Abstract
The insulin receptor (IR) and insulin-like growth factor-1 (IGF1) receptor (IGF1R) are receptor tyrosine kinases that participate in mitogenic and antiapoptotic signaling in normal and neoplastic epithelia. In the present study, immunoblotting and RT-PCR demonstrated expression of IGF1R and IR isoform A in acute myelogenous leukemia (AML) cell lines as well as >80% of clinical AML isolates. Treatment with insulin enhanced signaling through the Akt and MEK1/2 pathways as well as survival of serum-starved AML cell lines. Conversely, treatment with BMS-536924, a dual IGF1R/IR kinase inhibitor that is undergoing preclinical testing, inhibited constitutive receptor phosphorylation as well as downstream signaling through MEK1/2 and Akt. These changes inhibited proliferation and, in some AML cell lines, induced apoptosis at submicromolar concentrations. Likewise, BMS-536924 inhibited leukemic colony formation in CD34+ clinical AML samples in vitro. Collectively, these results not only indicate that expression of IGF1R and IR isoform A is common in AML, but also demonstrate that interruption of signaling from these receptors inhibits proliferation in clinical AML isolates. Accordingly, further investigation of IGF1R/IR axis as a potential therapeutic target in AML appears warranted.
Keywords: acute myelogenous leukemia, insulin receptor, insulin-like growth factor receptor, autocrine loop
INTRODUCTION
The insulin-like growth factor (IGF) signaling pathway plays an important role in various cellular processes, including proliferation and differentiation (1–3). Components of this pathway include the ligands IGF1 and IGF2, which exhibit a high degree of sequence homology to insulin; insulin-like growth factor-1 receptor (IGF1R), which contains an extracellular domain that binds both IGFs and an intracellular tyrosine kinase domain that initiates signaling; and IGF2R, which lacks a tyrosine kinase domain and binds IGF2 without initiating signal transduction (1, 3, 4).
The IGF1R and insulin receptor (IR) pathways are closely related. IGF1R and IR exhibit extensive sequence similarity, including sequence identity in their ATP binding clefts and at residues that contact the peptide ligands in their binding sites as well as 84% homology in the kinase domain (5). In both cases, ligand binding results in a conformational change that leads to autophosphorylation of the receptor tyrosine kinase domain; increased phosphorylation of the receptor substrates Shc, IRS-1 and IRS-2; and activation of the same downstream signaling cascades, including the mitogen-activated protein (MAP) kinase and phosphatidylinositol-3 kinase (PI3K)/Akt pathways (3–5). Indeed, IGF1R and IR are so similar that IGF1R and IR isoform A (IR-A), a splice variant that is overexpressed in certain carcinomas, can heterodimerize to form hybrid receptors that signal after binding IGF1, IGF2 or insulin at physiologic levels (6). Moreover, IR-A homodimers can bind IGF2 and, to a lesser extent, IGF1 (7–9), suggesting that proliferative IGF signaling can occur through IGF1R homodimers, IGF1R/IR-A heterodimers and IR-A homodimers (6, 9). In contrast, hybrid receptors containing IGF1R and IR isoform B are orders of magnitude less sensitive to IGF2 and insulin (6).
Uncontrolled IGF1R signaling, which stimulates proliferation and protects cells from apoptosis (1, 3, 4, 10), has been implicated in the development and maintenance of various neoplasms (1, 3, 4, 11–13). As a result, the IGF1/IGF1R axis has become a target for anticancer drug development. Agents currently undergoing preclinical or clinical testing include neutralizing IGF1 antibodies, antagonistic IGF1R antibodies, and small molecule IGF1R tyrosine kinase inhibitors (3, 4, 10, 14). In view of signaling by IGF1R/IR-A hybrids (6) and IR-A homodimers (7–9), as well as the high sequence homology of IGF1R and IR, there has also been recent interest in dual receptor inhibitors. The dual IGF1R/IR inhibitor BMS-554417 exhibited antiproliferative and proapoptotic activity in vitro and in vivo with modest effects on glucose tolerance (15). BMS-536924 (1H-benzoimidazol-2-yl-1H-pyridin-2-one), which is used in the present work, is likewise an ATP-competitive inhibitor of IGF1R and IR that has shown activity against neoplastically transformed cell lines (16, 17).
Although the role of the IGF1 system has been extensively investigated in various solid tumors, less is known about the role of IGF1R and IR in AML. Earlier reports demonstrated that IGF1 enhances colony formation by committed normal myeloid and erythroid progenitors (18, 19), but more primitive CD34+ normal progenitors lack IGF1R expression and are resistant to IGF1R downregulation (20). In addition, IGF1 enhances proliferation of human AML cell lines (21–24) and clonogenic growth of AML progenitors in vitro (25–27). More recently, expression of IR (28) as well as IGF1R (28, 29) was demonstrated in AML cell lines and a handful of primary AML specimens. In addition, it was reported that insulin and IGF1 both enhanced the proliferation of AML cells and that downregulation of either IR or IGFR modestly diminished proliferation of U937 cells (28), raising the possibility that IR signaling also contributes to survival and proliferation of AML cells. To build on these results, the present studies were designed to determine the frequency of IGF1R and IR expression in a larger series of AML specimens, establish the identity of the IR splice form expressed in AML cell lines and clinical specimens, and assess the effect of the dual IGF1R/IR inhibitor BMS-536924 on AML cell lines and clinical AML specimens in vitro.
MATERIALS AND METHODS
Materials
Antibodies that recognize the β-chains of IR (product #3020 and 3025) and IGF1R (#3027), as well as phospho-Tyr1131-IGF1R and phospho-Tyr1146-IR (product #3021), phospho-Ser473-Akt, Akt, phospho-ERK1/2 and ERK1/2 were obtained from Cell Signaling Technology (Beverly, MA). Oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA). 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethonyphenol)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and phenazine methosulfate were purchased from Promega (Madison, WI) and Sigma-Aldrich (St. Louis, MO), respectively. All other reagents were obtained as previously described (30, 31).
Tissue culture
AML lines were propagated at densities of <1 × 106 cells/ml in media containing 100 units/ml penicillin G, 100 µg/ml streptomycin and 2 mM glutamine. HL-60 (American Type Culture Collection), ML-1 cells (Michael Kastan, St. Jude Children’s Hospital, Memphis, TN) and K562 cells (Robert Abraham, Wyeth Pharmaceuticals) were maintained in medium A [RPMI 1640 with 10% (v/v) heat-inactivated fetal bovine serum (FBS)]. U937 cells (American Type Culture Collection) were maintained in medium A supplemented with 10 mM HEPES (pH 7.4), 1 mM sodium pyruvate, 1.5 g/L sodium bicarbonate and 4.5 g/L glucose (medium B). All experiments with BMS-536924 were performed in these serum-containing media.
MTS assay
Log phase cells were sedimented at 100 × g for 5 min, washed once in serum-free medium and resuspended in serum-free medium. Aliquots containing ~5 × 104 cells in 120 µl were incubated at 37° C with varying concentrations of insulin for 24–72 h. Alternatively, cells in medium A or medium B containing FBS were incubated with varying concentrations of BMS-536924 for 5 d. After reaction with MTS and phenazine methosulfate as instructed by the suppliers, plates were incubated for 3–6 h to obtain an absorbance of >0.5 at 490 nm in control samples.
Detection of apoptosis
Flow cytometry for DNA content (31, 32) and fluorescence microscopy for apoptotic nuclear morphological changes (33) were performed as described (31, 33, 34).
Clonogenic Assays
Aliquots containing 500–1000 HL-60 or U937 cells were plated in gridded 35-mm plates in the medium of Pike and Robinson (35) containing 0.3% (w/v) agar and the indicated concentrations of BMS-536924. After incubation for 10–14 d, colonies containing ≥50 cells were counted. Colony forming assays in MCF-7 cells were performed as described (30).
Leukemia samples
Under the aegis of Institutional Review Board-approved protocols, marrow samples were acquired from consenting patients with newly diagnosed AML prior to induction therapy. Mononuclear cells were isolated on ficoll-Hypaque gradients, washed with RPMI 1640 medium, and resuspended at 1.5 × 106/ml in Iscove’s modified Dulbecco’s medium containing 20% (v/v) FBS, 100 U/ml penicillin G, 100 µg/ml streptomycin, and 2 mM glutamine (medium C). Aliquots containing 600,000 cells were plated in gridded 35-mm culture dishes containing increasing BMS-536924 concentrations in growth factor-containing Methocult® methylcellulose medium (StemCell Technologies, Vancouver, BC), incubated for 14 d and scored at 25X magnification according to published morphological criteria (36).
RT-PCR
After total RNA was isolated using an RNeasy™ mini kit (Qiagen, Valencia, CA), cDNAs were synthesized using a Superscript™ III First-strand Synthesis kit (Invitrogen, Carlsbad, CA) following the supplier’s instructions. PCR reactions (50 µl) were performed using 3 µl cDNA product (5 µl for IR amplification), Promega (Madison, WI) Master Mix PCR reagents and the primers listed in Supplemental Table I. Amplification for IGF1, IGF2, IGF1R, IRS-2 and GAPDH involved a three-step program: 95°C for 60 sec; 35 cycles of 95°C for 30 sec, 55°C for 30 sec, 72°C for 60 sec; and 72°C for 7 min. Amplification of IR and IRS-1 was accomplished by nested PCR using the outer primers according to the conditions described above, then reamplifying 2% of the first PCR reaction using the inner primers listed in Supplemental Table I. Alternatively, amplification of IR nucleotides 2230–2867, a region that contains exon 11 in isoform B but not isoform A, was performed using primers and conditions specified by Pandini et al (6). Products were electrophoresed on a 2% (w/v) agarose gel containing 0.5 µg/ml ethidium bromide in 1X TAE buffer [30.7 mM Tris, 20 mM sodium acetate and 1 mM EDTA], visualized on a UV transilluminator, excised and sequenced using automated dye terminator technology.
Immunoprecipitation
All steps were performed at 4° C. Log phase cells were washed twice in PBS and lysed by incubation for 20 min in buffer consisting of 150 mM NaCl, 50 mM HEPES (pH 7.5), 10% (w/v) glycerol, 5 mM MgSO4, 1 mM EGTA, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 100 mM NaF, 1 mM PMSF, 10 µg/ml leupeptin, 10 µg/ml aprotinin, 1% (w/v) thiodiglycol and 1% (w/v) Triton X-100. After lysates were sedimented at 16000 × g for 5 min, supernatants were incubated for 16 h with rabbit monoclonal anti-IR or polyclonal anti-IGF1R on an end-over-end shaker. After addition of 50 µl of a 50% (v/v) suspension of protein A-Sepharose beads, samples were incubated for an additional 2 h. Receptor-antibody complexes were recovered by sedimentation at 4000 × g for 1 min, washed four times with wash buffer [150 mM NaCl, 20 mM HEPES (pH 7.5), 10% (w/v) glycerol, 0.1% (w/v) Triton X-100, 1% (w/v) thiodiglycol, 1 mM sodium orthovanadate], eluted in SDS sample buffer, and subjected to SDS-PAGE followed by immunoblotting.
Immunoblotting
Whole cell lysates were prepared from cell lines or clinical AML samples as previously described (37). Aliquots were resuspended in SDS sample buffer at 5 mg protein/ml (assayed by the bicinchoninic acid method)., separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with antibodies as described (38).
RESULTS
IGF1R, IR-A and IGF1 are expressed in AML cell lines
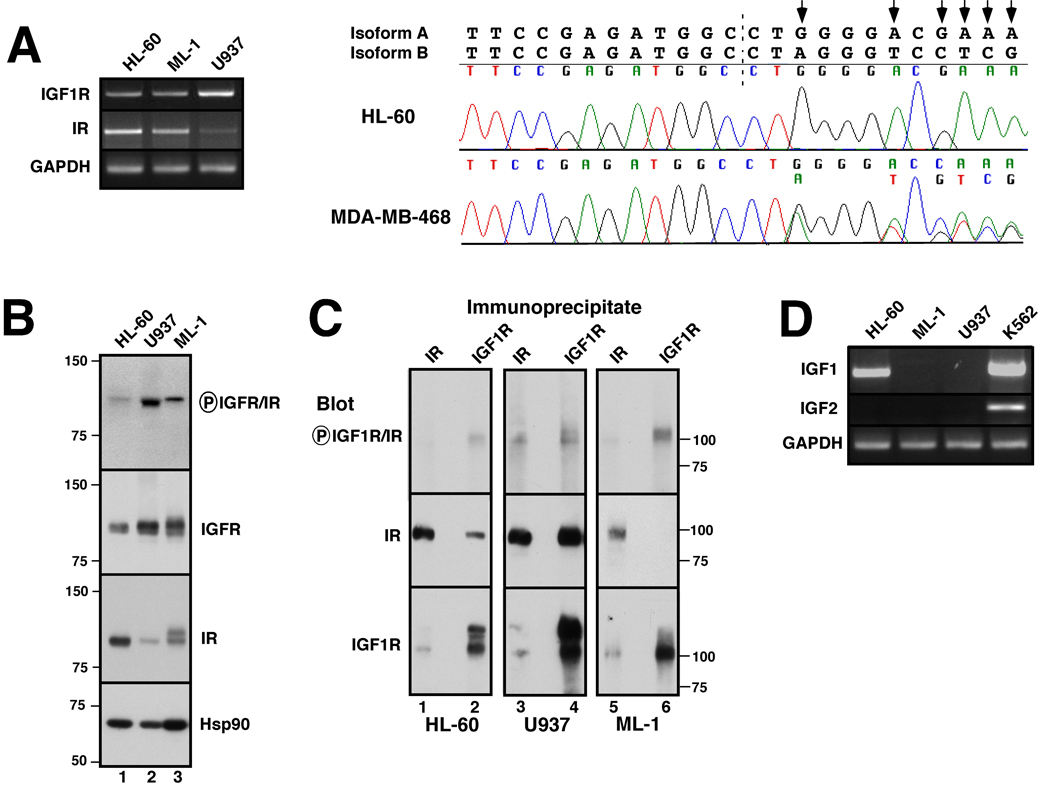
To assess expression of IGF1R and IR in human AML cells lines, RT-PCR and immunoblotting were performed using RNA and protein, respectively, from the HL-60, U937 and ML-1 human AML cell lines. As indicated in Fig. 1A, RT-PCR readily detected message encoding IGF1R, IR and the downstream signaling molecules IRS-1 and IRS-2 in each line. Sequencing demonstrated that the IR transcript in all three AML lines corresponds to isoform A (Fig. 1A, right panel and data not shown), an isoform that can bind IGF1 and IGF2 (7–9) as well as alter the ligand specificity of IGF1R (6). Immunoblotting (Fig. 1B) likewise demonstrated signals for IGF1R and IR at ~100 kDa, the expected molecular weight for the mature β chain of each receptor, in all three lines. Importantly, IGF1R was expressed at high levels in U937 and ML-1 cells, whereas IR was expressed at higher levels in HL-60. Immunoprecipitation followed by immunoblotting demonstrated the association of a portion of the total IR with IGF1R in HL-60 and U937 cells (Fig. 1C, lanes 2 and 4), suggesting the presence of IGF1R/IR-A heterodimers as well as IR-A homodimers depending upon the cell line. Analysis using affinity purified antibodies that recognize a conserved autophosphorylation site on IGF1R and IR also indicated that one or both receptors were phosphorylated under normal growth conditions (Fig. 1B and C, top panels). As indicated in Fig. 1D, message for IGF1 was detectable in HL-60 cells, but not in the U937 or ML-1 cell lines. IGF2 mRNA was not detectable in any of the three AML lines.
Figure 1. IGF-1R, IR, IGF1 and IGF2 expression in AML cell lines.
A, cDNA was amplified using primers specific for the β-chains of IGF-1R and IR as well as IRS-1, IRS-2 and, as a control, GAPDH. PCR products photographed under ultraviolet illumination (left panel) and sequenced to confirm their identities. Right panel, chromatograms obtained when IR cDNAs from HL-60 (upper) and MDA-MB-468 cells (lower) were sequenced across the splice junction (dashed line) between exons 12 and 11. Predicted sequences of isoforms A and B are shown above chromatograms. Arrows indicate nucleotides unique to the A isoform. MDA-MB-468 breast cancer cells, which express approximately equal amounts of both isoforms, served as a positive control for detection of both isoforms when present. B, Whole cell lysates were subjected to SDS-PAGE followed by immunoblotting with antibodies that recognize the indicated antigens. Heat shock protein 90 (Hsp90) served as a loading control. Numbers at left and in subsequent figures indicate molecular weight markers in kDa. C, Lysates from 2.5 × 107 log phase HL-60 (lanes 1, 2), U937 (lanes 3, 4), or ML-1 cells (lanes 5, 6) were prepared in the presence of protease and phosphatase inhibitors and subjected to immunoprecipitation with monoclonal anti-IR (lanes 1, 3, 5) or polyclonal anti-IGF1R antibodies (lanes 2, 4, 6) followed by SDS-PAGE and immunoblotting with reagents that recognize the indicated antigens. D, cDNA was amplified using primers specific for IGF-1 and IGF-2. K562 cells served as a positive control for IGF2 expression.
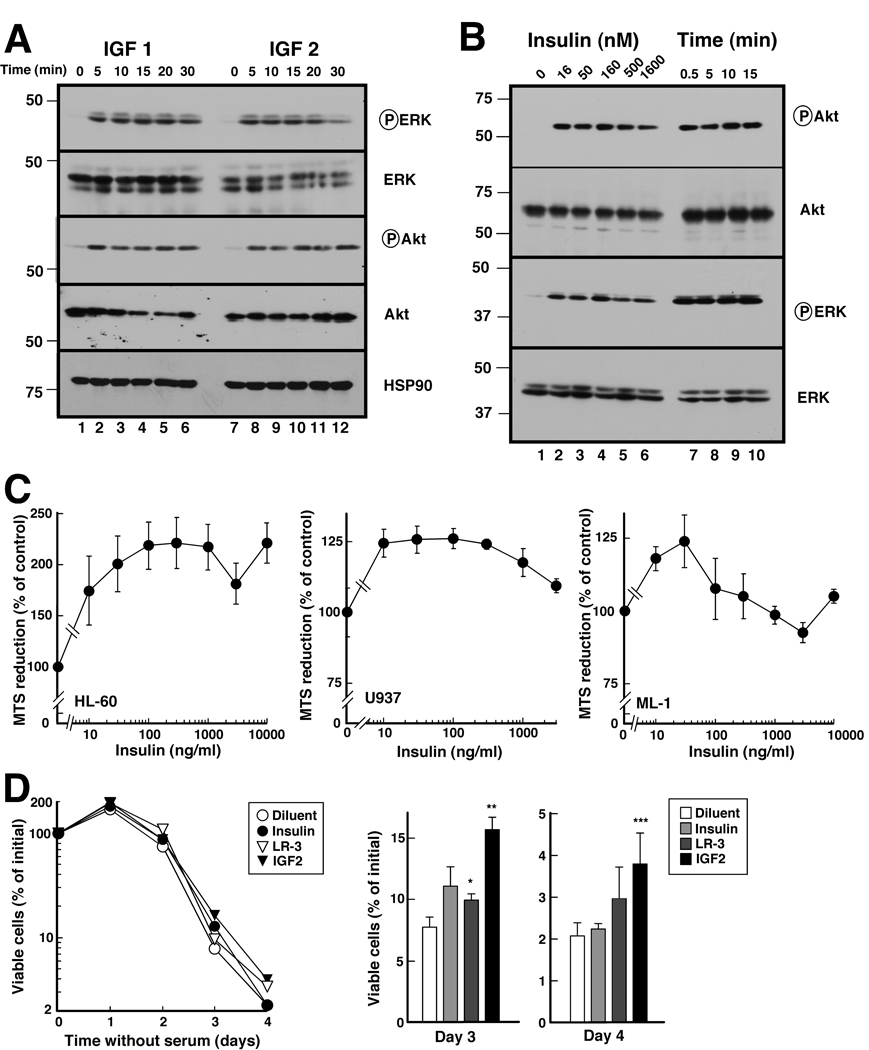
To determine whether these receptors were functional, cells were treated with IGF1, IGF2, or insulin. Activating phosphorylations of ERK and Akt were readily observed within 5 min after addition of 10 nM IGF1 or 13 nM IGF2 (Fig. 2A), indicating that the IGF1R signals in response to physiological ligand concentrations. Importantly, pathway activation was also observed within <1 min after insulin addition, suggesting that the IR expressed in these cells can signal (Fig. 2B). In further experiments, insulin at concentrations as low as 10 ng/ml (1.6 nM) enhanced the reduction of MTS, a common assay of viable cell mass, upon serum deprivation (Fig. 2C). The extent of this effect varied with the cell line, being highest in HL-60 cells (Fig. 2C). Because of concern that the MTS assay might reflect changes in glucose metabolism rather than cell viability, cell survival was also determined when cells were incubated with insulin, the IGF1 derivative LR-3 or IGF2 under serum-free conditions. As indicated in Fig. 2D for U937 cells, cell division continued over the first 24 h after serum withdrawal, reflecting completion of the cell cycle in the population that had passed the restriction point. Although cells subsequently died, LR-3 and particularly IGF-2 increased survival relative to diluent-treated cells. This effect was evident on day 3 and, to a lesser extent, day 4.
Figure 2. Effects of IGF1, IGF2 and insulin on downstream signaling and survival in AML cell lines.
A, Log phase U937 cells, which have high IGF1R levels (Fig. 1B), were incubated in serum-free medium for 23 h, then treated for 0–30 min at 37 °C with 10 nM IGF1 or 13 nM IGF2 and immediately diluted 4-fold into ice cold RPMI 1640 medium containing 10 mM HEPES (pH 7.4). After cells were lysed in 6 M guanidine hydrochloride under reducing conditions, aliquots containing 50 µg protein were subjected to SDS-PAGE gel and immunoblotting for the indicated antigens. B, HL-60 cells, which have high levels of IR (Fig. 1B), were treated with the indicated concentrations of insulin for 5 min (lanes 1–6) or with 170 nM insulin for the indicated length of time (lanes 7–10), then analyzed as in panel A. C, log phase cells were washed, resuspended in serum-free medium, exposed to the indicated insulin concentration for 48 h and analyzed by MTS assay. Error bars, ± 1 S.D. from eight replicate samples. Similar results were obtained in two additional independent assays. D, log phase U937 cells were washed; resuspended in serum-free medium; incubated for the indicated length of time under serum-free conditions in the absence or presence of 17 nM insulin, 10 nM IGF1 or 13 nM IGF2; and examined microscopically. Left panel is representative of 3 assays. Error bars in right panel, ± 1 S.D. from 3 independent assays. *, ** and ***, p = 0.024, 0.015 and 0.06, respectively, relative to diluent-treated sample by paired t test.
Collectively, the results presented in Fig. 1 and Fig. 2 indicate that human AML cell lines synthesize IR-A as well as IGF1R, that IGF1R/IR-A heterodimers can be detected in some of these cells, and that these receptors can signal under normal growth conditions.
BMS-536924 inhibits receptor signaling and proliferation in AML cell lines
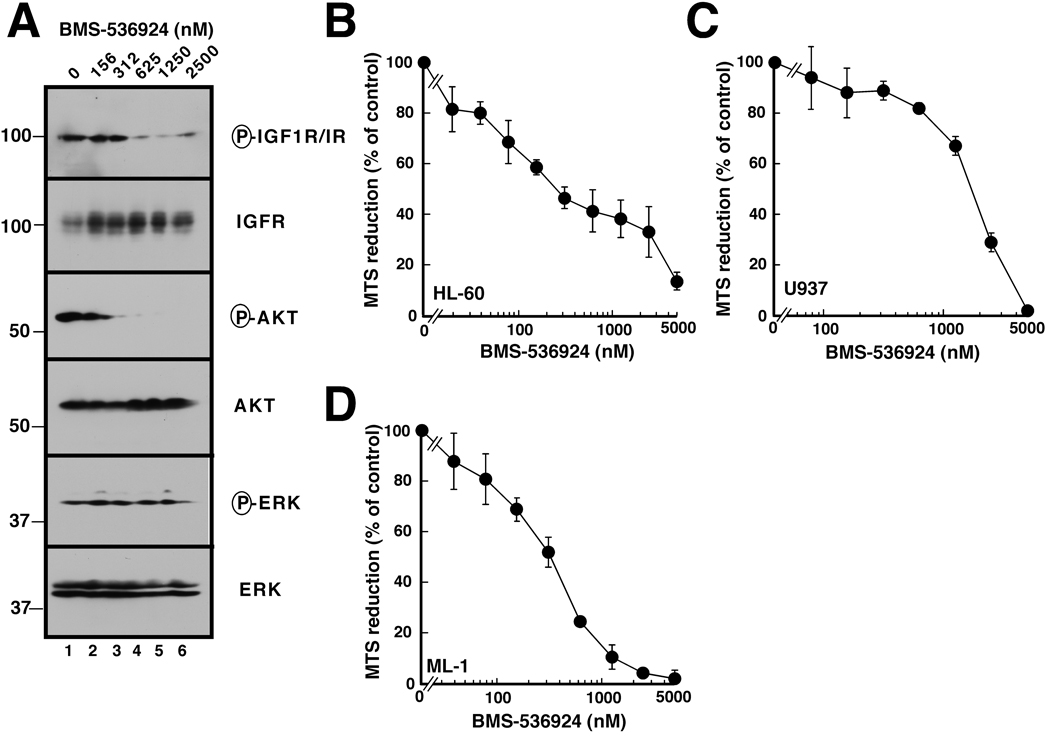
In light of the preceding results, we next assessed the effect of the dual IGF1R/IR inhibitor BMS-536924 (16) on receptor autophosphorylation and downstream signaling. As illustrated in Fig. 3A for ML-1 cells, submicromolar BMS-536924 concentrations induced a dose-dependent decrease in receptor phosphorylation that was accompanied by inhibition of Akt and, to a lesser extent, ERK phosphorylation (Fig. 3A). Interestingly, IGF1R levels increased in response to BMS-536924, possibly reflecting a feedback pathway.
Figure 3. Effect of BMS-536924 on IGFR1 phosphorylation and downstream signaling.
A, after log phase ML-1 cells in serum-containing medium were treated for 24 h with the indicated BMS-536924 concentration, whole cell lysates were subjected to immunoblotting with antibodies that recognize the indicated antigens. B–D, log phase cells were exposed to BMS-536924 for 5 d and analyzed by MTS assay. Error bars, ± 1 S.D. from eight replicate samples. Similar results were obtained in two additional independent assays.
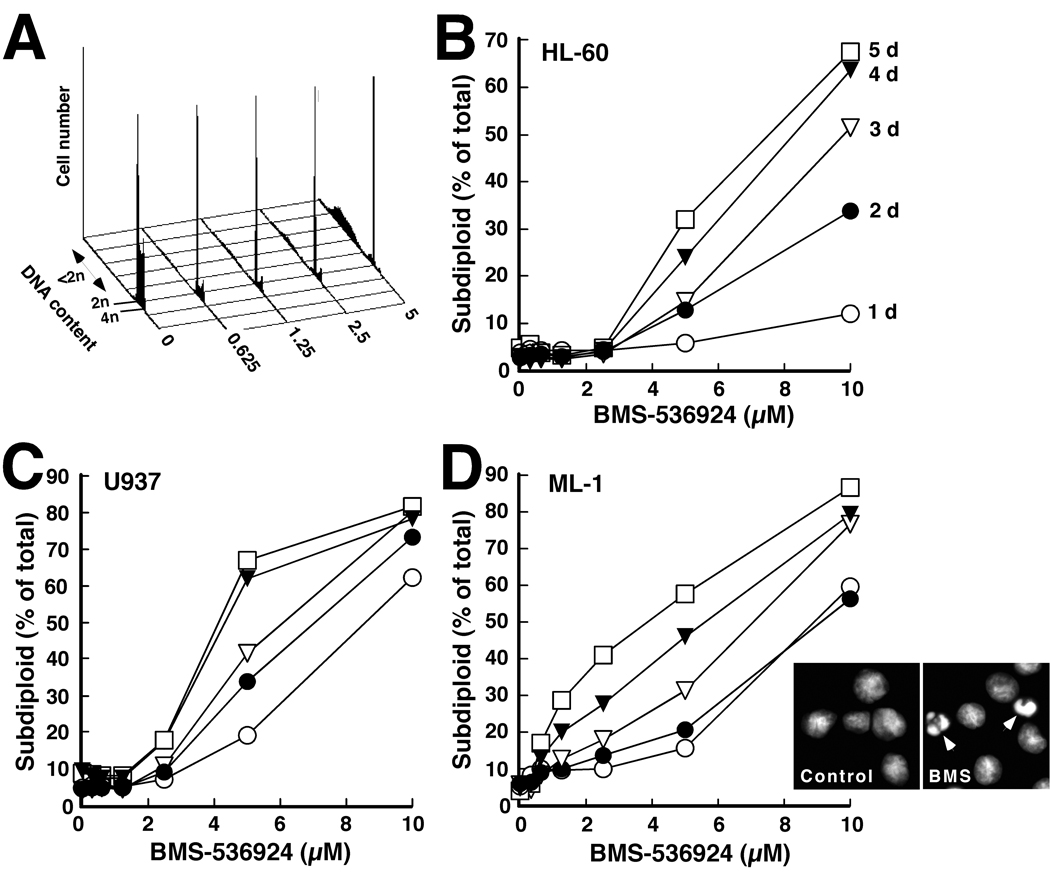
Subsequent experiments assessed the effect of BMS-536924 on AML cell proliferation and viability. When HL-60, U937 or ML-1 cells were incubated with BMS-536924 for 5 days, a dose-dependent decrease in viable cell mass was observed (Fig. 3B-D), with mean IC50 values ranging from 360 ± 130 nM (mean ± SD, n = 3) for ML-1 cells to 1800 ± 630 nM for U937. Because this decrease can reflect either diminished proliferation or increased apoptosis, cells were also treated with BMS-536924 and subjected to flow cytometry. As illustrated in Fig. 4A for ML-1 cells, BMS-536924 induced readily apparent G1 arrest. In addition, cells with “subdiploid” DNA content accumulated (Fig. 4A–4D), particularly in ML-1 cultures. Hoechst staining also demonstrated the presence of cells with fragmented nuclei after exposure to for 5 days (inset, Fig. 4D), confirming the induction of apoptosis.
Figure 4. BMS-536924 induces apoptosis in AML cell lines.
A, histograms showing PI staining of ML-1 cells treated for 4 d with the indicated BMS-536924 concentrations. The double-headed arrow indicates cells with <2n DNA. B–D, summary of results obtained by flow cytometry in the experiment shown in panel A when HL-60, U937, and ML-1 cells were treated with BMS-536924 of for 1 d (○), 2 d (●), 3 d (▽) 4 d (▼) or 5 d (□) in the presence of serumcontaining medium. Inset in D, morphology of ML-1 cells treated for 5 d with diluent or 1.25 µM BMS-536924. Arrows, apoptotic cells. Results in all panels are representative of 3 independent experiments.
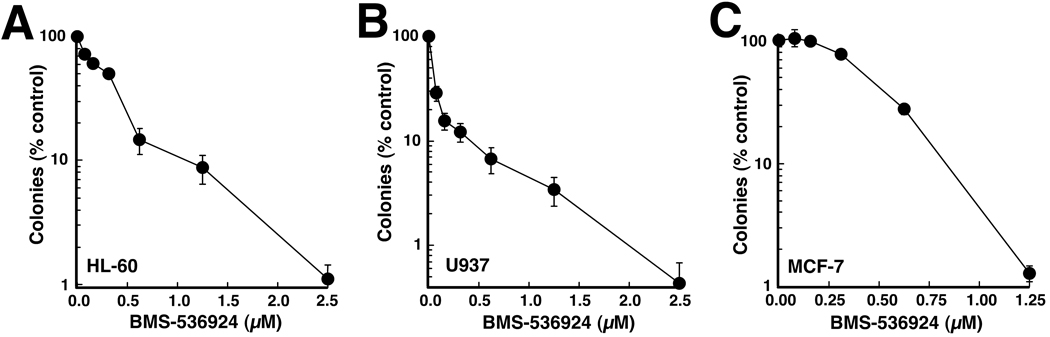
To provide an alternative method for assessing the cumulative effects of BMS-536924, clonogenic assays were performed using HL-60 and U937 cells, the two lines that form colonies in soft agar. BMS-536924 diminished colony formation in both cell lines, with IC50 values of 340 ± 50 nM (mean ± S.D., n= 3) in HL-60 cells and <80 nM (n=3) in U937 cells (Fig. 5A and B). In comparison, MCF-7 breast cancer cells, which are considered an IGF1-dependent solid tumor line (17, 39–41), exhibited an IC50 of 500 ± 100 nM (n=3) in colony forming assays (Fig. 5C), indicating that BMS-536924 induces long term antiproliferative effects in HL-60 and U937 cells at concentrations similar to those that inhibit proliferation of IGF1-dependent solid tumor cell lines.
Figure 5. BMS-536924 inhibits colony formation in AML cell lines.
Log phase HL-60 (A), U937 (B) or MCF-7 (C) cells were plated in medium containing 0.3% agar and varying concentrations of BMS-536924 (A,B) or allowed to adhere to tissue culture plates and continuously exposed to BMS-536924 (C). After a 10- to 14-day incubation, colonies were counted. Error bars, mean ± SD of quadruplicate samples.
Analysis of primary AML specimens
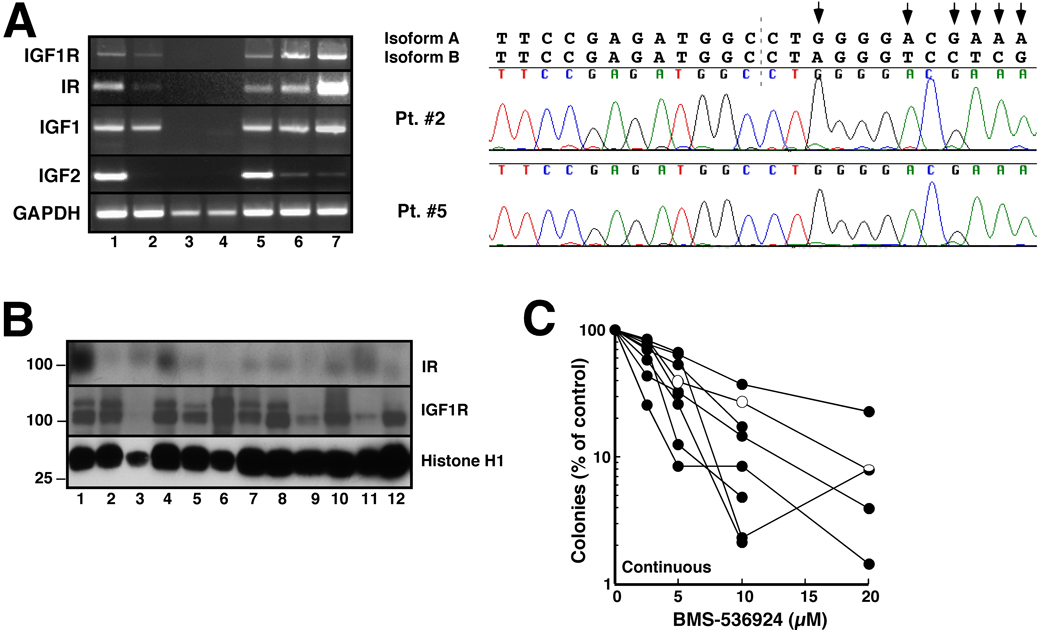
In view of the effects of BMS-536924 on human AML cell lines, we next examined IGF1R, IR, and IGF expression in clinical AML specimens. In a group of 21 pretreatment AML specimens (Fig. 6A and data not shown), mRNA encoding IGF1R, IR and IGF1 was detectable in 18 (86%); and mRNA for IGF2 was detectable in 15 (71%). When the IR message from the 18 positive AML samples was sequenced, only message encoding IR-A was detected (Fig. 6B and data not shown), as in the AML cell lines (Fig. 1A). In further experiments, blasts from 29 pretreatment AML marrows encompassing all FAB subtypes were examined by immunoblotting with antibodies that recognize IGF1R and IR. 26 of the 29 samples (90%) expressed IGF1R and 25 of 29 (86%) expressed IR at the protein level (Fig. 6C). Interestingly, IGF1R and IR were readily detected by immunoblotting in samples that contained low but detectable message levels (Fig. 6A, right panels), suggesting a poor correlation between mRNA and protein for these two receptors.
Figure 6. Examination of the IGF1R/IR axis and effects of BMS-536924 on colony formation in clinical AML samples.
A, blasts from untreated AML patients were subjected to RT-PCR with primers for the indicated message. GAPDH served as a positive control. Lanes 1–9 correspond to nine separate AML patients. In the inset, samples from patients 3 and 4 were subjected to 45 cycles of amplification (top) or immunoblotting for the indicated antigen (bottom). B, chromatograms obtained when IR cDNAs from patients 2 and 5 were sequenced across the splice junction between exons 12 and 11 (dashed line). Shown above the chromatogram are the predicted sequences of isoforms A and B. Arrows indicate nucleotides unique to A isoform. C, whole cell lysates from an additional patient cohort were subjected to immunoblotting with antibodies that recognize the indicated antigen or, as a control, histone H1. D, AML samples used in panel A were plated in Methocult® containing the indicated BMS-536924 concentration. Leukemic colonies were quantitated after 14 days as instructed by the supplier. Each line represents one clinical sample. Colors of lines indicate corresponding sample in panel A. Sample 7 in panel A did not form colonies. Conversely, the sample shown in black in panel D did not have enough cells for RNA isolation. Open symbols, sample with activating FLT3 mutation. Absence of lines beyond 10 µM BMS-536924 indicates lack of colonies at higher concentrations in several samples.
Because the majority of the leukemic marrow samples expressed IGF1R and/or IR-A, we examined the effect of BMS-536924 on blast cell proliferation using clonogenic assays. For these studies, nine CD34+ AML samples were examined. As indicated in Fig. 6D, blast cells in these specimens exhibited a dose-dependent decrease in colony formation upon exposure to BMS-536924, with IC50 values ranging from 1 to 7 µM.
DISCUSSION
Results of the present study provide the first evidence that human AML cells express exclusively isoform A, the proliferative form of the IR. Additional experiments demonstrate that the dual IGF1R/IR inhibitor BMS-536924 decreases autophosphorylation of its target receptors, diminishes signaling through the PI3K/Akt and MAP kinase pathways, and inhibits proliferation of AML cell lines and colony formation in clinical AML samples. Collectively, these results identify the IGF1R/IR axis as a possible contributor to AML cell survival and demonstrate for the potential utility of targeting both receptors in AML.
In earlier work, Tazzari et al. reported that IGF1 expression and IGF1R phosphorylation were increased in blast cells at the time of relapse in 4 AML patients (29). Doepfner et al. subsequently demonstrated expression of IR in addition to IGF1R in AML blasts (28). The present study extended these observations by showing that both IR and IGF1R are expressed at the protein and RNA level in >80% of AML samples (Fig. 6A and 6C). When primers that amplify across exon 11 of the IR (6) were utilized, no exon 11 sequence was detected in any of the AML cell lines (Fig. 1A) or clinical AML samples (Fig. 6A). In view of the ability of IR-A to bind IGF1 and IGF2 directly (7–9) as well as modify the ligand specificity of IGF1R (6), our demonstration that isoform A is the predominant IR expressed in AML has potential biological and therapeutic implications.
Previous studies have reported that the presence of IR-A not only provides a high affinity receptor for IGF2 (8), but also enhances the ability of insulin and IGF2 to signal through IGF1R (6). Consistent with these earlier reports, we observed that physiological concentrations of IGF1, IGF2 or insulin activated the MAP kinase and Akt pathways (Figs. 2A and 2B). These ligands also enhanced AML cell survival under serum-free conditions (Fig. 2C and 2D). The effects of insulin were more pronounced in HL-60 cells, which expressed the highest amount of IR, whereas the effects of LR-3 and IGF-2 were higher in U937 cells, which express more IGF1R (Fig. 1, Fig. 2 and data not shown).
Further studies demonstrated message for IGF1 and/or IGF2 in 70–90% of specimens from newly diagnosed AML patients (Fig. 6A). While the present study was not designed to rule out a possible contribution to these signals from small numbers of contaminating stromal cells, the presence of IGF1 and/or IGF2 message in a variety of AML lines, including HL-60, K562, THP.1, KG1a and HEL (Fig. 1D and data not shown), indicates that myeloid cells can express these ligands. Coupled with recent reports that these ligands are secreted by clinical AML samples (28, 42), these results raise the possibility of an autocrine or paracrine pathway involving IGF1R and IR-A in AML. In view of the ability of IGF1R and IR-A ligands to activate downstream signaling (Fig. 2A and 2B), such a pathway might contribute to the previously described constitutive activation of the MAP kinase (43–45) and PI3K/Akt pathways (46–48) in clinical AML specimens.
In further experiments, BMS-536924, an example of a new drug class that inhibits IGFR1R and IR almost equally (15, 16), diminished proliferation at concentrations as low as 200 nM (Fig. 3B–D) and induced apoptosis in susceptible AML lines (Fig. 4). Moreover, BMS-536924 inhibited colony formation in CD34+ clinical AML samples by as much as 98% (Fig. 6C). There was, however, variability among clinical samples, as indicated by the fact that 10 µM BMS-536924 inhibited colony formation by 60–98% (Fig. 6C).
This variability requires further investigation. No clear-cut correlation between different AML subtypes and BMS-536924 sensitivity is evident from the present studies, although the sample size was extremely small. On the other hand, we have observed differences in expression of some of the proteins that might be predicted to affect sensitivity, including IGF1R, IR, IRS1 and IRS2 (Fig. 6C and data not shown). Because expression of these polypeptides in the total blast population might not reflect levels in the cells that form colonies, it will be important to examine these polypeptides in specific AML subpopulations in the future. Other factors that might contribute to sensitivity differences include variability in expression of antiapoptotic Bcl-2 family members (15) or the transporter ABCG2, which has recently been shown to efflux BMS-536924 (49). Finally, it is conceivable that the ability or inability of cells to upregulate IGF1R and/or IR upon pathway inhibition might contribute to the variability. Future experiments will need to determine whether the BMS-536924-induced IGF1R upregulation observed in Fig. 3A reflects increased gene expression, message stabilization, and/or posttranslational changes, whether similar effects are observed in clinical leukemia specimens, and whether this upregulation affects drug sensitivity.
Additional studies are also required to determine whether a dual IGF1R/IR inhibitor such as BMS-536924 exhibits selectivity for AML as compared to normal progenitors. It is known that primitive CD34+ normal progenitors lack IGF1R expression and are resistant to IGF1R downregulation (20). Although it has been suggested that IR signaling might provide a survival signal for normal CD34+ cells (50), the paucity of marrow toxicity in mice treated with the IGF1R/IR inhibitor BMS-554417 (15), coupled with the limited effects of NVP-AEW541 on normal human CD34+ progenitors (28, 29) at concentrations that inhibit IR in addition to IGF1R, suggests that effects of dual IGF1R/IR inhibition on normal myeloid progenitors will be modest.
In summary, the present results not only demonstrate that IR-A, like IGF1R, is widely expressed in AML cell lines and clinical samples, but also show that the dual IGF1R/IR inhibitor BMS-536924 induces apoptosis in AML cell lines and profoundly inhibits colony formation in a subset of AML samples in vitro. Based on these results, further preclinical and possible clinical testing of therapies that simultaneously target IGF1R and IR in AML appear warranted.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully knowledge the assistance of the Mayo Clinic Flow Cytometry and Optical Morphology Shared Resource, advice of Karen Flatten and other members of the Kaufmann laboratory, and secretarial assistance of Deb Strauss.
Footnotes
Supported in part by grants from the National Institutes of Health (R01 CA69008 to SHK) and the General Mills Foundation to the Mayo Foundation Clinician Investigator Training Program (AEWH).
REFERENCES
- 1.Rubin R, Baserga R. Insulin-like growth factor-I receptor. Its role in cell proliferation, apoptosis, and tumorigenicity. Lab Invest. 1995;73:311–331. [PubMed] [Google Scholar]
- 2.Grothey A, Voigt W, Schober C, Muller T, Dempke W, Schmoll HJ. The role of insulin-like growth factor I and its receptor in cell growth, transformation, apoptosis, and chemoresistance in solid tumors. J Cancer Res Clin Oncol. 1999;125:166–173. doi: 10.1007/s004320050259. [DOI] [PubMed] [Google Scholar]
- 3.Kurmasheva RT, Houghton PJ. IGF-I mediated survival pathways in normal and malignant cells. Biochim Biophys Acta. 2006;1766:1–22. doi: 10.1016/j.bbcan.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 5.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 6.Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002;277:39684–39695. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi Y, Flier JS, Benecke H, Ransil BJ, Moller DE. Ligand-binding properties of the two isoforms of the human insulin receptor. Endocrinology. 1993;132:1132–1138. doi: 10.1210/endo.132.3.8440175. [DOI] [PubMed] [Google Scholar]
- 8.Frasca F, Pandini G, Scalia P, et al. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19:3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benyoucef S, Surinya KH, Hadaschik D, Siddle K. Characterization of insulin/IGF hybrid receptors: contributions of the insulin receptor L2 and Fn1 domains and the alternatively spliced exon 11 sequence to ligand binding and receptor activation. Biochem J. 2007;403:603–613. doi: 10.1042/BJ20061709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casa AJ, Dearth RK, Litzenburger BC, Lee AV, Cui X. The type I insulin-like growth factor receptor pathway: a key player in cancer therapeutic resistance. Front Biosci. 2008;13:3273–3287. doi: 10.2741/2925. [DOI] [PubMed] [Google Scholar]
- 11.Macaulay VM. Insulin-like growth factors and cancer. Br J Cancer. 1992;65:311–320. doi: 10.1038/bjc.1992.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 13.Miller BS, Yee D. Type I insulin-like growth factor receptor as a therapeutic target in cancer. Cancer Res. 2005:10123–10127. doi: 10.1158/0008-5472.CAN-05-2752. [DOI] [PubMed] [Google Scholar]
- 14.Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Molecular cancer therapeutics. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- 15.Haluska P, Carboni JM, Loegering DA, et al. In Vitro and In Vivo Antitumor Effects on the Dual Insulin-Like Growth Factor-l/Insulin Receptor Inhibitor, BMS 554417. Cancer Res. 2006;66:362–371. doi: 10.1158/0008-5472.CAN-05-1107. [DOI] [PubMed] [Google Scholar]
- 16.Wittman M, Carboni J, Attar R, et al. Discovery of a (1H-benzoimidazol-2-yl)-1H-pyridin-2-one (BMS-536924) inhibitor of insulin-like growth factor I receptor kinase with in vivo antitumor activity. J Med Chem. 2005;48:5639–5643. doi: 10.1021/jm050392q. [DOI] [PubMed] [Google Scholar]
- 17.Litzenburger BC, Kim HJ, Kuiatse I, et al. BMS-536924 reverses IGF-IR-induced transformation of mammary epithelial cells and causes growth inhibition and polarization of MCF7 cells. Clin Cancer Res. 2009;15:226–237. doi: 10.1158/1078-0432.CCR-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merchav S, Tatarsky I, Hochberg Z. Enhancement of human granulopoiesis in vitro by biosynthetic insulin-like growth factor I/somatomedin C and human growth hormone. J Clin Invest. 1988;81:791–797. doi: 10.1172/JCI113385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merchav S, Tatarsky I, Hochberg Z. Enhancement of erythropoiesis in vitro by human growth hormone is mediated by insulin-like growth factor I. Br J Haematol. 1988;70:267–271. doi: 10.1111/j.1365-2141.1988.tb02480.x. [DOI] [PubMed] [Google Scholar]
- 20.Ratajczak MZ, Kuczynski WI, Onodera K, et al. A reappraisal of the role of insulin-like growth factor I in the regulation of human hematopoiesis. J Clin Invest. 1994;94:320–327. doi: 10.1172/JCI117324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pepe MG, Ginzton NH, Lee PD, Hintz RL, Greenberg PL. Receptor binding and mitogenic effects of insulin and insulinlike growth factors I and II for human myeloid leukemic cells. J Cell Physiol. 1987;133:219–227. doi: 10.1002/jcp.1041330204. [DOI] [PubMed] [Google Scholar]
- 22.Sukegawa I, Hizuka N, Takano K, Asakawa K, Shizume K. Decrease in IGF-I binding sites on human promyelocytic leukemia cell line (HL-60) with differentiation. Endocrinol Jpn. 1987;34:365–372. doi: 10.1507/endocrj1954.34.365. [DOI] [PubMed] [Google Scholar]
- 23.Sinclair J, McClain D, Taetle R. Effects of insulin and insulin-like growth factor I on growth of human leukemia cells in serum-free and protein-free medium. Blood. 1988;72:66–72. [PubMed] [Google Scholar]
- 24.Reiss K, Porcu P, Sell C, Pietrzkowski Z, Baserga R. The insulin-like growth factor 1 receptor is required for the proliferation of hemopoietic cells. Oncogene. 1992;7:2243–2248. [PubMed] [Google Scholar]
- 25.Estrov Z, Meir R, Barak Y, Zaizov R, Zadik Z. Human growth hormone and insulin-like growth factor-1 enhance the proliferation of human leukemic blasts. J Clin Oncol. 1991;9:394–399. doi: 10.1200/JCO.1991.9.3.394. [DOI] [PubMed] [Google Scholar]
- 26.Zadik Z, Estrov Z, Karov Y, Hahn T, Barak Y. The effect of growth hormone and IGF-I on clonogenic growth of hematopoietic cells in leukemic patients during active disease and during remission--a preliminary report. J Pediatr Endocrinol. 1993;6:79–83. doi: 10.1515/jpem.1993.6.1.79. [DOI] [PubMed] [Google Scholar]
- 27.Frostad S, Bruserud O. In vitro effects of insulin-like growth factor-1 (IGF-1) on proliferation and constitutive cytokine secretion by acute myelogenous leukemia blasts. Eur J Haematol. 1999;62:191–198. doi: 10.1111/j.1600-0609.1999.tb01743.x. [DOI] [PubMed] [Google Scholar]
- 28.Doepfner KT, Spertini O, Arcaro A. Autocrine insulin-like growth factor-I signaling promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia. 2007;21:1921–1930. doi: 10.1038/sj.leu.2404813. [DOI] [PubMed] [Google Scholar]
- 29.Tazzari PL, Tabellini G, Bortul R, et al. The insulin-like growth factor-I recepto r kinase inhibitor NVP-AEW541 induces apoptosis in acute myeloid leukemia cells exhibiting autocrine insulin-like growth factor-I secretion. Leukemia. 2007;21:886–896. doi: 10.1038/sj.leu.2404643. [DOI] [PubMed] [Google Scholar]
- 30.Kottke TJ, Blajeski AL, Meng X, et al. Lack of Correlation Between Caspase Activation and Caspase Activity Assays in Paclitaxel-Treated MCF-7 Breast Cancer Cells. J Biol Chem. 2002;277:804–815. doi: 10.1074/jbc.M108419200. [DOI] [PubMed] [Google Scholar]
- 31.Meng X, Chandra J, Loegering D, et al. Central role of FADD in Apoptosis Induction by the Mitogen Activated Activated Protein Kinase Kinase Inhibitor CI1040 (PD184352) in Acute Lymphocytic leukemia Cell Lines in Vitro. J Biol Chem. 2003;278:47326–47339. doi: 10.1074/jbc.M304793200. [DOI] [PubMed] [Google Scholar]
- 32.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A Rapid and Simple Method for Measuring Thymocyte Apoptosis by Propidium Iodide Staining and Flow Cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 33.Martins LM, Mesner PW, Kottke TJ, et al. Comparison of Caspase Activation and Subcellular Localization in HL-60 and K562 Cells Undergoing Etoposide-Induced Apoptosis. Blood. 1997;90:4283–4296. [PubMed] [Google Scholar]
- 34.Mesa RA, Loegering D, Powell HL, et al. Heat Shock Protein 90 Inhibition Sensitizes Acute Myelogenous Leukemia Cells to Cytarabine. Blood. 2005;106:318–327. doi: 10.1182/blood-2004-09-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pike BL, Robinson WA. Human Bone Marrow Colony Growth in Agar-Gel. J Cell Physiol. 1970;76:77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- 36.Eaves C, Lambie K. Atlas of human hematopoietic colonies. Vancouver: StemCell Technologies Inc.; 1995. [Google Scholar]
- 37.Kaufmann SH, Svingen PA, Gore SD, Armstrong DK, Cheng Y-C, Rowinsky EK. Altered Formation of Topotecan-Stabilized Topoisomerase I-DNA Adducts in Human Leukemia Cells. Blood. 1997;89:2098–2104. [PubMed] [Google Scholar]
- 38.Kaufmann SH. Reutilization of Immunoblots After Chemiluminescent Detection. Anal Biochem. 2001;296:283–286. doi: 10.1006/abio.2001.5313. [DOI] [PubMed] [Google Scholar]
- 39.De Leon DD, Wilson DM, Powers M, Rosenfeld RG. Effects of insulin-like growth factors (IGFs) and IGF receptor antibodies on the proliferation of human breast cancer cells. Growth Factors. 1992;6:327–336. doi: 10.3109/08977199209021544. [DOI] [PubMed] [Google Scholar]
- 40.Neuenschwander S, Roberts CT, Jr, LeRoith D. Growth inhibition of MCF-7 breast cancer cells by stable expression of an insulin-like growth factor I receptor antisense ribonucleic acid. Endocrinology. 1995;136:4298–4303. doi: 10.1210/endo.136.10.7664648. [DOI] [PubMed] [Google Scholar]
- 41.Van den Berg CL, Cox GN, Stroh CA, et al. Polyethylene glycol conjugated insulin-like growth factor binding protein-1 (IGFBP-1) inhibits growth of breast cancer in athymic mice. Eur J Cancer. 1997;33:1108–1113. doi: 10.1016/s0959-8049(97)00071-3. [DOI] [PubMed] [Google Scholar]
- 42.Tamburini J, Chapuis N, Bardet V, et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood. 2008;111:379–382. doi: 10.1182/blood-2007-03-080796. [DOI] [PubMed] [Google Scholar]
- 43.Towatari M, Iida H, Tanimoto M, Iwata H, Hamaguchi M, Saito H. Constitutive Activation of Mitogen-Activated Protein Kinase Pathway in Acute Leukemia Cells. Leukemia. 1997;11:479–484. doi: 10.1038/sj.leu.2400617. [DOI] [PubMed] [Google Scholar]
- 44.Kim S-C, Hahn J-S, Min Y-H, Yoo N-C, Ko Y-W, Lee W-J. Constitutive Activation of Extracellular Signal-Regulated Kinase in Human Acute Leukemias: Combined Role of Activation of MEK, Hyperexpression of Extracellular Signal-Regulated Kinase, and Downregulation of a Phosphatase, PAC1. Blood. 1999;93:3893–3899. [PubMed] [Google Scholar]
- 45.Milella M, Kornblau SM, Estrov Z, et al. Therapeutic Targeting of the MEK/MAPK Signal Transduction Module in Acute Myeloid Leukemia. J Clin Invest. 2001;108:851–859. doi: 10.1172/JCI12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–980. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 47.Recher C, Dos Santos C, Demur C. mTOR, a new therapeutic target in acute myeloid leukemia. Cell Cycle. 2005;4:1540–1549. doi: 10.4161/cc.4.11.2159. [DOI] [PubMed] [Google Scholar]
- 48.Giles FJ, Albitar M. Mammalian target of rapamycin as a therapeutic target in leukemia. Current Molecular Medicine. 2005;5:653–661. doi: 10.2174/156652405774641034. [DOI] [PubMed] [Google Scholar]
- 49.Haluska P, Carboni JM, Asmann YW, Ten Eyck C, Attar RM, Tibodeau JD, Hou X, Nakanishi T, Ross DD, Kaufmann SH, Gottardis MM, Erlichman C. Drug efflux by breast cancer resistance protein (BCRP) is a mechanism of resistance to the insulin-like growth factor receptor/insulin receptor inhibitor, BMS-536924. Cancer Res. 2009;69(2 Suppl):2149. [Google Scholar]
- 50.Ratajczak J, Zhang Q, Pertusini E, Wojczyk BS, Wasik MA, Ratajczak MZ. The role of insulin (INS) and insulin-like growth factor-I (IGF-I) in regulating human erythropoiesis. Studies in vitro under serum-free conditions--comparison to other cytokines and growth factors. Leukemia. 1998;12:371–381. doi: 10.1038/sj.leu.2400927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.