Abstract
A large-scale study of narrative comprehension using fMRI was performed involving children ages 5–18 years old using a recently published method, Multivariate Autoregressive Modeling (MAR), modified for multi-subject analyses in order to investigate effective connectivity and its development with age. Feedback networks were found during a narrative processing task and involved effective connectivity from Broca’s area and the medial aspect of the superior frontal gyrus to the posterior aspects of the superior temporal gyrus bilaterally. The effective connectivity from Broca’s area to the superior temporal gyrus in the left hemisphere was shown to increase with age. The results demonstrate the feasibility of performing multi-subject MAR analyses to investigate effective connectivity in the absence of an a priori model.
Keywords: Speech Perception, Children, Brain Mapping, Magnetic Resonance Imaging
Introduction
The classic neural model of language involves two key areas within the left hemisphere. Wernicke’s area, in the posterior temporal lobe, is thought to serve speech processing and language comprehension. Expressive language skills have historically been assigned to frontal regions, although a role in the processing of morphosyntactic features has also been acknowledged for decades. Communication between these two anatomical regions has been attributed to the arcuate fasciculus, elegantly illustrated in a recent imaging study using diffusion tensor tractography [1]. The role of this fiber bundle has been considered a feed-forward mechanism, allowing information processed in Wernicke’s area to be made available to Broca’s area to serve such functions as preparing an oral reply to spoken information or in simply repeating words that have been heard [2].
More recent work has raised questions concerning this traditional view of the left-hemisphere language network. Additional work in functional neuroimaging has begun to assign additional roles beyond language formulation to cortex within Broca’s area and neighboring frontal areas. Left inferior frontal cortex activations are often seen in studies of verbal working memory [3], and verbal working memory is thought to be a necessary prerequisite to processing syntactically complex utterances [4]. If indeed it is the case that Broca’s area is supporting syntactic processing by holding incoming information in memory until the syntactic structure can be fully processed, then this implies that a functional mechanism must exist to pass information held in memory by Broca’s area back to Wernicke’s area for additional processing to fully derive the meaning. Furthermore, additional frontal areas appear to be necessary when meaning accrues over the course of sentences, as in the case of story processing [5–8].
Here we test the hypothesis that information is exchanged in a bi-directional manner between the superior temporal gyrus (including Wernicke’s area) and Broca’s areas using data from a large-scale pediatric functional neuroimaging study [7,8] involving over 300 children. One of the tasks used in this study required children to listen to stories constructed from sentences reflecting various syntactic forms, thought to place demands on verbal working memory (e.g., interrogatives, imbedded clauses). The story context in which these sentences occur requires children to build meaning across the entire time frame of the story, a skill thought to involve frontal regions neighboring Broca’s area [5]. Therefore, these stimuli require two types of verbal working memory that should both engage frontal cortex and require feedback of information to superior temporal areas in order to integrate information held in working memory with new information being processed by temporal lobe structures.
In order to investigate bi-directional connectivity between Broca’s area and temporal regions, a re-analysis of the narrative processing dataset was performed. The method chosen was Multivariate Autoregressive Modeling (MAR) [9], a data-driven and hypothesis-unconstrained method of analyzing effective connectivity. Effective connectivity, defined as the influence a neuronal population has on another [9], is distinguished from functional connectivity, which defined as the correlation between one neuronal population and another. An analysis of effective connectivity is therefore necessary to make directional inferences. While other methods such as linear Structural Equation Modeling (LSEM) are available [7,10], they suffer from the drawbacks of requiring an a priori hypothesis; moreover incorporation of bi-directional connectivity in the model may result in an unstable model, the result of loops. The MAR methodology, previously published only for a single-subject analysis, was modified in the present study for within- and between-subjects analyses as described in the Methods section.
Methods
302 children (145 boys, 157 girls) were successfully scanned for this study. Independent Component Analysis (ICA) [8] of this data set was published previously and was also used to define elements of an LSEM [7]. (ICA is also a data-driven model but is unable to provide measures of functional or effective connectivity, while LSEM suffers from the drawbacks mentioned above of the necessity of an accurate a priori model and possible difficulties with loops in models incorporating bi-directional connectivity.) Here the data is re-analyzed in order to investigate effective connectivity between frontal and temporal modules in the left and right hemisphere using a data-driven and hypothesis-unconstrained approach. A small number of datasets (11) were discarded from the original study due to subject compliance or image quality issues. The MAR approach necessitates each subject having the same number of frames (100) available, and that data be available from each region of interest. The most relevant details about the study are summarized below; we refer the reader to the originally-published study [8] for further details.
The study was approved by the local Institutional Review Board and informed consent was obtained from each child’s parent or guardian (assent was also obtained for children 8 years and older). All subjects were pre-screened for any conditions (such as the presence of orthodontic braces) which would prevent an MRI scan from being acquired. Children with previous neurological illness; learning disability; head trauma with loss of consciousness; current or past use of psychostimulant medication; pregnancy; birth at 37 weeks gestational age or earlier; or abnormal findings at a routine neurological examination performed by an experienced pediatric neurologist were excluded from the study. All subjects were native monolingual English speakers. 278 of the subjects were right-handed, 21 were left-handed, and 3 were ambidextrous according to the Edinburgh test for handedness. The racial/ethnic background of the subjects was: 269 Caucasian, 22 African-American, 2 Asian, 2 Hispanic, 1 Native American, 6 Multi-Ethnic. All subjects received the Wechsler Intelligence Scale for Children, Third Edition (WISC-III) or the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III). Mean age = 11.7 ± 3.74 yrs. (range = 4.9 – 18.9 yrs.); Mean Wechsler Full-Scale IQ = 111.5 ± 14.1 (range = 70 – 147). Three subjects had a Full-Scale IQ < 80; they were not excluded from the study population as there was no documented history of learning disability, and the frequency of such findings was not greater than expected given the total sample size.
MRI scans were obtained using a Bruker 3T Medspec (Bruker Medizintechnik, Karlsruhe, Germany) imaging system. An MRI-compatible audiovisual system was used for presentation of the stimuli as well as a movie during the preparation (e.g. shimming) and acquisition of the whole-brain anatomical scans. Details of the techniques used to obtain fMRI data from younger children, as well as the success rates, are given in [11]. For the task of narrative comprehension the success rate is approximately 50% in children 5 years of age, and improves to a success rate of over 90% in children 9 years of age and above. EPI-fMRI scan parameters were: TR/TE = 3000/38 ms; BW = 125 kHz; FOV = 25.6 × 25.6 cm; matrix = 64 × 64; slice thickness = 5 mm. Twenty-four slices were acquired, covering the entire cerebrum. 110 scans were acquired (the first 10 were discarded to allow the spins to reach relaxation equilibrium) for a total scan time of 5 min. 30 sec. Techniques detailed elsewhere [11] were used to acclimate the subjects to the MRI procedure and make them comfortable inside the scanner. An elastic strap was attached to either side of the head coil apparatus by means of Velcro strips and stretched over the subjects’ foreheads in order to minimize head motion. In addition to the fMRI scans, whole-brain T1-weighted MP-RAGE scans were acquired for anatomical coregistration.
The fMRI scan paradigm consisted of a 30 second on-off block design. One story, read by an adult female speaker, was presented during each 30 s task period. The stories contained 9, 10, or 11 sentences of contrasting syntactic constructions (e.g. conjoined sentences vs. center embeddings). The inclusion of complex syntactic structures was designed to increase the relative processing load for this aspect of language. During each 30 s control period, pure tones of 1 s duration were presented at unequal intervals of 1 to 3 s. The frequency of each tone was randomly selected from a choice of 150, 200, 250, 500, 700, 900, or 1000 Hz. The control condition was designed to control for sublexical auditory processing. The subject was instructed to listen to the stories so that he or she could answer questions about them after the scans. Performance data were obtained at the end of the scanning session by asking the subject to answer two multiple choice questions about each story.
Data was processed using in-house software written in IDL (Research Systems Inc., Boulder, CO). Nyquist ghosts and geometric distortion due to B0 field inhomogeneity were corrected for during reconstruction using a multi-echo reference scan [12]. Data was corrected for subject motion using a pyramid iterative algorithm [13]; all datasets met the criterion of median voxel displacement (median computed across all time frames) at the center of the brain < 2 mm. This criterion has been validated for acceptable motion via independent observation (W. Yuan et al., unpublished data). The fMRI data was subsequently transformed into Talairach space using a linear affine transformation, previously validated for the age range in our study [14,15].
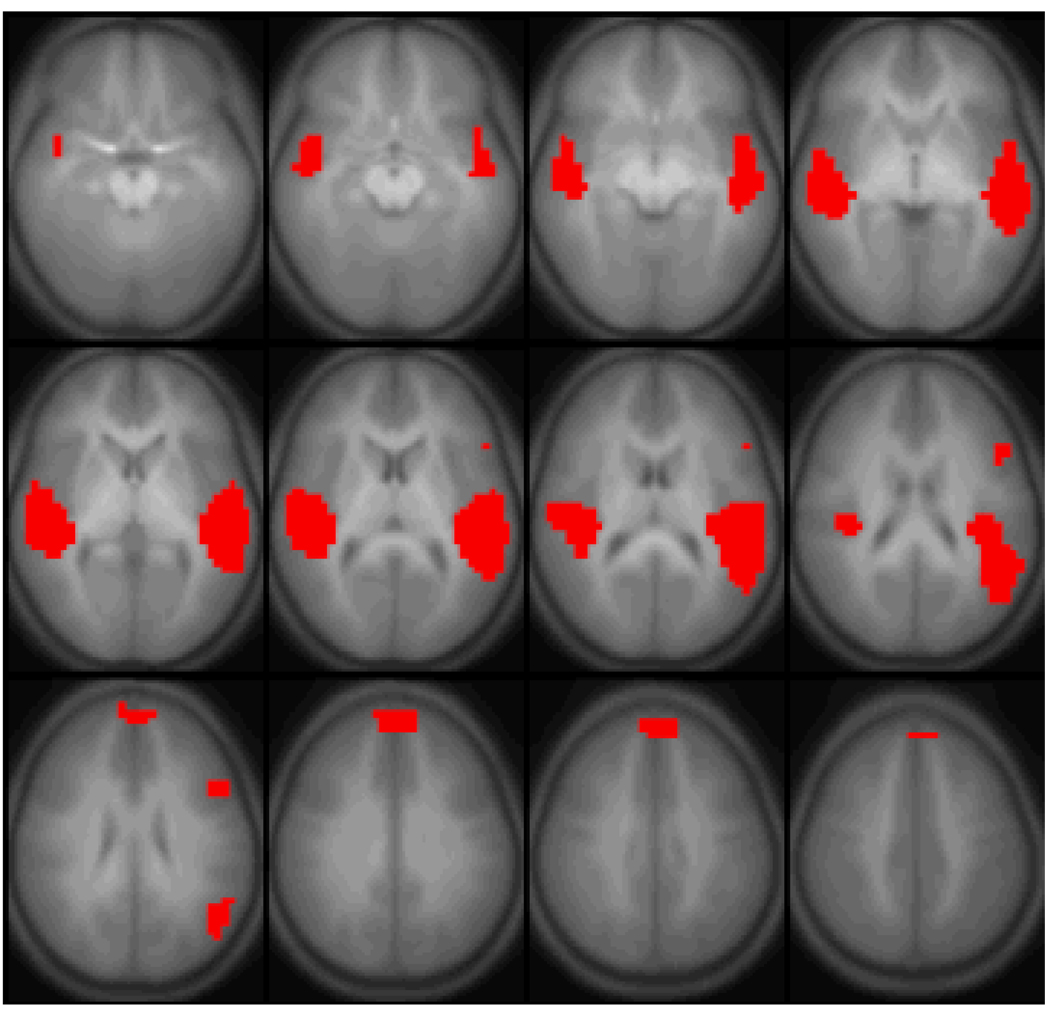
The General Linear Model (GLM) [16,17] was used to find regions with significant within-group functional activation. The threshold used was p < 1e-5 (Bonferroni-corrected). ROIs were then drawn around each of the four functional regions (Figure 1) found to exhibit functional activation (left superior temporal gyrus, right superior temporal gyrus, the medial aspect of the superior frontal gyrus, and left inferior frontal gyrus). Time courses were extracted from each ROI for each subject (normalized to percent change from the mean).
Figure 1.
Regions active (General Linear Model random-effects analysis, p < 1e-5, corrected) for the task of narrative processing performed by 302 normal children. Images are in radiologic orientation. Slice range: Z = −15 mm to Z = +40 mm.
Multivariate Autoregressive Modeling (MAR) [9] was then used to investigate effective connectivity between the regions. The MAR methodology, originally designed for single-subject analysis, was modified for group analysis. In the MAR approach, each time course is modeled as a linear sum of preceding time points from all regions. Since in our case the task consisted of a block design, the on-off task reference function was also incorporated as a regressor.
To determine the model order of the MAR, the analysis was performed multiple times with different model orders and the restricted log-likelihood functions calculated using the residuals for each time course from each subject. The Bayesian Information Criterion (BIC) was used to estimate an optimum model order of one. Using the Akaike’s Information Criterion (AIC) also yielded an optimum model order of one. While a more complicated Bayesian scheme for model order estimation was originally proposed [9], the very large number of subjects in our dataset make the BIC a suitable technique, due to the very large number of datapoints.
To modify the procedure for multi-subject analyses, the T-scores from the MAR regressions for each subject were used in a second-level analysis, analogous to what is typically done in GLM analyses. The T-scores from the regression parameters were entered into second-level analyses, investigating both within-subjects significance and correlations with subject age. The MAR analysis was repeated, incorporating interaction terms, as detailed in [9], to test for whether the connectivity strengths differ between conditions (active or control).
Results
The task performance in this population (# questions answered correctly out of 10; mean ± std.) was 7.76 ± 2.02. Over 85% (259) of the subjects answered 6 or more out of the 10 questions correctly (corresponding to p < 0.02 for rejection of the null hypothesis of responding by random chance). Thus, the children exhibited a high level of task compliance. There was a significant correlation (R = 0.29, p < 1e-5) of task performance with age. With regard to subject motion, there was also a high level of compliance (mean ± std. = 0.78 mm ± 0.39 mm; range = 0.18 – 1.97 mm). The majority (224 or 74%) of the subjects exhibited motion of 1 mm or less. There was however a significant correlation (Spearman’s R = −0.24, p < 1e-4) of motion with subject age.
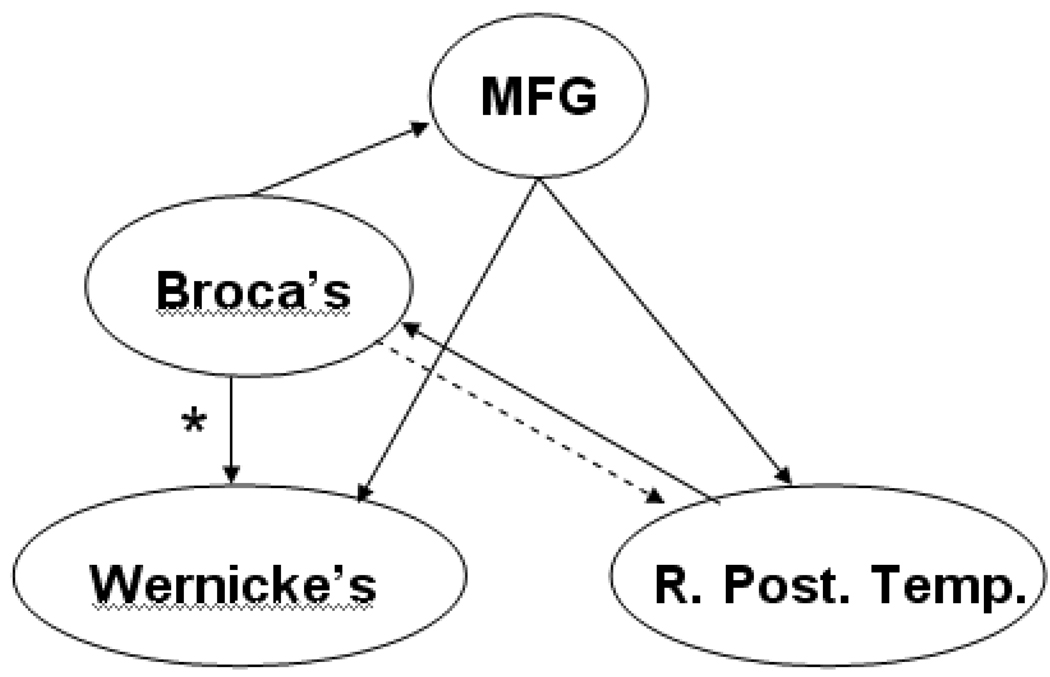
Areas with significant activation are shown in Figure 1. The regions found (Table 1) are: the superior temporal gyrus bilaterally, Broca’s area in the left hemisphere, and the medial aspect of the superior frontal gyrus. Via the across-subjects MAR analysis, the significant (p < 0.01, Bonferroni-corrected for the 12 connectivities tested) effective connectivities are: from Broca’s area to the superior temporal gyrus bilaterally, and the medial aspect of the superior frontal gyrus; and from the medial aspect of the superior frontal gyrus to the superior temporal gyrus bilaterally. The effect size (mean T-score/SEM) was also computed for each of the preceding connectivities and found to be greater than d = 0.4. A significant (p < 0.05, Bonferroni-corrected) effective connectivity was also seen from the right superior temporal gyrus to Broca’s area. A diagram of all significant effective connectivities is shown in Figure 2.
Table 1.
Activated regions and centroids (in Talairach coordinates) found from the task of narrative comprehension performed by 303 children, shown in Figure 1.
| Region | Talairach (X, Y, Z) | # Voxels |
|---|---|---|
| Broca’s Area | −47, 15, 21 | 13 |
| L. Superior Temporal Gyrus | −48, −29, 8 | 379 |
| R. Superior Temporal Gyrus | 46, −20, 5 | 245 |
| Superior Frontal Gyrus (Medial Aspect) | −2, 50, 32 | 42 |
Figure 2.
Feed-forward and feed-back networks found from multivariate autoregressive modeling (MAR) analysis. Solid lines: effective connectivities are significant with p < 0.01; dashed line: connectivity significant with p < 0.05. (R. STG = right posterior temporal cortex; L. STG = left superior temporal cortex; M. SFG = medial aspect of superior frontal gyrus). * = significantly correlated with subject age (R = 0.17; p < 0.05 Bonferroni-corrected).
Each of the effective connectivities was also examined for significant correlations with age and task performance (Table 2). The only connectivity with a significant correlation with age was that from Broca’s area to the left superior temporal gyrus (R = 0.17, p < 0.05, Bonferroni-corrected). This correlation remained significant even after task performance was partialled out (partial R = 0.16, p < 0.05) or after subject motion was partialled out (partial R = 0.15, p < 0.05) and thus it is verified to be an effect of age and not an artifact task performance or subject motion. None of the connectivities displayed a significant correlation with IQ or gender (determined via point-biserial correlation).
Table 2.
Correlations of the effective connectivity strengths (outlined in Figure 2) with: age (R Age), task performance (R Performance), and age covaried with performance (Partial R Age).
| Effective Connectivity | R Age | R Performance | Partial R Age |
|---|---|---|---|
| Broca’s → L. STG | 0.17* | 0.07 | 0.16* |
| Broca’s → R. STG | 0.06 | −0.01 | 0.07 |
| Broca’s → MSFG | 0.11 | 0.02 | 0.11 |
| MSFG → L. STG | 0.01 | −0.02 | 0.02 |
| MSFG → R. STG | 0.00 | −0.01 | 0.01 |
significant with Bonferroni-corrected p < 0.05.
(Abbreviations: STG = superior temporal gyrus; MSFG = medial aspect of the superior frontal gyrus).
When the interaction terms were incorporated into the model, there were no significant differences in connectivity strengths between active and control epochs. This indicates that our results might reflect a general organization of the brain (and maturation in terms of the connectivity from Broca’s area to superior temporal regions in the left hemisphere). Moreover, the Bayesian Information Criterion showed that the MAR model without inclusion of the interaction terms was more optimal. Hence there is not a significant difference between conditions and our results are reflective of correlated fluctuations between the brain regions.
Discussion
Our results provide functional MRI evidence supporting the presence of bi-directional connectivity between frontal and posterior temporal regions. The existence of such a pathway was suggested by the discovery that electrical stimulation of Broca’s area produced cortical evoked potentials in Wernicke’s area [18]. Here, an MAR approach substantiated the idea that an anterior-to-posterior connectivity between frontal and temporal language areas is employed during language processing. Results indicate that the activation in superior temporal regions is modulated by prior processing in Broca’s area, possibly related to the working memory load involved in either syntactic processing [19] or building meaning over the course of a story [6].
The effective connectivity from Broca’s area to the left superior temporal gyrus also displayed a significant age-related increase. This connectivity involves the arcuate fasciculus, where a previous diffusion tensor imaging (DTI) study displayed significant age-related increases in white matter anisotropy in the age range in this study [20]. The age-related increase in this effective connectivity may also be related to age-related increases in language lateralization, seen in a previous study [21], at least for semantic processing aspects of language. Our results corroborate a previous fMRI study [22], in which effective connectivity from the left inferior frontal gyrus for orthographic and phonographic tasks was found be to greater in adults as compared to children. This result was interpreted as evidencing the development of top-down cognitive control for language tasks in the left inferior frontal gyrus, modulating activity in temporal and parietal regions, in agreement with our results and hypothesis.
In addition to direct feedback from Broca’s area, our results suggest mediated feedback, using the medial aspect of the superior frontal gyrus. As shown in Figure 2, there is significant effective connectivity to the medial aspect of the superior frontal gyrus from Broca’s area, and there is also significant effective connectivity from the medial aspect of the superior frontal gyrus to the superior temporal gyrus bilaterally. The medial aspect of the superior frontal gyrus has been previously shown to be implicated in semantic processing [23] in normal adults. Thus, we would hypothesize that, in accordance with syntactic-semantic interaction [24], syntactic processing in Broca’s area is modulating activity in the superior frontal gyrus as well as in the superior temporal gyrus.
However, an unexpected finding was the lack of significant effective connectivity differences between the narrative comprehension task and the tones (control) task. It has been previously suggested that posterior superior temporal regions are more involved in spectral-temporal processing rather than language comprehension [25]; our results would seem to be in line with this hypothesis, with the left inferior frontal gyrus exercising top-down control over activity in superior temporal regions for auditory processing tasks in general. It is also possible that this top-down control and effective connectivity will persist even in the absence of any auditory stimulus; a future study involving completely silent control periods will be necessary to test this hypothesis.
A weaker effective feed-forward connectivity (dashed arrow in Figure 2) was seen from the right superior temporal gyrus to Broca’s area, while no significant effective connectivity was seen from the left superior temporal gyrus to Broca’s area in the left hemisphere. Previous results from our laboratory using a linear Structural Equation Modeling approach [7] support the ability of fMRI to detect such feed-forward networks, as would be expected from the classical Wernicke-Geschwind model. We expect that this discrepancy is simply an artifact of the time delay (3s) used in the MAR approach since the feed-forward connectivities occur on a shorter time scale.
A limitation with using the MAR approach on this dataset is the rather long TR, which allows analysis of information transfer in the brain only on a macroscopic time scale. The advantage of the MAR approach, however, lies in the ability to perform data-driven, hypothesis-unconstrained analyses of effective connectivity. The large effect size (d > 0.4) obtained for most of our effective connectivity strengths indicates that the multi-subject MAR technique may be quite feasible and yield sufficient sensitivity for studies involving smaller numbers of subjects. Data-driven analyses using an approach such as Structural Equation Modeling (SEM) [10] are subject to sample-specific confounds [26]. Moreover, unlike MAR, the SEM technique does not allow the simultaneous testing of feed-forward and feed-back connectivities between all regions of interest due to the presence of loops in the model.
Conclusion
A cohort of 302 children ages 5–18 performed the task of narrative processing by listening to 30 second short stories. Activation was detected in the superior temporal gyrus bilaterally, Broca’s area, and the medial aspect of the superior frontal gyrus. Analysis of the data via MAR resulted in feed-back networks being found, with a time delay of 3s, from Broca’s area to the superior temporal gyrus, both directly and mediated through the medial aspect of the superior frontal gyrus. The results corroborate previously proposed syntactic-semantic interactions as well as connectionist models of language processing in the brain.
Acknowledgments
Grant Support: National Institutes of Health R01 #HD38578
References
- 1.Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- 2.Geschwind N. Specializations of the human brain. Sci Am. 1979;241:180–201. doi: 10.1038/scientificamerican0979-180. [DOI] [PubMed] [Google Scholar]
- 3.Chein JM, Ravizza SM, Fiez JA. Using neuroimaging to evaluate models of working memory and their implications for language processing. Journal of Neurolinguistics. 2003;16:315–339. [Google Scholar]
- 4.Just MA, Carpenter PA, Keller TA. The capacity theory of comprehension: new frontiers of evidence and arguments. Psychol Rev. 1996;103:773–780. doi: 10.1037/0033-295x.103.4.773. [DOI] [PubMed] [Google Scholar]
- 5.Sirigu A, Cohen L, Zalla T, Pradat-Diehl P, Van Eeckhout P, Grafman J, et al. Distinct frontal regions for processing sentence syntax and story grammar. Cortex. 1998;34:771–778. doi: 10.1016/s0010-9452(08)70780-9. [DOI] [PubMed] [Google Scholar]
- 6.Virtue S, Haberman J, Clancy Z, Parrish T, Jung Beeman M. Neural activity of inferences during story comprehension. Brain Res. 2006;1084:104–114. doi: 10.1016/j.brainres.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 7.Karunanayaka PR, Holland SK, Schmithorst VJ, Solodkin A, Chen EE, Szaflarski JP, et al. Age-related connectivity changes in fMRI data from children listening to stories. Neuroimage. 2007;34:349–360. doi: 10.1016/j.neuroimage.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Schmithorst VJ, Holland SK, Plante E. Cognitive Modules Utilized for Narrative Comprehension in Children: A Functional Magnetic Resonance Imaging Study. Neuroimage. 2006;29:254–266. doi: 10.1016/j.neuroimage.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison L, Penny WD, Friston K. Multivariate autoregressive modeling of fMRI time series. Neuroimage. 2003;19:1477–1491. doi: 10.1016/s1053-8119(03)00160-5. [DOI] [PubMed] [Google Scholar]
- 10.Solodkin A, Hlustik P, Chen EE, Small SL. Fine modulation in network activation during motor execution and motor imagery. Cereb Cortex. 2004;14:1246–1255. doi: 10.1093/cercor/bhh086. [DOI] [PubMed] [Google Scholar]
- 11.Byars AW, Holland SK, Strawsburg RH, Bommer W, Dunn RS, Schmithorst VJ, et al. Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. J Child Neurol. 2002;17:885–890. doi: 10.1177/08830738020170122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20:535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thevenaz P, Unser M. A Pyramid Approach to Subpixel Registration Based on Intensity. IEEE Transactions on Image Processing. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- 14.Muzik O, Chugani DC, Juhasz C, Shen C, Chugani HT. Statistical parametric mapping: assessment of application in children. Neuroimage. 2000;12:538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- 15.Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Human Brain Mapping. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited–again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 17.Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, et al. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127:2316–2330. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- 19.Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD. Revisiting the role of Broca's area in sentence processing: Syntactic integration versus syntactic working memory. Hum Brain Mapp. 2005;24:79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of White Matter Diffusivity and Anisotropy Changes with Age During Childhood: A Cross-Sectional Diffusion Tensor Imaging Study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI Brain Activation Patterns in Children Performing a Verb Generation Task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- 22.Bitan T, Burman DD, Lu D, Cone NE, Gitelman DR, Mesulam MM, et al. Weaker top-down modulation from the left inferior frontal gyrus in children. Neuroimage. 2006;33:991–998. doi: 10.1016/j.neuroimage.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris GJ, Chabris CF, Clark J, Urban T, Aharon I, Steele S, et al. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006;61:54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Sakai KL, Homae F, Hashimoto R. Sentence processing is uniquely human. Neurosci Res. 2003;46:273–279. doi: 10.1016/s0168-0102(03)00122-6. [DOI] [PubMed] [Google Scholar]
- 25.Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, et al. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- 26.MacCallum RC, Roznowski M, Necowitz LB. Model modifications in covariance structure analysis: the problem of capitalization on chance. Psychol Bull. 1992;111:490–504. doi: 10.1037/0033-2909.111.3.490. [DOI] [PubMed] [Google Scholar]