Abstract
The mammalian target of rapamycin (mTOR) has emerged as an important cancer therapeutic target. Several mTOR inhibitors are currently being tested in cancer clinical trials. Both PI3K/Akt and MEK/ERK signaling regulate mTOR axis. However, inhibition of mTOR activates Akt survival signaling, which in turn attenuates mTOR inhibitors’ anticancer efficacy. We are interested in developing strategies for enhancing mTOR-targeted cancer therapy. In this study, we report that mTOR inhibition also induced activations of the MEK/ERK signaling pathway in some cancer cell lines after a prolonged treatment. The combination of rapamycin with the MEK inhibitor U0126 significantly enhanced growth inhibitory effects of cancer cells, suggesting that MEK/ERK activation may counteract mTOR inhibitors’ anticancer efficacy. Similarly, the combination of an mTOR inhibitor with the EGF receptor inhibitor erlotinib synergistically inhibited the growth of both human cancer cells in cell cultures and xenografts in nude mice. Moreover, the presence of erlotinib suppressed rapamycin-induced phosphorylation of Akt, ERK and eIF4E as well, implying that erlotinib can suppress mTOR inhibition-induced feedback activation of several survival signaling pathways including Akt, ERK and eIF4E. Thus, we suggest a therapeutic strategy for enhancing mTOR-targeted cancer therapy by preventing mTOR inhibition-induced feedback activation of several survival mechanisms.
Keywords: mTOR inhibitors, erlotinib, survival signaling, Akt, ERK, eIF4E
Introduction
The mammalian target of rapamycin (mTOR) is a phosphatidylinositol 3 kinase (PI3K)-related serine/theronine kinase that plays a central role in regulation of cell growth, proliferation and survival, in part by regulation of translation initiation through interaction with other proteins such as raptor and rictor 1-3. In response to mitogenic stimuli or nutrient availability, mTOR (i.e., mTOR/raptor complex) is activated 4, resulting in phosphorylation of p70S6K and 4E-BP1, and the subsequent enhanced translation of mRNAs that are critical for cell cycle progression and proliferation 1. The phosphorylation of both p70S6K and 4E-BP1 are sensitive to inhibition by mTOR inhibitors (e.g., rapamycin).
The PI3K/Akt pathway represents a major survival pathway and positively regulates mTOR (i.e., mTOR/raptor complex) 1, 5. However, the underlying molecular mechanism is still not fully elucidated. The tumor-suppressor proteins TSC1 (hamartin) and TSC2 (tuberin) form a heterodimer to inhibit cell growth and proliferation. TSC2 has GTPase-activating protein (GAP) activity towards the Ras family small GTPase Rheb, and the TSC1/2 complex antagonizes the mTOR signaling pathway via stimulation of GTP hydrolysis of Rheb 6, 7. It has been suggested that Akt associates with and inactivates the TSC1/2 complex through the phosphorylation of TSC2 8, 9. One model proposes that Akt-mediated phosphorylation destabilizes TSC1-TSC2 interactions, thereby inhibiting the formation of the TSC complex and activating mTOR kinase activity 6, 7. On the other hand, the recent discovery of mTOR/rictor complex as an Akt Ser473 kinase also places mTOR upstream of Akt 10.
The Raf/MEK/ERK signaling cascade plays a key role in the regulation of cell proliferation and differentiation 11, 12. This protein kinase cascade is initiated upon growth factor stimulation and subsequently activates Raf, MEK, ERK1/2 and ultimately certain downstream proteins such as c-Myc and Elk-1. A recent study has demonstrated that mitogenic stimuli or oncogenic Ras activates the Raf/MEK/ERK signaling cascade leading to phosphorylation of TSC2 primarily at Ser664 by ERK1/2 and the consequent functional inactivation of the TSC1-TSC2 complex. This signaling axis can therefore cooperate with the PI3K/Akt axis in inactivating TSC2 through the phosphorylation of distinct residues, leading to mTOR activation 13. Thus, the Raf/MEK/ERK pathway, like PI3K/Akt, can also function upstream of the TSC complex and modulate mTOR signaling.
mTOR signaling has recently emerged as an attractive therapeutic target for cancer therapy 1, 14. The potential applications of mTOR inhibitors for treating various types of cancer have been actively studied both pre-clinically and clinically. A recent study has shown encouraging results that the mTOR inhibitor CCI-779 improved overall survival among patients with metastatic renal-cell carcinoma 15. However, in most other tumor-types, the single agent activity of mTOR inhibitors has been modest, at best. 16-18. We and others previously reported that inhibition of mTOR (i.e., mTOR/raptor complex) with rapamycin or its related derivatives induces feedback activation of the Akt survival pathway in various types of cancer cells and cancer specimens exposed to an mTOR inhibitor 19, 20. Moreover, we also demonstrated that mTOR inhibitors increase Mnk-dependent phosphorylation of eIF4E while inhibiting 4E-BP1 phosphorylation 19, 21. Since prevention of Akt activation or eIF4E phosphorylation enhances mTOR inhibitors’ anticancer efficacy in our preclinical studies 19, 21, it is likely that the feedback activation of the survival signaling pathways such as Akt and eIF4E during mTOR-targeted cancer therapy may limit or attenuate the efficacy of mTOR inhibitors as single agents.
We have aimed at enhancing mTOR-targeted cancer therapy by developing mechanism-driven combination strategies through understanding the biological networks of the mTOR axis. In this study, we further show that inhibition of mTOR with mTOR inhibitors, particularly after prolonged treatment, induces feedback activation of the MEK/ERK signaling pathway in certain cancer cell lines. Accordingly, inhibition of MEK/ERK activation enhances rapamycin's growth inhibitory effects. Thus, our results suggest a novel strategy to enhance mTOR-targeted cancer therapy by preventing mTOR inhibition-induced MEK/ERK activation.
Materials and Methods
Reagents
Rapamycin, U0126 and erlotinib were purchased from LC Laboratories (Woburn, MA). They were dissolved in DMSO at a concentration of 20 mM, and aliquots were stored at −80°C. Stock solutions were diluted to the desired final concentrations with growth medium just before use. Formulated RAD001 and matched placebo control for animal studies were provided by Novartis Pharmaceuticals Corporation (East Hanover, NJ). Erlotinib suspension used in the animal study was made from Tarceva™ tablets, which were obtained from the Pharmacy of Winship Cancer Institute, with ethanol:PEG400:water (22.2:66.6:11.2). Rabbit polyclonal antibodies against Akt, p-Akt (Ser473), p-p70S6K (Thr389), p44/p42 (ERK), p-p44/p42 (p-ERK; Thr202/Tyr204), p-p90RSK (Ser380), p-eIF4E (Ser209), c-Myc, cyclin D1 and Mcl-1, respectively, were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Rabbit polyclonal anti-tubulin antibody was purchased from Sigma Chemical Co. (St. Louis, MO). Mouse anti-Bcl-2 monoclonal antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Cell Lines and Cell Treatment
The cell lines used in this study were originally purchased from the American Type Culture Collection (ATCC; Manassas, VA). They were grown as described previously 19. The parental A549 cell line (A549-P) and rapamycin-resistant cell line (A549-RR) were described previously 22. All treatments were done in mediums containing 5% FBS.
Western Blot Analysis
The procedures for preparation of whole-cell protein lysates and for Western blotting were described previously 19, 23. Some results were quantitated using NIH Image J (NIH, Bethesda, MA).
Growth Inhibition Assay
Cell number in monolayer culture was estimated by the sulforhodamine B (SRB) assay and the growth inhibition was calculated as previously described 24.
Colony Formation Assay
Cells (single cell suspension) were plated in 12-well plates at a density of 200 cells/well. On the second day, cells were treated with the given agents. Every 3 days, the medium was replaced with fresh medium containing the corresponding concentrations of the agents. After a 10-day treatment, the medium was removed and cell colonies were stained with crystal violet (0.1% in 20% methanol) and counted. Pictures were also obtained using a digital camera to record the result.
Cell Cycle Analysis
The procedure for analysis of cell cycle by flow cytometry was described previously 25.
Lung Cancer Xenografts and Treatments
Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University. Four-to 6-week old (about 20 g of body weight) female athymic (nu/nu) mice were ordered from Taconic (Hudson, NY) and housed under pathogen-free conditions in microisolator cages with laboratory chow and water ad libitum. A549 cells at 5 × 106 in serum-free medium were injected s.c. into the flank region of nude mice. When tumors reached certain size ranges (∼100 mm3), the mice were randomized into four groups (n = 6/group) according to tumor volumes and body weights for the following treatments: vehicle control, formulated RAD001 (3 mg/kg/day, og), erlotinib (80 mg/kg/day; og), and the combination of RAD001 and erlotinib . Tumor volumes were measured using caliper measurements once every two days and calculated with the formula V = π (length × width2)/6. After a 14-day treatment, the mice were sacrificed with CO2. The tumors were then removed and weighed.
Statistical Analysis
The statistical significance of differences in tumor sizes or weights between two groups was analyzed with two-sided unpaired Student's t tests when the variances were equal or with Welch's corrected t test when the variances were not equal, by use of Graphpad InStat 3 software (GraphPad Software, San Diego, CA). Data were examined as suggested by the same software to verify that the assumptions for use of the t tests held. Results were considered to be statistically significant at P < 0.05.
Results
Prolonged Treatment with Rapamycin Activates ERK Signaling
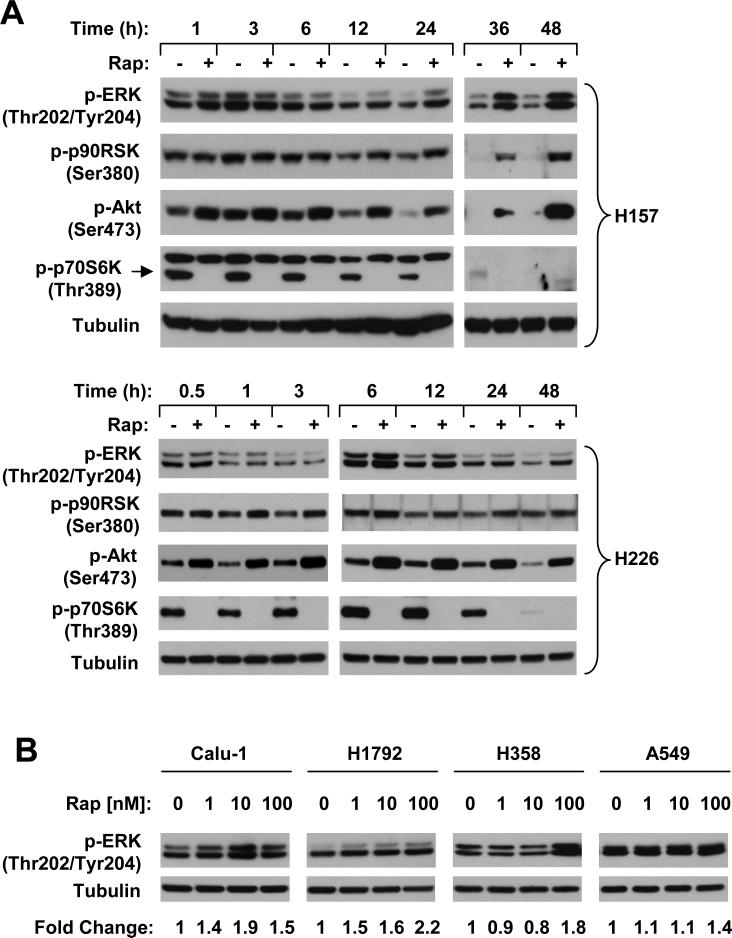
Given that MEK/ERK signaling is also involved in regulating mTOR activity 13, we determined whether inhibition of mTOR signaling using an mTOR inhibitor induces feedback activation of MEK/ERK signaling as we observed for PI3K/Akt activation 19. To this end, we treated two lung cancer cell lines (i.e., H157 and H226) with 10 nM rapamycin for a period of time ranging from 30 min to 48 h. In contrast to increase in Akt phosphorylation which occurred very rapidly (e.g., 30 min post treatment) and robustly, increase in p-ERK levels was observed in both cell lines exposed to rapamycin for 6 h (H226) or 12 h (H157) and sustained up to 48 h. Accordingly, phosphorylation of p90RSK, a well know substrate of ERK, was also increased. At the test conditions, p-p70S6K levels were inhibited (Fig. 1). These results suggest that prolonged inhibition of mTOR with rapamycin activates ERK signaling. To determine if mTOR inhibition-induced ERK activation is a common event, we further examined p-ERK levels in multiple human cancer cell lines exposed to rapamcyin for 24 h. As shown in Fig. 1B, rapamycin increased p-ERK levels in multiple lung cancer cell lines including Calu-1, H1792, H358 and A549 albeit with varied degrees. However, rapamycin did not increase p-ERK levels in HCT116 (colon), DU145 (prostate), MCF-7 (breast), MDA-MB435 (breast) and 686LN (head and neck) cancer cells (data not shown). These results suggest that rapamycin activates ERK signaling only in certain types of cancer cells (e.g., lung cancer cells).
Fig. 1. Prolonged treatment with rapamycin increases ERK phosphorylation.
A, The indicated cell lines were treated with DMSO (-) or 10 nM rapamycin (Rap; +) for the given times. B, The indicated cells lines were treated with the indicated concentrations of rapamycin for 24 h. After the aforementioned treatments, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis.
Inhibition of ERK Activation Enhances Rapamycin's Growth Inhibitory Effect
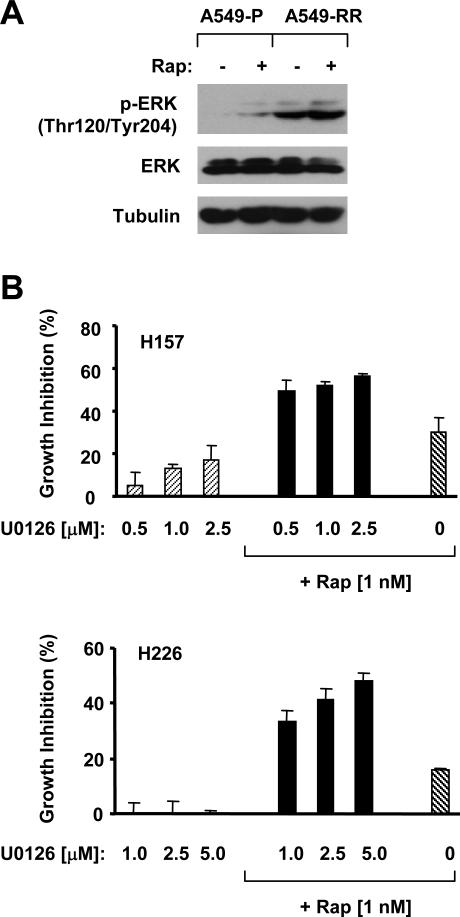
To understand the biological significance of the activation of MEK/ERK signaling during mTOR inhibition, we further compared the p-ERK levels between A549-P (rapamycin-sensitive) and A549-RR (rapamycin-resistant) cells. As shown in Fig. 2A, A549-RR cells exhibited much higher basal levels of p-ERK than A549-P cells, suggesting that elevated MEK/ERK signaling during mTOR inhibition may be associated with development of rapamycin-resistance. Following this observation, we studied whether inhibition of the activation of the MEK/ERK signaling during mTOR inhibition enhances the anticancer activity of mTOR inhibitors. To this end, we combined rapamycin with the MEK inhibitor U0126 for their growth inhibitor effects. As presented in Fig. 2B, the combination of rapamycin and U0126 was more potent than each single agent alone in inhibiting the growth of human lung cancer cells. Thus, we conclude that blockage of the activation of the MEK/ERK signaling during mTOR inhibition indeed enhances mTOR inhibitors’ anticancer efficacy.
Fig. 2. Rapamycin-resistant cells have elevated levels of p-ERK (A) and rapamycin combined with the MEK inhibitor U0126 enhances growth-inhibitory effects (B).
A, The indicated cell lines were plated in 10-cm diameter cell culture dishes and treated on the second day with DMSO or 10 nM rapamycin for 24 h. The cells were then harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. Rapamycin was removed from the culture medium for at least 24 h before A549-RR cells were used in the experiments. B, The indicated cell lines were plated in 96-well plates and then treated with 1 nM rapamycin alone, the different concentrations of U0126 alone, or their respective combinations as indicated on the second day. After 3 days, the cell numbers were estimated using the SRB assay. Each point represents a mean ± SD of four replicate determinations.
The EGFR Inhibitor Erlotinib Enhances mTOR Inhibitors’ Anticancer Efficacy
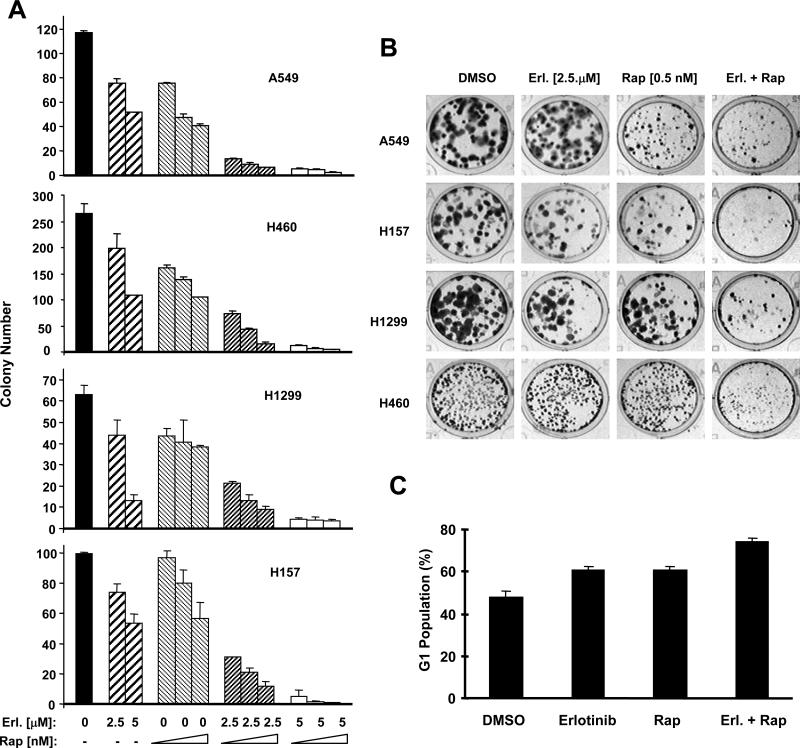
The current study on rapamycin activation of the MEK/ERK signaling together with our previous findings on the activation of PI3K/Akt and eIF4E survival pathways by mTOR inhibitors 19, 21 indicate that mTOR inhibition induces feedback activation of multiple survival signaling pathways which may counteract the efficacy of the mTOR-targeted cancer therapy. Given that EGFR inhibitors inhibit both PI3K/Akt and MEK/ERK signaling pathways 26, we determined whether rapamycin combined with an EGFR inhibitor results in enhanced anticancer efficacy. For this, we examined the effects of rapamycin and erlotinib combination on the growth of human lung cancer cells. As presented in Figs. 3A and 3B, the combination of rapamycin with erlotinib was much more effective than each single agent in inhibiting the growth of multiple human lung cancer cell lines tested in a 10-day colony formation assay. Moreover, the combination of rapamycin and erlotinib induced more cells arrested in G1 than either rapamcyin or erlotinib alone after a 48 h exposure (Fig. 3C). We did not detect increased number of apoptotic cells when the cells were treated with rapamycin and erlotinib combination even for a 3-day exposure (data not shown). Collectively, we suggest that the combination of rapamycin and erlotinib primarily enhances G1 arrest, leading to inhibition of human cancer cells.
Fig. 3. The combination of mTOR inhibition and EGFR inhibition results in enhanced growth-inhibitory effects (A and B) and G1 arrest (C).
A and B, The indicated cell lines at a density of approximately 200 cells/well were seeded in 12-well plates. On the second day, cells were treated with different concentrations of rapamycin (Rap) alone (0.25, 0.5 and 1 nM), indicated concentrations of erlotinib (Erl.) alone, and their respective combinations. The same treatments were repeated every 3 days. After 10 days, the plates were stained for the formation of cell colonies with crystal violet dye (A). The picture of the colonies was then taken using a digital camera (B). C, H460 cells were treated with DMSO, 1 nM rapamycin alone, 5 μM erlotinib alone, or the combination of rapamycin and erlotinib. After 2 days, the cells were harvested for flow cytometric analysis of cell cycle. The data are means ± SE of duplicate experiments. The similar results were also generated in H157 cells (data not shown).
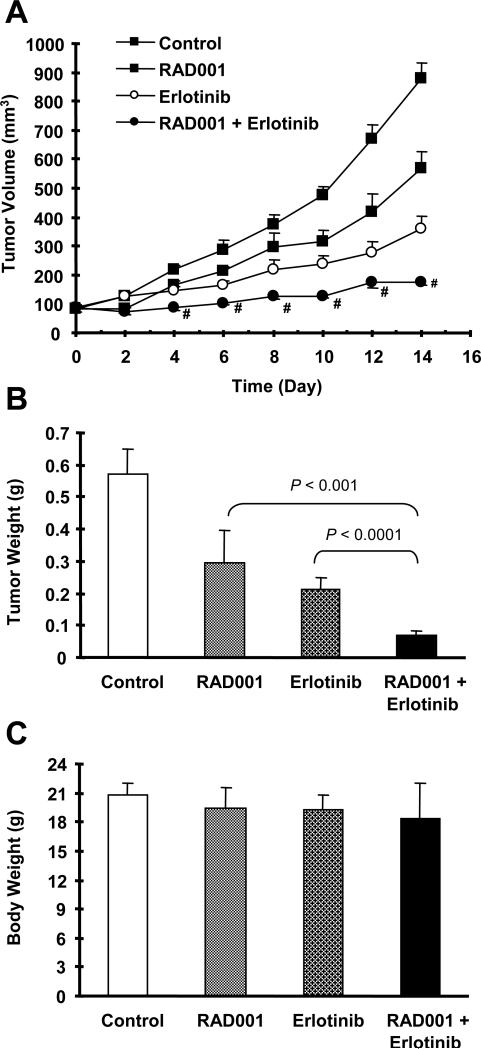
Moreover, we evaluated the efficacy of this combination regimen in lung cancer xenografts in nude mice. In this experiment, we used RAD001, a derivative of rapamycin with identical mTOR-inhibitory activity to rapamycin, in combination with erlotinib. As presented in Fig. 4A, both RAD001 alone and erlotinib alone moderately but significantly inhibit the growth of A549 lung cancer xenografts. However, their combination exhibited the best inhibitory effect on the growth of the lung xenografts, which was superior to either agent alone. At the end of the experiment, we obtained identical results by directly measuring tumor weights (Fig. 4B). This combination did not significantly reduce mouse body weight (Fig. 4C), indicating that the combination of RAD001 and erlotinib at the tested conditions does not have apparent toxicity.
Fig. 4. The combination of mTOR inhibition and EGFR inhibition exhibits enhanced anticancer efficacy in vivo.
Four groups of mice with either A549 xenografts were treated with vehicle control, RAD001 (3 mg/kg/day) alone, erlotinib (80 mg/kg/day) alone, and RAD001 plus erlotinib on the same day after grouping. After 14 days, the mice were sacrificed and weighed (C). The tumors were then removed and weighed (B). During the treatments, tumor sizes were measured once every two days (A). Tumor sizes treated with erlotinib alone and RAD001 plus erlotinib were significantly smaller than vehicle control treatment at all measurement times (p < 0.01). Tumor sizes treated with RAD001 alone were significantly smaller than vehicle control treatment at day 10, 12 and 14 (p < 0.01). # p < 0.05 compared to either RAD001 or erlotinib alone treatment. The data are means ± SE of 6 tumors from 6 mice.
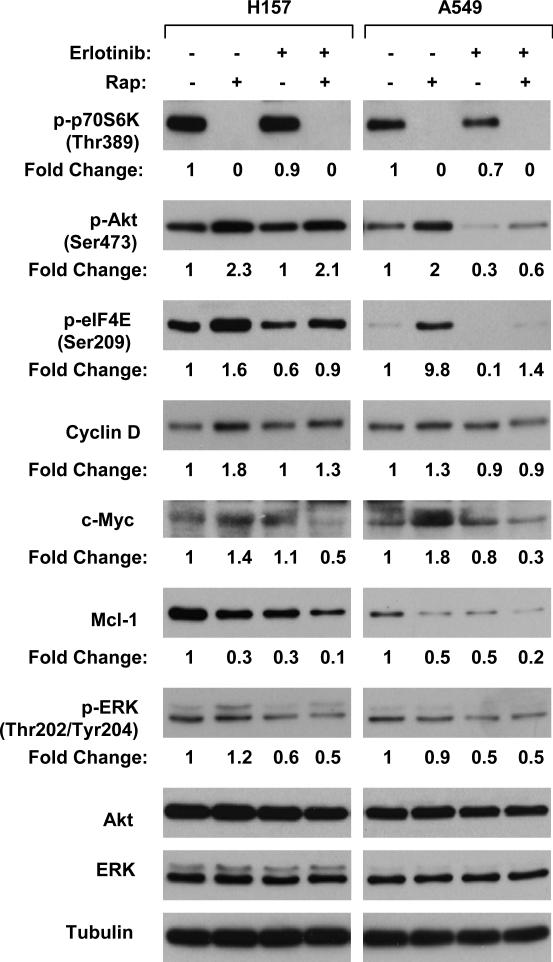
Erlotinib Interrupts mTOR Inhibition-induced Feedback Activation of Survival Signaling Pathways
To gain insight into the mechanisms by which erlotinib enhances the anticancer effects of mTOR inhibitors, we then compared the modulatory effects of rapamycin on the phosphorylation of Akt, ERK and eIF4E as well as the expression of cyclin D1, c-Myc and Mcl-1, which are regulated by cap-dependent protein synthesis, in the absence and presence of erlotinib. As expected, rapamycin alone abolished p70S6K phosphorylation, indicating inhibition of mTOR signaling. Concurrently, both p-Akt and p-eIF4E levels were substantially elevated in rapamycin-treated cancer cell lines. In the cells exposed to the combination of rapamycin and erlotinib, the increases in both p-Akt and p-eIF4E were inhibited, particularly in A549 cells (Fig. 5). Accordingly, cyclin D1 and c-Myc expression levels were elevated in rapamycin-treated cell lines, but not or only weakly in cells exposed to the rapamycin and erlotinib combination. Although rapamycin did not increase Mcl-1 levels, the combination of rapamycin and erlotinib was more potent than each single agent alone in reducing the levels of Mcl-1. p-ERK levels were increased more in H157 cells exposed to rapamycin alone than those treated with the combination of rapamycin and erlotinib (Fig. 5). Collectively, these results indicate that presence of the tyrosine kinase inhibitor erlotinib attenuates or block mTOR-inhibition-induced feedback activation of multiple survival signaling pathways, particularly those involving Akt or eIF4E.
Fig. 5. The combination of mTOR inhibition and EGFR inhibition disrupts feedback activation of multiple survival signaling pathways induced by mTOR inhibition.
The indicated cell lines were treated with 1 nM rapamycin, 5 μM erlotinib or their combination for 24 h. The cells were then subjected to preparation of whole cell protein lysates for the Western blot analysis of the indicated proteins.
Discussion
In this study, we show that prolonged treatment with rapamycin increased the levels of p-ERK and p-p90RSK in some human lung cancer cell lines while inhibiting mTOR signaling, suggesting that sustained inhibition of the mTOR signaling with an mTOR inhibitor activates MEK/ERK survival signaling. While we submitted this manuscript for publication, Carracedo et al 27 also reported that inhibition of mTORC1 activates MAPK pathway through a PI3K-dependent feedback loop in human cancer. Together with our previous findings that mTOR inhibitors increase phosphorylation of both Akt and eIF4E 19, 21, it appears that mTOR inhibition with an mTOR inhibitor induces feedback activation of multiple cell survival mechanisms including PI3K/Akt, Mnk/eIF4E and MEK/ERK.
MEK/ERK activation is often associated with cell proliferation and survival 12. In our study, we found that the basal levels of p-ERK were substantially higher in rapamycin-resistant A549 cells (A549-RR) than rapamycin-sensitive A549 parental cells (A549-P). Moreover, the combination of rapamycin with the MEK inhibitor U0126 showed enhanced growth-inhibitory effects (Fig. 2). In agreement, Carracedo et al 27 also demonstrated that pharmacological inhibition of the MAPK pathway enhances mTOR inhibitors’ anticancer effects. Together, these results suggest that activation of the MEK/ERK signaling during mTOR inhibition in some cell lines may attenuate or counteract mTOR inhibitors’ anticancer efficacy. Given that mTOR inhibition-induced Akt activation and increase in eIF4E phopshorylation also counteract mTOR inhibitors's anticancer activity as demonstrated in our previous studies 19, 21, it is reasonable to suggest that mTOR inhibitors induce feedback activation of multiple survival signaling pathways, which in turn attenuate mTOR inhibitors’ anticancer activity.
Thus, enhancing mTOR-targeted cancer therapy can be based on the strategies that prevent mTOR inhibition-induced feedback activation of the multiple survival signaling pathways. We have recently demonstrated that the RAD001 combined with the PI3K inhibitor LY294002 inhibits increase in Akt phosphorylation and enhances anticancer efficacy in vivo 22. In this study, we examine the effects of the combination of rapamycin or RAD001 with the EGFR tyrosine kinase inhibitor erlotinib, which supposes to inhibit both PI3K/Akt and MEK/ERK signaling pathways, on the growth of human lung cancer cells both in cell cultures and in a mouse xenograft model. As reported in other types of cancer cells 28-31, the combination was more effective than each single agent alone in inhibiting the growth of lung cancer cells and xenografts (Figs. 3 and 4). We have noted that the human lung cancer cell lines used in our study have wild-type EGFR and are in general insensitive to EGFR inhibitors 32, 33. Thus, from a therapeutic point of view, our findings have important clinical significance because the combination regimen of an mTOR inhibitor with an EGFR tyrosine kinase inhibitor may be effective in lung cancer patients without EGFR mutations. In our study, we did not detected induction of apoptosis by the combination, rather G1 arrest (Fig. 3B). Thus, the combination of an mTOR inhibitor with an EGFR inhibitor is cytostatic, at least in our systems.
The reasons underlying the synergy between mTOR inhibition and EGFR inhibition has not been elucidated. In our study, the presence of erlotinib substantially inhibited rapamycin-induced Akt phosphorylation in one cell line (A549), but minimally in another cell line (H157). Similarly, rapamycin-induced increase in p-ERK was inhibited by the presence of erlotinib only in H157 cells. Thus, it is possible that the inhibition of Akt and ERK activation may be important for the enhanced growth inhibition of the combination only in some cancer cell lines. In addition to Akt and ERK phosohorylation, mTOR inhibitors induce Mnk-dependent eIF4E phosphorylation as we documented previously 19, 21. It is well known that eIF4E plays a critical or rate-limiting role in regulation of cap-dependent translation initiation 34. Recently, it has been shown that eIF4E phosphorylation is absolutely required for its oncogenic function and apoptosis resistance 35. In the current study, the presence of erlotinib drastically suppresses rapamycin-induced increase in eIF4E phosphorylation as well as increases in cyclin D1 and c-Myc (Fig. 5), both of which are subjected to regulation by the cap-dependent protein translation involving eIF4E. Given the importance of cyclin D1 and c-Myc in regulation of cell proliferation 34, it is reasonable to suggest that inhibition of mTOR-inhibition-induced eIF4E phopshorylation may be an important mechanism underlying the synergy between mTOR inhibition and EGFR inhibition.
Mcl-1 is a Bcl-2 family protein with antiapoptotic function and frequently overexpressed in malignant tissues 36. It has been recently shown that Mcl-1 plays a critical role in mediating eIF4E-mediated oncogenesis and apoptosis resistance 35. In our study, we did not find that rapamycin increased Mcl-2 levels in both lung cancer cell lines tested; rather, it decreased the levels of Mcl-1. Like rapamycin, erlotinib alone also reduced Mcl-1 expression. Importantly, the combination of rapamycin and erlotinib resulted in further reduction of Mcl-1 expression (Fig. 5). Since we did not observe increased induction of apoptosis by the combination of rapamycin and erlotinib, the importance of Mcl-2 downregulation in mediating the synergy between mTOR inhibition and EGFR inhibition against the growth of cancer cells needs further investigation.
In summary, the current study demonstrates that prolonged mTOR inhibition activates MEK/ERK survival signaling in addition to activation of PI3K/Akt and Mnk/eIF4E survival signaling pathways. Combined inhibition of both mTOR and EGFR signaling pathways generates enhanced anticancer activity both in cell cultures and in animal experiments. Inhibition of mTOR inhibition-induced feedback activation of the multiple survival pathways, particularly eIF4E and its regulated oncogenic proteins, may accounts for the enhanced anticancer efficacy by the combination of mTOR inhibition and EGFR inhibition.
Acknowledgement
This study is supported by NIH RO1 CA118450-01 (S-Y. S.) and PO1 CA116676-01 (Project 1 to F.R. K and S-Y. S), Georgia Cancer Coalition Distinguished Cancer Scholar award (S-Y. S.), DOD IMPACT award W81XWH-05-0027 (Project 5 to F.R. K. and S-Y. S.) and BATTLE award W81XWH-06-1-0303 (Project 4 to F.R. K. and S-Y. S.). SY Sun, SS Ramalingam, H Fu, and FR Khuri are Georgia Cancer Coalition Distinguished Cancer Scholars.
References
- 1.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–48. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 2.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–61. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 4.Manning BD, Cantley LC. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem Soc Trans. 2003;31:573–8. doi: 10.1042/bst0310573. [DOI] [PubMed] [Google Scholar]
- 5.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Corradetti MN, Inoki K, Guan KL. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci. 2004;29:32–8. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 8.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci U S A. 2002;99:13571–6. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 2001;15:1383–92. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 11.Chambard JC, Lefloch R, Pouyssegur J, Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773:1299–310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 13.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–93. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 14.Sawyers CL. Will mTOR inhibitors make it as cancer drugs? Cancer Cell. 2003;4:343–8. doi: 10.1016/s1535-6108(03)00275-7. [DOI] [PubMed] [Google Scholar]
- 15.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O'Toole T, Lustgarten S, Moore L, Motzer RJ. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England journal of medicine. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 16.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med. 2007;13:433–42. doi: 10.1016/j.molmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res. 2007;13:3109–14. doi: 10.1158/1078-0432.CCR-06-2798. [DOI] [PubMed] [Google Scholar]
- 19.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E Survival Pathways by Rapamycin-Mediated Mammalian Target of Rapamycin Inhibition. Cancer Res. 2005;65:7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 20.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Yue P, Chan CB, Ye K, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Fu H, Khuri FR, Sun SY. Inhibition of mammalian target of rapamycin induces phosphatidylinositol 3 kinase-dependent and Mnk-mediated eIF4E phosphorylation. Mol Cell Biol. 2007 doi: 10.1128/MCB.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Yue P, Kim YA, Fu H, Khuri F, Sun SY. Enhnacing mTOR-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008 doi: 10.1158/0008-5472.CAN-08-1522. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun SY, Yue P, Wu GS, El-Deiry WS, Shroot B, Hong WK, Lotan R. Mechanisms of apoptosis induced by the synthetic retinoid CD437 in human non-small cell lung carcinoma cells. Oncogene. 1999;18:2357–65. doi: 10.1038/sj.onc.1202543. [DOI] [PubMed] [Google Scholar]
- 24.Sun SY, Yue P, Dawson MI, Shroot B, Michel S, Lamph WW, Heyman RA, Teng M, Chandraratna RA, Shudo K, Hong WK, Lotan R. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 1997;57:4931–9. [PubMed] [Google Scholar]
- 25.Sun SY, Yue P, Shroot B, Hong WK, Lotan R. Induction of apoptosis in human non-small cell lung carcinoma cells by the novel synthetic retinoid CD437. J Cell Physiol. 1997;173:279–84. doi: 10.1002/(SICI)1097-4652(199711)173:2<279::AID-JCP36>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, Greene MI. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest. 2007;117:2051–8. doi: 10.1172/JCI32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008 doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buck E, Eyzaguirre A, Brown E, Petti F, McCormack S, Haley JD, Iwata KK, Gibson NW, Griffin G. Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and breast tumors. Mol Cancer Ther. 2006;5:2676–84. doi: 10.1158/1535-7163.MCT-06-0166. [DOI] [PubMed] [Google Scholar]
- 29.Bianco R, Garofalo S, Rosa R, Damiano V, Gelardi T, Daniele G, Marciano R, Ciardiello F, Tortora G. Inhibition of mTOR pathway by everolimus cooperates with EGFR inhibitors in human tumours sensitive and resistant to anti-EGFR drugs. Br J Cancer. 2008;98:923–30. doi: 10.1038/sj.bjc.6604269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birle DC, Hedley DW. Signaling interactions of rapamycin combined with erlotinib in cervical carcinoma xenografts. Mol Cancer Ther. 2006;5:2494–502. doi: 10.1158/1535-7163.MCT-05-0504. [DOI] [PubMed] [Google Scholar]
- 31.Rao RD, Mladek AC, Lamont JD, Goble JM, Erlichman C, James CD, Sarkaria JN. Disruption of parallel and converging signaling pathways contributes to the synergistic antitumor effects of simultaneous mTOR and EGFR inhibition in GBM cells. Neoplasia. 2005;7:921–9. doi: 10.1593/neo.05361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das AK, Sato M, Story MD, Peyton M, Graves R, Redpath S, Girard L, Gazdar AF, Shay JW, Minna JD, Nirodi CS. Non-small-cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res. 2006;66:9601–8. doi: 10.1158/0008-5472.CAN-06-2627. [DOI] [PubMed] [Google Scholar]
- 33.Amann J, Kalyankrishna S, Massion PP, Ohm JE, Girard L, Shigematsu H, Peyton M, Juroske D, Huang Y, Stuart Salmon J, Kim YH, Pollack JR, Yanagisawa K, Gazdar A, Minna JD, Kurie JM, Carbone DP. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res. 2005;65:226–35. [PubMed] [Google Scholar]
- 34.Graff JR, Konicek BW, Carter JH, Marcusson EG. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 2008;68:631–4. doi: 10.1158/0008-5472.CAN-07-5635. [DOI] [PubMed] [Google Scholar]
- 35.Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Teruya-Feldstein J, Pelletier J, Lowe SW. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21:3232–7. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warr MR, Shore GC. Unique biology of Mcl-1: therapeutic opportunities in cancer. Curr Mol Med. 2008;8:138–47. doi: 10.2174/156652408783769580. [DOI] [PubMed] [Google Scholar]