioprine instead. With cyclophosphamide treatment, the results were slightly but not significantly better with intrafamilial renal transplantation. In addition, there was improvement in the results after orthotopic liver transplantation. However, there was a similar slight balancing superiority under azathioprine protection in the recipients of cadaveric kidney grafts.
In the second group of patients, azathioprine was discontinued at varying times after transplantation and replaced with cyclophosphamide. It was not possible in the vast majority of patients to identify either subsequent deterioration or improvement in homograft function. Even in those patients who had alterations in function, these changes were usually attributable to adjustments in steroid dosage. A valid generalization was that patients doing well before changing from azathioprine to cyclophosphamide did well afterward. In contrast, those with pre-existing infection or graft deterioration that had developed under azathioprine were not benefited merely by instituting cyclophosphamide. Instead, the outcome was dependent upon the success with which antibiotics were used or with which prednisone dosages were manipulated.
The remarkably comparable effectiveness of cyclophosphamide and azathioprine under both these general conditions of clinical testing is of some theoretic interest since their pharmacologic actions are thought to be dissimilar. Cyclophosphamide, an alkylating agent, has been said by Wheeler (18) to exert its primary cytotoxic effect by blocking the G2 phase of the cell cycle. In contrast, the purine analogue azathioprine has been shown by Elion (2) to inhibit deoxyribonucleic acid production during the S phase of the cycle. Yet, the events of rejection, its reversibility, and eventually the feasibility, in many instances, to lighten maintenance immunosuppression have not been perceptibly different with either of the drugs as compared with the other.
The newest observations with cyclophosphamide provide one more example of how nonspecific immunosuppression in the continuous presence of homograft antigen can lead by undefined mechanisms to a characteristic cycle of recovery and eventual graft acceptance, which implies a profound alteration in the host-graft relationship (17). The reliability with which this can be achieved with the use of either azathioprine or cyclophosphamide in the triple drug therapeutic regimen has become so great with intrafamilial renal transplantation that a high degree of benefit can be achieved with 80 per cent, or more, of such operations with, or without, discriminating histocompatibility matching. In contrast, the cycle of graft acceptance cannot be so readily negotiated after transplantation from unrelated donors, making cadaveric transplantation a more dangerous and unpredictable undertaking under any conditions of treatment known today, as was illustrated by the results in both our cyclophosphamide and azathioprine series.
From a practical point of view, the fact that cyclophosphamide and azathioprine have been shown to be capable of essentially identical roles implies that sweeping improvements in clinical immunosuppression probably will not be made possible by the wider routine use of cyclophosphamide. Instead, the most important immediate use for cyclophosphamide in most established transplantation centers will probably prove to be a potent replacement for azathioprine for special indications. Although azathioprine has been remarkably free of side reactions, the suspicion of liver injury or other forms of toxicity should warrant its discontinuance and a trial of cyclophosphamide as a diagnostic procedure. In several of our patients, derangements in liver function were thereby improved, and in another recipient, a high fever of unexplained cause disappeared within a few days.
It is our intention to continue to use azathioprine as a primary immunosuppressant in many future instances of renal and cardiac homotransplantation, if only because of the acquisition of ten years of satisfactory experience with this agent. In view of the encouraging results, it is also planned to continue the evaluation of cyclophosphamide in such new recipients. Moreover, a substantial number of patients undergoing liver transplantation will be given cyclophosphamide from the beginning. After liver transplantation, it is crucial as well as exceptionally difficult to distinguish homograft rejection from other causes of postoperative hepatic dysfunction of which drug toxicity, biliary duct obstruction, and viral hepatitis are the most important. The use of an agent which has little known intrinsic hepatotoxicity in these recipients will carry an obvious advantage.
SUMMARY
Cyclophosphamide in conjunction with prednisone and horse antilymphocyte globulin was administered daily in low dosages as a primary immunosuppressant to 40 recipients of 37 kidneys, six livers and one heart. Rejection was controlled with about the same frequency and effectiveness as in a companion series of 31 recipients of kidneys or livers who were treated in the same way except that azathioprine was used instead of cyclophosphamide. The incidence of side-effects, including bone marrow depression, serious infection, and gastrointestinal morbidity, was not significantly different with these two agents.
Fifty-four additional patients including 49, four, and one recipients of kidneys, livers, and a heart, respectively, had therapy with azathioprine discontinued and cyclophosphamide was begun from one and one-half to 94 months after transplantation. Since the antilymphocyte globulin injections usually had been stopped earlier, the drug switch in the majority of patients was made when the only other immunosuppressive agent being used was prednisone. Subsequent to the drug change, the clinical courses usually were not obviously different from those preceding the substitution. In a few instances in which hepatic dysfunction or fever were present, these abnormalities resolved.
These studies have demonstrated that cyclophosphamide is an immunosuppressant with a potency, safety, and therapeutic role similar to that of azathioprine. Since these two cytotoxic agents are thought to act at different phases of the cell cycle, the fact that they can be used interchangeably is of theoretic interest. From a practical point of view, the findings indicate that either agent can serve as the cornerstone for combination drug therapy and that substitution of one for the other can be freely considered if there is suspicion of specific pharmacologic toxicity.
Acknowledgments
This work was supported by research grants from the Veterans Administration, by Grant Nos. RR-00051 and RR-00069 from the general clinical research centers program of the Division of Research Resources, National Institutes of Health, and by Grant Nos. AI-10176-01, AI-AM-08898, AM-07772, and HE-09110 of the U.S. Public Health Service.
REFERENCES
- 1.BUCKLEY CE, DURHAM NC, GILLS JP. Cyclophosphamide therapy of peripheral uveitis. Arch. Intern. Med. 1969;124:29. [PubMed] [Google Scholar]
- 2.ELION GP. Biochemistry and pharmacology of purine analogues. Fed. Proc. 1967;26:898. [PubMed] [Google Scholar]
- 3.FOSDICK WM, PARSONS JL, HILL DF. Long-term cyclophosphamide (CP) therapy in rheumatoid arthritis; a progress report, six years' experience. Arthritis Rheum. 1969;12:663. doi: 10.1002/art.1780110205. [DOI] [PubMed] [Google Scholar]
- 4.Fox M. Suppression of tissue immunity by cyclophosphamide. Transplantation. 1964;2:475. doi: 10.1097/00007890-196407000-00004. [DOI] [PubMed] [Google Scholar]
- 5.GOODWIN WE, KAUFFMAN JJ, MIMS MM, TURNER RD, GLASSOCK R, GOLDMAN R, MAXWELL MM. Human renal transplantation—I, clinical experiences with 6 cases of renal homotransplantation. J. Urol. 1963;89:13. doi: 10.1016/S0022-5347(17)64491-4. [DOI] [PubMed] [Google Scholar]
- 6.GREENSPAN EM. Cyclophosphamide—prednisone therapy of lethal midline granuloma. J. A. M. A. 1965;193:74. doi: 10.1001/jama.1965.03090010080031. [DOI] [PubMed] [Google Scholar]
- 7.LAROS RK, PENNER JA. “Refractory” thrombocytopenic purpura treated successfully with cyclophosphamide. J. A. M. A. 1971;215:445. [PubMed] [Google Scholar]
- 8.MICKEY MR, KREISLER M, ALBERT ED, TANAKA N, TERASAKI PI. Analysis of HL-A incompatibility in human renal transplants. Tissue Antigens. 1971;1:57. doi: 10.1111/j.1399-0039.1971.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 9.MONCRIEFF MM, WHITE RHR, OGG CS, CAMERON JS. Cyclophosphamide therapy in the nephrotic syndrome in childhood. Br. Med. J. 1969;1:666. doi: 10.1136/bmj.1.5645.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NOVACK SN, PEARSON CM. Cyclophosphamide therapy in Wegener's granulomatosis. N. Engl. J. Med. 1971;284:938. doi: 10.1056/NEJM197104292841703. [DOI] [PubMed] [Google Scholar]
- 11.PARSONS FM, FOX M, ANDERSON CK, MARKLAND MA, CLARK PB, RAPER FP. Cyclophosphamide in renal homotransplantation. Br. J. Urol. 1966;38:673. doi: 10.1111/j.1464-410x.1966.tb09776.x. [DOI] [PubMed] [Google Scholar]
- 12.REAMS GB. Use of cyclophosphamide in an attempt to modify the canine renal homograft response. Nature. 1963;197:713. doi: 10.1038/197713a0. [DOI] [PubMed] [Google Scholar]
- 13.SANTOS GW, BURKE PJ, SENSENBRUNNER LL, OWENS AH., JR. Rationale for the use of cyclophosphamide as an immunosuppressant for marrow transplants in man. In: Bertelli A, Monaco AP, editors. Pharmacological Treatment in Organ and Tissue Transplantation. Excerpta Medica Foundation; Amsterdam: 1970. p. 24. [Google Scholar]
- 14.SEAH CS, WONG KH, CHEW AG, JAYARATNAM FJ. Cyclophosphamide in treatment of systemic lupus erythematosus. Br. Med. J. 1966;1:333. doi: 10.1136/bmj.1.5483.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.STARZL TE, HALGRIMSON CG, PENN I, MARTINEAU G, SCHROTER G, AMEMIYA H, PUTNAM CW, GROTH CG. Cyclophosphamide and human organ transplantation. Lancet. 1971;2:70. doi: 10.1016/s0140-6736(71)92046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.STARZL TE, MARCHIORO TL, PORTER KA, IWASAKI Y, CERILLI GJ. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation and in human renal homotransplantation. Surg. Gynecol. Obstet. 1967;124:301. [PMC free article] [PubMed] [Google Scholar]
- 17.STARZL TE, MARCHIORO TL, WADDELL WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg. Gynecol. Obstet. 1963;117:385. [PMC free article] [PubMed] [Google Scholar]
- 18.WHEELER GP. Some biochemical effects of alkylating agents. Fed. Proc. 1967;26:885. [PubMed] [Google Scholar]
- 19.WHITE RHR, CAMERON JS, TROUNCE JR. Immunosuppressive therapy in steroid-resistant proliferative glomerulonephritis accompanied by the nephrotic syndrome. Br. Med. J. 1966;2:853. doi: 10.1136/bmj.2.5518.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WONG VG. Immunosuppressive therapy of ocular inflammatory disease. Arch. Ophthalmol. 1969;81:628. doi: 10.1001/archopht.1969.00990010630006. [DOI] [PubMed] [Google Scholar]