Abstract
To determine whether increased mitochondrially localized catalase was radioprotective, a human catalase transgene was cloned into a small pSVZeo plasmid and localized to the mitochondria of 32D cl 3 cells by adding the mitochondrial localization sequence of MnSOD (mt-catalase). The cell lines 32D-Cat and 32D-mt-Cat had increased catalase biochemical activity as confirmed by Western blot analysis compared to the 32D cl 3 parent cells. The MnSOD-overexpressing 32D cl 3 cell line, 2C6, had decreased baseline catalase activity that was increased in 2C6-Cat and 2C6-mt-Cat subclonal cell lines. 32D-mt-Cat cells were more radioresistant than 32D-Cat cells, but both were radioresistant relative to 32D cl 3 cells. 2C6-mt-Cat cells but not 2C6-Cat cells were radioresistant compared to 2C6 cells. Intratracheal injection of the mt-catalase-plasmid liposome complex (mt-Cat-PL) but not the catalase-plasmid liposome complex (Cat-PL) increased the resistance of C57BL/6NHsd female mice to 20 Gy thoracic irradiation compared to MnSOD-plasmid liposomes. Thus mitochondrially targeted overexpression of the catalase transgene is radioprotective in vitro and in vivo.
INTRODUCTION
There is increasing evidence that ionizing radiation-induced apoptosis in normal cells and tissues is mediated in part through alteration of the redox balance, leading to oxidative stress-induced injury (1–9). Ionizing radiation induces rapid production of radical oxygen species (ROS) within cells, including the formation of superoxide, hydroxyl and nitric oxide radicals (1).
Three forms of superoxide dismutase convert superoxide to hydrogen peroxide. These include two copper/zinc metalloenzymes found in the cytoplasm and extracellularly, respectively, and a manganese metalloenzyme localized to the mitochondria by a 22-amino acid mitochondrial targeting sequence (8, 9). Hydrogen peroxide also contributes to oxidative stress and is inactivated by catalase and glutathione peroxidase (7). In addition to the antioxidant enzyme system (including superoxide dismutases, catalase and glutathione peroxidase), cellular antioxidant stores, including thiols and glutathione, contribute to the neutralization of ROS and maintain the cellular redox balance (6). Mitochondrially localized manganese superoxide dismutase converts radiation-induced superoxide to hydrogen peroxide, which is then converted to water and oxygen by catalase or glutathione peroxidase. Increased concentrations of mitochondrially targeted MnSOD have been demonstrated to be radioprotective for hematopoietic and epithelial cells in vitro and in vivo; however, toxicity of the hydrogen peroxide product may limit radioprotection.
Recent evidence indicates that irradiated cells and tissues continue to generate ROS for prolonged periods after irradiation (10–12). The distal steps in the cellular injury response after the production of ROS include production of inflammatory cytokines and pro-apoptosis signal transduction cascades that then induce both extracellular and intracellular mechanisms of apoptosis (13–18). Strategies that can push the redox balance within irradiated cells toward a state of enhanced antioxidant stores can facilitate cellular and tissue repair and thus ameliorate radiation damage in vitro and in vivo (10–12, 19).
One approach to increasing the antioxidant capacity of cells and tissues has been organ-specific gene therapy, including increased production of manganese superoxide dismutase (20–24). Previous studies have shown that mitochondrial localization of SOD is associated with radiation resistance of cells in vitro and protection of specific organs in vivo, including lung (21), esophagus (20), oral cavity (22, 23), and bladder (24). The incomplete radiation protection afforded by the elevation of MnSOD alone suggested that the hydrogen peroxide product of the action of SOD may have continued to contribute to radiation-induced cellular damage (11). Recently, a mitochondrially targeted transgene for catalase, which contains the mitochondrial targeting sequence obtained from the MnSOD transgene, has been developed (7). In the present studies, we tested the effect of overexpression of the mitochondrially targeted catalase compared to the native catalase transgene using plasmid liposome transfer to cells in vitro and by intratracheal administration to the lung in vivo. We sought to determine whether mitochondrial targeting of the transgene product catalase enhanced its radioprotective effect, as is the case for MnSOD. The results demonstrate a significant radiation protective capacity of mitochondrially targeted catalase in vitro and in vivo.
MATERIALS AND METHODS
Cells and Cell Culture
32D cl 3, an IL-3-dependent hematopoietic progenitor cell line, has been described previously (6). Cells were grown in WEHI-3 cell conditioned medium as a source of IL-3 at 37°C in a 93% air/7% CO2 incubator in McCoy’s 5A medium supplemented with 15% fetal bovine serum, Penicillin and streptomycin and were passaged weekly (6). Subclones of 32D cl 3 overexpressing transgenes for MnSOD (2C6) and mitochondrially targeted catalase (32D-mt-Cat) and catalase (32D-Cat) and subclones of 2C6 overexpressing each catalase transgene (2C6-m-Cat and 2C6-Cat) were cloned by co-transfection with a G418 (neo-resistance) plasmid with a 200-fold excess of each p-Cat or Cat transgene (6). Clones were selected by undergoing expansion in 50 μM G418 and demonstrated by RT-PCR to contain the mt-Cat or Cat transgene. Colonies were selected according to published methods (6).
Radiation Survival Curves
32D cl 3 cells and their transgene-expressing subclones were irradiated with 137Cs γ rays using a J. L. Shepherd Mark IV irradiator at a dose rate of 80 cGy/min and were transferred immediately to medium containing 0.8% methylcellulose at densities of 500, 1000 or 5000 cells per ml. Cells were grown in 10% WEHI-3 cell conditioned medium as a source of IL-3, and colonies with more than 50 cells were scored at day 7 according to published methods (2, 6). Triplicate experiments were performed (6). Data points were fitted to curves using the single-hit, multitarget and linear-quadratic models as described previously (6).
Measurement of Mitochondrial Membrane Polarity
As an indication of mitochondrial stability in transfected pools of cells and in clonal cell lines, mitochondrial membrane depolarization was determined by JC-1 fluorescence relative to complete membrane collapse using 10 μM carbonyl cyanide 3-cholorphenylhydraxone (CCCP) (Invitrogen, Carlsbad, CA). Cells (~1 × 106) were irradiated with 10 Gy and at various times after irradiation were incubated with 2 μM JC-1 at 37°C for 10 min, then washed with PBS and resuspended in 2 ml phosphate-buffered saline (PBS) for fluorescence measurements using a Shimadzu RF-5301PC. The ratio of 590 nm to 535 nm fluorescence emission (using 485 nm excitation) was performed on three or four samples in three separate experiments. The data were combined, and standard errors and P values (using Student’s t test) were obtained using KaleidaGraph (Synergy Software, Reading, PA).
Assays for Catalase, Superoxide Dismutase, Glutathione Peroxidase and Glutathione
Western blot analysis for catalase protein production was carried out according to published procedures (18) and was standardized by densitometry to the housekeeping gene product Actin according to published methods (18). Biochemical catalase activity was quantified by using a Catalase Assay Kit (EMD Chemicals, La Jolla, CA).
The assays for glutathione peroxide (GPX) and glutathione (GSH) were carried out using a Glutathione Assay Kit and a Glutathione Peroxidase Kit (EMD Chemicals) according to published methods (23). The method is based on catalase reacting with methanol in the presence of H2O2, resulting in the formation of formaldehyde, which reacts with purpald, resulting in a purple color that is measured spectrophotometrically. The results are expressed as units of catalase activity, where 1 unit is defined as the amount of catalase that will cause the formation of 1.0 nmol formaldehyde per minute. Catalase activity was measured in whole cells or in isolated subcellular fractions including the cytoplasm or mitochondria.
To separate cytoplasm from mitochondria, cells were pelleted and frozen in dry ice. The cells were then rapidly thawed and frozen three times followed by centrifugation for 10 min at 500g to remove nonlysed cells, nuclei and cell debris. The supernatant was then centrifuged for 30 min at 10,000g. The resulting supernatant was confirmed to contain cytoplasm. Mitochondria were localized to the pellet and were confirmed by electron microscopy using published methods (6). GPX activity was reported as mU where 1 mU = 1 nmol NADPH/min per mg protein = (A340/min)/0.00622 as described in the GPX Cellular Assay Kit.
Mouse Lung Irradiation
Female C57BL/6NHsd mice (20–25 g) that were 8–10 weeks old were housed five per cage. Mice were immobilized by Nembutal and irradiated according to published methods (21) with the head and abdomen shielded to achieve a dose of 20 Gy to both lungs.
Intratracheal injection of plasmid liposome complexes, prepared according to published methods, was carried out as described previously (25).
Mice received intratracheal injection of 100 μl containing 100 μg of mt-Cat-PL, Cat-PL or MnSOD-PL 24 h prior to irradiation. Control mice received empty plasmid liposomes with no vector insert. All animals were monitored for pulmonary toxicity including lethargy, weight loss and dehydration. Moribund or dying mice were euthanized by carbon dioxide inhalation according to institutional IACUC policies. The lungs were expanded with OCT (Optimum Cutting Temperature, ThermoFisher, Waltham, MA), frozen in OCT, sectioned and stained with hematoxylin and eosin. The percentage of the lung displaying organizing alveolitis (fibrosis) was calculated as described previously (21).
Animal Care
All animal protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Veterinary care was provided by the Division of Laboratory Animal Research of the University of Pittsburgh.
Statistics
Data from radiation survival curves were analyzed using the linear-quadratic and single-hit, multitarget models (6). A long rank test was used to analyze the data from the in vivo lung irradiation experiments (21). A Student’s t test was used to determine the significance of differences between groups in the assays for catalase activity, GSH and GPX determination, and JC-1 uptake (23).
RESULTS
Mt-Catalase Transgene Overexpression Confers Radiation Resistance to 32D cl 3 Cells In Vitro
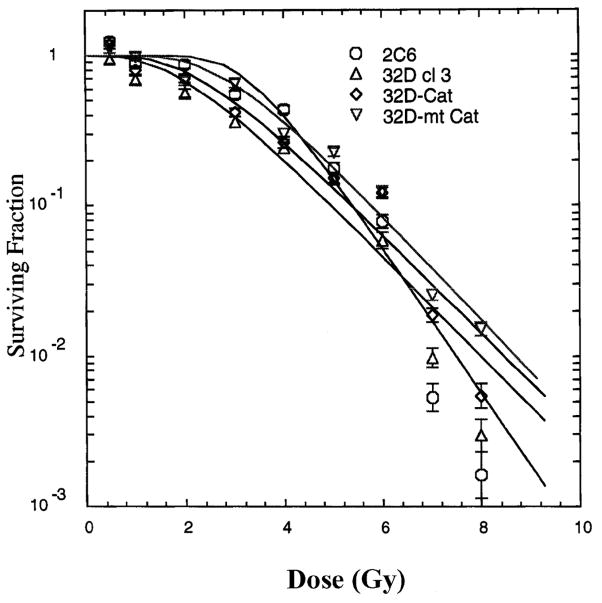
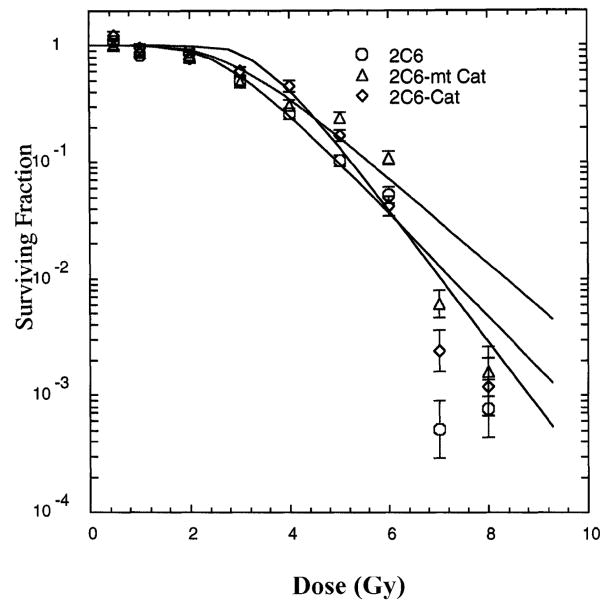
The radiation survival curves for subclonal lines of 32D cl 3 cells expressing mt-Cat or Cat were compared to those of cells of the MnSOD-overexpressing subclonal line 2C6 and the parent cell line 32D cl 3 (Fig. 1). 32D-mt-Cat cells demonstrated radiation resistance. Cells overexpressing comparable quantities of catalase after transfection with the non-mitochondrially targeted catalase transgene, 32D-Cat, demonstrated less radioresistance. Cells expressing both MnSOD (2C6) and mt-catalase (2C6-mt-Cat) were more radioresistant (Fig. 2, Table 1). The radioresistance induced by mt-Cat in 32D-mt-Cat cells was comparable to that in the MnSOD-overexpressing subclonal 2C6 cells (Fig. 2, Table 1).
FIG. 1.
Survival curves for 32D cl 3 cells and sublines transfected with MnSOD (2C6), catalase (32D-Cat) or mitochondrial catalase (32D-mt-Cat). Values are means ± SD from three experiments. Data were analyzed using the linear-quadratic model and the single-hit, multitarget model (shown on figure). Fitting parameters are shown in Table 1.
FIG. 2.
Survival curves for coexpression of mt-catalase and MnSOD. 2C6 cells overexpressing MnSOD were stably transfected with either the catalase or mitochondrial catalase transgene. Values are means ± SD from three experiments. Survival curve data were analyzed by the linear-quadratic model and the single-hit, multitarget model (shown on the figure). Fitting parameters are shown in Table 1.
TABLE 1.
Overexpression of Mitochondrially Targeted Catalase Transgene Increases the Radioresistance of Cell Lines In Vitro
| Cell line | D0 | ñ | α | β | Plating efficiency |
|---|---|---|---|---|---|
| 32D cl 3 | 1.26 + 0.13 | 2.9 + 1.06 | 0.118 + 0.043 | 0.059 + 0.008 | 55 + 9% |
| 32D-mt-Cat | 1.05 + 0.16 | 10.3 + 0.5 (P = 0.0134)* | 0.017 + 0.017 | 0.067 + 0.001 | 46 + 8.0% |
| 32D-Cat | 1.25 + 0.03 | 5.9 + 0.2 (P = 0.048)* | 0.036 + 0.035 | 0.078 + 0.004 | 49 + 5% |
| 2C6 | 0.97 + 0.1 | 12.6 + 6.3 | 0.000 + 0.000 | 0.094 + 0.001 | 42 + 3% |
| 2C6-mt-Cat | 1.18 + 0.01 (P = 0.001)# | 12.2 + 0.6 | 0.000 + 0.000 | 0.075 + 0.002 | 37 + 6% |
| 2C6-Cat | 0.76 + 0.12 | 10.7 + 1.5 | 0.000 + 0.000 | 0.087 + 0.001 | 33 + 4% |
Notes. Results are the means + SEM of at least three experiments with each cell line. Linear-quadratic and single-hit, multitarget models were used to analyze the survival curve data. Significant differences were determined using a Student’s t test.
Significant difference compared to 32D cl 3 cells.
Significant difference compared to 2C6 cells.
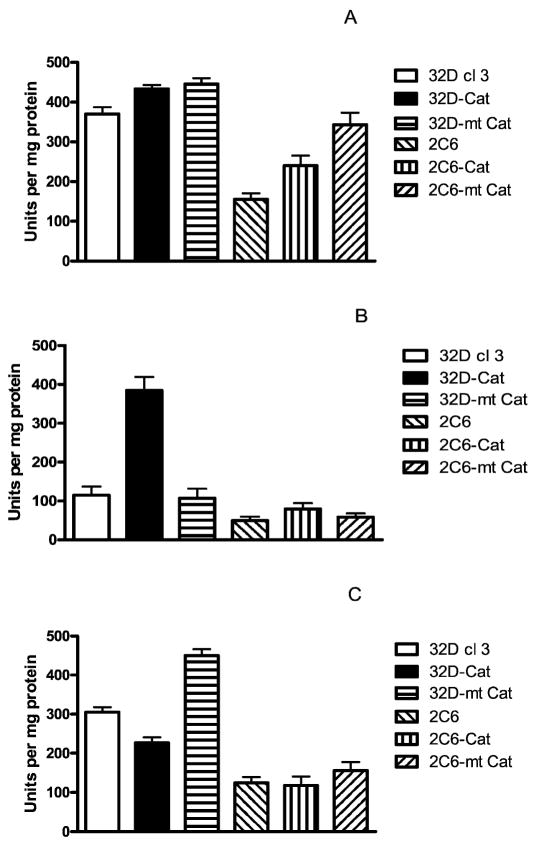
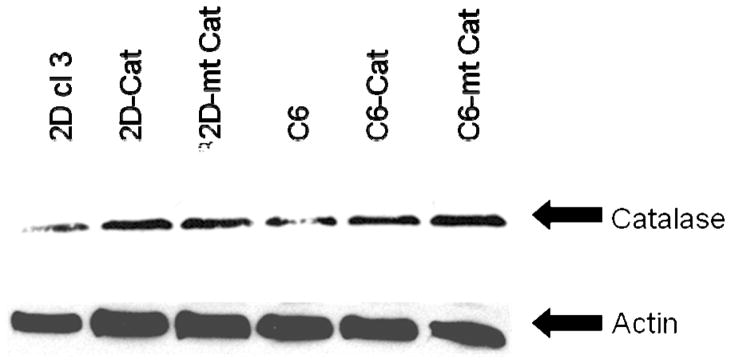
Catalase levels in the clonal cell lines were assayed by biochemical assay (Fig. 3) and by Western blot analysis (Fig. 4). There was a decrease in catalase in 2C6 cells compared to 32D cl 3 cells (P < 0.001). Western blot analysis demonstrated increased catalase activity relative to control 32D cl 3 cells in both the mt-Cat and Cat transgene-transfected cells.
FIG. 3.
Catalase activity in the whole cell (panel A), cytoplasm (panel B) and mitochondria (panel C) of 32D cl 3, 32D-Cat, 32D-mt Cat, 2C6, 2C6-Cat and 2C6-m-Cat cells. Values are means ± SEM of four experiments. Catalase activity is expressed as units of catalase activity per mg protein. In the whole cells (panel A), catalase activity was significantly increased in 32D Cat and 32D-mt-Cat cells compared to 32D cl 3 cells (P = 0.034 and 0.013, respectively) and decreased in 2C6 cells compared to 32D cl 3 cells (P < 0.001). Catalase activity was increased in 2C6-Cat and 2C6-mt-Cat cells compared to 2C6 cells (P = 0.001 and < 0.001). In the cytoplasm (panel B), catalase activity increased in 32D-Cat and 2C6-Cat cells compared to either 32D cl 3 or 2C6 cells (P < 0.001 or = 0.001, respectively) and decreased in 2C6 cells compared to 32D cl 3 (P < 0.001). In the mitochondria (panel C), catalase activity increased in 32D-mt-Cat and 2C6-mt-Cat cells compared to 32D cl 3 and 2C6 cells (P < 0.001). Mitochondria of 32D cl 3 cells had more catalase activity than mitochondria of 2C6 cells (P < 0.001). Values are means ± SEM. Significance of differences was determined with Student’s t test.
FIG. 4.
Western blot analysis of catalase and actin levels. Representative blot from three experiments.
2C6 cells, which overexpress MnSOD, demonstrated a baseline decrease in catalase activity that was persistent and stable in five separate experiments over 6 months. The catalase activity was increased after transfection of 2C6 cells with the mt-Cat or Cat transgene (Fig. 3A).
Measurement of Mitochondrially Localized Catalase Transgene Product
Separation of the cytoplasm from the mitochondria demonstrated that cell lines transfected with plasmid containing the control catalase transgene had catalase activity localized to the cytoplasm (Fig. 3B). In contrast, cells transfected with the mt-catalase transgene plasmid had increased catalase activity localized to the mitochondria (Fig. 3C). Western blot analysis confirmed the presence of higher levels of transgene protein in whole cell pellets from mt-Cat- or Cat-transfected cells (Fig. 4).
Mitochondrial Stability is Increased in Cells Lines that Overexpress the Catalase Transgene Product
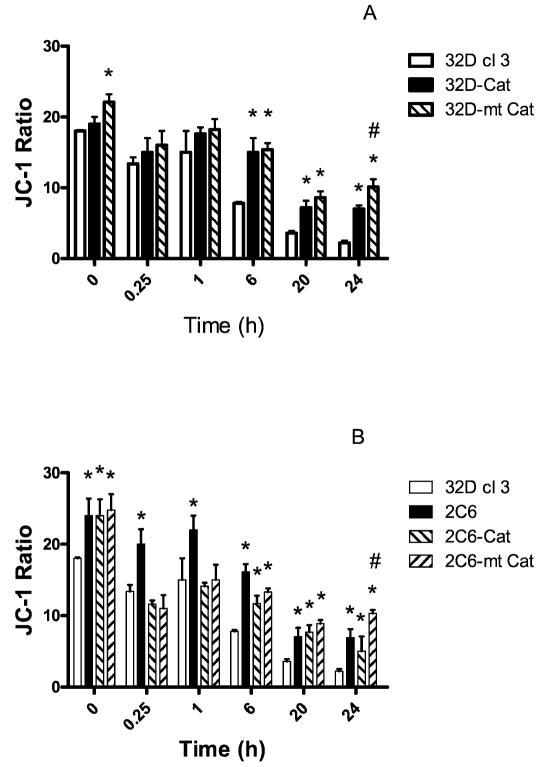
The baseline JC-1 ratios (nonirradiated time-zero controls) indicated that all the 32D cl 3 subclones including those overexpressing MnSOD (2C6) had mitochondria with greater membrane polarity (ΔΨ) than their 32D cl 3 counterparts (P < 0.05) (Fig. 5). Soon after irradiation there was a decrease in ΔΨ that was significant (P < 0.05) in all cell lines. MnSOD overexpression in 2C6 protected against the initial change in ΔΨ. The addition of the transgene for catalase (mitochondrial or cytosolic) to 2C6 cells did not further reduce the rapid radiation-induced decrease in ΔΨ at 0.25 and 1 h (Fig. 5B). At longer times after irradiation (20 to 24 h), the cells that contained mitochondrial catalase (32D-mt-Cat or 2C6-mt-Cat) were better protected against the ΔΨ decrease compared to other cell lines (P < 0.05). These data indicate that overexpression of MnSOD is important for mitochondrial stability early after irradiation while mitochondrial catalase is important at later times. Overexpression of both MnSOD and mitochondrially targeted catalase together did not improve radiation protection more than overexpression of either one alone.
FIG. 5.
Mitochondrial function in 32D cl 3, 32D-Cat and 32D-mt-Cat cells (panel A) and in 32D c1 3, 2C6, 2C6-Cat and 2C6-mt-Cat cells (panel B) as measured by uptake of JC-1 a measure of mitochondrial membrane stability) after irradiation with 10 Gy. Values are means ± SEM from three experiments. * indicates a statistically significant increase in the JC-1 ratio, which indicates better mitochondrial membrane stability compared to 32D cl 3 cells (P < 0.05). # indicates a significantly increased JC-1 ratio (P < 0.05) compared to 32D-Cat and 2C6-Cat cells, respectively, at 24 h after irradiation. Student’s t test was used to determine statistical significance.
Glutathione Peroxidase and Glutathione Levels are Decreased in Antioxidant Transgene-Overexpressing Subclonal Lines
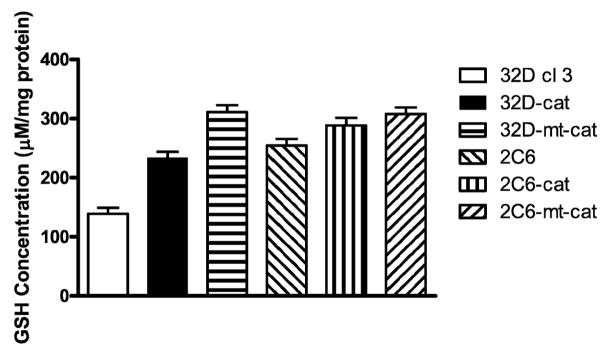
Total antioxidant stores are reflected by the glutathione level within cell packs, and glutathione levels have been demonstrated to decrease after the oxidative stress associated with irradiation of cells in culture (2). Levels of glutathione (GSH) (Fig. 6) and glutathione peroxidase (GPX) (Fig. 7) were stably increased in 2C6, 2C6-mt-Cat, 2C6-Cat, 32D-mt-Cat and 32D-Cat cells compared to the parent 32D cl 3 cells. These results confirm previous results showing increased antioxidant capacity in MnSOD-overexpressing 2C6 cells (2). Cells overexpressing MnSOD (2C6) had a decrease in GPX levels, while 32D cl 3 cells expressing the mitochondrial or non-localized catalase (32D-m-Cat or 32D-Cat, respectively) demonstrated an increase in GPX activity (Fig. 7).
FIG. 6.
Glutathione levels (GSH) in the experimental cell lines. Values are means ± SEM from four experiments. There was an increase in GSH levels in all transfected cell lines compared to 32D cl 3 cells (Student’s t test, P < 0.001 for all cell lines).
FIG. 7.
Glutathione peroxidase (GPX) levels in the experimental cell lines. Values are means ± SEM. There was increased GPX activity in 32D-Cat and 32D-mt-Cat cells compared to 32D cl 3 cells (Student’s t test, P = 0.0002 or P < 0.0001, respectively). In all cell lines overexpressing MnSOD, there was decreased GPX activity compared to 32D cl 3 cells (Student’s t test, P < 0.001).
Protection of Mouse Lung from Radiation-Induced Damage by Overexpression of Mitochondrially Targeted Catalase
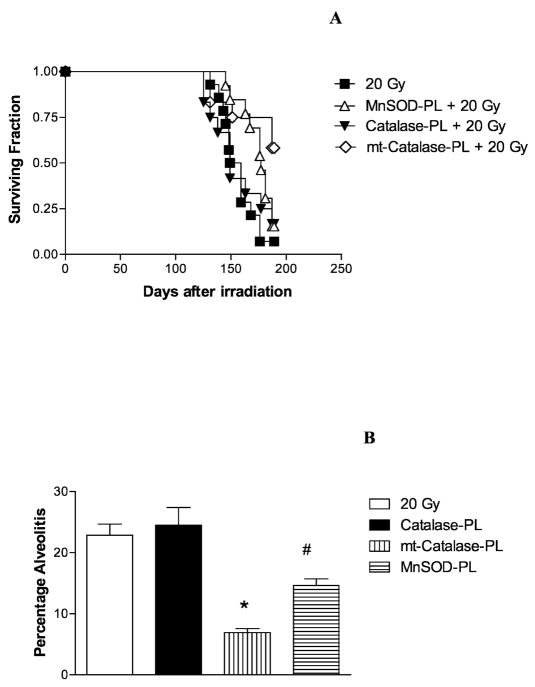
We next determined whether the increased in vitro radioresistance induced in 32D cl 3 cells by transfection with the mt-Cat-plasmid liposome construct was translated to an in vivo radioprotective effect in the mouse lung using an established model of radiation-induced organizing alveolitis (21). Groups of 20 mice received 100 μl containing 100 μg of mt-Cat-plasmid liposomes, Cat-plasmid liposomes or empty vector-liposomes 24 h prior to whole-lung irradiation with 20 Gy. The C57BL/6NHsd mouse model has been shown to be an accurate system for detecting radiation-induced organizing alveolitis/fibrosis at 120–150 days (21). Mice were followed for evidence of pulmonary damage. As shown in Fig. 8, irradiated control mice demonstrated significant pulmonary toxicity beginning at around day 100, and all mice were dead or required euthanasia by day 150. Mice receiving non-mitochondrially targeted catalase (Cat-PL) showed no significant improvement in survival. Mice receiving MnSOD-PL as a positive control showed a significant increase in survival, confirming prior results (21). Mice receiving mt-Cat-PL demonstrated a significant increase in survival, with over 70% of mice alive at day 150.
FIG. 8.
Radioprotective capacity of mt-Cat transgene product in mouse lungs in vivo. Groups of 20 C57BL/6NHsd mice were injected intratracheally with 100 μl liposomes containing 100 μg of plasmid DNA as either MnSOD-PL complex, Cat-PL complex or mt-Cat-PL complex. Twenty-four hours later, treated and control mice were irradiated with 20 Gy to the lungs. The mice were followed for the development of symptoms of severe organizing alveolitis/fibrosis, at which time they were euthanized (panel A). Mice injected with mt-Cat-PL or MnSOD-PL had increased survival compared to irradiated control mice (P = 0.004 or P = 0.017, respectively) as determined by a log rank test. Panel B: Percentage of the lung displaying organizing alveolitis/fibrosis at the time of euthanasia. Mice injected with mt-Cat-PL or MnSOD-PL had significantly less organizing alveolitis/fibrosis than control mice. (Student’s t test, * P < 0.001 or # P = 0.004, respectively).
At the time of necropsy, the percentage of the lung showing organizing alveolitis/fibrosis was measured (Fig. 8). In mice receiving mt-Cat-PL or MnSOD-PL, less organizing alveolitis was seen than in irradiated mice. There were no statistically significant differences between the control irradiated mice and the Cat-PL-treated mice. These data show that expression of the transgene product for mitochondrially targeted catalase was radioprotective when it was administered prior to total-lung irradiation in C57BL/6NHsd mice.
DISCUSSION
The present studies demonstrate that mitochondrial targeting of the antioxidant enzyme catalase had a significant radioprotective effect in vitro and in vivo. This effect was dependent on mitochondrial localization of comparable levels of catalase transferred by a vector that was mitochondrially targeted. In contrast, elevation of catalase levels in the cytoplasm by a non-targeted vector was not radioprotective in vitro or in vivo. Mitochondrially targeted catalase transgene product was confirmed in mitochondria isolated from cells in vitro. These cell lines also demonstrated increased mitochondrial stabilization by a fluorescence assay for JC-1 conversion of dimers to momers. These data add further support to the notion that mitochondrial targeting of an antioxidant enzyme activity in a gene therapy approach to radioprotection is a rational strategy given that oxidative stress at the mitochondrial membrane is involved in radiation-induced apoptosis (6).
Overexpression of catalase has been shown to decrease oxidative stress from a variety of injuries, including inflammatory cytokine damage, hyperbaric oxygen and aging in long-term marrow culture (26). The alteration in redox balance that occurs from depletion of antioxidant stores after irradiation or these other forms of oxidative stress may explain in part the success of mt-catalase as a radioprotector (6, 26).
The present studies revealed that 32D cl 3 mouse hematopoietic progenitor cells overexpressing MnSOD (2C6) (6) have an intrinsic down-regulation of catalase. This result was unexpected since overexpression of MnSOD was predicted to result in further dismutation of superoxide to hydrogen peroxide, providing more hydrogen peroxide in cells and potentially requiring greater catalase levels (27). The lower levels of catalase in 2C6 cells were not associated with a detectable up-regulation of glutathione peroxidase, an enzyme associated with hydrogen peroxide degradation. Other enzyme systems for hydrogen peroxide detoxification may be at work in both 2C6 cells and 32D cl 3 cells, where overexpression of either mt-Cat or Cat by plasmid liposome transfer did result in an increase in catalase activity.
Mitochondrially targeted catalase delivered by plasmid liposomes protected mice against lung damage from ionizing radiation. Whether addition of mt-Cat to MnSOD could provide additive or synergistic radioprotection in vivo (as we detected in vitro) is not known.
The present studies demonstrated that 2C6 cells overexpressing both MnSOD and mt-Cat but not Cat showed further radiation resistance. These data suggest that, when appropriately targeted to the mitochondria, mt-Cat may be an additive radioprotector along with MnSOD. The data do not immediately translate to in vivo radioprotection, because the same cells would have to receive both transgene products. The transfection efficiency of MnSOD-PL has been quantified by an epitope-tagged hemagglutinin-MnSOD construct and is around 60% for pulmonary cells after intratracheal injection (25). If mt-Cat-PL transfection was of comparable efficiency, then delivery of both plasmids in a single inoculum might result in some but not a majority of pulmonary cells overexpressing both enzymes. Construction of a vector containing both transgenes is in progress to effectively demonstrate a superior protective role of both transgene products in the mitochondria of the same cells in vivo.
The present data add another transgene product to the armamentarium of potential agents for use as radioprotectors for normal tissues during cancer therapy and potentially also in radiation counterterrorism.
Acknowledgments
This work was supported by NIH research grant U19-A1068021.
References
- 1.Spitz DR, Azzam EL, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 2.Epperly MW, Osipov AN, Martin I, Kawai K, Borisenko GG, Jefferson M, Bernarding M, Greenberger JS, Kagan VE. Ascorbate as a “redox-sensor” and protector against irradiation-induced oxidative stress in 32D cl 3 hematopoietic cells and subclones overexpressing human manganese superoxide dismutase. Int J Radiat Oncol Biol Phys. 2004;58:851–861. doi: 10.1016/j.ijrobp.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell JB, Krishna MC. Nitroxides as radiation protectors. Mil Med. 2002;167:49–50. [PubMed] [Google Scholar]
- 4.Hahn SM, Krishna MC, DeLuca AM, Coffin D, Mitchell JB. Evaluation of the hydroxylamine tempol-H as an in vivo radioprotector. Free Radic Biol Med. 2000;28:953–958. doi: 10.1016/s0891-5849(00)00176-3. [DOI] [PubMed] [Google Scholar]
- 5.Weiss JF, Landauer MR. Radioprotection by antioxidants. Ann NY Acad Sci. 2000;899:44–60. [PubMed] [Google Scholar]
- 6.Epperly MW, Gretton JE, Bernarding M, Nie S, Rasul B, Greenberger JS. Mitochondrial localization of copper/zinc superoxide dismutase (Cu/ZnSOD) confers radioprotective functions in vitro and in vivo. Radiat Res. 2003;160:568–578. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 7.Connor KM, Regan KJ, Subbaram S, Nelson KK, Bartholomew PJ, Flores SC, Tai YT, Aplin AE, Mazurkiewicz JE, Melendez JA. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem. 2005;280:16916–16924. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
- 8.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 9.Kang SK, Rabbani ZN, Folz RJ, Golson ML, Huang H, Yu D, Samulski TS, Dewhirst MW, Anscher MS, Vujaskovic Z. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2003;57:1056–1066. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- 10.Greenberger JS, Greenberger MW, Gretton J, Jefferson M, Nie S, Bernarding M, Kagan V, Guo HL. Radioprotective gene therapy. Curr Gene Ther. 2003;3:183–195. doi: 10.2174/1566523034578384. [DOI] [PubMed] [Google Scholar]
- 11.Greenberger JS, Epperly MW. Radioprotective antioxidant gene therapy: Potential mechanisms of action. Gene Ther Mol Biol. 2004;8:31–44. [Google Scholar]
- 12.Greenberger JS, Epperly MW. Antioxidant therapeutic approaches toward amelioration of ionizing irradiation-induced pulmonary injury. Curr Respir Med Rev. 2007;7:29–37. [Google Scholar]
- 13.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB. Imatinib mesylate inhibits the profibrogenic activity of TGF-B and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1311. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhowmick NA, Ghiassi M, Aakre M, Brown K, Singh V, Moses HL. TGF-beta-induced RhoAand p160ROCK activation is involved in the inhibition of Cdc25A with resultant cell-cycle arrest. Proc Natl Acad Sci USA. 2003;100:15548–15553. doi: 10.1073/pnas.2536483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez AM, Takagawa S, Sekosan M, Jaffe HA, Varga J, Roman J. Smad3 deficiency ameliorates experimental obliterative bronchiolitis in a heterotopic tracheal transplantation model. Am J Pathol. 2004;165:1223–1232. doi: 10.1016/S0002-9440(10)63382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flanders KC, Sullivan CD, Fuji M, Sowers A, Anzaro MA, Arabshahi A, Major C, Deng C, Russo A, Roberts AB. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing irradiation. Am J Pathol. 2002;160:1057–1068. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flanders KC, Major CD, Arabshahi A, Aburime EE, Okada MH, Fujii M, Blalock TD, Schultz GS, Sowers A, Roberts AB. Interference with transforming growth factor-beta/Smad 3 signaling results in accelerated healing of wounds in previously irradiated skin. Am J Pathol. 2003;163:2247–2257. doi: 10.1016/s0002-9440(10)63582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epperly MW, Gretton JA, Defilippi SJ, Sikora CA, Liggitt D, Koe G, Greenberger JS. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD-PL) gene therapy. Radiat Res. 2001;155:2–14. doi: 10.1667/0033-7587(2001)155[0002:morice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stickle RL, Epperly MW, Klein E, Bray JA, Greenberger JS. Prevention of irradiation-induced esophagitis by plasmid/liposome delivery of the human manganese superoxide dismutase (MnSOD) transgene. Radiat Oncol Invest Clin Basic Res. 1999;7:204–217. doi: 10.1002/(SICI)1520-6823(1999)7:4<204::AID-ROI2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter M, Epperly MW, Agarwal A, Nie S, Hricisak L, Niu Y, Greenberger JS. Inhalation delivery of manganese superoxide dismutase-plasmid/liposomes (MnSOD-PL) protects the murine lung from irradiation damage. Gene Ther. 2005;12:685–690. doi: 10.1038/sj.gt.3302468. [DOI] [PubMed] [Google Scholar]
- 22.Guo HL, Seixas-Silva JA, Epperly MW, Gretton JE, Shin DM, Greenberger JS. Prevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (MnSOD) transgene. Radiat Res. 2003;159:361–370. doi: 10.1667/0033-7587(2003)159[0361:porioc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Epperly MW, Wegner R, Kanai AJ, Kagan V, Greenberger EE, Nie S, Greenberger JS. Irradiated murine oral cavity orthotopic tumor antioxidant pool destabilization by MnSOD-plasmid liposome gene therapy mediates tumor radiosensitization. Radiat Res. 2007;267:289–297. doi: 10.1667/RR0761.1. [DOI] [PubMed] [Google Scholar]
- 24.Kanai AJ, Zeidel ML, Lavelle JP, Greenberger JS, Birder LA, deGroat SC, Apodaca GL, Meyers SA, Ramage R, Epperly MW. Manganese superoxide dismutase gene therapy protects against irradiation-induced cystitis. Am J Physiol Renal Physiol. 2002;44:1152–1160. doi: 10.1152/ajprenal.00228.2002. [DOI] [PubMed] [Google Scholar]
- 25.Epperly MW, Guo HL, Jefferson M, Wong S, Gretton J, Bernarding M, Bar-Sagi D, Greenberger JS. Cell phenotype specific duration of expression of epitope-tagged HA-MnSOD in cells of the murine lung following intratracheal plasmid liposome gene therapy. Gene Ther. 2003;10:163–171. doi: 10.1038/sj.gt.3301852. [DOI] [PubMed] [Google Scholar]
- 26.Gupta R, Karpatkin S, Basch RS. Hematopoiesis and stem cell renewal in long-term bone marrow cultures containing catalase. Blood. 2006;107:1837–1846. doi: 10.1182/blood-2005-03-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8:722–730. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 28.Bai J, Rodriguez AM, Melendez JA, Cederbai AI. Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injury. J Biol Chem. 1999;274:26217–26224. doi: 10.1074/jbc.274.37.26217. [DOI] [PubMed] [Google Scholar]
- 29.Epperly MW, Osipov AN, Martin I, Kawai K, Borisenko GG, Jefferson M, Bernarding M, Greenberger JS, Kagan VE. Ascorbate as a “redox-sensor” and protector against irradiation-induced oxidative stress in 32D cl 3 hematopoietic cells and subclones overexpressing human manganese superoxide dismutase. Int J Radiat Oncol Biol Phys. 2004;58:851–861. doi: 10.1016/j.ijrobp.2003.10.022. [DOI] [PubMed] [Google Scholar]