Abstract
Background
Hepatitis E virus (HEV) is prevalent and causes disease worldwide, but its epidemiological profile is only partially understood.
Methods
We used an enzyme immunoassay to measure anti-HEV immunoglobulin G antibodies in 18,695 serum samples collected in the Third National Health and Nutrition Examination Survey. We calculated estimates of HEV seroprevalence and examined associations with putative risk factors.
Results
The seroprevalence of HEV in the civilian noninstitutionalized United States (US) population during the period from 1988 through 1994 was 21.0% (95% confidence interval [CI], 19.0%–22.9%). Among US-born individuals, males, non-Hispanic whites, and individuals residing in the Midwest and/or in metropolitan areas had the highest seroprevalence estimates. Having a pet in the home (odds ratio [OR], 1.19 [95% CI, 1.01–1.40]) and consuming liver or other organ meats more than once per month (OR, 1.38 [95% CI, 1.01–1.88]) were significantly associated with increased odds of HEV seropositivity.
Conclusions
Exposure to HEV is common in the US population, although hepatitis E is rarely reported. Having pets and consuming organ meats may play a role in HEV transmission in the United States, but other mechanisms of transmission may also exist. HEV may be considered a possible etiologic agent of acute and chronic hepatitis in US patients reporting no travel history.
Hepatitis E virus (HEV) is a major cause of acute viral hepatitis in developing countries [1, 2]. HEV, which is commonly spread by contaminated water in developing countries [3], causes both sporadic cases and large epidemics of hepatitis E. HEV infection is also associated with significant maternal and fetal morbidity and mortality, particularly during the third trimester of pregnancy, in developing countries [4–6].
In industrialized countries, HEV is occasionally implicated as the cause of sporadic cases of acute hepatitis. Some of these cases can be traced to travel to developing countries, but others have occurred among individuals reporting no foreign travel or contact with travelers [7–11]. Although, to date, autochthonous cases in industrialized countries remain few in absolute number, they have generated considerable interest in the scientific community. In contrast to travel-related cases, which have been primarily associated with HEV genotypes 1 and 2 (the principal causes of waterborne hepatitis E in developing countries), autochthonous cases of hepatitis E in industrialized countries are generally caused by HEV genotypes 3 and 4 [7–10]. In addition to causing occasional cases of human disease, these genotypes also circulate widely in swine populations [12] and can cause mild hepatitis in experimentally infected nonhuman primates [13]. These findings, along with other lines of evidence [13–17], suggest that some cases of autochthonous hepatitis E in industrialized countries could reflect zoonotic transmission. Questions persist, however, regarding the origin of autochthonous hepatitis E among persons in developed countries who do not report any exposure to animal HEV reservoirs [7, 8, 18–20]. Furthermore, sero-surveys have documented substantial HEV seroprevalence in blood donors in developed countries [21–26]; the mode of exposure and clinical significance of these infections are not well understood.
To better understand the epidemiological profile of HEV in developed countries, we tested a nationally representative sample of the United States (US) population for anti-HEV immunoglobulin G (IgG) antibodies, by use of a highly sensitive and specific enzyme immunoassay (EIA). We documented overall and subgroup-specific HEV seroprevalences in the United States and examined associations between HEV sero-positivity and putative risk factors.
MATERIALS AND METHODS
Study population
From 1988 through 1994, the National Center for Health Statistics (NCHS) conducted the Third National Health and Nutrition Examination Survey (NHANES III). NHANES III was a cross-sectional study of the civilian noninstitutionalized US population that was designed to provide national statistics on the health and nutritional status of the general household population through household interviews, standardized physical examinations, and the collection of biological samples in special mobile examination centers. To ensure adequate sample sizes of specific subgroups of the US population, Mexican Americans, non-Hispanic blacks, children, and elderly individuals were oversampled. Details of the design and methods of NHANES III are available elsewhere [27].
Of the 24,713 examined NHANES III study participants ≥6 years of age, 18,695 participants had serum samples available for evaluation in this study. Study participants for whom serum samples were not available were more likely to be male (P < .01), younger (P < .01), and of a different race/ethnicity (P < .01) than participants for whom serum samples were available. Written informed consent was obtained from all NHANES III study participants, and the institutional review boards of the NCHS and the Johns Hopkins Bloomberg School of Public Health (hereafter referred to as “Johns Hopkins”) approved the present study before it was initiated.
Laboratory methods
In the present study, we used an “in-house” EIA developed at the US National Institutes of Health to test NHANES III serum samples for anti-HEV IgG antibodies. The assay, which uses a truncated 56-kDa recombinant HEV capsid protein expressed in insect cells as antigen, was performed as described elsewhere [28], with modifications [29]. The assay has been used extensively for measuring anti-HEV in studies of HEV infection in humans and swine and for evaluation of the efficacy of hepatitis E vaccine in nonhuman primates [30–33]. The antibodies measured by this assay include the principal, if not only, neutralizing antibody to the virus [34].
In brief, wells of polystyrene microwell plates (Nunc 468667) were coated with recombinant HEV antigen diluted in a carbonate- bicarbonate (pH 9.6) buffer and allowed to bind to the solid phase at room temperature for ~18 h. The antigen concentration was ~0.025 µg/well (a concentration slightly lower than that used in previous investigations). Next, unbound antigen was removed, and the wells were washed twice with a wash solution (KPL 50-63-00) that contained 0.02% Tween 20 in 0.002 mol/L imidazole-buffered saline by use of an automated plate washer. Wells were blocked with 1% bovine serum albumin/gelatin (KPL 50-61-00) at 37°C for 1 h to prevent nonspecific binding. The buffer was then suctioned off, and capture plates were stored at −80°C with desiccant in plastic bags.
Immediately before use, plates were washed twice. All NHANES III serum samples and negative control samples were pre-diluted with blocking buffer to a net starting dilution of 1:20 in a separate noncoated microwell plate, and 10 µL of each diluted sample was transferred to corresponding test plate wells that contained 90 µL of blocking buffer, for a final testing dilution of 1:200. The test plates were incubated at 37°C for 30 min. After plates were washed 4 times to remove unbound material, 100 µL of horseradish peroxidase–labeled anti-IgG (KPL 074-1006) was added to each well, and plates were incubated at 37°C for 30 min. After this incubation, unbound conjugate was removed by washing. To start the enzymatic color reaction, 100 µL of ABTS substrate (KPL 50-66-00) was added, and absorbance was read at 405 nm every 2 min for 30 min. A time point was selected that most closely matched our acceptability criteria for the slope, intercept, and R2 value of a secondary anti-HEV standard series and that had valid values for the blank and negative control samples.
Negative control serum samples were obtained from HEV-naive chimpanzees. The anti-HEV standard series consisted of serial dilutions of a secondary anti-HEV standard, which is modeled to match the World Health Organization (WHO) 95/584 anti-HEV preparation (available from the National Institute for Biological Standards and Control, Hertfordshire, England). The cutoff level for each test run was set by the 0.01–WHO unit sample in the anti-HEV standard series, which is ~2.5 times the mean optical density measurement of the negative control samples. NHANES III serum sample values were expressed, after subtraction of background optical density values, as the ratio of the NHANES III sample optical density value to the 0.01–WHO unit sample optical density value. Samples with ratios >1.0 were classified as seropositive.
All NHANES III serum samples were tested at Johns Hopkins by a single trained technician. The samples were tested only once. After testing at Johns Hopkins was completed, a random sample of 109 of the putatively seropositive serum samples was sent to the Hepatitis Viruses Section, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health for supplemental testing. This supplemental testing included retesting of samples by use of the EIA system described above and conducting a variation of an anti-HEV antigen–specific blocking assay described elsewhere [35]. In brief, each sample and the control samples were preincubated with 1.0 µg/mL of either phosphate-buffered saline or HEV antigen (Sar55) at 25°C for ~18 h. Next, the samples were tested using the standard anti-HEV EIA procedure described above. Optical density values for phosphate-buffered saline–treated versus HEV antigen–treated samples were compared, and the samples that showed ≥40% blocking were classified as reactive and specific. We chose the criterion of ≥40% blocking on the basis of the average blocking rate of a low-level (0.004–WHO unit) positive standard.
Of the 109 samples found to be putatively seropositive by the technician at Johns Hopkins, 88 were confirmed to have positive results by use of the blocking assay, 7 were considered to have indeterminate results, and 14 were found to have negative results. On the basis of this information and a comparison of the EIA results, a decision was made to adjust the seropositivity cutoff level slightly upward, to increase specificity at a cost to sensitivity. This higher cutoff level for seropositivity translated to a mean of 0.016 WHO units (standard deviation [SD], 0.0025 WHO units). Of the 109 samples originally designated as being seropositive, 84 had ratios greater than the higher cutoff level for seropositivity. Of these 84 samples, 77 were confirmed to have positive results by use of the blocking assay, 4 were considered to have indeterminate results, and 3 were found to have negative results. We estimated that the testing performed at Johns Hopkins, using the higher seropositivity cutoff level, had a positive predictive value of 92% (results were positive for 77 of 84 samples), compared with the blocking assay conducted at the Hepatitis Viruses Section. All laboratory testing was conducted in a blinded fashion: that is, laboratory results were linked to the NHANES III data set only after the completion of all laboratory testing.
Definitions
Race/ethnicity was defined by self-report and was categorized as non-Hispanic white, non-Hispanic black, and Mexican American. Persons not fitting these categories were classified as belonging to the “Other” race/ethnicity category. Region was defined by standard US Census Bureau categories: Northeast, Midwest, South, and West. Residence in central or fringe counties of metropolitan areas with a population of ≥1 million was defined as metropolitan residence. Nonmetropolitan residence was defined as residence in all other areas not defined as a metropolitan area. This definition, developed by the US Department of Agriculture, was used by the NCHS to assign a metropolitan or nonmetropolitan residence category to each NHANES III participant. Poverty was defined as a poverty income ratio of <1.0. A poverty income ratio was computed by NCHS for each NHANES III participant, by use of the observed family income category, the age of the family reference person, the calendar year, and the Census Bureau poverty threshold value. Because of nonresponses regarding income, the potential for bias in the poverty income ratio may be high. A crowding index variable was created by dividing the number of household residents by the total number of reported household rooms (excluding bathrooms) and was expressed as the number of persons per room.
Statistical methods
We calculated the overall seroprevalence of HEV as well as seroprevalence estimates specific to sex, race/ethnicity, geographic region, and country of birth. Seroprevalence estimates were weighted (1) to denote the total civilian noninstitutionalized US household population in the age groups covered, (2) to account for oversampling, and (3) to account for nonresponse to the household interview and physical examination but not for nonresponse to phlebotomy. The weights were further ratio adjusted (by age, sex, and race/ethnicity) to the US population control estimates from the Current Population Survey adjusted for undercounts [36]. We tested bivariate associations of sex, race/ethnicity, country of birth, and geographic region with HEV seropositivity, using unadjusted logistic regression models. We plotted HEV seroprevalence by age within the following strata: sex, race/ethnicity, country of birth, and geographic region.
We calculated age-adjusted HEV seroprevalence estimates for selected demographic variables for the total US-born population, and we stratified those estimates by race/ethnicity. Age-adjustment procedures used the 2000 US census population as the standard population, as recommended by the NCHS. We tested associations between selected demographic variables and HEV seropositivity for the US-born population and for racial/ethnic groups in separate logistic regression models, adjusting only for age (8 age groups).
We calculated HEV seroprevalence estimates for selected risk factors for the US-born population and tested associations between risk factors and HEV seropositivity in separate logistic regression models, adjusting for age (8 age groups), sex, race/ethnicity, and geographic region of residence. All statistical analyses were performed using SAS software (version 9.1; SAS Institute) SURVEY procedures. These procedures allow for specification of NHANES III design parameters as well as sample weights. Standard errors associated with these procedures were estimated via Taylor series linearization.
RESULTS
On the basis of the testing of 18,695 NHANES III study participants ≥6 years of age, the seroprevalence of HEV in the civilian noninstitutionalized US population from 1988 through 1994 was 21.0% (95% confidence interval [CI], 19.0%–22.9%). Seroprevalence estimates specific to sex, race/ethnicity, country of birth, and geographic region are shown in table 1. As seen in table 1, the seroprevalence of HEV among individuals born outside the United States was significantly higher than that among individuals born in the United States.
Table 1.
Prevalence of antibody to hepatitis E virus (anti-HEV) for selected demographic variables among participants (age, ≥6 years) in the Third National Health and Nutrition Examination Survey, 1988–1994.
| Variable | Samples tested, no. (n = 18,695) |
Positive for anti-HEV,a % (95% CI) |
|---|---|---|
| Sex | ||
| Female | 10,124 | 20.4 (18.3–22.5) |
| Male | 8571 | 21.6 (19.3–23.9) |
| Race/ethnicity | ||
| Non-Hispanic | ||
| White (reference) | 7052 | 22.1 (19.8–24.4) |
| Black | 5312 | 14.5 (13.1–15.9)b |
| Mexican American | 5527 | 20.3 (18.3–22.3) |
| Other | 804 | 20.2 (16.5–23.9) |
| Country of birthc | ||
| United States (reference) | 15,051 | 20.1 (18.1–22.0) |
| Mexico | 2357 | 30.9 (28.9–32.9)b |
| Other | 1233 | 26.2 (22.9–29.5)b |
| Region of residence | ||
| South (reference) | 8168 | 14.7 (12.3–17.0) |
| Northeast | 2372 | 20.8 (16.5–25.1)b |
| Midwest | 3655 | 26.6 (22.4–30.8)b |
| West | 4500 | 25.0 (20.9–29.1)b |
NOTE. Seroprevalence estimates were weighted (1) to denote the total civilian noninstitutionalized US household population in the age groups covered, (2) to account for oversampling, and (3) to account for nonresponse to the household interview and physical examination but not for nonresponse to phlebotomy. CI, confidence interval; reference, reference group.
Of 18,695 samples tested, the percentage (95% CI) of samples that were anti-HEV positive was 21.0% (19.0%–22.9%).
P < .05 compared with the reference group.
The total no. of samples shown is <18,695, because of incomplete reporting.
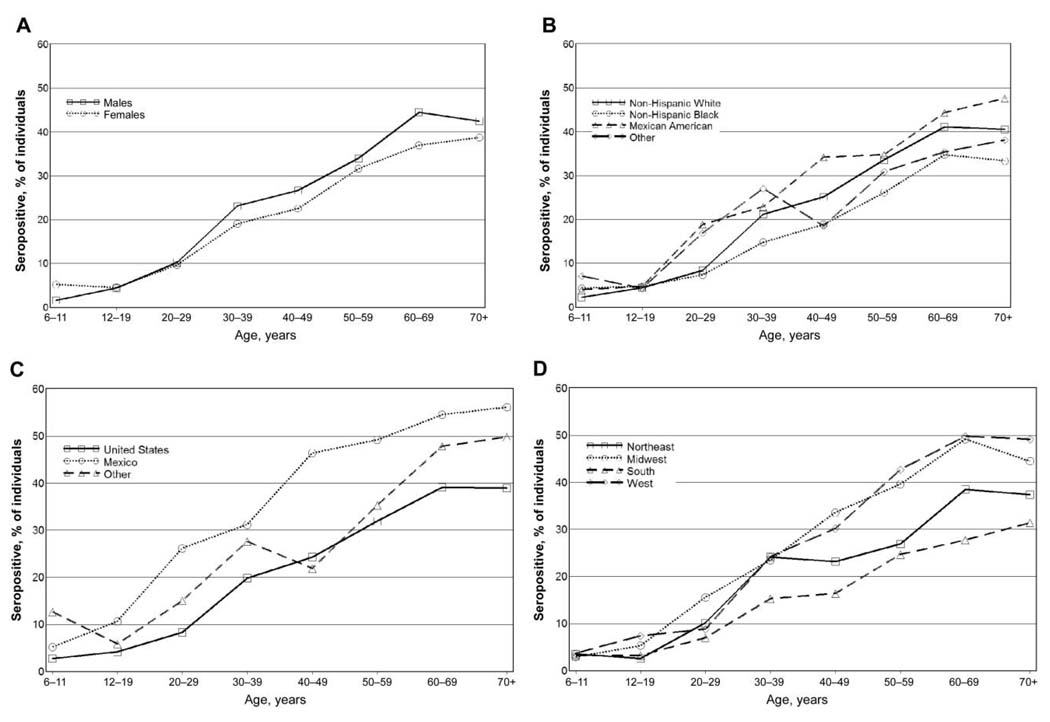
Figure 1 shows the seroprevalence of HEV by age group for individuals in different sex, race/ethnicity, country of birth, and geographic region strata. As seen in each panel of figure 1, HEV seropositivity was generally rare among children and generally increased with age. Among individuals born in the United States, the increase with age was most marked between the age group of 20 to 29 year olds and that of 30 to 39 year olds. Among individuals born in Mexico, the first marked increase in seropositivity occurred between the age group of 12 to 19 year olds and that of 20 to 29 year olds.
Figure 1.
Hepatitis E virus seroprevalence, by age group, for individuals in different sex (A), race/ethnicity (B), country of birth (C), and geographic region (D) strata.
Table 2 shows age-adjusted seroprevalence estimates for the total US-born population and for non-Hispanic whites, non- Hispanic blacks, and Mexican Americans, for selected demographic variables. As seen in table 2, males had higher seroprevalence estimates than did females in each racial/ethnic group analyzed. Individuals living in the South had the lowest regional seroprevalence estimates, and individuals living in the Midwest had the highest regional seroprevalence estimates in each racial/ethnic group analyzed. An equally consistent finding is that seroprevalence estimates were higher among individuals living in metropolitan areas than among those living in non-metropolitan areas, for each racial/ethnic group analyzed.
Table 2.
Age-adjusted prevalence of antibody to hepatitis E virus (anti-HEV) for selected demographic variables among only United States–born participants (age, ≥6 years) in the Third National Health and Nutrition Examination Survey, 1988–1994, according to race/ethnicity.
| Non-Hispanic participants | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total study populationa |
White | Black | Mexican American participants |
|||||
| Variable | Tested,b no. |
Positive for anti-HEV, % (95% CI) |
Tested,b no. |
Positive for anti-HEV, % (95% CI) |
Tested,b no. |
Positive for anti-HEV, % (95% CI) |
Tested,b no. |
Positive for anti-HEV, % (95% CI) |
| Sex | ||||||||
| Female | 8256 | 18.6 (16.7–20.5) | 3681 | 19.4 (17.2–21.5) | 2783 | 16.0 (14.7–17.3) | 1673 | 14.6 (11.4–17.8) |
| Male | 6795 | 20.7 (18.5–22.9)c | 3026 | 21.1 (18.6–23.6) | 2199 | 17.2 (14.9–19.5) | 1461 | 16.9 (14.2–19.6) |
| Region of residence | ||||||||
| South (reference) | 7055 | 13.8 (11.7–16.0) | 2669 | 13.9 (11.3–16.5) | 2877 | 12.8 (11.0–14.7) | 1463 | 13.9 (11.8–16.1) |
| Northeast | 1880 | 19.4 (15.2–23.6)c | 1107 | 19.4 (15.0–23.9)c | 675 | 20.9 (16.3–25.5)c | 15 | …d |
| Midwest | 3263 | 24.6 (20.4–28.8)c | 1905 | 24.9 (20.4–29.3)c | 1078 | 21.4 (18.3–24.5)c | 260 | 21.6 (14.2–29.0)c |
| West | 2853 | 22.8 (18.4–27.3)c | 1026 | 24.5 (19.9–29.2)c | 352 | 18.2 (14.2–22.3)c | 1396 | 16.1 (11.9–20.2) |
| Persons per room, no. | ||||||||
| <0.8 (reference) | 10,568 | 19.5 (17.5–21.5) | 5828 | 20.0 (17.8–22.2) | 3065 | 16.5 (15.0–18.0) | 1546 | 16.9 (13.7–20.2) |
| 0.8–1.2 | 3344 | 20.1 (16.7–23.4) | 783 | 22.3 (17.5–27.1) | 1462 | 16.6 (14.0–19.3) | 1042 | 11.8 (8.2–15.5)c |
| >1.2 | 1102 | 18.3 (12.7–24.0) | 75 | …d | 444 | 14.6 (9.2–20.1)c | 542 | 15.8 (10.0–21.7) |
| Residence | ||||||||
| Nonmetropolitane | 8415 | 15.6 (13.3–17.9) | 4254 | 16.1 (13.4–18.7) | 2388 | 13.2 (10.9–15.4) | 1691 | 13.6 (11.2–16.0) |
| Metropolitanf | 6636 | 24.4 (21.6–27.2)c | 2453 | 25.4 (22.4–28.5)c | 2594 | 19.2 (17.3–21.2)c | 1443 | 17.7 (13.6–21.8) |
| Poverty index | ||||||||
| At or above poverty line | 11,699 | 19.8 (17.8–21.8) | 6051 | 20.3 (18.1–22.5) | 3349 | 16.4 (14.7–18.1) | 2133 | 16.1 (13.1–19.2) |
| Below poverty line | 3352 | 16.4 (14.1–18.7)c | 656 | 16.8 (13.1–20.5) | 1633 | 16.4 (13.5–18.1) | 1001 | 13.6 (10.9–19.2) |
NOTE. Seroprevalence estimates were weighted (1) to denote the total civilian noninstitutionalized US household population in the age groups covered, (2) to account for oversampling, and (3) to account for nonresponse to the household interview and physical examination but not for nonresponse to phlebotomy. CI, confidence interval; reference, reference group.
Includes individuals whose race/ethnicity was “Other.”
Totals vary because of incomplete reporting.
P < .05 compared with the reference group.
No estimate was available because of the small sample size.
Defined as residence in all other areas not defined as a metropolitan area.
Defined as residence in central or fringe counties of metropolitan areas with a population of ≥1 million.
Table 3 shows the seroprevalence estimates for the total US-born population and the adjusted odds ratios (ORs) for selected risk factors in relation to HEV seropositivity. Having a well as the source of tap water (OR, 0.78 [95% CI, 0.63–0.97]) and having hepatitis A virus (HAV) seropositivity (OR, 0.80 [95% CI, 0.70–0.92]) were associated with significantly lower odds of HEV seropositivity. Having a pet in the household (OR, 1.19 [95% CI, 1.01–1.40]), having a dog in the household (OR, 1.22 [95% CI, 1.04–1.43]), consuming liver or other organ meats more than once per month (OR, 1.38 [95% CI, 1.01–1.88]), and having hepatitis C virus seropositivity (OR, 1.71 [95% CI, 1.07–2.74]) were associated with significantly higher odds of HEV seropositivity. No other statistically significant associations were observed between risk factors and HEV seropositivity.
Table 3.
Prevalence and relative odds of antibody to hepatitis E virus (anti-HEV) for selected risk factor variables among only United States–born participants (age, ≥6 years) in the Third National Health and Nutrition Examination Survey, 1988–1994.
| Variable | Participants tested,a no. |
Positive for anti-HEV, % (95% CI) |
ORb (95% CI) |
|---|---|---|---|
| Military servicec | |||
| No | 9922 | 20.8 (18.7–22.9) | 1.0 |
| Yes | 1998 | 33.0 (28.6–37.4) | 1.21 (0.99–1.48) |
| Source of tap water | |||
| Water company | 11,782 | 20.3 (18.2–22.5) | 1.0 |
| Well | 1691 | 18.9 (14.3–23.5) | 0.78 (0.63–0.97) |
| Spring | 40 | 18.6 (0.05–31.7) | 0.64 (0.30–1.37) |
| Sex partners in lifetime,c no. | |||
| 1–10 | 5944 | 18.8 (16.5–21.2) | 1.0 |
| >10 | 1604 | 18.9 (15.5–22.3) | 0.91 (0.73–1.14) |
| Ever had male-to-male sexc | |||
| No | 5034 | 23.9 (21.2–26.6) | 1.0 |
| Yes | 258 | 23.1 (15.2–30.9) | 1.09 (0.68–1.74) |
| Ever used cocaine or crackc | |||
| No | 10,968 | 23.6 (21.4–25.9) | 1.0 |
| Yes | 998 | 16.8 (13.2–20.4) | 0.95 (0.75–1.21) |
| Have a pet | |||
| Any | |||
| No | 9589 | 20.2 (18.1–22.3) | 1.0 |
| Yes | 5461 | 20.0 (17.4–22.4) | 1.19 (1.01–1.40) |
| Dog | |||
| No | 11,616 | 19.7 (17.8–21.7) | 1.0 |
| Yes | 3433 | 20.9 (18.0–23.8) | 1.22 (1.04–1.43) |
| Cat | |||
| No | 12,709 | 20.0 (17.9–22.1) | 1.0 |
| Yes | 2340 | 20.2 (17.0–23.5) | 1.12 (0.90–1.38) |
| Eat meat, no. of times/monthd | |||
| Bacon, sausage, or processed meats | |||
| 0–10 | 9581 | 22.2 (20.0–24.6) | 1.0 |
| 11–20 | 1754 | 17.8 (14.8–20.9) | 0.96 (0.75–1.21) |
| >20 | 1965 | 19.8 (16.2–23.4) | 0.89 (0.65–1.22) |
| Liver or other organ meats | |||
| 0 | 9875 | 20.4 (18.3–22.5) | 1.0 |
| 1 | 1763 | 25.2 (21.5–28.9) | 1.15 (0.96–1.36) |
| >1 | 1656 | 26.5 (21.1–31.8) | 1.38 (1.01–1.88) |
| Pork or ham | |||
| 0–5 | 10,600 | 21.9 (19.5–24.3) | 1.0 |
| 6–10 | 1710 | 18.3 (17.1–24.4) | 0.98 (0.75–1.27) |
| >10 | 992 | 16.1 (13.3–19.0) | 0.78 (0.60–1.02) |
| HCV antibody | |||
| No | 14,540 | 19.8 (17.8–21.8) | 1.0 |
| Yes | 292 | 29.1 (20.1–38.0) | 1.71 (1.07–2.74) |
| HBV core antibody | |||
| No | 14,020 | 19.7 (17.6–21.6) | 1.0 |
| Yes | 845 | 28.5 (22.2–34.8) | 1.37 (1.00–1.86) |
| HAV antibody | |||
| No | 9170 | 18.0 (15.7–20.3) | 1.0 |
| Yes | 5693 | 25.6 (23.5–27.7) | 0.80 (0.70–0.92) |
NOTE. Seroprevalence estimates were weighted (1) to denote the total civilian noninstitutionalized US household population in the age groups covered, (2) to account for oversampling, and (3) to account for nonresponse to the household interview and physical examination but not for nonresponse to phlebotomy. CI, confidence interval; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; OR, odds ratio.
Totals vary because of incomplete reporting.
Adjusted for age, sex, race/ethnicity, and region of residence.
Among participants ≥17 years of age.
Among participants ≥12 years of age.
To better understand the negative association between HAV and HEV seropositivity, we tested for statistical interactions between HAV and each adjustment variable (e.g., age, sex, race/ethnicity, and region of residence). None of the interaction terms associated with these tests was statistically significant (data not shown).
DISCUSSION
The results of the present study suggest that exposure to HEV is common in the general US population. The seroprevalence estimate observed in the present study is similar to estimates observed in studies of US blood donors presented elsewhere [21, 22], but it is higher than estimates reported from other developed countries [23–26, 37]. Two factors may help explain these differences, although further research on this topic is needed. First, the sensitivity and specificity of anti-HEV assays vary significantly. Previous studies found a higher prevalence of anti-HEV by use of the EIA used in this study than by use of commercial assays [38, 39]. This finding may have been the result of the fact that the anti-HEV EIA used in the present study uses a recombinant open-reading frame 2–derived capture antigen that detects antibody to HEV genotypes 1 and 3 equally well [30]. This assay may also detect remote infections better than commercial assays that were developed to diagnose acute HEV infection [39]. Second, the geographic differences in the HEV seroprevalence within and between developed countries may be pronounced. Evidence of significant geographic heterogeneity has been noted elsewhere [25, 37], as well as in this study.
Despite the high HEV seroprevalence observed in the United States in this and in previous studies, clinical hepatitis E of autochthonous origin is rarely reported in the United States [7, 11, 40]. Several hypotheses may help explain this apparent discrepancy, although further research is needed. First, it is likely that some of the HEV seroprevalence observed in this study stemmed from exposure to autochthonous HEV genotype 3; it has been hypothesized that HEV genotype 3 may be less virulent than HEV of other genotypes [41, 42]. Genotype 3 viruses circulate widely in US swine populations [12], and infectious genotype 3 virus has been isolated from pig livers sold at US grocery stores [43], suggesting that exposures to this genotype could be common. Second, it is possible that HEV causes clinical symptoms in a dose-dependent manner and that US individuals are generally exposed to only low doses of virus; evidence of dose-dependent infection and viral replication has been previously observed in experimentally infected swine [13]. Last, it is also possible that autochthonous hepatitis E is underreported in the United States, in part because there is no US-licensed diagnostic test for anti-HEV.
We observed that birth outside the United States was significantly associated with HEV seropositivity. This finding is consistent with the hypothesis that HEV infection is common in many developing countries (most foreign-born individuals in the United States emigrate from developing countries). We also observed elevated, albeit not significant, odds of seropositivity associated with military service. Military service in the United States often entails travel to developing countries and to remote areas. Together, these 2 findings suggest that some of the HEV seroprevalence observed in the present study is attributable to HEV infections acquired in developing countries. It is also possible that other travel to developing countries contributed to the HEV seroprevalence observed in the present study; the NHANES III questionnaire did not include questions regarding foreign travel.
Consistent with previous studies [8, 9, 16, 18, 44], we found evidence that HEV seropositivity was positively associated with having pets and dogs in the household and with consumption of liver or other organ meats in the US-born population. Antibody to HEV has been detected in a wide variety of animal species [45–47], suggesting that exposures to pets and other animals could play a role in HEV transmission cycles. However, detection of HEV RNA in biological samples collected from animals other than swine has not been common, suggesting that pets may be accidental rather than primary hosts. It is also possible that common causes could explain the association between pets and HEV seropositivity. The association between consumption of liver or other organ meats and seropositivity, however, is highly plausible, consistent with the findings of previous compelling studies [16, 17].
It is possible that some of the HEV seroprevalence observed in this study was not associated with travel to developing countries and zoonotic exposures. Transfusion-transmitted HEV infections have been documented elsewhere [48, 49], and the significant association observed in the present study between HEV seropositivity and antibody to hepatitis C virus is consistent with a hypothesis of transfusion-associated HEV transmission. However, use of cocaine or crack, a surrogate marker for injection drug use in NHANES III, was not associated with HEV seropositivity, a finding that is consistent with the findings of a previous study [22]. The estimates of HEV seroprevalence observed in this study varied markedly by race/ethnicity, geographic region, and metropolitan residence status; numerous factors could explain these differences. The Midwestern region of the United States had the largest swine population at the time of NHANES III and the highest observed seroprevalence. Residents of metropolitan areas may be more likely to travel to developing countries.
In bivariate analysis, we observed a positive association between HAV and HEV seropositivity among US-born participants. After we adjusted for age in the multivariable model to control for the confounding effect of age, we observed a negative association between HAV and HEV seropositivity. The negative association between HAV and HEV seropositivity is consistent with the negative association observed between poverty and HEV seropositivity among US-born participants, but more research is needed to better understand the meaning of this finding. However, this association is unlikely to be due to HAV vaccination; the NHANES III study was conducted before widespread use of HAV vaccine in the United States.
The present study has some limitations. First, there was some misclassification of anti-HEV serostatus. The diagnostic testing conducted at Johns Hopkins was not perfectly predictive, compared with testing conducted at the Hepatitis Viruses Section. Given the blinded nature of this study, however, we believe that all misclassification was nondifferential. Second, this study did not have sufficient statistical power to examine associations between uncommon exposures and HEV seropositivity. For example, the numbers of NHANES III participants reporting rodents and farm animals as pets and consumption of liver or other organ meats >5 times per month were too small for meaningful analysis. Third, the HEV seropositivity measured in this study reflects exposure to all HEV genotypes; therefore, a precise picture of HEV genotype–specific epidemiological profiles was not possible in this investigation. Last, the NHANES III questionnaire did not include information on several topics of potential interest (e.g., vegetarianism, history of blood transfusion, and injection drug use).
The current study represents, to our knowledge, the largest population-based study of HEV yet conducted and notably includes significant numbers of children and elderly individuals. These results do not, however, shed light on long-term epidemiological trends of HEV infection in the United States. However, they are consistent with results obtained in Denmark, where a similar age-specific prevalence of anti-HEV was observed [50]. In that study, clear evidence for a cohort effect was obtained, suggesting that the incidence of HEV infection was higher in the past, a phenomenon also widely observed for HAV infection. In contrast, evidence from Japan suggests that HEV infection rates are not decreasing markedly over time in that country [26, 51], possibly because exposure remains higher there than in many Western countries. Until the epidemiological profile of HEV is better understood, HEV may be considered to be a possible etiologic agent of acute and chronic hepatitis in US patients reporting no travel history.
Acknowledgments
We thank Gregory Armstrong, Jan Drobeniuc, Morgan Marks, Homayoon Farzadegan, Stacey Meyerer, Jonathan Samet, and Robert Davidson for their contributions to this project.
Financial support: Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) (R.H.P. and R.E.E.); NIAID, NIH (grant 1R21AI067449-01A1 to K.E.N.)
Footnotes
Potential conflicts of interest: none reported.
Presented in part: Viral Hepatitis Prevention Board Meeting: Hepatitis A and E, Update on Prevention and Epidemiology, Antwerp, Belgium, 13 March 2009; 13th International Symposium on Viral Hepatitis and Liver Disease, Washington, DC, 21 March 2009 (abstract PL-1).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/The Agency for Toxic Substances and Disease Registry.
References
- 1.Labrique AB, Thomas DL, Stoszek SK, Nelson KE. Hepatitis E: an emerging infectious disease. Epidemiol Rev. 1999;21:162–179. doi: 10.1093/oxfordjournals.epirev.a017994. [DOI] [PubMed] [Google Scholar]
- 2.Panda SK, Thakral D, Rehman S. Hepatitis E virus. Rev Med Virol. 2007;17:151–180. doi: 10.1002/rmv.522. [DOI] [PubMed] [Google Scholar]
- 3.Emerson SU, Purcell RH. Running like water—the omnipresence of hepatitis E. N Engl J Med. 2004;351:2367–2368. doi: 10.1056/NEJMp048285. [DOI] [PubMed] [Google Scholar]
- 4.Patra S, Kumar A, Trivedi SS, Puri M, Sarin SK. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med. 2007;147:28–33. doi: 10.7326/0003-4819-147-1-200707030-00005. [DOI] [PubMed] [Google Scholar]
- 5.Tsega E, Hansson BG, Krawczynski K, Nordenfelt E. Acute sporadic viral hepatitis in Ethiopia: causes, risk factors, and effects on pregnancy. Clin Infect Dis. 1992;14:961–965. doi: 10.1093/clinids/14.4.961. [DOI] [PubMed] [Google Scholar]
- 6.Rab MA, Bile MK, Mubarik MM, et al. Water-borne hepatitis E virus epidemic in Islamabad, Pakistan: a common source outbreak traced to the malfunction of a modern water treatment plant. Am J Trop Med Hyg. 1997;57:151–157. doi: 10.4269/ajtmh.1997.57.151. [DOI] [PubMed] [Google Scholar]
- 7.Amon JJ, Drobeniuc J, Bower WA, et al. Locally acquired hepatitis E virus infection, El Paso, Texas. J Med Virol. 2006;78:741–746. doi: 10.1002/jmv.20617. [DOI] [PubMed] [Google Scholar]
- 8.Dalton HR, Thurairajah PH, Fellows HJ, et al. Autochthonous hepatitis E in southwest England. J Viral Hepat. 2007;14:304–309. doi: 10.1111/j.1365-2893.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 9.Widdowson MA, Jaspers WJ, van der Poel WH, et al. Cluster of cases of acute hepatitis associated with hepatitis E virus infection acquired in The Netherlands. Clin Infect Dis. 2003;36:29–33. doi: 10.1086/345439. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Iwata K, Watanabe N, et al. Full-genome nucleotide sequence of a hepatitis E virus strain that may be indigenous to Japan. Virology. 2001;287:9–12. doi: 10.1006/viro.2001.1017. [DOI] [PubMed] [Google Scholar]
- 11.Tsang TH, Denison EK, Williams HV, Venczel LV, Ginsberg MM, Vugia DJ. Acute hepatitis E infection acquired in California. Clin Infect Dis. 2000;30:618–619. doi: 10.1086/313730. [DOI] [PubMed] [Google Scholar]
- 12.Meng XJ, Purcell RH, Halbur PG, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng XJ, Halbur PG, Shapiro MS, et al. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J Virol. 1998;72:9714–9721. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colson P, Kaba M, Bernit E, Motte A, Tamalet C. Hepatitis E associated with surgical training on pigs. Lancet. 2007;370:935. doi: 10.1016/S0140-6736(07)61441-X. [DOI] [PubMed] [Google Scholar]
- 15.Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16:5–36. doi: 10.1002/rmv.482. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda H, Okada K, Takahashi K, Mishiro S. Severe hepatitis E virus infection after ingestion of uncooked liver from a wild boar. J Infect Dis. 2003;188:944. doi: 10.1086/378074. [DOI] [PubMed] [Google Scholar]
- 17.Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371–373. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- 18.De Silva AN, Muddu AK, Iredale JP, Sheron N, Khakoo SI, Pelosi E. Unexpectedly high incidence of indigenous acute hepatitis E within South Hampshire: time for routine testing? J Med Virol. 2008;80:283–288. doi: 10.1002/jmv.21062. [DOI] [PubMed] [Google Scholar]
- 19.Lewis H, Morgan D, Ijaz S, Boxall E. Indigenous hepatitis E virus infection in England and Wales. BMJ. 2006;332:1509–1510. doi: 10.1136/bmj.332.7556.1509-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colson P, Borentain P, Motte A, et al. First human cases of hepatitis E infection with genotype 3c strains. J Clin Virol. 2007;40:318–320. doi: 10.1016/j.jcv.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Meng XJ, Wiseman B, Elvinger F, et al. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40:117–122. doi: 10.1128/JCM.40.1.117-122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas DL, Yarbough PO, Vlahov D, et al. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J Clin Microbiol. 1997;35:1244–1247. doi: 10.1128/jcm.35.5.1244-1247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buti M, Dominguez A, Plans P, et al. Community-based seroepide-miological survey of hepatitis E virus infection in Catalonia, Spain. Clin Vaccine Immunol. 2006;13:1328–1332. doi: 10.1128/CVI.00255-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalton HR, Fellows HJ, Gane EJ, et al. Hepatitis E in New Zealand. J Gastroenterol Hepatol. 2007;22:1236–1240. doi: 10.1111/j.1440-1746.2007.04894.x. [DOI] [PubMed] [Google Scholar]
- 25.Mansuy JM, Legrand-Abravanel F, Calot JP, et al. High prevalence of anti-hepatitis E virus antibodies in blood donors from South West France. J Med Virol. 2008;80:289–293. doi: 10.1002/jmv.21056. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka E, Matsumoto A, Takeda N, et al. Age-specific antibody to hepatitis E virus has remained constant during the past 20 years in Japan. J Viral Hepat. 2005;12:439–442. doi: 10.1111/j.1365-2893.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- 27.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994 Jul;:1–407. [PubMed] [Google Scholar]
- 28.Tsarev SA, Tsareva TS, Emerson SU, et al. ELISA for antibody to hepatitis E virus (HEV) based on complete open-reading frame-2 protein expressed in insect cells: identification of HEV infection in primates. J Infect Dis. 1993;168:369–378. doi: 10.1093/infdis/168.2.369. [DOI] [PubMed] [Google Scholar]
- 29.Yu C, Engle RE, Bryan JP, Emerson SU, Purcell RH. Detection of immunoglobulin M antibodies to hepatitis E virus by class capture enzyme immunoassay. Clin Diagn Lab Immunol. 2003;10:579–586. doi: 10.1128/CDLI.10.4.579-586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engle RE, Yu C, Emerson SU, Meng XJ, Purcell RH. Hepatitis E virus (HEV) capsid antigens derived from viruses of human and swine origin are equally efficient for detecting anti-HEV by enzyme immunoassay. J Clin Microbiol. 2002;40:4576–4580. doi: 10.1128/JCM.40.12.4576-4580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fix AD, Abdel-Hamid M, Purcell RH, et al. Prevalence of antibodies to hepatitis E in two rural Egyptian communities. Am J Trop Med Hyg. 2000;62:519–523. doi: 10.4269/ajtmh.2000.62.519. [DOI] [PubMed] [Google Scholar]
- 32.Meng XJ, Dea S, Engle RE, et al. Prevalence of antibodies to the hepatitis E virus in pigs from countries where hepatitis E is common or is rare in the human population. J Med Virol. 1999;59:297–302. [PubMed] [Google Scholar]
- 33.Purcell RH, Nguyen H, Shapiro M, et al. Pre-clinical immunogenicity and efficacy trial of a recombinant hepatitis E vaccine. Vaccine. 2003;21:2607–2615. doi: 10.1016/s0264-410x(03)00100-2. [DOI] [PubMed] [Google Scholar]
- 34.Zhou YH, Purcell RH, Emerson SU. An ELISA for putative neutralizing antibodies to hepatitis E virus detects antibodies to genotypes 1, 2, 3, and 4. Vaccine. 2004;22:2578–2585. doi: 10.1016/j.vaccine.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Tsarev SA, Tsareva TS, Emerson SU, et al. Experimental hepatitis E in pregnant rhesus monkeys: failure to transmit hepatitis E virus (HEV) to offspring and evidence of naturally acquired antibodies to HEV. J Infect Dis. 1995;172:31–37. doi: 10.1093/infdis/172.1.31. [DOI] [PubMed] [Google Scholar]
- 36.Mohadjer L, Montaquila J, Waksberg J. National Health and Nurtition Examination Survey III: weighting and examination methodology. Hyattsville, MD: Westat for the National Center for Health Statistics; 1996. [Google Scholar]
- 37.Li TC, Zhang J, Shinzawa H, et al. Empty virus-like particle-based enzyme-linked immunosorbent assay for antibodies to hepatitis E virus. J Med Virol. 2000;62:327–333. doi: 10.1002/1096-9071(200011)62:3<327::aid-jmv4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Ghabrah TM, Tsarev S, Yarbough PO, Emerson SU, Strickland GT, Purcell RH. Comparison of tests for antibody to hepatitis E virus. J Med Virol. 1998;55:134–137. [PubMed] [Google Scholar]
- 39.Mast EE, Alter MJ, Holland PV, Purcell RH Hepatitis E Virus Antibody Serum Panel Evaluation Group. Evaluation of assays for antibody to hepatitis E virus by a serum panel. Hepatology. 1998;27:857–861. doi: 10.1002/hep.510270331. [DOI] [PubMed] [Google Scholar]
- 40.Kwo PY, Schlauder GG, Carpenter HA, et al. Acute hepatitis E by a new isolate acquired in the United States. Mayo Clin Proc. 1997;72:1133–1136. doi: 10.4065/72.12.1133. [DOI] [PubMed] [Google Scholar]
- 41.Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48:494–503. doi: 10.1016/j.jhep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Ohnishi S, Kang JH, Maekubo H, et al. Comparison of clinical features of acute hepatitis caused by hepatitis E virus (HEV) genotypes 3 and 4 in Sapporo, Japan. Hepatol Res. 2006;36:301–307. doi: 10.1016/j.hepres.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Feagins AR, Opriessnig T, Guenette DK, Halbur PG, Meng XJ. Detection and characterization of infectious hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J Gen Virol. 2007;88:912–917. doi: 10.1099/vir.0.82613-0. [DOI] [PubMed] [Google Scholar]
- 44.Borgen K, Herremans T, Duizer E, et al. Non-travel related hepatitis E virus genotype 3 infections in The Netherlands; a case series 2. BMC Infect Dis. 2008;8:61. doi: 10.1186/1471-2334-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arankalle VA, Joshi MV, Kulkarni AM, et al. Prevalence of anti-hepatitis E virus antibodies in different Indian animal species. J Viral Hepat. 2001;8:223–227. doi: 10.1046/j.1365-2893.2001.00290.x. [DOI] [PubMed] [Google Scholar]
- 46.Kabrane-Lazizi Y, Fine JB, Elm J, et al. Evidence for widespread infection of wild rats with hepatitis E virus in the United States. Am J Trop Med Hyg. 1999;61:331–335. doi: 10.4269/ajtmh.1999.61.331. [DOI] [PubMed] [Google Scholar]
- 47.Okamoto H, Takahashi M, Nishizawa T, Usui R, Kobayashi E. Presence of antibodies to hepatitis E virus in Japanese pet cats. Infection. 2004;32:57–58. doi: 10.1007/s15010-004-3078-0. [DOI] [PubMed] [Google Scholar]
- 48.Mitsui T, Tsukamoto Y, Yamazaki C, et al. Prevalence of hepatitis E virus infection among hemodialysis patients in Japan: evidence for infection with a genotype 3 HEV by blood transfusion. J Med Virol. 2004;74:563–572. doi: 10.1002/jmv.20215. [DOI] [PubMed] [Google Scholar]
- 49.Boxall E, Herborn A, Kochethu G, et al. Transfusion-transmitted hepatitis E in a ‘nonhyperendemic’ country. Transfus Med. 2006;16:79–83. doi: 10.1111/j.1365-3148.2006.00652.x. [DOI] [PubMed] [Google Scholar]
- 50.Christensen PB, Engle RE, Hjort C, et al. Time trend of the prevalence of hepatitis E antibodies among farmers and blood donors: a potential zoonosis in Denmark. Clin Infect Dis. 2008;47:1026–1031. doi: 10.1086/591970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukuda S, Ishikawa M, Ochiai N, et al. Unchanged high prevalence of antibodies to hepatitis E virus (HEV) and HEV RNA among blood donors with an elevated alanine aminotransferase level in Japan during 1991–2006. Arch Virol. 2007;152:1623–1635. doi: 10.1007/s00705-007-0996-z. [DOI] [PubMed] [Google Scholar]