Abstract
Objectives:
Our aim was to determine if (1) Hybrid Capture 2 and a PCR-based method were comparable for detection of high-risk HPVs, (2) clinician-collected and self-collected samples were equally efficient to detect HPV and cervical cancer precursor lesions and (3) if participation rates improved with home-based vs. clinic-based self collection.
Methods:
Samples were selected from women participating in a cervical cancer screening study according to human papillomavirus (HPV), visual inspection with acetic acid (VIA), or Pap smear screening results. From 432 of 892 selected women, split sample aliquots were tested for HPV DNA using both the Hybrid Capture 2 assay and the Roche prototype line blot assay. Women from a subset of villages were recruited at two separate time points for clinic-based self-collection and home-based self-collection, and participation rates were compared.
Results:
Pairwise agreement between self- and clinician-collected samples was high by both hc2 (90.8% agreement, kappa=0.7) and PCR (92.6% agreement, kappa=0.8), with significantly increased high-risk HPV detection in clinician-collected specimens (McNemar's p<0.01). Ability to detect precursor lesions was highest by PCR testing of clinician-collected samples and lowest by Hybrid Capture 2 testing of self-collected samples (11/11 and 9/11 cases of cervical intraepithelial neoplasia grade 2/3 and cancer detected, respectively). Participation in home-based screening was significantly higher than clinic-based screening (71.5% and 53.8%, respectively; p<0.001) among women 30-45 years old.
Conclusion:
The combination of improved screening coverage and a high single test sensitivity afforded by HPV DNA testing of home-based self-collected swabs may have a greater programmatic impact on cervical cancer mortality reduction compared to programs requiring a pelvic exam.
INTRODUCTION
Cervical cancer screening via cytology (i.e., Pap smears) and treatment of identified precursor lesions are rightly credited with a marked decrease in cervical cancer incidence in industrialized nations. Although a single Pap smear has relatively low sensitivity, frequent testing over the course of a woman's lifetime assures the identification of the precursor lesions. The requirement for repeat testing and multiple follow-up visits make cytology screening strategies virtually impossible to implement in developing nations, which bear the major burden of cervical cancer.
The recognition that infection of the genital tract with high-risk human papillomaviruses (HPV) is the primary cause of cervical cancer has led to the investigation of using HPV assays as an alternative to cytology in screening for cervical cancer. These studies have found that the presence of high-risk HPV (HR-HPV) in cervical swabs collected from the transformation zone of the cervix in the course of a pelvic examination has a higher sensitivity, but lower specificity, for the detection of cervical cancer precursors when compared to cervical cytology (reviewed in (1)). However, screening based on physician-directed cervical sampling still requires a clinic visit by the woman, and a speculum-assisted pelvic exam by a health care provider. These requirements limit broad access to and acceptability of the method in many regions of the developing world. An alternative strategy is screening for HPV from a vaginal swab taken by the women herself, so that the first stage of screening can occur outside of a clinical setting. Studies evaluating the comparability of self-collected vaginal versus clinician-collected cervical swabs have generally shown good agreement for their ability to detect HPV (2-6) but the two methods have not been adequately compared for their performance in detecting cervical neoplasia in a screening setting.
We are conducting studies on HPV and cervical cancer in Medchal Mandal, a peri-urban community in Andhra Pradesh, 45 km north of Hyderabad. The CATCH Study (Community Access to Cervical Health) aims to evaluate the effectiveness of several cervical cancer screening methods (conventional Pap smear, visual inspection after application of acetic acid (VIA), HPV DNA testing from clinician-collected cervical swabs and from self-collected vaginal swabs) incorporated into a health delivery system. In order to assess the suitability of self-collected specimens for primary screening, we conducted a nested analysis of paired clinician-collected cervical and self-collected vaginal samples from 432 women enrolled in the CATCH study to answer three questions: (1) is the detection of high-risk HPVs by the commercially available Hybrid Capture 2 (hc2) test comparable to that by the Roche prototype PCR line blot method; (2) are the clinician-collected samples comparable to the self-collected samples with respect to detection of HPV and detection of cervical intraepithelial neoplasia (CIN) and (3) does home-based self-sample collection increase the rate of participation of the rural women.
METHODS
Study design and population
Using a census list of the entire Medchal Mandal community in Ranga Reddy District in Andhra Pradesh State, India, we approached all eligible women from 42 villages to participate in the CATCH Study from January 2005 to July 2007. Individual house-to-house recruitment with personal invitation was conducted in 35 villages; village level invitation was used in the remaining 7 villages. Women were eligible if they were 25 years or older, had an intact uterus, were mentally competent, and were able and willing to provide informed consent. Information on basic demographics, reproductive, and contraceptive histories, as well as tobacco use, was collected from all consenting women by standardized interviews. Women underwent a standard pelvic examination which included (in order) collection of a conventional Pap smear using an Ayre's spatula and endocervical swab, collection of a cervical swab sample for HPV testing using the Digene cervical sampler, and VIA. Pap smears were fixed immediately in ethanol. Women were also asked to provide a 10cc blood sample and to collect a vaginal self-swab sample for HPV testing. Even though provision of the blood and self-collected swab were optional, 98.6% and 99.96% of women agreed to provide blood and self-swab samples, respectively. All study procedures were reviewed and approved by the institutional review boards at all participating institutions in India and the US.
For the present study, a nested sample enriched for HPV-positive women and women with precursor lesions was selected from the first 896 women enrolled. Selection included all 220 women who had a positive screening test result from one or more of the 3 assays compared in the study (i.e., HPV DNA, Pap smear, and VIA). An additional 250 women who were negative by all 3 tests were randomly selected for a total of 470 women. Of these, 38 were excluded because of missing data (n=8 for hc2 results, n=7 for PCR results) or because the sample was inadequate for PCR analysis (i.e., beta-globin internal control could not be amplified, n=23). The final dataset represents paired clinician-collected and self-collected samples from 432 women, of whom 87 (20.1%) were positive for hc2, 115 (26.6%) had abnormal Pap and/or VIA test results but were hc2 negative, and 230 (53.2%) were negative by all 3 screening assays.
Vaginal self-swab collection
Women were provided verbal and printed diagrammatic instructions for collecting the vaginal swab by the study nurse. The women were asked to insert the swab into their vagina until it met with resistance, turn it 3 times, and remove it. After providing the instructions, the nurse opened the collection kit and handed the swab to the participant, who went unaccompanied into a private room and collected the sample. After sample collection, the participant handed the swab back to the study nurse, who placed it into 1.0 ml of the labeled Digene sample transport medium. The self-swabs used in this analysis were collected immediately prior to the clinician-collected swabs on the same day.
Specimen processing
Both clinician and self-collected specimens were kept at 4°C until processed within 24 hours of collection. After vigorous vortex mixing, the samples were subaliquotted into cryovials and stored at −80°C. One 200 μl aliquot of the clinician-collected sample was sent directly to the Center for DNA Fingerprinting and Diagnostics (CDFD) in Hyderabad for immediate hc2 testing. The remaining aliquots were stored at −80°C until removed for batch testing by PCR (both clinician- and self-collected samples) and hc2 (self-collected sample).
Hybrid Capture 2 testing
The hc2 test was performed according to the manufacturer's instructions except where indicated. In order to preserve the sample for future DNA analyses, a 70 μl aliquot of each sample was denatured in 35 μl of denaturation solution. Seventy-five microliters of the denatured sample was then used as per manufacturer's instructions. Positive results were defined as samples with relative light units per control sample (RLU/CO) ≥ 1.0.
PCR testing
Aliquots were thawed and 90μl was digested in a proteinase K/laureth-12 solution at 65°C for 1 hour. Proteinase was heat inactivated at 95°C for 10 minutes, and DNA precipitated in an ethanol/ammonium acetate solution overnight at −20°C. DNA was pelleted by centrifugation at maximum rpm for 30 minutes at 4°C and supernatant removed with a disposable fine-tip transfer pipette. DNA was air dried and then resuspended in 50 μl of loTE (20 mM Tris-HCl, 1 mM EDTA, pH 8.5). A five microliter aliquot of the extracted DNA was added to a 100μl final volume PCR master mix including the PGMY09/11 consensus primers as previously described (7). Amplification products were hybridized to a reverse line blot allowing detection of the presence and discrimination of 37 HPV genotypes, including HPV 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51-56, 58, 59, 61, 62, 64, 66-73, 81, 82, 82v, 83, 84, and 89 (8).
Participation in home-based screening
Beginning in January 2007, all women, regardless of previous participation in the CATCH Study, were recruited to provide a home-based self-swab sample from 8 randomly selected villages. Women who had not previously enrolled in the study were consented in the field. Effective coverage was estimated on the basis of participation rates for screening (i.e., clinic-based and/or self-sample provision) and follow-up visit of HPV positive participants (i.e., clinic-based colposcopic exam).
Statistical Analysis
The hc2 results reflect a binary outcome defined as ‘positive’ if the sample contains one or more HR- HPV types and ‘negative’ if the sample does not contain a HR- HPV. In order to make the type-specific results for both high and low risk HPVs detected by the Roche prototype line blot assay comparable to the hc2 results, the type-specific data were similarly categorized into a binary outcome. Any PCR test result which included one or more of the HPV types targeted by the hc2 kit (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) and/or HPV 66 were considered to be PCR HR-HPV positive. Samples containing none of these types, regardless of the presence of other low-risk HPV types were defined as PCR HR-HPV negative. HPV 66 was included in the definition of HR-HPV by PCR because HPV66 is considered a bona fide HR-HPV and is easily detected by the hc2 assay via probe cross-hybridization (9). Exclusion of HPV66 from the PCR HR-HPV definition did not substantially change the agreement.
We calculated overall agreement in pairwise comparisons of hc2 versus PCR in clinician-collected and in self-collected samples, and in pairwise comparisons of clinician-collected versus self-collected samples tested by hc2 and by PCR for the detection of HR-HPV. In the pairwise comparison of clinician-collected versus self-collected samples by PCR testing, we also calculated the overall agreement in detection of any HPV (including low risk). For all pairwise comparisons, the agreement beyond chance was estimated with the kappa statistic and corresponding 95% confidence intervals. We used the McNemar's test to detect significant differences in the discordant results (p<0.05), indicating systematic differences by assay or sample type in the detection of HPV.
The correlation between the relative viral load in clinician-collected and self-collected sample pairs was estimated using Pearson's correlation coefficient from the log-transformed RLU/CO values obtained by hc2 testing which are a semi-quantitative estimate of HPV load. Differences in log viral load in cervical and vaginal samples were tested using a paired t-test.
RESULTS
Table 1 summarizes the demographics of the nested sample selected for this sub study. Women in the sub study ranged in age from 25 to 85 years (median age 32 years), 69% had not received a formal education, 87% were currently married, and 96% reported no history of previous Pap screening. In all these respects, they were very similar to the total population of 896 women from which they were sampled.
Table 1.
Demographics of study population (N=432).
| Age (in years) | ||
| 25-29 | 138 (32.9) | |
| 30-34 | 96 (22.9) | |
| 35-39 | 50 (11.9) | |
| 40-44 | 39 (9.3) | |
| 45-49 | 31 (7.4) | |
| 50-54 | 32 (7.6) | |
| 55+ | 34 (8.1) | |
| Religion | ||
| Hindu | 386 (89.3) | |
| Muslim | 28 (6.5) | |
| Christian | 18 (4.2) | |
| Educational status (in years) | ||
| None | 292 (69.4) | |
| 1-8 | 73 (17.3) | |
| 9+ | 56 (13.3) | |
| Marital status | ||
| Currently married | 736 (88.5) | |
| Currently not married | 49 (11.5) | |
| Previous Pap history | ||
| No | 413 (95.8) | |
| Yes | 9 (2.1) | |
| Don't know | 9 (2.1) | |
Missing data: 12 missing due to age; 11 missing due to educational status; 7 missing due to marital status; 1 missing due to pap history.
The agreement between results of hc2 and consensus PCR assays for the detection of HR-HPV is shown in Table 2 for both clinican- and self-collected samples. The overall agreement between the assays was 94.4% (k=0.8) for both clinician- and self-collected samples. PCR was slightly more likely to be HR-HPV positive in the self-collected samples (p=0.04), but PCR and hc2 were equally likely to result in a positive HPV test from the clinician-collected samples (p=0.68).
Table 2.
Agreement in HR-HPV DNA detection: hc2 vs. consensus PCR in clinician (CC) and self-collected (SC) samples.
| N | hc2+ | PCR+ | hc2+/PCR+ | hc2+/PCR− | hc2−/PCR+ | hc2−/PCR− | % agreement |
kappa | McNemar's p-value |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Clinician | 432 | 87 (20.1%) | 89 (20.6%) | 76 (17.6%) | 11 (2.5%) | 13 (3.0%) | 332 (76.9%) | 94.4% | 0.8 | 0.68 |
| Self | 432 | 61 (14.1%) | 71 (16.4%) | 54 (12.5%) | 7 (1.6%) | 17 (3.9%) | 354 (81.9%) | 94.4% | 0.8 | 0.04 |
The overall agreement between clinician-collected and self-collected samples for detection of HR-HPV was similarly high by both hc2 and consensus PCR (90.3% (k=0.7) and 92.6% (k=0.8), respectively). However, clinician-collected specimens were more frequently positive for HR-HPV (20.6% by PCR and 20.1% by hc2) than self-collected specimens (16.4% by PCR and 14.1% by hc2; Table 3, McNemar's p<0.01). This difference was not seen when comparing the detection of any high or low risk HPV by PCR in the paired samples (McNemar's p=0.45, Table 3). At the type-specific level, the PCR resulted in complete type agreement between the clinician-collected cervical and self-collected vaginal specimens in 82.6% of the PCR-positive pairs, and partial agreement (i.e., multiple infections with at least one type in common) in 5.6% of the PCR-positive pairs. There was no agreement in 51 (11.8%) clinician-collected/self-collected pairs.
Table 3.
Agreement in HR-HPV DNA detection: clinician (CC) vs. self-collected (SC) specimens by hc2 and PCR.
| N | CC | SC | CC+/SC+ | CC+/SC− | CC−/SC+ | CC−/SC− | % agreement |
kappa | McNemar's p-value |
|
|---|---|---|---|---|---|---|---|---|---|---|
| hc2 | 432 | 87 (20.1%) | 61 (14.1%) | 53 (12.3%) | 34 (7.9%) | 8 (1.9%) | 337 (78.0%) | 90.3% | 0.7 | <0.01 |
| PCR (HR) | 432 | 89 (20.6%) | 71 (16.4%) | 64 (14.8%) | 25 (5.8%) | 7 (1.6%) | 336 (77.8%) | 92.6% | 0.8 | <0.01 |
| PCR (any type) |
432 | 117 (27.1%) | 112 (25.9%) | 92 (21.3%) | 25 (5.8%) | 20 (4.6%) | 295 (68.3%) | 89.6% | 0.7 | 0.45 |
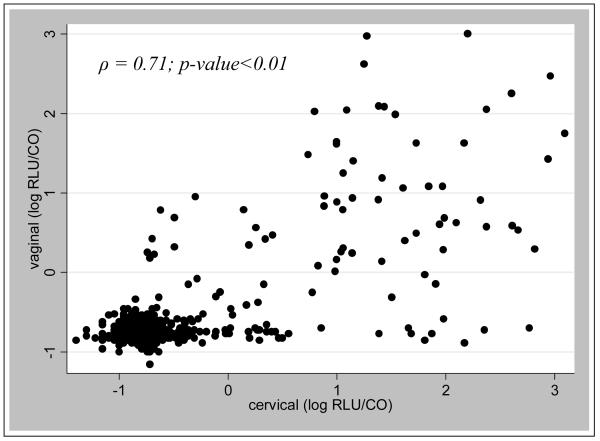
Figure 1 is a scatterplot of the correlation of the relative viral load (measured by hc2 relative light unit per control, or RLU/CO) in clinician-collected and self-collected sample pairs. These results show good correlation in general between the two viral load measures (ρ=0.71, p<0.01). For specimens which were hc2 –positive in both samples (N=53), the median log RLU/CO for cervical specimens was 1.4 fold greater than that for vaginal specimens (p=0.004). There were 12 pairs which had clinician-collected cervical specimens with RLU/COs greater than 10 (median 69.04 RLU/CO) and self-collected vaginal specimens that were negative (Table 4). There were no pairs in which the vaginal specimen had an RLU/CO of 10 or greater but had negative cervical specimen.
Figure 1.
Scatterplot correlation of cervical and vaginal RLU/CO values
Table 4.
Hybrid Capture viral load and genotype signal strength for the 12 clinician-collected cervical positive/self-collected vaginal negative discordant results. RLU/CO indicates a semi-quantitative measure of viral load. HPV type indicated; (n) indicates the relative strength of the hybridization signal; 1=very weak positive, 2=weak positive, 3=strong positive, 4=very strong positive.
| RLU/CO, cervical | HPV type, cervical |
RLU/CO, vaginal | HPV type, vaginal |
|---|---|---|---|
| 24.36 | 52 (3) | 0.17 | HPV negative |
| 31.73 | 31 (4) | 0.49 | 31 |
| 45.4 | IS39 (4) | 0.2 | 51 (1), IS39 (4) |
| 48.19 | 16 (4) | 0.17 | 42 (4) |
| 63.79 | 16 (4) | 0.14 | HPV negative |
| 64.02 | 33 (4) | 0.94 | 33 (2) |
| 74.06 | 16 (3) | 0.17 | HPV negative |
| 80.29 | 16 (3) | 0.72 | 16 (2) |
| 95.74 | 16 (4) | 0.26 | 16 (4) |
| 147.88 | 16 (4) | 0.13 | HPV negative |
| 225.32 | 39 (2) | 0.19 | 64 (2) |
| 582.5 | 35 (4), 70 (1) | 0.2 | HPV negative |
Ability to identify CIN2/3 and cervical cancer
The purpose of screening is to identify high grade CIN and cervical cancer cases. Among the 432 women participating in this study, a total of 11 cases of biopsy-proven high grade disease were identified including 4 cases of CIN2, 3 cases of CIN3, and 4 invasive cancers. The majority of cases were detected by any of the combination of sampling and HPV testing methods, ranging from 100% detection of high grade lesions by PCR from clinician-collected samples to 81.8% detection by hc2 from self-collected samples (Table 5).
Table 5.
Hybrid Capture and PCR genotyping results for 11 cases diagnosed with CIN2/3 or invasive cancer. RLU/CO indicates a semi-quantitative measure of viral load. HPV type indicated; (n) indicates the relative strength of the hybridization signal; 1=very weak positive, 2=weak positive, 3=strong positive, 4=very strong positive.
| Sample | Histology diagnosis |
RLU/CO, cervical |
HPV type, cervical | RLU/CO, vaginal |
HPV type, vaginal |
|---|---|---|---|---|---|
| 1 | CIN2 | 0.32 | 16 (2) | 2.08 | 16 (1) |
| 2 | CIN2 | 97.11 | 16 (4), 18 (4) | 4.88 | 16 (3) , 31 (3) |
| 3 | CIN2 | 5.91 | 51 (3) | 0.56 | 51 (3) |
| 4 | CIN2 | 208.55 | 16 (4) | 8.17 | 16 (4) |
| 5 | CIN3 | 406.92 | 16 (3) , 82 (4) | 3.9 | 16 (2) , 82* |
| 6 | CIN3 | 93.3 | 16 (4) | 12.14 | 16 (4) |
| 7 | CIN3 | 652.46 | 16 (3) | 1.96 | 16 (3) |
| 8 | Invasive cancer | 147.88 | 16 (4) | 0.13 | HPV negative |
| 9 | Invasive cancer | 53.44 | 16 (4) | 42.59 | 16 (3) , 56 (4) |
| 10 | Invasive cancer | 157.97 | 16 (4) , 52 (1) | 1008.98 | 16 (4) , 51 (1) |
| 11 | Invasive cancer | 2.56 | 16 (3) | 2.96 | 16 (3) |
Comparison of participation in clinic vs. home-based screening
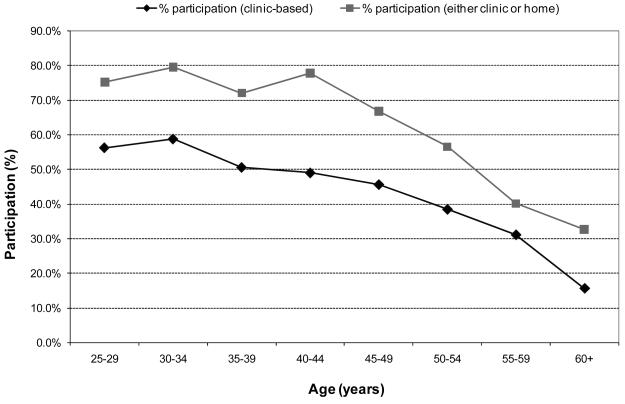
In the 8 villages where women were recruited to provide a home-based self-collected sample regardless of previous participation, clinic based screening participation was 45.6% (257/564) and home-based participation was 57.5% (324/564). Screening participation was dependent on age (Figure 2); women over the age of 45 were less likely to participate in either clinic or home based screening compared to women 45 years and younger (44.8% vs. 76.6%; p<0.001). Among women likely to be targeted for a once- or twice-in-a-lifetime screening program (i.e., those aged 30-45 years), participation in home based screening (71.5%) was significantly higher than clinic based screening (53.8%; p<0.001). Total screening coverage for women 30-45 years by either clinic or home based self collected swab for HPV DNA testing was 76.3%.
Figure 2.
Participation in cervical cancer screening among the 8 villages where clinic and home-based participation among total eligible population was assessed; clinic-only (circles) versus clinic or home-based (squares).
Discussion
The major aim of this study was to assess the suitability of HPV DNA analysis on home-based self-collected swabs as a primary screening strategy for cervical cancer screening in developing nations. The study was population-based and conducted in a peri-urban community in Andhra Pradesh, India. The women had virtually no previous history of cervical cancer screening and few had any formal education or financial resources. The self-collected swabs were taken by the women, without any supervision, in the privacy of their homes or in private in the clinic. The conditions of the study were similar to what will be encountered in implementing any future cervical cancer screening strategy in rural communities in developing nations.
The women showed no hesitation in accepting self-collection; virtually all women who had agreed to clinic-based, physician-directed examination agreed to self collection and the laboratory data show that self-collection was successfully performed (as indicated by detection of human DNA) by nearly all (96.5%) participants.
One aim of the study was to examine if the more complex PCR assay offers any advantage over the simpler, commercially available hc2 assay. In specimens collected at both vaginal and cervical sites, DNA detection rates by the two assays were very similar, suggesting that either signal or target amplified technologies are compatible with field based self-collection protocols.
Numerous previous studies have concluded that HPV DNA detection in cervical swabs has a high sensitivity for detection of cervical cancer precursor lesions; the sensitivity estimates for HPV DNA detection in the cervix are 66-100% as compared to 44-78% for cervical cytology (1). A second aim of our study was to assess how HPV DNA detection in self-collected swabs compares with HPV DNA detection in clinician-collected swabs. Although there was a good correlation between positive and negative results at the two sites, there were some systematic differences. The DNA detection rate in self-collected specimens was 25-42% less than that in clinician-collected specimens, and the amount of virus in HPV-positive self-collected specimens was about 1.4 fold lower than that in the paired HPV-positive clinician-collected specimens. Most of the discordant results could be attributed to Poisson sampling error due to low viral load; 8/8 (100%) of hc2 negative clinician-collected /hc2 positive self-collected samples and 21/33 (63.6%) of hc2-positive clinician-collected /hc2 negative self-collected samples had an RLU/CO<10.0. This variability is likely to occur on repeat clinician sampling. However, 36.4% of clearly hc2-positive clinician-collected samples were missed in the self-collected sample (nearly half of which were also PCR-negative). These discrepancies represent a systematic difference in the sampling methods, which likely reflect a cervical infection that was insufficiently sampled by the self-collected vaginal swab. The self-collected sample contains a sample of vulvar and vaginal cells, as well as a sample of cervical cells that are obtained either directly from the brush touching the cervix, or indirectly from pools of exfoliated cervical cells in the vaginal vault. As such, the self-collected specimen is usually, but not always, a good representation of cervical infection (4). Such ‘false negative’ self-collected vaginal samples will impact cervical cancer screening programs only if they are disproportionately occurring in cases of high grade neoplasia and cancer. In our study, tests of clinician-collected samples of the 11 women who had cervical precursor lesions or cervical cancer, the PCR assay was positive in all instances and the hc2 assay was positive in all but one instance; in one case of HPV16-positive CIN2, the hc2 assay was negative. When testing the self-collected samples, the viral load was systematically lower compared to the clinician-collected samples; one HPV51 positive CIN2 was missed by hc2 and one invasive cancer was missed by both hc2 and PCR. While the sample size was small for precise estimation of the test sensitivities, our data suggests that even the least sensitive option (81.8% from self-collected sample tested by hc2) remains significantly better than the Pap reference standard. In addition, a similar number of women screened positive, suggesting that the specificity of the test will remain acceptably high.
The absolute reduction in cervical cancer mortality through screening is dependent not only on test sensitivity, but also on the population level coverage (i.e., how many women are screened and treated). Coverage among women 30-45 years increased from 53.8% to 71.5% when offering home-based self-collection versus clinic-based cervical collection in the 8 villages where we examined this question. Using a published model of reduction of lifetime risk of cervical cancer as a function of screening coverage and test sensitivity in twice-in-a-lifetime screening programs (10), our clinic-based program (53.8% coverage, estimated 90.9% sensitivity by hc2) would predict an ∼20% reduction in lifetime risk of cervical cancer. Comparatively, our home-based program using self-collected samples (71.5% coverage, estimated 81.8% sensitivity by hc2) would predict a nearly 30% reduction in lifetime cancer risk. Therefore, a loss in sensitivity combined with significant increases in screening coverage when using self-collected samples for HPV testing may yield comparable, if not improved, reductions in cervical cancer mortality compared to clinic-based HPV screening programs. These results will, of course, be contingent on the ability of such programs to provide appropriate follow-up and treatment of screen-positive women.
At present, the cost of the HPV DNA test is the primary factor prohibiting broader application in cervical cancer screening programs, using either clinician- or self-collected samples. Recently, a new low cost HPV DNA test, careHPV (Qiagen) showed comparable performance to the hc2 test used in our study in both self- and clinician-collected samples (11). Broader availability of this or other validated and inexpensive HPV tests may make HPV-based screening programs increasingly more feasible. In our population which has a 10% HR-HPV prevalence, 90% of women would provide a simple self-collected sample with no further clinical follow-up. Available resources could be then appropriately targeted to the HPV positive women at highest risk.
Acknowledgements
We would like to thank Y.S. Chowdry, Madhu Mohan, M.K. Agarwal, Vijay Yeldandi, Surendar Reddy, Malakonda Reddy, and the administration and staff at Mediciti Institute of Medical Sciences for their support and cooperation; Dorothy Rosenthal, Doug Clark, and Kathy Johnson for cytopathology training; Neerja Bhatla, Dr. B.M. Nene, and Dr. R. Sankaranarayanan for advice and VIA training; Vivian Go, Sudha Sivaram, Linda Groetzinger, and C Sethu Laksmi and YRG CARE for consultation and training in qualitative research; D. Sunita for educational artwork; Dinesh Gupta and Digene Corporation for discounted reagents; and Janet Kornegay, Sean Boyle, and Roche Molecular Systems for PCR reagents. We also express our gratitude to the community health volunteers (CHVs) and women of Medchal Mandal for their cooperation and participation in this study.
Financial Support: This study was funded under the joint INDO-US collaborative program of Department of Biotechnology, Ministry of Science and Technology, Government of India and the NIH, USA (BT/IN/US/CRHR/PP/2002), and the NIH SPORE P50 CA98252.
References
- 1.Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of Human Papillomavirus-Based and Other Novel Options for Cervical Cancer Screening in Developed and Developing Countries. Vaccine. 2008;26:K29–K41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Ogilvie GS, Patrick DM, Schulzer M, et al. Diagnostic accuracy of self collected vaginal specimens for human papillomavirus compared to clinician collected human papillomavirus specimens: a meta-analysis. Sex Transm Infect. 2005;81:207–12. doi: 10.1136/sti.2004.011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petignat P, Faltin DL, Bruchim I, Tramer MR, Franco EL, Coutlee F. Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecol Oncol. 2007;105:530–5. doi: 10.1016/j.ygyno.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Winer RL, Feng Q, Hughes JP, et al. Concordance of self-collected and clinician-collected swab samples for detecting human papillomavirus DNA in women 18 to 32 years of age. Sex Transm Dis. 2007;34:371–7. doi: 10.1097/01.olq.0000240315.19652.59. [DOI] [PubMed] [Google Scholar]
- 5.Jones HE, Allan BR, van de Wijgert JH, et al. Agreement between self- and clinician-collected specimen results for detection and typing of high-risk human papillomavirus in specimens from women in Gugulethu, South Africa. J Clin Microbiol. 2007;45:1679–83. doi: 10.1128/JCM.02369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safaeian M, Kiddugavu M, Gravitt PE, et al. Comparability of self-collected vaginal swabs and physician-collected cervical swabs for detection of human papillomavirus infections in Rakai, Uganda. Sex Transm Dis. 2007;34:429–36. doi: 10.1097/01.olq.0000243623.67673.22. [DOI] [PubMed] [Google Scholar]
- 7.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castle PE, Solomon D, Wheeler CM, Gravitt PE, Wacholder S, Schiffman M. Human Papillomavirus Genotype Specificity of Hybrid Capture 2. J Clin Microbiol. 2008 doi: 10.1128/JCM.00824-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldie SJ, Kim JJ, Myers E. Chapter 19: Cost-effectiveness of cervical cancer screening. Vaccine. 2006;24(Suppl 3):S164–70. doi: 10.1016/j.vaccine.2006.05.114. [DOI] [PubMed] [Google Scholar]
- 11.Qiao Y-l, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. The Lancet Oncology. 2008;9:929–36. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]